Abstract
Chylous ascites is a rare, potentially sinister complication in post donor nephrectomy patients which may cause significant morbidity in form of severe malnutrition and an immuno-compromised state. We present two patients with post donor nephrectomy-related chylous leaks who failed conservative treatment. In both cases, lymphangiography was done first to detect the chylous leak site in the left renal fossa, and thereafter transvenous retrograde approach via left subclavian vein with selective lymphatic duct embolization of chylous leak was done with coils and glue successfully. Chylous ascites resolved in both patients after the embolization. Hence retrograde transvenous embolization technique appears to be an effective management option for refractory chylous leaks.
Keywords: Chylous ascites, Chylous leak, Lymphangiography, Embolization, Thoracic duct
Introduction
Chylous ascites (CA) is an uncommon, however, potentially fatal complication after donor nephrectomy which causes crucial immunological deficits and protein-energy malnutrition [1–3]. Conservative treatment such as fat-free diet, salt restriction, total parenteral nutrition, percutaneous drain placement, and subcutaneous octreotide administration remains the primary therapy for most of the low-volume leaks [2]. A drain output of more than 1000 mL per day is regarded as refractory CA and requires surgical ligation or embolization of the thoracic duct leak site. Lymphangiography using lipiodol as a contrast agent is a safe and effective technique for diagnosing active chylous leaks as well as also offers some therapeutic benefit due to its inflammatory and granulomatous effects [3]. Percutaneous trans-abdominal embolization and retrograde transvenous embolization with N-butyl cyanoacrylate (NBCA) are the major techniques applied to control high volume chylous leak (CL) apart from surgical management [1]. The percutaneous technique remains difficult in obese patients and raises the chance of visceral injury. We hereby present two cases with refractory chylous ascites as a complication to donor nephrectomy, treated with selective lymphatic duct embolization at the site of leakage via retrograde transvenous approach. Both the patients have given consent for using their case details and images for this case report.
Case 1
A 50-year-old female who underwent laparoscopic donor nephrectomy on the left side developed abdominal pain with distention on the third postoperative day. Sonography suggested moderate ascites for which large-volume abdominal paracentesis was done and drain was placed which revealed milky white fluid with a high triglyceride level of 780 mg/dL, confirming the diagnosis of CA. Despite conservative treatment and after percutaneous drain placement, the abdominal pain persisted, and drain output remained high (>1000 mL per day) suggesting refractory CA. Further, intranodal lymphangiography with a 25 G lumbar puncture (LP) needle was done by placing it at the cortico-medullary interphase of the left inguinal lymph node. The LP needle was then connected to an injection pump filled with lipiodol set at a rate of 0.2–0.4 mL/minute [3]. The ascent of contrast with the opacification of retroperitoneal lymphatics was monitored by taking fluoroscopic images every 5–10 minutes and leak point was visualized in the left renal fossa. Retrograde transvenous approach was used wherein, left basilic vein access was taken followed by advancement of a 5 Fr SHK (Shepherd Hook) 1.0 catheter (Boston Scientific, USA) up to the left jugular-subclavian angle and the lymph venous junction was successfully cannulated. Thereafter a 2.4 Fr micro catheter (Direxion™, Boston Scientific, USA) with 0.014 guide wire (TransendTM) was advanced into the thoracic duct (Fig 1C, D). The micro catheter was then successfully advanced into a selective renal lymphatic duct via the thoracic duct. Ductogram showed leak in the form of extravasation from the left renal lymphatic channel (Fig.1E). The micro catheter was advanced beyond the leak site with deployment of two fibred micro-coils (Nester® Embolization Coil, 18-14-3, COOK, USA) followed by injection of 0.5 mL mixture of Lipiodol and N-butyl cyanoacrylate (NBCA) in the ratio of 3:1 proximal to the coils (Fig 1F, G). This embolization mixture was noted traversing into the site of the leak and in the renal lymphatic branch of the thoracic duct, which was selectively embolized, stopping the active CL. The patient was put on a fat-free diet and subcutaneous injection octreotide for the next 3 days. The patient was symptom-free and there were no ascites on 1- and 3-weeks follow-ups.
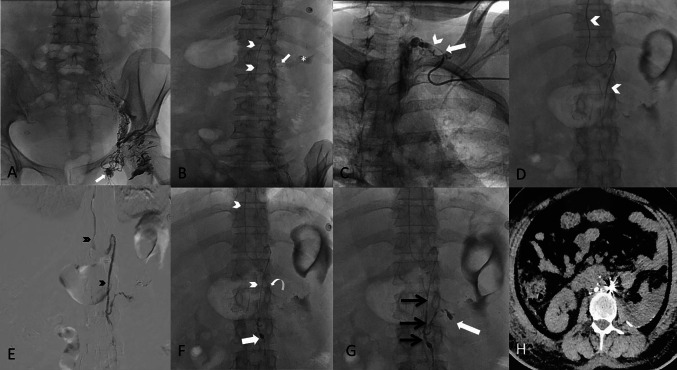
Fig. 1.
A Post intranodal lymphangiography from left inguinal node (white arrow), shows gradual opacification of lymphatic channels cranially with lipiodol reaching upto the lower abdomen. B The lumbar lymphatic duct (arrowheads) is visualized along with the site of leak (arrow) and the extravasation (asterisk). C Using retrograde left subclavian approach, the venolymphatic junction is cannulated (arrow) and microcatheter is progressed into thoracic duct (arrowhead). D Microcatheter (arrowheads) is advanced upto the leak site by selectively cannulating the left lumbar lymphatic branch. E A ductogram is taken with iodinated contrast demonstrating the lymphatic duct (black arrowheads) and extravasation in the left renal fossa. F Microcatheter (arrowheads) is advanced beyond the site of leak (curved arrow) and coil embolization done (white arrow). G Glue embolization done with 33% N-butyl cyanoacrylate and the glue cast is appreciated in the lymphatic duct (black arrows) and extending into the site of leak (white arrow). H Axial computed tomography image at mid abdomen level, the next day demonstrating post embolization glue accumulation with coils at the leakage site, showing steak artifact with minimal residual ascites
Case 2
A 51-year-old woman, status post laparoscopic left donor nephrectomy developed massive chylous ascites on the 2nd postoperative day and the fluid showed high triglyceride levels (1722 mg/dL) on biochemical examination. Conservative treatment remained unsuccessful and the patient’s abdominal girth increased and pain increased with drain output >1000 mL suggesting refractory CA. Therefore, Left inguinal intranodal lymphangiography with lipiodol was done which revealed the site of an active leak in the left renal fossa. Similar to the previous case, the left brachial vein was punctured and the thoracic duct was cannulated with a 5 F Cobra catheter (Cook, USA), further 2.4 Fr micro catheter (Cantata®, Cook, USA) was advanced beyond the site of leakage point and 0.5 mL a mixture of lipiodol and NBCA in a ratio of 2:1 was injected at the site of the leak and in lymphatic duct embolizing the leak (Fig. 2E, F). Thereafter, she was conservatively managed and showed no CA on follow-up.
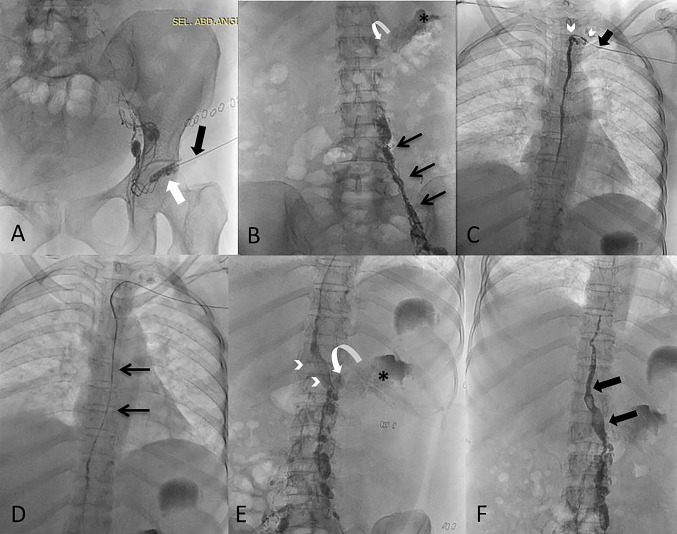
Fig. 2.
A Intranodal lymphangiography from left inguinal node (white arrow) with 25 G needle (black arrow). B Figure shows gradual opacification of lymphatic channels cranially (black arrows) with lipiodol reaching upto the lower abdomen. The site of leak (curved arrow) is visualized along with and the extravasation (asterisk). C Using retrograde left subclavian approach, the venolymphatic junction is cannulated (black arrow) and microcatheter is progressed into thoracic duct (arrowheads) and contrast was injected into thoracic duct. D Microcatheter (arrows) is advanced beyond into the abdominal part of thoracic duct. E Glue embolization done with N-butyl cyanoacrylate in the selective lymphatic duct (arrowheads) and extending into the site of leak (curved arrow) with visualization of site of leak (asterisk). F Post embolization glue cast (black arrows) can be appreciated in the lymphatic, however no active chylous leak was noted in the left renal fossa
Discussion
Thoracic duct (TD) originates from cisterna chyli at L1-L2 vertebral level and ascends cranially in retrocrucal plane between the azygos vein and aorta, and commonly drains in the left internal jugular vein (IJV), however, can also drain into the left subclavian vein, or less commonly into right IJV, left innominate vein, azygous vein or external jugular vein. TD may have multiple terminal branches and is vulnerable to injury due to its intricate relationship with critical structures. Thoracic duct or selective lymphatic duct embolization is a favorable treatment for stopping chylous leaks. CL may occur due to injury to cisterna chyli or commonly to its lymphatic tributaries while performing donor nephrectomy [4, 5]. The proposed etiology of CL post laparoscopic nephrectomy is failure to serially tie hilar tissue and to dissect and clear all the perihilar fatty tissue around the renal vessels. Larger lymphatics need to be dissected into smaller channels, before being clipped to avoid leakage. Laparoscopic management of CL includes maneuvers such as clipping the perihilar area, argon beam coagulation, fibrin glue application, intracorporeal suturing, and bipolar electro cauterization.
Bipedal and inguinal nodal lymphangiography are the techniques that involve opacification of the lymphatic ducts and cisterna chyli with lipiodol facilitating visualization of leak point which can be further embolized [3]. CA is a whitish-colored triglyceride-rich fluid, which results due to the formation of a lymphatic-peritoneal fistula secondary to operative injury to lymphatic channels or due to obstructive disruptive causes such as malignancy, trauma, congenital lymphatic malformations or chronic liver disease [3, 5–7]. A triglyceride level of more than 200 mg/dL is diagnostic of CA [7]. Minor CA may remain asymptomatic in absence of drain and can settle with conservative treatment in most of the donor nephrectomy patients and should always be tried first, however, a high-volume leak (> 1000 mL per day) will often cause abdominal distention and symptoms such as vomiting, dyspnea and further cause malnutrition and needs immediate treatment [6].
CA represents a rare complication in post donor nephrectomy patients with incidence up to 0.013 to 5.9% and predominantly on the left side as the left ascending lymphatic trunk lies in close relation to left renal fossa and can usually be controlled with conservative treatment only [6, 7]. In our institute, 5 out of 135 patients who underwent laparoscopic donor nephrectomy over the last 3 years (incidence ~3.7%) developed refractory CA of which 3 underwent surgical ligation whereas 2 were managed with retrograde transvenous lymphatic embolization. Conservative management remains the first line of treatment and includes various measures such as bed rest, drain suction, diet changes (medium-chain triglycerides with fat deficient and high protein diet), drain placement, total parental nutrition, pressure dressing, and subcutaneous octreotide administration, but they primarily control the low output chylous leak (<1000 mL per day). Whereas, high output leaks (> 1000 mL per day) are better controlled with embolization is done using coils and NBCA [3–5]. Ratio of NBCA: Lipiodol is kept between 1:2 or 1:3 to achieve a polymerization time of up to 4 to 7 seconds which is appropriate for this maneuver.
Percutaneous access to the lymphatic duct is often challenging due to its small caliber, depth, and also due to anatomical variations. Both our patients were clinically obese, hence percutaneous embolization was avoided so as to avoid any visceral injury and a retrograde route was preferred. Transvenous retrograde embolization is a minimally invasive, effective, and safe technique performed by cannulating the thoracic duct via a left subclavian vein. This technique demands a high level of expertise and is being practiced only at a few tertiary level hospitals in India (<1%). The embolization should be done proximal and distal to the leak and selective embolization of the leak point is done avoiding the rest of the lymphatic channels [5]. Localizing the exact site of the leak has a better probability of stopping the CL. [8]. The lymphatic channel ligation and peritoneal–venous bypass form important surgical interventions in case of failure of the above measures, however, are rarely practiced due to the high incidence of complications such as sepsis and disseminated intravascular coagulation [6, 9, 10]. A combination of NBCA (glue) and coils works the best embolization of the CL. Coils provide a good matrix base assisting glue polymerization and safety.
In our first case, the cervical part of the thoracic duct formed a plexiform network and it was difficult to cross over into the thoracic duct, a similar problem was also encountered by Shuji et al [9]. The reverse Trendelenburg position, due to the gravitational effects causes better opacification of the thoracic duct [11]. Diagnostic lymphangiography can also have therapeutic effects in controlling CL due to the inflammatory and granulomatous effects of lipiodol [12].
Mild complications such as pain, infection, allergies, or extravasation of lipiodol have been associated with a pedal or nodal lymphangiography. Very rare complications such as pulmonary hemorrhage, pulmonary and cerebral embolism have also been reported with the use of lipiodol [13]. Hence it should be avoided in patients with right to left cardiac shunts [14]. Limiting the lipiodol volume to less than 20 mL has not been associated with major complications [12, 15]. Potential complications of using NBCA (glue) include non-target embolization, venous egress; premature polymerization causing catheter blockage or adherence hence it should be used by an experienced operator only. However, NBCA is an excellent embolization agent for refractory chylous leaks, in cases that have failed with conservative management and lymphangiography.
Conclusion
Lymphangiography with retrograde transvenous selective lymphatic duct embolization is a minimally invasive, safe, and effective interventional technique in controlling refractory chylous ascites where conservative treatments fail. The therapeutic effect of lipiodol lymphangiography in minor leaks is also well known. Overall, it promises no significant morbidity or mortality with good results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morikawa K, Takenaga S, Hasumi J, et al. Retrograde transvenous lymphatic embolization for postoperative chylous ascites: a report of three cases and literature review. Radiol Case Rep. 2020;15(9):1623–1628. doi: 10.1016/j.radcr.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aerts J, Matas A, Sutherland D, Kandaswamy R. Chylous ascites requiring surgical intervention after donor nephrectomy: case series and single center experience. Am J Transplant. 2010;10(1):124–128. doi: 10.1111/j.1600-6143.2009.02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee EW, Shin JH, Ko HK, Park J, Kim SH, Sung KB. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol. 2014;15(6):724–732. doi: 10.3348/kjr.2014.15.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guglielmo N, Melandro F, Nudo F, et al. Chylous leakage after a laparoscopic live-donor nephrectomy: case report and literature review. Exp Clin Transplant. 2016;14(3):338–340. doi: 10.6002/ect.2014.0123. [DOI] [PubMed] [Google Scholar]
- 5.Mittleider D, Dykes TA, Cicuto KP, Amberson SM, Leusner CR. Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites. J Vasc Interv Radiol. 2008;19(2 Pt 1):285–290. doi: 10.1016/j.jvir.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Capocasale E, Iaria M, Vistoli F, et al. Incidence, diagnosis, and treatment of chylous leakage after laparoscopic live donor nephrectomy. Transplantation. 2012;93(1):82–86. doi: 10.1097/TP.0b013e31823b2d8e. [DOI] [PubMed] [Google Scholar]
- 7.Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A. Review article: the diagnostic approach and current management of chylous ascites. Aliment Pharmacol Ther. 2017;46(9):816–824. doi: 10.1111/apt.14284. [DOI] [PubMed] [Google Scholar]
- 8.Nadolski GJ, Chauhan NR, Itkin M. Lymphangiography and lymphatic embolization for the treatment of refractory chylous ascites. Cardiovasc Intervent Radiol. 2018;41(3):415–423. doi: 10.1007/s00270-017-1856-1. [DOI] [PubMed] [Google Scholar]
- 9.Kariya S, Nakatani M, Ueno Y, et al. Transvenous retrograde thoracic ductography: initial experience with 13 consecutive cases. Cardiovasc Intervent Radiol. 2018;41(3):406–414. doi: 10.1007/s00270-017-1814-y. [DOI] [PubMed] [Google Scholar]
- 10.Leibovitch I, Mor Y, Golomb J, Ramon J. The diagnosis and management of postoperative chylous ascites. J Urol. 2002;167(2 Pt 1):449–457. doi: 10.1097/00005392-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139(3):584–590. doi: 10.1016/j.jtcvs.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki R, Sugimoto K, Fujii M, et al. Therapeutic effectiveness of diagnostic lymphangiography for refractory postoperative chylothorax and chylous ascites: correlation with radiologic findings and preceding medical treatment. AJR Am J Roentgenol. 2013;201(3):659–666. doi: 10.2214/AJR.12.10008. [DOI] [PubMed] [Google Scholar]
- 13.Dupont H, Timsit JF, Souweine B, Gachot B, Bedos JP, Wolff M. intra-alveolar hemorrhage following bipedal lymphography. Intensive Care Med. 1996;22(6):614–615. doi: 10.1007/BF01708114. [DOI] [PubMed] [Google Scholar]
- 14.Kusumoto S, Imamura A, Watanabe K. Case report: the incidental lipid embolization to the brain and kidney after lymphography in a patient with malignant lymphoma: CT findings. Clin Radiol. 1991;44(4):279–280. doi: 10.1016/s0009-9260(05)80199-0. [DOI] [PubMed] [Google Scholar]
- 15.Guermazi A, Brice P, Hennequin C, Sarfati E. Lymphography: an old technique retains its usefulness. Radiographics. 2003;23(6):1541–1560. doi: 10.1148/rg.236035704. [DOI] [PubMed] [Google Scholar]