Abstract
A 75-year-old man with fever was diagnosed with alveolar hemorrhage. Antineutrophil cytoplasmic antibodies for myeloperoxidase and proteinase 3 were absent. He received corticosteroid therapy, which immediately improved his symptoms and chest radiological findings. After the discontinuation of corticosteroids, fever and general fatigue relapsed, and renal function deteriorated with hematuria and proteinuria. A nerve conduction study revealed mononeuritis multiplex. Renal biopsy demonstrated focal necrotizing crescentic glomerulonephritis with endocapillary proliferative lesions, immunofluorescence C3 deposits, and electron-microscopic subepithelial hump-like deposits. Nephritis-associated plasmin receptor (NAPlr) and plasmin activity, biomarkers of infection-related glomerulonephritis, were positive in glomeruli. Although pathological findings suggested infection-related glomerulonephritis (IRGN), clinical manifestations, such as alveolar hemorrhage and mononeuritis multiplex, suggested systemic small vessel vasculitis. After corticosteroid therapy, systemic symptoms disappeared, and the gradual amelioration of hematuria and proteinuria was observed. Based on the clinical symptoms for which steroid therapy was effective, the patient was considered to have systemic small vessel vasculitis, the etiology of which may have been associated with infection.
Keywords: Small vasculitis, Nephritis-associated plasmin receptor (NAPlr), Plasmin activity, Infection-related glomerulonephritis (IRGN)
Introduction
Previous cases of postinfectious glomerulonephritis mainly occurred in childhood following streptococcal infections and termed post-streptococcal glomerulonephritis (PSGN). The clinical course, causative pathogens, infection site, and pathological findings of PSGN were recently reported to vary, and PSGN is now referred to as infection-related glomerulonephritis (IRGN). The proportion of elderly patients is increasing, and the causative infection of IRGN is frequently reported to be non-streptococcal in adults [1].
Nephritis-associated plasmin receptor (NAPlr) was isolated from group A Streptococcus and is a potential cause of PSGN. Although the glomerular deposition of NAPlr is frequently observed in patients with early-phase PSGN [2], it is also detected in other glomerular diseases. Thus, a subgroup of glomerulonephritis (GN) induced by streptococcal infection and the glomerular deposition of NAPlr was proposed [3]. The deposition of NAPlr and plasmin activity were observed in IRGN induced not only by Streptococcus, but also by other species of bacteria [4, 5], thus they are regarded as general biomarkers of bacterial IRGN [6].
We report a case of suspected antineutrophil cytoplasmic antibody (ANCA)-negative systemic small vessel vasculitis with alveolar hemorrhage, mononeuritis multiplex, and rapidly progressive glomerulonephritis (RPGN). Renal biopsy revealed crescentic necrotizing GN with histological features of IRGN, such as segmental endocapillary proliferative lesions on light microscopy, C3-dominant glomerular deposition on immunofluorescence microscopy, hump-like subepithelial electron-dense deposits on electron microscopy, and glomerular-positive staining for NAPlr and plasmin activity. Plasmin activity was also positive in a small vessel. Therefore, IRGN and small vessel vasculitis may have been combined in the present case. However, infection may also have been involved in the development of small vessel vasculitis.
Case report
A 75-year-old man was admitted to an outside hospital with a 2-month history of fever, general fatigue, anorexia, and weight loss. His past history were prostatic hyperplasia and varices. He used daily flavoxate hydrochloride and magnesium oxide. Laboratory examination revealed that the white blood cell (WBC) count and C-reactive protein (CRP) level increased to 11,800 /µL and 11.8 mg/dL, respectively. On chest computed tomography (CT), a granular shadow was observed in the bilateral upper and middle lung fields. He was diagnosed with bronchial pneumonia and treated using piperacillin-tazobactam. Although blood examination demonstrated slight improvement of inflammation, the bilateral lung shadow persisted. One week later, he was referred to our hospital for further examinations and treatment. His WBC count and CRP level deteriorated to 9300 /µL and 4.68 mg/dL, respectively. His serum creatinine level was 0.96 mg/dL and eGFR was 58.8 mL/min/1.73m2. Proteinuria and hematuria were absent. Myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA were negative. Diffuse ground-glass opacities were noted in the bilateral upper and middle lung fields on CT (Fig. 1a). Bronchoscopy was performed and bronchoalveolar lavage (BAL) samples suggested alveolar hemorrhage. He received intravenous methylprednisolone for 3 days at a dose of 250 mg daily, followed by oral prednisolone at a dose of 1 mg/kg daily. This treatment immediately improved his symptoms, CRP level, and chest radiological findings. No microorganisms grew on a BAL fluid culture. Prednisolone was tapered and discontinued after 2 weeks.
Fig. 1.
Radiological findings from chest computed tomography. Diffuse ground-glass opacities were observed in the bilateral upper and middle lung fields on the first hospitalization (a). Opacities disappeared by the second hospitalization (b)
Three days later, symptoms, such as fever and general fatigue, relapsed. Renal function also deteriorated with hematuria and proteinuria 1 month after the discontinuation of prednisolone, thus he was readmitted to our hospital. On physical examination, his temperature was 37.4 ℃, and livedo reticularis and paresthesia were detected in the legs. His serum creatinine level increased from 0.96 to 1.49 mg/dL within 2 months (Table 1). Urinalysis revealed glomerular hematuria and proteinuria of 1.22 g/gCr. MPO-ANCA, PR3-ANCA, and the anti-glomerular basement membrane antibody were absent. Serum C3 and C4 levels were normal. Anti-streptolysin O (ASO) and anti-streptokinase antibody (ASK) were within normal ranges. There were no abnormal findings in the lungs on chest CT (Fig. 1b). Nerve conduction study demonstrated mononeuritis multiplex. Although ANCA was negative, we suspected small vessel vasculitis based on the systemic clinical findings, RPGN, alveolar hemorrhage, and mononeuritis multiplex.
Table 1.
Laboratory data at the time of renal biopsy
| Urinalysis | Blood test | ||||||
|---|---|---|---|---|---|---|---|
| (Qualitative) | (Hematology) | (Biochemistry) | (Serology) | ||||
| Specific gravity | 1.015 | WBC | 10,500/μL | TP | 5.7 g/dL | CRP | 6.37 mg/dL |
| pH | 7.0 | Neut | 75.8% | Alb | 1.8 g/dL | IgG | 2067 mg/dL |
| Protein | 1 + | Lymp | 18.4% | AST | 32 IU/L | IgA | 205 mg/dL |
| Blood | 2 + | Eos | 2.0% | ALT | 26 IU/L | IgM | 64 mg/dL |
| (Biochemistry) | RBC | 325 × 104/μL | LDH | 201 IU/L | C3 | 105 mg/dL | |
| Protein | 1.22 g/gCr | Hb | 8.6 g/dL | ALP | 260 IU/L | C4 | 26 mg/dL |
| (Sediment) | Hct | 27.0% | BUN | 27.1 mg/dL | CH50 | 52.7 IU/mL | |
| RBC | 20–29/HPF | Plt | 52.6 × 104/μL | Cr | 1.49 mg/dL | MPO-ANCA | < 1.0 IU/mL |
| WBC | < 1/HPF | eGFR | 36.2 mL/min/1.73m2 | PR3-ANCA | < 1.0 IU/mL | ||
| Granular casts | 1–4/WF | ESR | 63 mm/hr | UA | 2.9 mg/dL | anti-GBM antibody | < 2.0 IU/mL |
| Na | 129 mEq/L | anti-nuclear antibody | < 40 × | ||||
| K | 4.4 mEq/L | ASO | 28 IU/mL | ||||
| Cl | 96 mEq/L | ASK | 160 × | ||||
| Glu | 96 mg/dL | RF | 3.0 IU/mL | ||||
| HbA1c | 6.2% | HBs-Ag | − | ||||
| HCV-Ab | − | ||||||
ESR erythrocyte sedimentation rate, MPO-ANCA myeloperoxidase-anti-neutrophil cytoplasmic antibody, PR3 proteinase 3, GBM glomerular basement membrane, RF rheumatoid factor
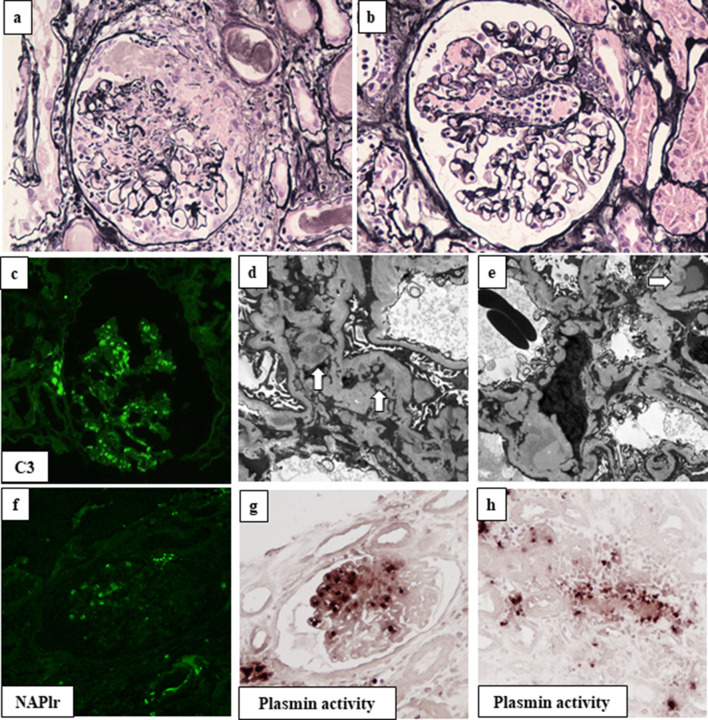
Renal biopsy was performed to evaluate the pathology of RPGN. The tissue specimen contained 24 glomeruli, with 9 exhibiting global sclerosis. Four glomeruli had cellular crescents (Fig. 2a). Two glomeruli exhibited segmental endocapillary proliferation with neutrophils and tuft necrosis (Fig. 2a, b). There was no evidence of fibrinoid necrosis in the arteries. On immunofluorescence analysis, granular staining of C3 (2 +) in the mesangial area and along the glomerular capillaries was observed, with negative staining for IgG, IgA, IgM, and C1q (Fig. 2c). Electron microscopy revealed mesangial, basement membrane, and subepithelial electron dense deposits, some of which exhibited a subepithelial hump-shaped appearance (Fig. 2d, e). The combined presence of subepithelial hump-like deposits and positive C3 staining suggested the involvement of infection in the pathogenesis. Therefore, we performed further immunostaining for NAPlr and in situ zymography for plasmin activity. NAPlr and plasmin activity were similarly positive in the glomerulus (Fig. 2f, g). Positive staining for plasmin activity was also observed in a small vessel (Fig. 2h). The final histopathological diagnosis was crescentic necrotizing GN with subepithelial C3 depositions; the etiology was considered to be associated with an infectious process, i.e., IRGN.
Fig. 2.
Pathological findings of renal biopsy. In renal biopsy samples, 24 glomeruli were obtained, 4 of which showed cellular crescents (a: silver stain, × 400). Glomeruli exhibited segmental endocapillary proliferation with neutrophils and tuft necrosis (b: silver stain, × 400). Immunofluorescence (IF) microscopy showed the granular staining of C3 in the mesangial area and along the glomerular capillaries (c). Electron microscopy showed mesangial (arrow in d) and subepithelial electron dense deposits, some of which had a hump-shaped appearance (arrow in e). IF staining for nephritis-associated plasmin receptor (NAPlr) and in situ zymography for plasmin activity were similarly positive in the segmental area in the glomerulus (f, g). Plasmin activity was also found in small vessel walls (h)
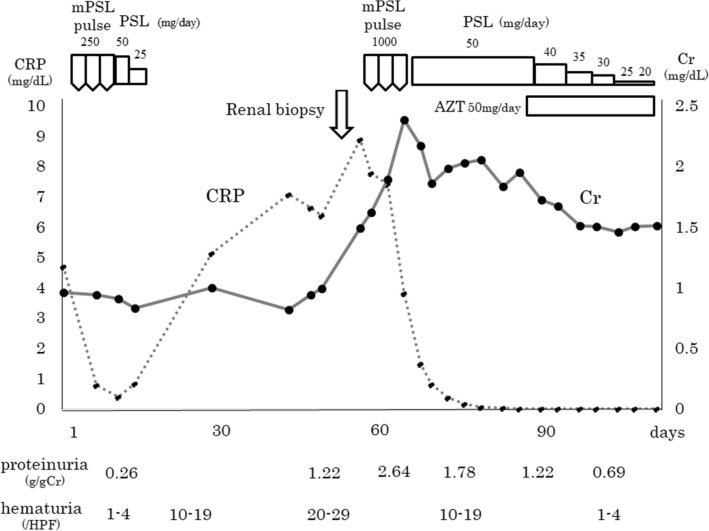
After renal biopsy, his serum creatinine level increased to 2.3 mg/dL. Although pathological findings suggested IRGN, according to clinical manifestations, the patient was considered to have systemic small vessel vasculitis. A 3-day course of intravenous methylprednisolone pulse therapy followed by oral prednisolone at a dose of 1 mg/kg daily was initiated (Fig. 3). After induction therapy, systemic symptoms, such as fever, general fatigue, and livedo reticularis disappeared. Paresthesia in the legs did not improve. The gradual amelioration of hematuria and proteinuria was observed, and his serum creatinine level decreased to 1.5 mg/dL. Azathioprine was added for maintenance immunosuppression and prednisolone was then tapered. Although the dose of prednisolone was gradually tapered to 5 mg daily, his renal function has been maintained without the recurrence of hematuria or proteinuria for 2 years.
Fig. 3.
Clinical course. mPSL methylprednisolone, PSL prednisolone, AZT azathioprine
Discussion
We presented a case of crescentic necrotizing GN with the glomerular deposition of C3, NAPlr, and plasmin activity. Although MPO and PR3-ANCA were negative and pathological findings suggested IRGN, the patient exhibited the typical features of small vessel vasculitis such as microscopic angiitis (MPA), including persistent fever and inflammation, alveolar hemorrhage, mononeuritis multiplex, and RPGN. The present case was atypical of MPA due to the negativity of ANCA and notable deposition of C3. However, up to one-third of reported patients with pauci-immune GN had negative ANCA serology [7].
Renal biopsy generally demonstrates pauci-immune necrotizing and crescentic GN in MPA [8]. Of note, ‘pauci-immune’ should not be interpreted as indicating a completely negative immunofluorescence. ‘Pauci-immune’ should be defined as 2 + or less staining for any immunoglobulin and absence of immune complex-type electron dense deposits on electron microscopy [9, 10]. In the present case, immune complex-type was not found on renal biopsy, only C3 deposits. Sethi et al. evaluated 13 patients diagnosed with pauci-immune necrotizing and crescentic GN: 4 were ANCA-negative, 5 had PR3 antibodies, and 4 had MPO antibodies. Immunofluorescence staining of glomeruli was essentially negative in the PR3-ANCA and MPO-ANCA groups, whereas mild C3 staining was observed in ANCA-negative patients with mesangial and few subepithelial deposits [10]. Moreover, a major differential diagnosis of IRGN is ANCA-induced pauci-immune necrotizing and crescentic GN. Nasr et al. reported that 8% of elderly patients with IRGN were seropositive for ANCA (perinuclear, MPO, PR3, and unknown pattern) [11]. Therefore, ANCA testing is recommended in patients with IRGN whose biopsy specimen exhibits diffuse crescents and/or any lesion of fibrinoid necrosis [12]. We pathologically diagnosed this case of RPGN as IRGN because NAPlr and plasmin activity were also positive. Although extra renal symptoms were consistent with MPA, renal pathological findings did not match the diagnosis of MPA. After steroid treatment, the hematuria, proteinuria, serum creatinine level, and systemic symptoms improved. Therefore, IRGN and ANCA-negative small vessel vasculitis may have been combined.
In the present case, renal biopsy demonstrated the isolated deposition of C3 and subepithelial hump-like deposits accompanied by focal segmental neutrophil-dominant endocapillary proliferation lesions. These findings suggested the involvement of infection in the pathogenesis of GN in this patient. Therefore, additional staining for NAPlr and plasmin activity was performed. NAPlr was isolated from group A Streptococcus and confirmed to bind plasmin. The glomerular deposition of NAPlr is frequently observed in patients with early-phase PSGN, and was identified as a potential cause of PSGN [2]. NAPlr accumulates on glomeruli and then maintains the activity of plasmin, which may induce glomerular damage by degrading the glomerular basement membrane. Plasmin activity may also mediate neutrophil and macrophage infiltration [3]. NAPlr is the same substance as streptococcal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [2]. GAPDH is universally expressed among bacterial species and the amino acid sequences of GAPDH from some types of bacteria have high similarity to the sequence of NAPlr (streptococcal GAPDH); therefore, positive staining for NAPlr is also observed in cases of IRGN induced by bacteria other than Streptococcus such as Streptococcus pneumoniae [4] and Mycoplasma pneumoniae [5]. Furthermore, these bacterial GAPDH molecules possess the ability to bind plasmin and will therefore be positive on staining for plasmin activity [6]. The glomerular deposition of NAPlr was also detected in patients with glomerular diseases other than PSGN, including those with Henoch-Schönlein nephritis [13], dense deposit disease [14], and granulomatosis with polyangiitis [15]. In the present case, NAPlr and plasmin activity were positive in the same part of the glomeruli. Moreover, plasmin activity was positive in a small vessel. IRGN and small vessel vasculitis may have coexisted by chance, but these findings suggested that infection was involved in the development of vasculitis in the present case. Although staining for NAPlr was negative in the vessels of renal biopsy tissue, this may have been due to the inevitable use of paraffin sections, which have poor sensitivity, instead of frozen sections. However, ASO and ASK titers were not high, and there were no findings of streptococcal infection. In contrast to children in whom the latent period between infection and onset of renal disease is typically 1–6 weeks, in a significant percentage of adults and elderly patients in particular, the infection is only discovered at the time of IRGN diagnosis [12]. The sites of infection in the present case were not clarified. Slight improvements in inflammatory markers following the administration of antibiotics early in the disease course suggested that respiratory infection by some species of bacteria other than Streptococcus induced the development of GN.
Yano et al. reported a case of PSGN accompanied by ANCA-negative small vessel vasculitis [16]. Renal pathology revealed endocapillary proliferative glomerulonephritis with cellular crescents, diffuse peritubular capillaritis, and small arteriolitis. Although there was a lack of clear findings of preceding infection and C3 deposits in the 1st biopsy, NAPlr and plasmin activity were similarly positive in the glomerulus. They discussed that streptococcus infection might have concurrently provoked vasculitis. Moreover, there are some case reports about which streptococcal infection might involve the development of other than renal manifestation of arteritis. Traverso et al. reported a case of mononeuropathy multiplex due to necrotizing arthritis associated with beta-haemolitic streptococcal infection [17]. Yoshida et al. reported a case of concomitant PSGN and diffuse alveolar hemorrhage for which methylprednisolone therapy was effective [18]. Association between streptococcal infection and arthritis was unclear in these reports. Okabe et al. reported a case of infection-related eosinophilic glomerulonephritis and respiratory symptoms [19]. Interestingly, NAPlr and plasmin activity were positive not only in the glomerulus as well as in the interstitium, but also in walls of the pulmonary artery in their case. Even in our case, plasmin activity was positive in a renal small vessel. These findings suggest that infection itself might involve the development of systemic small vasculitis.in our case.
Infection has also been reported to be associated with the pathogenesis of ANCA associated vasculitis (AAV). Although the mechanisms by which infection induces AAV remain unclear, several explanations were proposed. The propensity to develop ANCA depends on a variety of predisposing factors including microbial infection. These factors are usually associated with the production of proinflammatory cytokines with the capacity to prime neutrophils [20]. Moreover, it was reported that neutrophil extracellular traps (NETs) which are released by ANCA-stimulated neutrophils and triggers vasculitis, may be further enhanced by bacterial infections with Staphylococcus aureus [21]. Kain et al. [22] reported that molecular mimicry is the fundamental mechanism in the development of pauci-immune focal necrotizing glomerulonephritis (FNGN) with ANCA. Bacterial adhesin FimH-triggered autoimmunity to human lysosomal membrane protein-2 (LAMP-2), whose autoantibodies are a new ANCA subtype, may be responsible for the initiation and development of injury in pauci-immune FNGN. Although MPO-ANCA and PR3-ANCA were negative in the present case, vasculitis may have developed due to autoantibody production other than these ANCA or molecular mimicry by infection. We only performed the ANCA detection test using enzyme-linked immunosorbent assay (ELISA) kits. Although we should have performed minor ANCA detection test using the indirect immunofluorescence, we could not perform due to lack of serum.
In conclusion, we reported a case of crescentic necrotizing GN with the glomerular deposition of C3. Positive staining for NAPlr and plasmin activity was observed in glomeruli. Although pathological findings suggested IRGN, the patient exhibited typical features of small vessel vasculitis clinically. The possibility of coincidental coexistence of IRGN and small vessel vasculitis remains to be clarified. However, based on crescentic necrotizing GN and positive staining for plasmin activity in a small vessel, we suspected infection to be involved in the development of vasculitis in the present case.
Declarations
Conflict of interest
All the authors have declared no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained for the publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yamakami K, Yoshizawa N, Wakabayashi K, Takeuchi A, Tadakuma T, Boyle MD. The potential role for nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Methods. 2000;21:185–197. doi: 10.1006/meth.2000.0990. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizawa N, Yamakami K, Fujino M, Oda T, Tamura K, Matsumoto K, et al. Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: characterization of the antigen and associated immune response. J Am Soc Nephrol. 2004;15:1785–1793. doi: 10.1097/01.ASN.0000130624.94920.6B. [DOI] [PubMed] [Google Scholar]
- 3.Oda T, Yoshizawa N, Yamakami K, Sakurai Y, Takechi H, Yamamoto K, et al. The role of nephritis-associated plasmin receptor (NAPlr) in glomerulonephritis associated with streptococcal infection. J Biomed Biotechnol. 2012 doi: 10.1155/2012/417675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odaka J, Kanai T, Ito T, Saito T, Aoyagi J, Betsui H, et al. A case of post-pneumococcal acute glomerulonephritis with glomerular depositions of nephritis-associated plasmin receptor. CEN Case Rep. 2015;4:112–116. doi: 10.1007/s13730-014-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano D, Oda T, Ito A, Yamada A, Kakegawa D, Miwa S, et al. Glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma pneumoniae induces infection-related glomerulonephritis. Clin Nephrol. 2019;92:263–272. doi: 10.5414/CN109786. [DOI] [PubMed] [Google Scholar]
- 6.Uchida T, Oda T. Glomerular deposition of nephritis-associated plasmin receptor (naplr) and related plasmin activity: key diagnostic biomarkers of bacterial infection-related glomerulonephritis. Int J Mol Sci. 2020;21:2595. doi: 10.3390/ijms21072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Kallenberg CG, Zhao MH. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol. 2009;5:313–318. doi: 10.1038/nrneph.2009.67. [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol. 2017;12:1680–1691. doi: 10.2215/CJN.02500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk RJ, Jennette JC. ANCA small-vessel vasculitis. J Am Soc Nephrol. 1997;8:314–322. doi: 10.1681/ASN.V82314. [DOI] [PubMed] [Google Scholar]
- 10.Sethi S, Zand L, De Vriese AS, Specks U, Vrana JA, Kanwar S, et al. Complement activation in pauci-immune necrotizing and crescentic glomerulonephritis: results of a proteomic analysis. Nephrol Dial Transplant. 2017;32:i139–i145. doi: 10.1093/ndt/gfw299. [DOI] [PubMed] [Google Scholar]
- 11.Nasr SH, Fidler ME, Valeri AM, Cornell LD, Sethi S, Zoller A, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011;22:187–195. doi: 10.1681/ASN.2010060611. [DOI] [PubMed] [Google Scholar]
- 12.Nasr SH, Radhakrishnan J, D'Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi Y, Yoshizawa N, Oda T, Imakiire T, Suzuki S, Miura S. Streptococcal origin of a case of Henoch-Schoenlein purpura nephritis. Clin Nephrol. 2006;65:124–128. doi: 10.5414/CNP65124. [DOI] [PubMed] [Google Scholar]
- 14.Sawanobori E, Umino A, Kanai H, Matsushita K, Iwasa S, Kitamura H, et al. A prolonged course of group A streptococcus-associated nephritis: a mild case of dense deposit disease (DDD)? Clin Nephrol. 2009;71:703–707. doi: 10.5414/CNP71703. [DOI] [PubMed] [Google Scholar]
- 15.Kohatsu K, Suzuki T, Yazawa M, Yahagi K, Ichikawa D, Koike J, et al. Granulomatosis With polyangiitis induced by infection. Kidney Int Rep. 2019;4:341–345. doi: 10.1016/j.ekir.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano K, Suzuki H, Oda T, Ueda Y, Tsukamoto T, Muso E. Crescentic poststreptococcal acute glomerulonephritis accompanied by small vessel vasculitis: case report of an elderly male. BMC Nephrol. 2019;20:471. doi: 10.1186/s12882-019-1663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traverso F, Martini F, Banchi L, Maritato F, Fazio B. Vasculitic neuropathy associated with beta-haemolytic streptococcal infection: a case report. Ital J Neurol Sci. 1997;18:105–107. doi: 10.1007/BF01999571. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Yamakawa H, Yabe M, Ishikawa T, Takagi M, Matsumoto K, et al. Diffuse alveolar hemorrhage in a patient with acute poststreptococcal glomerulonephritis caused by impetigo. Intern Med. 2015;54:961–964. doi: 10.2169/internalmedicine.54.3838. [DOI] [PubMed] [Google Scholar]
- 19.Okabe M, Takamura T, Tajiri A, Tsuboi N, Ishikawa M, Ogura M, et al. A case of infection-related glomerulonephritis with massive eosinophilic infiltration. Clin Nephrol. 2018;90:142–147. doi: 10.5414/CN109415. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hussain T, Hussein MH, Conca W, Al Mana H, Akhtar M. Pathophysiology of ANCA-associated vasculitis. Adv Anat Pathol. 2017;24:226–234. doi: 10.1097/PAP.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 21.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]