Abstract
In geographical areas with a low incidence of tuberculosis, recurrent tuberculosis is generally due to reactivation of the disease. However, the relative contribution of tuberculosis reinfection increases in parallel with the incidence of disease and is likely to depend on the epidemiological context: factors such as the spread of multidrug resistance, human immunodeficiency virus (HIV) infection, and immigration from developing countries could modify disease transmission in areas at low risk for tuberculosis. A molecular epidemiology study was performed in Lombardy, Northern Italy, where the incidence of tuberculosis is 17.5 cases per 100,000 persons. A total of 2,452 cases of culture-confirmed tuberculosis in 2,127 patients were studied. A group of 32 patients (1.5%), each of whom had two episodes of tuberculosis with cure as the outcome of the first episode and with more than 6 months between the two episodes, were studied by means of restriction fragment length polymorphism DNA fingerprinting analysis. For 5 of the 32 patients (16%), the DNA fingerprinting patterns of Mycobacterium tuberculosis strains responsible for the second episode did not match those of the corresponding isolates of the first episode, indicating exogenous reinfection. Two of these patients developed multidrug-resistant tuberculosis during the second episode, and in three cases the isolates belonged to clusters of M. tuberculosis strains spreading in the community. A fourfold-increased risk for reinfection was observed in immigrant patients compared to Italian subjects. In contrast, a higher risk of relapse rather than reinfection was evidenced in HIV-positive subjects and in patients infected with multidrug-resistant tuberculosis. Episodes of tuberculosis reinfection in areas with a low incidence of tuberculosis are rare compared to those in high-incidence geographical regions. In populations that have immigrated from high-risk areas, reinfection may represent a considerable contributor to the rate of recurrent tuberculosis. This finding emphasizes the importance of containing the spread of epidemic strains in close communities, in order to prevent changes in global tuberculosis trends for developed countries.
Active tuberculosis in patients with prior tuberculous infection can occur following endogenous reactivation or exogenous reinfection. For decades, the issue of the role of exogenous reinfection has been debated. Recent models based on estimates of the annual risk of infection and the incidence of tuberculosis have suggested that the relative contribution of exogenous reinfection increases in parallel with the incidence of the disease (2, 26). Molecular biology-based methods have been shown useful in differentiating Mycobacterium tuberculosis strains, demonstrating whether a new episode of tuberculosis is caused by infection with the same strain as the previous episode or by a different strain (7, 20). Recently, a study carried out in a metropolitan area of South Africa, using DNA fingerprinting to examine isolates of M. tuberculosis, found evidence that exogenous reinfection can have a dominant role in the pathogenesis of postprimary tuberculosis in an area with a high incidence of the disease (24). A different scenario could be evoked for a population with a low risk of infection, where the likelihood of reexposure is small and thus most cases of recurrence probably result from relapse. However, specific phenomena, such as the emergence of human immunodeficiency virus (HIV)-related tuberculosis outbreaks or immigration from high-incidence countries, could modify the evolution of tuberculosis transmission. Since 1993, Northern Italy has been our point of observation for investigating some aspects of this issue by means of molecular epidemiology techniques. The occurrence of tuberculosis in the Lombardy region was characterized by a general increase in the early 1990s, with immigrants and HIV-infected patients as the most important sources of tuberculosis among the younger age groups. The resurgence of tuberculosis in this area has also been accompanied by alarming outbreaks of the disease caused by organisms resistant to multiple antituberculous drugs (9, 10, 14). A large proportion of patients infected with these organisms have been coinfected with HIV, and the fatality rate has been extremely high.
More recently, the global incidence of tuberculosis has begun to decrease again, but cases among immigrants are still on the increase in both absolute and relative terms. During the period between 1993 and 1996, the approximate annual rates for Italian-born and immigrant patients were 14.4 per 100,000 and 109 per 100,000, respectively (4).
In this study we determined the relative frequencies of relapse and exogenous reinfection, using DNA fingerprinting for patients with episodes of recurrent tuberculosis in a geographic area where the risk of infection is low but where tuberculosis outbreaks among HIV-infected patients, caused by organisms resistant to multiple antituberculous drugs, have been reported in recent years. We also considered the possible role of immigrants from developing countries in maintaining a high rate of tuberculosis reinfection in the population.
MATERIALS AND METHODS
Patient population and data collection.
All tuberculosis cases, confirmed by examination of cultures, from the Reference Centre for Tuberculosis Control of Lombardy, Villa Marelli, and from two representative infectious diseases in Lombardy wards (L. Sacco Hospital of Milan and Spedali Civili of Brescia) were included in this study. Lombardy is the most highly populated region of Northern Italy (about 9 million inhabitants) and accounts for as many as 30% of Italian AIDS cases, with a considerable concentration of immigrants from developing countries. In 1995 we initiated a prospective survey study in which all collected cultures positive for M. tuberculosis were genotyped by restriction fragment length polymorphism (RFLP) fingerprinting and spoligotyping analysis (for M. tuberculosis isolates with fewer than five bands). A database of the results was also established. Patients included in this study had at least two episodes of tuberculosis, with cure as the outcome of the first episode, within the study period (between January 1995 and December 1999). Cure was defined as the completion of a course of 6 months of combination therapy, a sputum culture positive for M. tuberculosis at diagnosis, and at least one negative sputum culture at the end of treatment. Recurrence was defined as development of a culture positive for M. tuberculosis and symptoms consistent with tuberculosis after the patient had completed a course of treatment and had been confirmed culture negative and clinically recovered. Patients who needed treatment for a subsequent episode but who did not meet criteria for cure, since less than 6 months had elapsed between the two episodes, were considered “not cured” and were excluded from the study. A patient whose M. tuberculosis isolates from the first and second episodes were different upon RFLP analysis was considered to have tuberculosis due to a new, exogenous reinfection. Clinical records of the patients were obtained, including data on age, sex, country of birth, date of arrival in Italy (for the immigrants), medical status with regard to HIV infection, drug susceptibility, treatment, and outcome.
Clinical specimen collection.
Primary mycobacterial isolation was performed using Löwenstein-Jensen slant cultures and a radiometric method (BACTEC; Middlebrook 7H12; Becton Dickinson, Diagnostic Instrumental System, Towson, Md.). Drugs and concentrations used for susceptibility testing for M. tuberculosis isolates included isoniazid (1 mg/μl), ethambutol (5 mg/μl), rifampin (1 mg/μl), and streptomycin (10 mg/μl).
RFLP DNA fingerprinting analysis.
DNA extraction, digestion, and Southern blotting were carried out as described previously (25). M. tuberculosis isolates were genotyped by RFLP fingerprinting analysis using the IS6110 probe as a genetic marker, as previously described by van Embden et al. (23).
Spoligotyping method.
Spoligotyping, a PCR method based on the polymorphic direct-repeat (DR) region containing 36-bp direct repeats and interspersed 35- to 41-bp variable spacer sequences, was performed on genomic DNA by standard methods as previously described by Kamerbeek and others (13, 22).
Computer-assisted analysis of the patterns.
GelCompar software, version 4.1 (Applied Maths BVBA, Kortrijk, Belgium), was used to compare the hybridization patterns obtained by RFLP DNA fingerprinting and spoligotyping. After the results of the analysis, each patient's isolate were compared with our database, which contains more than 3,000 DNA patterns derived from tuberculosis cases analyzed from January 1994 to March 2000, to determine whether the isolates belonged to a cluster or were unique within the communities studied.
Statistical analysis.
Differences in rates of tuberculosis reinfection or relapse between groups were assessed by Fisher's exact test and the chi-square test with the Pearson correlation coefficient. Univariate and multivariate analyses were performed, evaluating crude and adjusted risks through a logistic regression model. Statistical significance was defined as a P value of <0.05.
RESULTS
During the study period (January 1995 through December 1999) a total of 2,452 M. tuberculosis cultures from 2,127 patients were available for RFLP analysis. Italian citizens accounted for 86% of the episodes, and immigrants accounted for 14% of the episodes. Among the 2,127 patients, 448 (21%) were known to be infected with HIV. Thirty-two of the 2,127 patients (1.5%) met the selection criteria, in that they had two successive episodes of pulmonary tuberculosis within the study period with more than 6 months between those two episodes. The median age was 49 years. The median interval between cure and subsequent diagnosis was 19 months (range, 7 to 60 months). Of the 32 patients with tuberculosis relapse, 6 were immigrants from developing countries (1 from Senegal, 2 from Peru, 1 from China, 1 from Morocco, and 1 from Brazil) (Table 1).
TABLE 1.
Characteristics of 32 patients with tuberculosis recurrence
| Patient no. | Age (yr)/sexa | Interval between episodes (mo) | Birth country | HIV statusb | Drug resistancec
|
No. of secondary cases
|
Exogenous reinfection | ||
|---|---|---|---|---|---|---|---|---|---|
| 1st episode | 2nd episode | 1st episode | 2nd episode | ||||||
| 1 | 28/M | 39 | Morocco | Neg | Rif, Inh, Str | Rif, Inh, Str | Not clustered | Not clustered | No |
| 2 | 39/F | 24 | Italy | Pos | Rif, Inh, Str | Rif, Inh, Str | 0 | 1 | No |
| 3 | 52/M | 10 | Italy | Neg | Inh | Inh | Not clustered | Not clustered | No |
| 4 | 56/M | 8 | Italy | Pos | Inh, Str | Rif, Inh, Emb, Str | Not clustered | 1 | Yes |
| 5 | 36/F | 9 | Italy | Neg | Susceptible | Susceptible | 1 | 1 | No |
| 6 | 43/M | 17 | China | Neg | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 0 | 1 | No |
| 7 | 66/M | 13 | Italy | Neg | Susceptible | Susceptible | Not clustered | Not clustered | No |
| 8 | 56/M | 9 | Italy | Neg | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 0 | 9 | No |
| 9 | 51/M | 25 | Senegal | Neg | Susceptible | Susceptible | Not clustered | Not clustered | Yes |
| 10 | 50/M | 7 | Italy | Pos | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 165 | 176 | No |
| 11 | 34/M | 13 | Italy | Pos | Str | Str | 1 | 3 | No |
| 12 | NDd/M | 60 | Italy | Neg | Susceptible | Susceptible | Not clustered | Not clustered | Yes |
| 13 | 35/M | 13 | Italy | Pos | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 107 | 176 | No |
| 14 | 37/M | 24 | Italy | Pos | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 4 | 7 | No |
| 15 | 33/F | 12 | Italy | Pos | Rif, Inh | Rif, Inh | 3 | 4 | No |
| 16 | 22/M | 7 | Brazil | Pos | Susceptible | Susceptible | 1 | 3 | No |
| 17 | 73/F | 12 | Italy | Neg | Inh, Str | Inh, Str | Not clustered | Not clustered | No |
| 18 | 31/F | 6 | Peru | Neg | Rif, Inh, Str (2)e | Susceptible | 1 | 2 | Yes |
| 19 | 76/F | 12 | Italy | Neg | Susceptible | Susceptible | 0 | 1 | No |
| 20 | 60/M | 39 | Italy | Pos | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 8 | 15 | No |
| 21 | NDd/F | 17 | Italy | Neg | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 1 | 1 | No |
| 22 | 45/F | 26 | Italy | Neg | Rif | Rif | 1 | 1 | No |
| 23 | 43/M | 12 | Italy | Neg | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 1 | 1 | No |
| 24 | 55/M | 13 | Italy | Neg | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | 2 | 3 | No |
| 25 | 41/M | 21 | Italy | Neg | Susceptible | Susceptible | 0 | 1 | No |
| 26 | 53/M | 60 | Italy | Neg | Rif, Inh, Str | Rif, Inh, Str | 0 | 2 | No |
| 27 | 69/M | 24 | Italy | Neg | Inh | Inh | 0 | 1 | No |
| 28 | 58/M | 7 | Italy | Neg | Rif, Inh, Emb | Rif, Inh, Emb | Not clustered | Not clustered | No |
| 29 | 64/M | 24 | Italy | Neg | Susceptible | Rif, Inh | Not clustered | 39 | Yes |
| 30 | 28/M | 9 | Peru | Pos | Inh | Inh | Not clustered | Not clustered | No |
| 31 | 70/M | 13 | Italy | Neg | Rif, Inh, Emb, Str | Rif, Inh, Emb, Str | Not clustered | Not clustered | No |
| 32 | 57/M | 24 | Italy | Neg | Inh | Inh | Not clustered | Not clustered | No |
M, male; F, female.
Neg, negative; Pos, positive.
Rif, rifampin; Inh, isoniazid; Str, streptomycin; Emb, ethambutol.
ND, not determined
(2), two episodes of TB with the same MDR strain.
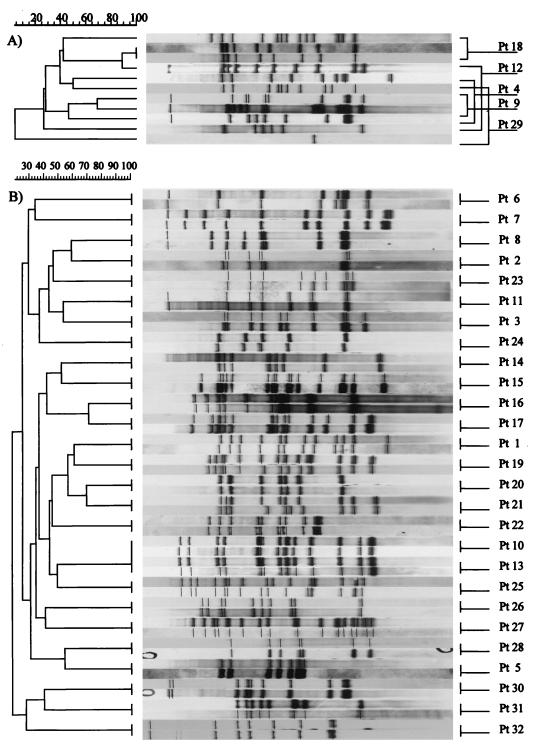
In 5 cases (16%) the RFLP patterns of the M. tuberculosis strains responsible for the episode of recurrence did not match those of the corresponding isolates of the first episode, indicating exogenous reinfection, whereas 27 patients (84%) had the same DNA fingerprinting for both episodes of tuberculosis (Fig. 1).
FIG. 1.
RFLP analysis drendograms showing the genotypic relationship among strains isolated from 32 patients with recurrence of tuberculosis. (A) Patients with tuberculosis reinfection; (B) patients with tuberculosis reactivation. Pt, patient.
Three of the five patients with presumed exogenous reinfection were Italian (one of whom was positive for HIV infection), and two were HIV-negative immigrants from developing countries (Senegal and Peru). Two patients whose initial isolates had been drug-sensitive strains had organisms resistant to isoniazid and rifampin during the second, genotypically different episode. Two patients had episodes caused by two different drug-sensitive strains. The last patient initially had a drug-resistant isolate that caused two successive episodes of pulmonary tuberculosis; 8 months after the beginning of the second episode, a drug-sensitive strain of M. tuberculosis showing a different RFLP type was isolated. Isolates from the five patients with presumed reinfection were studied in relation to the complete RFLP database. Of the M. tuberculosis isolates responsible for the first episode, only one belonged to a cluster of strains present in the community. The isolates responsible for reinfection in three of five cases belonged to clusters of M. tuberculosis strains present in the community; for the remaining two isolates no matching strains were identified in the database. One of the clustered strains isolated from an HIV-negative immigrant patient was a drug-sensitive strain belonging to a cluster containing only two other strains, both found in HIV-negative Italian patients. The other two clustered strains responsible for exogenous reinfection were multidrug resistant: the first, found in an Italian HIV-positive patient, matched another strain isolated from a 92-year-old Italian HIV-negative patient; the other was a multidrug-resistant strain of Mycobacterium bovis isolated from an Italian HIV-negative patient. Genotypic analysis performed by both IS6110 RFLP and spoligotyping showed that this strain originated from a large outbreak which occurred in an Infectious Diseases Department in Lombardy between March 1993 and May 1995, involving 37 HIV-positive patients and 3 HIV-negative patients.
The other 27 patients (84%) showed two identical RFLP patterns for the two episodes, suggesting endogenous reactivation. Nine patients (33%) were HIV positive. Four patients (15%) were immigrants from developing countries: two HIV-positive patients (from Brazil and Peru) and two HIV-negative patients (from Morocco and China). For 15 patients with the same isolates at the initial and the second episode (56%), the isolates responsible for both episodes showed multidrug resistance. The multidrug-resistant isolates responsible for reactivation in 12 of 15 cases belonged to clusters of strains present in the community. The molecular definition of exogenous reinfection used in this study excludes the possibility of exogenous reinfection with the identical strain. Therefore, it is possible that some of the patients considered to have relapses actually had new, exogenous reinfection with the same strain. Our results may thus underestimate the extent of exogenous reinfection. Among the 12 cases of infection with multidrug-resistant strains, 7 isolates associated with different nosocomial epidemics spreading in our region mainly involving HIV-positive patients. Among the drug-sensitive strains responsible for endogenous reactivation, 7 out of 12 belonged to clusters. Two were isolated from HIV-positive patients (a Brazilian and an Italian patient) sharing the same RFLP typing with other Italian patients with HIV infection (four patients for each cluster). The drug-sensitive strains isolated from five Italian patients without HIV infection were included in different clusters containing only isolates from Italian patients (both HIV positive and HIV negative) (two patients for each cluster).
The rates of reinfection were analyzed for different groups: HIV-positive versus HIV-negative patients, Italian residents versus immigrants, drug-susceptible versus drug-resistant isolates. Reinfection occurred in 3 (11.5%) of 26 Italian patients and in 2 (33.3%) of 6 immigrant patients. Reinfection was less frequent in patients with multidrug-resistant strains responsible for the first episode (11.8%) than in those who had a first episode sustained by susceptible strains (20%). Reinfection was less common among HIV patients (10%) than among patients without HIV infection (18.2%) (Table 2).
TABLE 2.
Comparison of rates of reinfection between groups by Fisher's exact test
| Characteristica | Reinfection | Reactivation | P |
|---|---|---|---|
| Patient characteristics | |||
| Age (median) | 53.5 yr | 47.5 yr | 0.708 |
| IDC (no./total) | 2/5 | 4/27 | 0.228 |
| HIV infection (no./total) | 1/5 | 9/27 | 1.000 |
| Interval (median) | 24 mo | 13 mo | 0.357 |
| M. tuberculosis strain characteristics | |||
| MDR∗ (no./total) | 2/5 | 15/27 | 0.645 |
| Clustered with HIV∗ (no./total) | 2/3 | 12/19 | 1.000 |
| Clustered with MDR∗ (no./total) | 1/3 | 11/19 | 0.571 |
| Clustered with IDC∗ (no./total) | 0/3 | 4/19 | 1.000 |
IDC, immigrants from developing countries; MDR, multidrug resistant. Asterisks refer to strains responsible for the second episode.
Results of multivariate analysis did not show any significant association. However, the analysis evidenced a fourfold risk of reinfection among immigrant patients (adjusted risk, 4.7; 95% confidence interval [95% CI], 0.48 to 45.9) compared to Italian patients. HIV infection was associated with a one-half risk of reinfection (adjusted risk, 0.45; 95% CI, 0.036 to 5.46), and the isolation of multidrug-resistant strains in the first episode was correlated with a one-third risk of reinfection (adjusted risk, 0.35; 95% CI, 0.0098 to 12.8) (Table 3).
TABLE 3.
Univariatea and multivariate risk factor analysis for tuberculosis reinfection
| Risk factor | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| Crude risk | 95% CI | P | Adjusted risk | 95% CI | P | |
| Immigration | 3.83 | 0.478–30.700 | 0.20 | 4.71 | 0.484–45.911 | 0.18 |
| HIV infection | 0.50 | 0.048–5.153 | 0.56 | 0.44 | 0.036–5.460 | 0.52 |
| MDR-TBb strains | 0.53 | 0.076–3.724 | 0.52 | 0.35 | 0.009–12.802 | 0.57 |
Chi-square test with the Pearson correlation coefficient.
MDR-TB, multidrug-resistant tuberculosis.
DISCUSSION
The possibility of persons previously infected with M. tuberculosis being exogenously reinfected has been debated for decades. However, it was supposed to occur rarely because of the immunity conferred by initial infection. The extent to which exogenous reinfection occurs depends on the prevalence of disease—the higher the prevalence, the greater the likelihood of exogenous reinfection (8, 19). On the few occasions on which exogenous reinfection has been documented in areas with a low incidence of the disease, it has involved only selected populations, for example, alcoholic residents of a homeless shelter or patients with advanced HIV infection (3, 11, 16, 21).
Our study confirmed that reinfection in areas with a low incidence of tuberculosis is possible, although less common than in high-incidence geographical regions, indicating that higher prevalence of M. tuberculosis represents the major risk for tuberculosis reinfection (24). Relapse of a previous infection remains, in our region, the more probable cause of recurrence. However, this scenario could be susceptible to change in the future, due to social, microbiological, and epidemiological factors. Using multivariate analysis we found no significant association between HIV, immigration, multidrug resistance, and the rate of reinfection. However, we observed a fourfold higher risk of reinfection in immigrant patients compared to Italian subjects. Cohesiveness within ethnic communities, overcrowding, and poor hygienic conditions allow for an elevated frequency of close contacts, with a consequent high circulation of M. tuberculosis strains, that could explain the increased risk of reinfection. This phenomenon plays a major role for immigrants coming from areas with a high incidence of tuberculosis. In a previous study on immigrants from developing countries, we demonstrated that the M. tuberculosis clustering ratio varies among immigrant groups, reflecting mostly the duration of stay in Italy and the social behaviors of the immigrants (4).
Recurrence of tuberculosis is a frequent event among HIV populations (6, 15, 17). We showed that HIV-infected patients were more prone to recurrences than people without HIV infection. The HIV patients had twice the risk of reactivation of a previous M. tuberculosis episode, demonstrated by correspondence of the RFLP genotypes. It is well known that the rate of relapse of infection due to the same M. tuberculosis strain is high in HIV-positive populations, especially in association with a low CD4+ cell count and with a duration of treatment shorter than 9 months (1, 12, 18, 27). Even though recent studies have shown similar rates of tuberculosis relapse in HIV-positive and HIV-negative patients after successful directly observed therapy (5), the higher occurrence of relapse in our HIV-positive population could be first explained by the low compliance of our patients (frequently intravenous drug users) with the antituberculosis treatment. Due to inadequate drug adherence, an apparent clinical and microbiological response was observed after the first episode, regardless of incomplete elimination of M. tuberculosis bacilli. Moreover, in our region, the emergence of multidrug-resistant tuberculosis outbreaks among HIV-positive patients had been a critical issue. The difficulty of finding sustained successful treatments for those particular strains associated with the immunological decline of HIV-positive patients has represented a key factor for the increased risk of reactivation of multidrug-resistant disease.
The importance of multidrug-resistant strains was also evaluated in our study, which detected an increased risk of relapse in patients whose first episode was attributable to multidrug-resistant strains, compared to those whose first episode was caused by susceptible strains. This observation confirms the difficulty of treating and eradicating multidrug-resistant tuberculosis, mainly when associated with HIV infection and low compliance of patients.
In conclusion, our data seem to confirm that reinfection is possible among people in developed countries, but at rates lower than those in high-risk areas. However, this scenario could be modified in the future by the presence of high-risk populations. In particular, for immigrant populations from high-risk areas, especially when they live in poor socioeconomic conditions, reinfection may be a major contributor to the overall rate of tuberculosis in adults.
ACKNOWLEDGMENTS
We thank Bianca Ghisi for computer counseling, Stefano Rusconi for valuable help, Elizabeth Kaplan and Alan Michael Rosen for professional language assistance, and Mauro Moroni for helpful discussion.
This work was supported by a grant from the Italian National Institute of Health, 2nd National Tuberculosis Project.
REFERENCES
- 1.Ackah A N, Coulibaly D, Digbeu H, Diallo K, Vetter K M, Coulibaly I M, Greenberg A E, De Cock K M. Response to treatment, mortality, and CD4 lymphocyte counts in HIV-infected persons with tuberculosis in Abidjan, Cote d'Ivoire. Lancet. 1995;345:607–610. doi: 10.1016/s0140-6736(95)90519-7. [DOI] [PubMed] [Google Scholar]
- 2.Canetti G, Sutherland I, Svandova E. Endogenous reactivation and exogenous reinfection: their relative importance with regard to the development of non-primary tuberculosis. Bull Int Union Tuberc. 1972;47:116–134. [PubMed] [Google Scholar]
- 3.Chaves F, Dronda F, Alonso-Sanz M, Noriega A R. Evidence of exogenous reinfection and mixed infection with more than one strain of Mycobacterium tuberculosis among Spanish HIV-infected inmates. AIDS. 1999;13:615–620. doi: 10.1097/00002030-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 4.Codecasa L R, Porretta A D, Gori A, Franzetti F, Degli Esposti A, Lizioli A, Carreri V, Di Proietto M C, Perozziello F, Besozzi G. Tuberculosis among immigrants from developing countries in the province of Milan, 1993–1996. Int J Tuberc Lung Dis. 1999;3:589–595. [PubMed] [Google Scholar]
- 5.Connolly C, Reid A, Davies G, Sturm W, McAdam K P, Wilkinson D. Relapse and mortality among HIV-infected and uninfected patients with tuberculosis successfully treated with twice weekly directly observed therapy in rural South Africa. AIDS. 1999;13:1543–1547. doi: 10.1097/00002030-199908200-00015. [DOI] [PubMed] [Google Scholar]
- 6.Daley C L, Small P M, Schecter G F, Schoolnik G K, McAdam R A, Jacobs W R, Jr, Hopewell P C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Chan S L, Allen B W, Mitchison D A, Lawrie D B. Application of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis patients in Hong Kong before, during and after short-course chemotherapy. Tuber Lung Dis. 1993;74:48–51. doi: 10.1016/0962-8479(93)90068-9. [DOI] [PubMed] [Google Scholar]
- 8.Fine P E M, Small P M. Exogenous reinfection in tuberculosis. N Engl J Med. 1999;341:1226–1227. doi: 10.1056/NEJM199910143411609. [DOI] [PubMed] [Google Scholar]
- 9.Franzetti F, Gori A, Iemoli E, Meraviglia P, Mainini F, Quirino T, degli Esposti A, degl'Innocenti M, Grassini A, Nardi G, Cargnel A. Outcome of multidrug-resistant tuberculosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;29:553–560. doi: 10.1086/598633. [DOI] [PubMed] [Google Scholar]
- 10.Gori A, Marchetti G, Catozzi L, Nigro C, Ferrario G, Rossi M C, Degli Esposti A, Orani A, Franzetti F. Molecular epidemiology characterization of a multidrug-resistant Mycobacterium bovis outbreak among HIV-positive patients. AIDS. 1998;12:445–446. [PubMed] [Google Scholar]
- 11.Horn D L, Hewlett Jr D, Haas W H, Butler W R, Alfalla C, Tan E, Levine A, Nayak A, Opal S M. Superinfection with rifampin-isoniazid-streptomycin-ethambutol (RISE)-resistant tuberculosis in three patients with AIDS: confirmation by polymerase chain reaction fingerprinting. Ann Intern Med. 1994;121:115–116. doi: 10.7326/0003-4819-121-2-199407150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Johnson J, Okwera A, Vjecha M J, Byekwaso F, Nakibali J, Nyole S, Milberg J, Aisu T, Whalen C C, Mugerwa R D, Elner J J. Risk factors for relapse in human immunodeficiency virus type 1-infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1:446–453. [PubMed] [Google Scholar]
- 13.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moro M L, Gori A, Errante I, Infuso A, Franzetti F, Sodano L, Iemoli E the Italian Multidrug-Resistant Tuberculosis Outbreak Study Group. An outbreak of multidrug-resistant tuberculosis involving HIV-infected patients of two hospitals in Milan, Italy. AIDS. 1998;12:1095–1102. [PubMed] [Google Scholar]
- 15.Murray J, Sonnenberg P, Shearer S C, Godfrey-Faussett P. Human immunodeficiency virus and the outcome of treatment for new and recurrent pulmonary tuberculosis in African patients. Am J Respir Crit Care Med. 1999;159:733–740. doi: 10.1164/ajrccm.159.3.9804147. [DOI] [PubMed] [Google Scholar]
- 16.Nardell E, McInnis B, Thomas B, Weidhaas S. Exogenous reinfection with tuberculosis in a shelter for the homeless. N Engl J Med. 1986;315:1570–1575. doi: 10.1056/NEJM198612183152502. [DOI] [PubMed] [Google Scholar]
- 17.Nunn P P, Elliott A M, McAdam K P. Tropical respiratory medicine. 2. Impact of human immunodeficiency virus on tuberculosis in developing countries. Thorax. 1994;49:511–518. doi: 10.1136/thx.49.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulido F, Pena J M, Rubio R, Moreno S, Gonzalez J, Guijarro C, Costa J R, Vazquez J J. Relapse of tuberculosis after treatment in human immunodeficiency virus-infected patients. Arch Intern Med. 1997;157:227–232. [PubMed] [Google Scholar]
- 19.Romeyn J A. Exogenous reinfection in tuberculosis. Am Rev Respir Dis. 1970;101:923–927. doi: 10.1164/arrd.1970.101.6.923. [DOI] [PubMed] [Google Scholar]
- 20.Sahadevan R, Narayanan S, Paramasivan C N, Prabhakar R, Narayanan P R. Restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, India, by use of direct-repeat probe. J Clin Microbiol. 1995;33:3037–3039. doi: 10.1128/jcm.33.11.3037-3039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small P M, Shafer R W, Hopewell P C, Singh S P, Murphy M J, Desmond E, Sierra M F, Schoolnik G K. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med. 1993;328:1137–1144. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
- 22.Sola C, Horgen L, Maisetti J, Devallois A, Goh K S, Rastogi N. Spoligotyping followed by double-repetitive-element PCR as rapid alternative to IS6110 fingerprinting for epidemiological studies of tuberculosis. J Clin Microbiol. 1998;36:1122–1124. doi: 10.1128/jcm.36.4.1122-1124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Embden J D, Cave M D, Crawford J T, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rie A, Warren R, Richardson M, Victor T C, Gie R P, Enarson D A, Beyers N, van Helden P D. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–1179. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 25.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D. Occurence and stability of insertion sequences in Mycobacterium tuberculosis complex strains; evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vynnycky E, Fine P E M. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whalen C, Okwera A, Johnson J, Vjecha M, Hom D, Wallis R, Huebner R, Mugerwa R, Ellner J. Predictors of survival in human immunodeficiency virus-infected patients with pulmonary tuberculosis. The Makerere University-Case Western Reserve University Research Collaboration. Am J Respir Crit Care Med. 1996;153:1977–1981. doi: 10.1164/ajrccm.153.6.8665064. [DOI] [PubMed] [Google Scholar]