Abstract
Background and Purpose
In this study we aimed to find the association between neuropsychological performance and body mass index (BMI) in patients with mild cognitive impairment (MCI). In addition, we investigated the effects of the apolipoprotein E (APOE) genotype in the relationship between the BMI and cognition in MCI.
Methods
We enrolled a cohort of 3,038 subjects with MCI aged 65–90 from the Clinical Research Center for Dementia of South Korea and a dementia cohort of the Ewha Womans University Mokdong Hospital. MCI patients were classified into three subgroups according to the Asian standard of BMI. We compared cognitive performances between groups by one-way analysis of variance. To investigate the effects of the APOE genotype, we used multivariate linear regression models after adjusting for possible confounders.
Results
Even though normal BMI groups were younger, had more females, and had less comorbidities, the higher BMI groups had better cognitive functions. Among subjects with APOE ε4 carriers, there was a positive relationship between the BMI and the memory task alone.
Conclusions
Our findings suggested that higher BMI in patients with MCI were associated with better cognitive performance. The effects of the APOE ε4 genotype in the associations between BMI and cognition were distinguishing. Therefore, according to physical status, APOE ε4 genotype-specific strategies in the assessments and treatments may be necessary in elderly patients with MCI.
Keywords: Body Mass Index, Apolipoprotein E, Cognitive Function, Mild Cognitive Impairment
INTRODUCTION
Mild cognitive impairment (MCI) represents a state in which cognitive function, including memory, is impaired and is below the normal range in an objective test, but the daily living ability is largely preserved. The prevalence of MCI is reported to be 6.7%–25%, increasing with age.1 Although about 15% of MCI subjects ultimately progress to dementia after two years,1 no effective pharmacological treatments have been available to prevent cognitive impairment in MCI. Inevitably, it is important to identify and intervene with modifiable risk factors in the MCI state in order to prevent progression to dementia.
By means of interventions with 12 modifiable risk factors, including obesity, about 40% of dementia could be prevented or delayed.2,3 Mid-life obesity is consistently associated with an increased risk for dementia. However, late-life underweight and weight loss may precede dementia,4,5,6,7,8 and slower cognitive decline5,9,10 in normal elderly people has been known as the “obesity paradox.” Furthermore, obesity has been shown to be related to structural change in subcortical brain volume,11 medial temporal lobe volume,12 and amygdala and hippocampus volume,13 as well as to cognitive deficits.14,15,16 In studies investigating the association between obesity and dementia, the body mass index (BMI) is the most commonly used physical marker to assess obesity.
The apolipoprotein E (APOE) genotype is the important genetic risk factor in Alzheimer’s disease (AD), accounting for up to about a 10 times increased risk for developing dementia. However, not all carriers develop AD in their lifetime.17,18 Although the association between the APOE ε4 and BMI has been investigated to look for gene-physical interactions in dementia, it remains unclear. One study found that MCI patients with high BMI at baseline are less likely to progress to AD in the presence of APOE ε4.19 On the contrary, other studies failed to show a link between BMI and the APOE genotype.20,21 Furthermore, because sex has distinct biological properties, such as chromosomes or sex hormones, sex may interact with BMI or the APOE. Specifically, the “estrogen hypothesis,” highlighting the neuroprotective role of estrogen, has been proposed to explain sex differences caused by gonadal steroids in AD.22 In addition, psychosocial and environmental factors also contribute to sex differences in AD. Given that females who are APOE ε4 carriers exhibit a significantly greater risk than do males, the interaction between the APOE genotype and BMI may differ between males and females.16 In this context, the effect of BMI on cognition should be further analyzed according to the APOE genotype and sex, but there have been few studies focusing on the interaction between the three. Therefore, in this study we aimed to find out whether late-life BMI is associated with cognitive function in MCI patients. Second, we investigated the interaction between BMI and the APOE ε4 genotype as well as sex.
METHODS
Participants
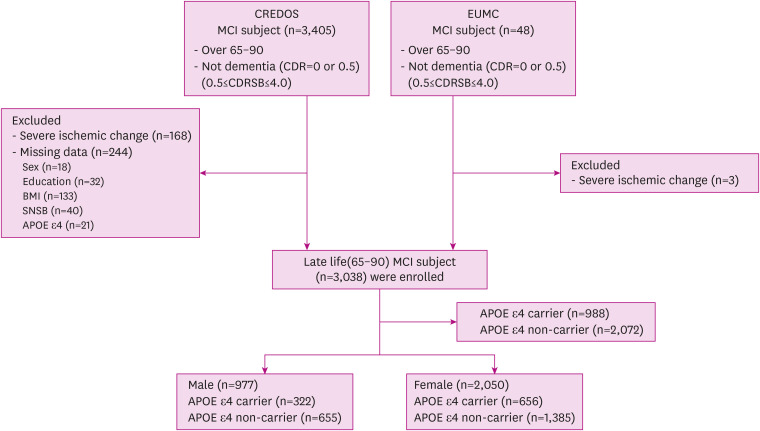
We included MCI patients from 65 to 90 years old from the Clinical Research Center for Dementia of South Korea (CREDOS) cohort23 and the Memory Disorder Clinic in Ewha Womans University Mokdong Hospital (EUMC). We enrolled 3,038 subjects with MCI, including 2,993 from the CREDOS cohort and 45 from EUMC who fulfilled these criteria in this study (Fig. 1). MCI was defined according to Peterson’s24 criteria:
Fig. 1. Flow chart showing the process of selecting the study population.
CREDOS: Clinical Research Center for Dementia of South Korea, MCI: mild cognitive impairment, CDR: clinical dementia rating, CDRSB: clinical dementia rating sum of boxes, EUMC: Ewha Womans University Medical Center, BMI: body mass index, SNSB: Seoul Neuropsychological Screening Battery, APOE: apolipoprotein E.
1) cognitive impairment, especially subjective memory complaint by patients or caregivers, 2) evidence of objective memory or other cognitive function impairment in neuropsychological tests, lower than expected for the patient's age and educational level, 3) largely maintained activities of daily living and minimally impaired instrumental activity of daily living (IADL), and 4) absence of dementia in the individual.
Additionally, we included the Clinical Dementia Rating (CDR) and CDR sum of boxes (CDRSB) as inclusion criteria. The CDR score is 0 or 0.5, and the CDRSB should be in the range 0.5–4.0. Patients with a global CDR score of 0 were selected as those with a CDRSB of 0.5, because one or more cognitive domains were lowered to 1SD or less in an objective neuropsychological test. A CDR score of 0.5 indicates a decline in cognitive function, but the IADL remains within the normal range (<0.43). Subjects were excluded for the following reasons:
1) history of significant hearing or visual impairment rendering participation in the interview difficult, 2) history of following neurologic disorder(brain tumor, subarachnoid hemorrhage, epilepsy, encephalitis, and metabolic encephalopathy), 3) severe white matter hyperintensities that might be vascular rather than degenerative, 4) disorder of thyroid gland, syphilis, or vitamin B12 deficiency, 5) history of a psychiatric disorder, including schizophrenia, bipolar disorder, and major depressive disorder and other, 6) history of using psychoactive substances other than alcohol, 7) missing data, such as for sex, education, BMI, neuropsychological tests, and the APOE ε4 genotype.
Clinical evaluations
All participants were evaluated by means of comprehensive interviews, neurological tests, and laboratory results. We obtained the demographic data about the patients and caregivers, lifestyle and family history, and past medical history, including vascular risk factors, such as hypertension (HTN), hyperlipidemia (HL), diabetes mellitus (DM), cardiac disease, previous stroke history, and brain trauma. Laboratory tests included complete blood counts, chemistry, electrolytes, urinalysis, syphilis serology, thyroid function test, and vitamin B12/folate. We assessed APOE genotype by polymerase chain reaction.25 We classified the APOE genotype into ε4 carrier and non-carrier according to the presence of the ε4 allele, and categorized the ε4 carrier group into homozygote and heterozygote. We also evaluated the severity of white-matter hyperintensities (WMH) using the T2 or fluid-attenuated inversion recovery axial images on brain magnetic resonance imaging. These two ratings were combined into three groups of WMH severity: minimal, moderate and severe.26
BMI
We calculated BMI as weight in kilograms divided by height in meters squared (kg/m2). We classified all patients into four BMI subgroups (Less than 18.5 kg/m2 = underweight, 18.5–23 kg/m2 = normal, 23–25 kg/m2 = overweight, and >25 kg/m2 = obesity) based on the World Health Organization (WHO) guidelines for Asian populations.27 Given that we had too few patients for the underweight group (n=128), we divided the subjects into three groups (normal, overweight, and obese) by combining the underweight and the normal-weight group.
Neuropsychological assessment
All subjects received a standardized neuropsychological battery, the Seoul Neuropsychological Screening Battery,28 and only patients who received all the tests below were included in the analysis. We assessed global cognition using the Korean Mini-Mental State Examination (K-MMSE),29 the CDR, and the CDRSB.30 We used the Korean version of the Boston Naming Test (K-BNT),31 the Rey-Osterrieth Complex Figure Test (RCFT), the Seoul Verbal Learning Test (SVLT), phonemic and semantic Controlled Oral Word Association Test (COWAT, phonemic/animal fluency), and the Stroop test to assess each cognitive domain. The raw score of the neuropsychological tests was used for the analysis.
Statistical analysis
We analyzed the frequencies and calculated the mean values of the clinical characteristics by the total sample (Table 1). We presented age, education, and BMI corresponding to continuous variables as mean and standard deviations. We presented sex, APOE ε4, CDR, comorbid conditions, and ischemic change corresponding to categorical variables as numbers and percentages. We conducted clinical comparisons between BMI categories using one-way analysis of variance for continuous variables and the chi-squared test for categorical variables. We examined differences between the three groups according to the BMI using analyses of the covariate, and applied a Bonferroni correction to the post hoc tests in neuropsychological measures (K-BNT, RCFT copy, SVLT/RCFT delay recall, animal/phonemic fluency, Stroop test, K-MMSE, CDRSB) performance among MCI patients, after adjusting for the covariates, including age, sex, education, HTN, HL, DM, cardiac disease, previous stroke, and APOE ε4. Last, we used multiple linear regression to investigate the association between various neuropsychological tests and BMI after adjusting for possible confounders, such as age, education, HTN, HL, DM, cardiac disease, and previous stroke; we did additional analysis of the effects of APOE genotype and sex, separately. Considering the influence of APOE ε4, we further analyzed it by dividing it into homozygote and heterozygote groups. We computed statistical data with SPSS, version 20.0 (IBM, Chicago, IL). We considered results statistically significant for p<0.05.
Table 1. Demographics and clinical characteristics of mild cognitive impairment subjects.
| Variable | Total | Normal weight (BMI<23) | Overweight (23≤BMI<25) | Obese (BMI≥25) | p-value | |
|---|---|---|---|---|---|---|
| No. | 3,038 | 1,317 (43.4) | 802 (26.4) | 919 (30.3) | ||
| Age (yr) | 73.55±5.13 | 74.09±5.35 | 73.24±5.27 | 73.05±5.23 | <0.001 | |
| Sex | ||||||
| Female | 2,041 (67.2) | 885 (67.2) | 514 (64.1) | 642 (69.9) | 0.039 | |
| Education (yr) | 7.78 (5.44) | 7.81 (5.38) | 8.02 (5.56) | 7.52 (5.42) | 0.156 | |
| No education | 529 (17.4) | 212 (16.1) | 144 (18.0) | 173 (18.8) | ||
| ≤6 yr | 1,037 (34.1) | 466 (35.4) | 251 (31.3) | 320 (34.8) | ||
| 7–12 yr | 916 (30.2) | 405 (30.8) | 240 (29.9) | 271 (29.5) | ||
| ≥13 yr | 556 (18.3) | 234 (17.8) | 167 (20.8) | 155 (16.9) | ||
| Family history | ||||||
| Dementia | 343 (11.3) | 144 (21.2) | 79 (18.9) | 120 (24.1) | 0.153 | |
| Stroke | 300 (10.0) | 124 (9.5) | 68 (8.6) | 108 (12.0) | 0.190 | |
| APOE ε4 | ||||||
| APOE ε4(+) | 988 (32.5) | 464 (35.2) | 242 (30.2) | 282 (30.7) | 0.020 | |
| BMI (kg/m2) | 23.66 (3.13) | 20.94 (1.63) | 24.01 (0.58) | 27.27 (2.10) | <0.001 | |
| Comorbid conditions | ||||||
| Hypertension | 1,557 (51.3) | 549 (41.8) | 409 (51.1) | 599 (65.3) | <0.001 | |
| Hyperlipidemia | 639 (21.0) | 213 (16.2) | 172 (21.5) | 254 (27.8) | <0.001 | |
| Diabetes mellitus | 684 (22.5) | 254 (19.3) | 198 (24.8) | 232 (25.4) | 0.001 | |
| Cardiac disease | 484 (15.9) | 169 (12.9) | 139 (17.4) | 176 (19.3) | <0.001 | |
| Stroke | 97 (3.2) | 31 (2.4) | 21 (2.6) | 45 (4.9) | 0.002 | |
| Brain trauma | 78 (2.6) | 36 (2.8) | 18 (2.3) | 24 (2.7) | 0.788 | |
| CDR | ||||||
| 0 | 170 (5.6) | 61 (4.6) | 56 (7.0) | 53 (5.8) | ||
| 0.5 | 2,868 (94.4) | 1,256 (95.4) | 746 (93.0) | 866 (94.2) | 0.071 | |
| Ischemic change | ||||||
| Mild | 2,631 (86.6) | 1,150 (88.1) | 691 (87.1) | 790 (86.7) | 0.591 | |
| Moderate | 378 (12.4) | 155 (11.9) | 102 (12.9) | 121 (13.3) | 0.591 | |
Data are shown as mean±standard deviation or number (%).
BMI: body mass index, APOE: apolipoprotein E, CDR: clinical dementia rating.
Ethics statement
All participants and their caregivers provided written informed consent, and the study protocol was approved by the Institutional Review Board (IRB) of Ewha Womans University Mokdong Hospital (IRB EUMC 2018-08-005).
RESULTS
The demographics of the 3,038 MCI patients are summarized in Table 1. The three BMI groups showed statistically significant differences in sex, age, APOE ε4, HTN, HL, DM, cardiac disease, and stroke. The normal-weight group had lower comorbidities, more females, and an older population than did the overweight and obese group. The APOE ε4 carriers in the overweight and the obesity groups accounted for about 30%, that in the normal-weight group about 35%.
When the APOE ε4 carriers and the non-carriers were compared, the APOE ε4 carrier group had significantly lower BMI than did the non-carriers (23.40 kg/m2 vs 23.78 kg/m2, p=0.002). The proportion of family history of dementia (13.6% vs 9.9%, p=0.011) and of HL (23.2% vs 19.7%, p=0.027) was more common in the APOE 4 carriers than in the non-carriers.
Comparisons of cognitive function between the three groups, adjusting for age, sex, education level, HTN, HL, DM, cardiac disease, previous stroke, and presence of APOE ε4, are presented in Table 2. There were significant differences between the three BMI groups in K-BNT, RCFT copy, SVLT delay recall, RCFT delay recall, animal fluency, phonemic fluency, Stroop test, MMSE, and CDRSB. The overweight group had higher scores in the K-BNT, RCFT delay recall, phonemic fluency, Stroop test, MMSE and CDRSB than did those in the normal weight group. The obese group performed better in the K-BNT, SVLT delay recall, RCFT delay recall, Stroop test and K-MMSE and had lower score in the CDRSB than did the normal-weight group.
Table 2. Comparison of neuropsychological test performance between BMI subgroups.
| Cognitive function | Normal (BMI<23) | Overweight (23≤BMI<25) | Obese (BMI≥25) | p-value | |
|---|---|---|---|---|---|
| No. | 1,317 | 802 | 919 | ||
| Language | |||||
| K-BNT | 36.78±0.27 | 37.94±0.33*,§ | 38.50±0.32‡,∥ | <0.001 | |
| Visuospatial function | |||||
| RCFT copy | 27.05±0.20 | 27.48±0.26 | 27.60±0.25 | 0.185 | |
| Verbal memory | |||||
| SVLT delay recall | 2.77±0.72 | 2.98±0.91 | 3.14±0.09†,∥ | 0.005 | |
| Visual memory | |||||
| RCFT delay recall | 7.10±0.16 | 7.78±0.21*,§ | 7.89±0.20†,∥ | 0.004 | |
| Frontal/executive function | |||||
| Animal fluency | 11.57±0.11 | 11.90±0.14 | 11.86±0.13 | 0.100 | |
| Phonemic fluency | 16.57±0.26 | 17.88±0.33†,§ | 17.20±0.32 | 0.008 | |
| Stroop test | 59.20±0.71 | 63.57±0.90‡,§ | 63.11±0.85†,∥ | <0.001 | |
| General index | |||||
| MMSE | 23.82±0.10 | 24.17±0.12*,§ | 24.34±0.12†,∥ | 0.002 | |
| CDRSB | 1.76±0.03 | 1.64±0.04†,§ | 1.61±0.04†,∥ | 0.001 | |
Data are shown as adjusted mean±standard deviation.
SD: standard distribution, BMI: body mass index, K-BNT: Korean version of the Boston Naming Test, RCFT: Rey-Osterrieth Complex Figure Test, SVLT: Seoul Verbal Learning Test, MMSE: Mini-Mental State Examination, CDRSB: clinical dementia rating sum of boxes.
*p<0.05, †p<0.01, ‡p<0.001, adjusted by age, sex, years of education, hypertension, hyperlipidemia, diabetes mellitus, previous stroke, apolipoprotein E ε4 genotype; §Bonferroni’s post hoc analyses comparing normal and overweight group; ∥Bonferroni’s post hoc analyses comparing normal and obese group.
To find the relationships between BMI, APOE genotype, sex, and cognitive function, we did a multiple regression analysis while controlling for extra variables. The results are presented in Table 3. As BMI increased, scores of all neurocognitive tests were significantly increased (RCFT copy, p=0.047; animal fluency, p=0.039; phonemic fluency, p=0.013; SVLT delay recall, p=0.005; RCFT delay recall, K-MMSE, p=0.001; K-BNT and Stroop test, p<0.001), and CDRSB (p=0.001) was decreased.
Table 3. Association between BMI and cognitive function according to APOE ε4 genotype.
| B (SE) | Total | APOE ε4 carrier | APOE ε4 non-carrier | p-value for BMI × APOE | |
|---|---|---|---|---|---|
| Language | |||||
| K-BNT | 0.259 (0.058)‡ | 0.088 (0.100) | 0.336 (0.071)‡ | 0.388 | |
| Visuospatial function | |||||
| RCFT copy | 0.091 (0.044)* | 0.077 (0.080) | 0.094 (0.053) | 0.355 | |
| Verbal memory | |||||
| SVLT delay recall | 0.044 (0.015)† | 0.065 (0.027)* | 0.031 (0.018) | 0.018 | |
| Visual memory | |||||
| RCFT delay recall | 0.114 (0.035)‡ | 0.185 (0.061)† | 0.078 (0.043) | 0.003 | |
| Frontal/executive function | |||||
| COWAT animal fluency | 0.049 (0.023)* | 0.003 (0.040) | 0.069 (0.029)* | 0.947 | |
| COWAT phonemic fluency | 0.139 (0.058)* | 0.083 (0.101) | 0.166 (0.071)* | 0.418 | |
| Stroop test | 0.579 (0.156)‡ | 0.526 (0.287) | 0.604 (0.186)‡ | 0.067 | |
| General index | |||||
| MMSE | 0.074 (0.021)‡ | 0.059 (0.037) | 0.082 (0.025)‡ | 0.117 | |
| CDRSB | −0.021 (0.006)‡ | −0.015 (0.011) | −0.024 (0.007)‡ | 0.199 | |
Multivariate linear regression analysis was performed after adjusting for age, education, hypertension, hyperlipidemia, diabetes mellitus, cardiac disease, previous stroke.
SE: standard error, BMI: body mass index, APOE: apolipoprotein E, K-BNT: Korean version of the Boston Naming Test, RCFT: Rey-Osterrieth Complex Figure Test, SVLT: Seoul Verbal Learning Test, COWAT: Controlled Oral Word Association Test, MMSE: Mini-Mental State Examination, CDRSB: clinical dementia rating sum of boxes.
*p < 0.05, †p < 0.01, ‡p < 0.001.
For the effect of the APOE genotype, in the APOE ε4 carrier group, there was a significant relationship only with memory tasks. As BMI increased in the APOE ε4 carriers, scores significantly increased in the SVLT delay recall (p=0.017) and RCFT delay recall (p=0.002) tasks. In the APOE ε4 non-carrier group, there was a significant positive association between BMI and K-BNT (p<0.001), animal fluency (p=0.022), phonemic fluency (p=0.018), Stroop test (p=0.002), and K-MMSE (p=0.002), but CDRSB (p=0.003) had a negative association with BMI. The linear regression showed that the BMI × APOE ε4 status interaction revealed significant differences only in memory. This result suggested that BMI affects the memory of APOE carriers as a modifying factor. The effect of sex was further analyzed, but we did not find the significant results we expected.
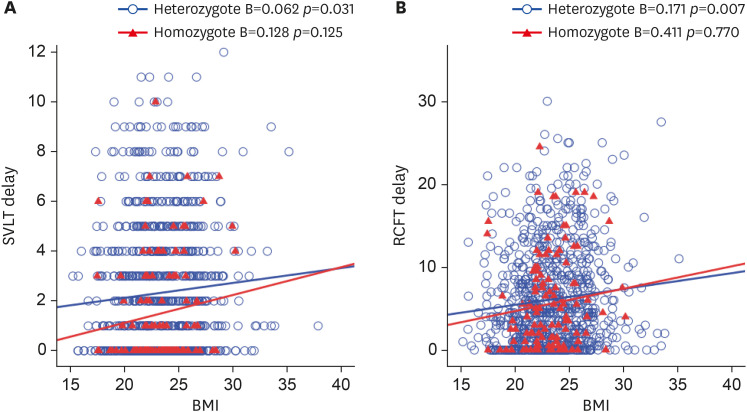
Next, we analyzed the association between homozygote (n=123) and heterozygote (n=865) in the APOE ε4 carrier group (Fig. 2). Positive associations between BMI and verbal memory (p=0.031) and visual memory (p=0.007) were significant only in the heterozygote group.
Fig. 2. Regression analysis for the association of BMI and memory test among apolipoprotein E ε4 heterozygote and homozygote groups. (A, B), values of the X axis represent BMI (kg/m2) and values of the Y axis represent SVLT delay and RCFT delay, respectively.
BMI: body mass index, SVLT: Seoul Verbal Learning Test, RCFT: Rey-Osterrieth Complex Figure Test.
DISCUSSION
In this study we report relationships between BMI and cognitive function with modifying effects of APOE ε4 genotype and sex. First, higher BMI was associated with better cognitive functions in late-life MCI patients. Second, in the APOE ε4 carriers, there was a positive association only between the BMI and the memory task, and performance in memory was improved as BMI increased, but a sex difference was not observed. Specifically, a significant association between the APOE ε4 gene and memory was found only in the heterozygote group. Conversely, cognitive functions (language, frontal function, and general index) excluding memory were improved as BMI increased in the APOE ε4 non-carrier group
We found that neuropsychological test performance is improved as BMI increases in MCI patients. This corresponds with previous studies’ demonstration that in MCI patients, the overweight and obese had less risk of cognitive decline than did those with normal BMI.32,33 The underweight MCI group had an increased risk for progression to AD34,35 in longitudinal follow-up studies. Furthermore, patients with low BMI correlated with structural alterations, including more advanced medial temporal atrophy and more microbleeds.36 In contrast to people with low BMI, normal older adults with high BMI had a better cognitive function, a slow rate of cognitive decline, and a decreased risk of developing dementia.5,8,37 Our finding suggested that a high BMI index is a predictor of cognitive function and good health status in late life, as evidenced by the “obesity paradox.”
We also found that APOE ε4 carriers were associated with only memory tests, because BMI increased regardless of sex. Not surprisingly, a significant positive relationship between BMI and memory was found only in the APOE ε4 heterozygote group. These results were consistent with some previous studies. Overweight and obese patients performed better on memory recall tasks than did the underweight.33 Moreover, in one longitudinal study, those in their 50s who had significantly decreased memory scores over the next decade were associated with weight loss, and this trend was more pronounced among the underweight.38 The obese APOE ε4 carriers had a higher cognitive function score at baseline and less cognitive decline over time than did the underweight group.39 Another study found that cognitive decline was less pronounced in the obese APOE ε4 groups than in those with normal BMI33 The longitudinal study also demonstrated that low BMI was a risk factor for cognitive decline and dementia progression only in APOE carriers.40
Expanding upon these previous studies, our study has suggested that BMI is a significant modefying factor for the risk of dementia, even allowing for the APOE ε4 gene effect in MCI. The pathophysiological mechanism by which BMI triggers cognitive alteration has not yet been fully elucidated. Low body weight may be associated with poor intake of nutrition and with metabolic disturbances. The accumulation of Aβ in the APOE ε4 transporter is regulated by leptin signaling in the hypothalamus and promotes weight loss.39 High BMI, reflecting healthy nutritional status,41 associated with higher levels of leptin and adiponectin, may bring about oxidative damage to the neuronal cell membrane and facilitate hippocampal synaptic plasticity and improved cognitive function.42,43,44 Healthy lifestyle prevented cognitive decline and diminished the risk of AD even given the effect of APOE ε4.45,46,47 Therefore, high BMI in older MCI patients can indicate a healthy physical condition. It seems essential to maintain healthy body weight to stably preserve cognitive function in patients.
On the other hand, the positive association between BMI and verbal/visual memory was found only in the heterozygote group. Generally, having one ε4 allele increases the risk of developing AD three times, and having two ε4 alleles is twelve times more likely to develop AD than are non-carriers48 and accelerates the age of onset.49 The APOE ε4 homozygote carriers showed more severe memory loss than did the heterozygote group.50,51 These results indicate that the genetic influence of APOE ε4/ε4 is strong, and environmental factors as a protective factor have insignificant effects on individual cognitive function and dementia risk. Hence specific intervention is required according to the APOE ε4 subtype.
Last, we found no sex difference in the effect of BMI and APOE status on cognitive function, despite distinct effects of the APOE according to sex. One study revealed that increased BMI was associated with diminished cognitive function in males but not in females,52 whereas Hayes et al.12 found no sex difference in the association. This finding requires further study and confirmation, because sex-dependent relationships in the risk of developing AD and cognitive decrease according to APOE ε4 have shown conflicting results.
There are some limitations to this study. First, BMI is a proxy to measure an individual's obesity; in order to measure obesity precisely, variables such as waist circumference, percent body fat, and lean muscle mass should be included. Second, we did not use cerebrospinal fluid analysis, amyloid PET imaging, or other pathological studies to established AD pathology in this study. However, MCI was defined by the clinical criteria as well as by comprehensive neuropsychological test batteries. Some previous studies have reported that cognitive function declines when the BMI is lower than 18.5 kg/m2 or higher than 30 kg/m2. However, the same results were not obtained in this study (Supplementary Table 1), because the data for this study did not consider the sample size of the BMI subgroup. Also, Asians follow the BMI standards of the WHO-Westen Pacific Regional Office (WHO-WPRO) and are classified as overweight or obese differently from Westerners; So, there are not many elderly people with severely obese BMI over 30 in Korea. Therefore, further research on the difference between the underweight and severely obese groups is needed to show how high an individual's BMI is appropriate. In addition, longitudinal changes should be looked at together to clarify their causal relationship.
In conclusion, we investigated the association between BMI and comprehensive neuropsychological test batteries using a large sample of as an older MCI patients. The results highlight the importance of high BMI, which as a marker of good physical status may indicate improved cognitive function, and furthermore distinguished a subgroup of APOE ε4 allele carriers associated with BMI and cognition. Therefore, physical status and APOE ε4 genotype-specific strategies in the assessments and treatments may be necessary for elderly patients with MCI.
Footnotes
Funding: This research was supported by the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2018M3C7A1057137 and NRF2020M3E5D2A01084721) and by the grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HU20C0271 and HU21C0016).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Mun YS, Park HK.
- Data curation: Mun YS, Kim J.
- Formal analysis: Mun YS, Kim J.
- Funding acquisition: Choi SH.
- Investigation: Mun YS, Park HK.
- Methodology: Mun YS, Park HK.
- Project administration: Mun YS, Park HK.
- Supervision: Jeong JH, Choi SH.
- Validation: Jeong JHs, Choi SH.
- Visualization: Mun YS.
- Writing - original draft: Mun YS, Park HK, Jeong JH.
- Writing - review & editing: Mun YS, Park HK, Kim J, Yeom J, Kim GH, Chun MY, Lee HA, Yoon SJ, Park KW, Kim EJ, Yoon B, Jang JW, Hong JY, Choi SH, Jeong JH.
SUPPLEMENTARY MATERIAL
Comparison of neuropsychological test performance between BMI subgroups
References
- 1.Petersen RC, Lopez O, Armstrong MJ, Getchius TS, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 6.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72:1741–1746. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45:14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 8.Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow-up of a birth cohort. Diabetes Care. 2011;34:1986–1991. doi: 10.2337/dc10-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008;56:111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank study. Radiology. 2019;291:763–771. doi: 10.1148/radiol.2019181012. [DOI] [PubMed] [Google Scholar]
- 12.Hayes JP, Moody JN, Roca JG, Hayes SM Alzheimer's Disease Neuroimaging Initiative. Body mass index is associated with smaller medial temporal lobe volume in those at risk for Alzheimer's disease. Neuroimage Clin. 2020;25:102156. doi: 10.1016/j.nicl.2019.102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zonneveld MH, Noordam R, van der Grond J, van Heemst D, Mooijaart SP, Sabayan B, et al. Interplay of circulating leptin and obesity in cognition and cerebral volumes in older adults. Peptides. 2021;135:170424. doi: 10.1016/j.peptides.2020.170424. [DOI] [PubMed] [Google Scholar]
- 14.Doorduijn AS, Visser M, van de Rest O, Kester MI, de Leeuw FA, Boesveldt S, et al. Associations of AD biomarkers and cognitive performance with nutritional status: the NUDAD project. Nutrients. 2019;11:1161. doi: 10.3390/nu11051161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer's disease, and dementia. J Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 16.Moser VA, Pike CJ. Obesity and sex interact in the regulation of Alzheimer's disease. Neurosci Biobehav Rev. 2016;67:102–118. doi: 10.1016/j.neubiorev.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers RH, Schaefer EJ, Wilson PW, D'Agostino R, Ordovas JM, Espino A, et al. Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neurology. 1996;46:673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- 18.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell SP, Liu D, Samuels LR, Shah AS, Gifford KA, Hohman TJ, et al. Late-life body mass index, rapid weight loss, apolipoprotein E ε4 and the risk of cognitive decline and incident dementia. J Nutr Health Aging. 2017;21:1259–1267. doi: 10.1007/s12603-017-0906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Ajnakina O, Steptoe A, Cadar D. Higher risk of dementia in English older individuals who are overweight or obese. Int J Epidemiol. 2020;49:1353–1365. doi: 10.1093/ije/dyaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman A, Jackson H, Hristov H, Isaacson RS, Saif N, Shetty T, et al. Sex and gender driven modifiers of Alzheimer's: the role for estrogenic control across age, race, medical, and lifestyle risks. Front Aging Neurosci. 2019;11:315. doi: 10.3389/fnagi.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HK, Na DL, Han SH, Kim JY, Cheong HK, Kim SY, et al. Clinical characteristics of a nationwide hospital-based registry of mild-to-moderate Alzheimer's disease patients in Korea: a CREDOS (Clinical Research Center for Dementia of South Korea) study. J Korean Med Sci. 2011;26:1219–1226. doi: 10.3346/jkms.2011.26.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 25.Hong YJ, Yoon B, Shim YS, Cho AH, Shin HE, Kim YI, et al. APOE ε4 allele status in Korean dementia patients with severe white matter hyperintensities. J Alzheimers Dis. 2011;24:519–524. doi: 10.3233/JAD-2011-101611. [DOI] [PubMed] [Google Scholar]
- 26.Noh Y, Lee Y, Seo SW, Jeong JH, Choi SH, Back JH, et al. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities. J Stroke Cerebrovasc Dis. 2014;23:636–642. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Shiwaku K, Anuurad E, Enkhmaa B, Kitajima K, Yamane Y. Appropriate BMI for Asian populations. Lancet. 2004;363:1077. doi: 10.1016/S0140-6736(04)15856-X. [DOI] [PubMed] [Google Scholar]
- 28.Kang Y, Jahng S, Na DL. Seoul Neuropsychological Screening Battery. (SNSBII): Professional Manual. Incheon: Human Brain Research and Consulting; 2012. [Google Scholar]
- 29.Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study) Arch Gerontol Geriatr. 2008;47:302–310. doi: 10.1016/j.archger.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Choi SH, Lee BH, Kim S, Hahm DS, Jeong JH, Yoon SJ, et al. Interchanging scores between clinical dementia rating scale and global deterioration scale. Alzheimer Dis Assoc Disord. 2003;17:98–105. doi: 10.1097/00002093-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 32.Doruk H, Naharci MI, Bozoglu E, Isik AT, Kilic S. The relationship between body mass index and incidental mild cognitive impairment, Alzheimer's disease and vascular dementia in elderly. J Nutr Health Aging. 2010;14:834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- 33.Rajan KB, Skarupski KA, Rasmussen HE, Evans DA. Gene-environment interaction of body mass index and apolipoprotein E ε4 allele on cognitive decline. Alzheimer Dis Assoc Disord. 2014;28:134–140. doi: 10.1097/WAD.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joo SH, Yun SH, Kang DW, Hahn CT, Lim HK, Lee CU. Body mass index in mild cognitive impairment according to age, sex, cognitive intervention, and hypertension and risk of progression to Alzheimer's disease. Front Psychiatry. 2018;9:142. doi: 10.3389/fpsyt.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye BS, Jang EY, Kim SY, Kim EJ, Park SA, Lee Y, et al. Unstable body mass index and progression to probable Alzheimer's disease dementia in patients with amnestic mild cognitive impairment. J Alzheimers Dis. 2016;49:483–491. doi: 10.3233/JAD-150556. [DOI] [PubMed] [Google Scholar]
- 36.Verhaar BJ, de Leeuw FA, Doorduijn AS, Fieldhouse JL, van de Rest O, Teunissen CE, et al. Nutritional status and structural brain changes in Alzheimer's disease: The NUDAD project. Alzheimers Dement (Amst) 2020;12:e12063. doi: 10.1002/dad2.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 38.Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes. 2015;39:1383–1389. doi: 10.1038/ijo.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blautzik J, Kotz S, Brendel M, Sauerbeck J, Vettermann F, Winter Y, et al. Relationship between body mass index, ApoE4 status, and PET-based amyloid and neurodegeneration markers in amyloid-positive subjects with normal cognition or mild cognitive impairment. J Alzheimers Dis. 2018;65:781–791. doi: 10.3233/JAD-170064. [DOI] [PubMed] [Google Scholar]
- 40.Sachs-Ericsson NJ, Sawyer KA, Corsentino EA, Collins NA, Blazer DG. APOE \xcf\xb54 allele carriers: biological, psychological, and social variables associated with cognitive impairment. Aging Ment Health. 2010;14:679–691. doi: 10.1080/13607860903292594. [DOI] [PubMed] [Google Scholar]
- 41.Jang JW, Kim Y, Choi YH, Lee JM, Yoon B, Park KW, et al. Association of nutritional status with cognitive stage in the elderly Korean population: the Korean Brain Aging Study for the early diagnosis and prediction of Alzheimer's disease. J Clin Neurol. 2019;15:292–300. doi: 10.3988/jcn.2019.15.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forny-Germano L, De Felice FG, Vieira MN. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer's disease. Front Neurosci. 2019;12:1027. doi: 10.3389/fnins.2018.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sergi G, De Rui M, Coin A, Inelmen EM, Manzato E. Weight loss and Alzheimer's disease: temporal and aetiologic connections. Proc Nutr Soc. 2013;72:160–165. doi: 10.1017/S0029665112002753. [DOI] [PubMed] [Google Scholar]
- 45.Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuźma E, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322:430–437. doi: 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer's disease, and dementia: from FINGER to world-wide FINGERS. J Prev Alzheimers Dis. 2020;7:29–36. doi: 10.14283/jpad.2019.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon A, Turunen H, Ngandu T, Peltonen M, Levälahti E, Helisalmi S, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462–470. doi: 10.1001/jamaneurol.2017.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 50.Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, et al. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66:329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hestad K, Engedal K, Horndalsveen P, Strand BH. Cognition in patients with memory difficulties and dementia relative to APOE e4 status. Front Psychol. 2021;12:686036. doi: 10.3389/fpsyg.2021.686036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M. Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Transl Psychiatry. 2018;8:18. doi: 10.1038/s41398-017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of neuropsychological test performance between BMI subgroups