Abstract
Background
The purpose of this study was to investigate the safety of outpatient and inpatient total shoulder arthroplasty (TSA) and to investigate changes over time.
Methods
Patients undergoing primary TSA during 2006-2019 as part of the American College of Surgeons National Surgical Quality Improvement Program were identified. Patients were divided into an early cohort (2006-2016, 12,401 patients) and a late cohort (2017-2019, 12,845 patients). Outpatient procedures were defined as those discharged on the day of surgery. Patient comorbidities and rate of adverse events within 30 days postoperatively were compared with adjustment for baseline characteristics using standard multivariate regression.
Results
There was a significant reduction in complications over time when considering all cases (5.69% in the early cohort vs. 3.67% in the late cohort, adjusted relative risk [RR] = 0.65, 95% confidence interval [CI] = 0.58-0.73, P < .001). The rate of complications decreased over time among inpatients (5.80% vs. 3.90%, adjusted RR = 0.68, 95% CI = 0.60-0.76, P < .001). However, there was no difference in the rate of complications among outpatients over time (1.98% vs. 1.38%, adjusted RR = 0.64, 95% CI = 0.28-1.47, P = .293). There were significantly more complications among inpatients vs. outpatients in both the early and late cohorts (early: 5.80% vs. 1.98%, adjusted RR = 2.57, 95% CI = 1.24-5.34, P = .011, late: 3.90% vs. 1.38%, adjusted RR = 2.28, 95% CI = 1.39-3.74, P = .001). TSA became more common in elderly patients over 70 years of age over time in both the inpatient and outpatient cohorts, whereas fewer young patients (aged 18-59 years) underwent TSA in the late cohorts than in the early cohorts for both the inpatient and outpatient samples (P < .001).
Conclusion
The overall complication rate of TSA has decreased over time as outpatient TSA has become increasingly common. When contemporary data are examined, the complication rate of outpatient procedures has remained constant over time while that of inpatient procedures decreased, despite the changing demographics of patients undergoing TSA. This indicates that outpatient TSA remains a safe procedure as patient selection criteria have evolved, while the safety of inpatient TSA continues to improve.
Keywords: Total shoulder arthroplasty, Reverse total shoulder arthroplasty, Outpatient surgery, Inpatient surgery, Clinical outcomes, Complications over time
The rate of total shoulder arthroplasty (TSA) is rapidly increasing.8,19,27 By 2025, the volume of shoulder arthroplasty performed in the United States is expected to exceed the expected growth of total hip (47% increase) and knee (22% increase) arthroplasty over the same time period.29 The increased volume of TSA is partly attributable to United States Food and Drug Administration approval of the reverse TSA in 2004 and its expanding indications.29 With time, the confidence in the reverse TSA has expanded indications for its utilization. Not surprisingly, reverse TSA is expected to see the greatest increase of all shoulder arthroplasty procedures in the upcoming years.
Recent advances in surgical technique, implant design, pain management strategies, and perioperative care have led to decreased postoperative complications and shortened hospital length of stay after TSA.11,26 These developments have spurred interest in performing TSA in the outpatient setting, which has been shown to be safe and effective.1 Approximately 2%-3% of TSA procedures were performed outpatient in the early 2010s, and that proportion has steadily increased, with the number of TSAs performed in ambulatory surgery centers doubling over a 5-year span.3,9,23 Leroux et al found high levels of patient satisfaction with outpatient TSA, as 97% of patients described their experience as good or excellent, and over 94% said they would choose outpatient TSA again.21 In addition, multiple studies have shown outpatient TSA is more cost-effective than inpatient, with two studies reporting over 40% cost savings, even after excluding inpatient-specific charges and accounting for confounding variables.9,17,23 With the implementation of the Bundled Payment for Care Improvement program by Medicare in 2011 and subsequent declines in TSA reimbursement, recent emphasis has been placed on reducing episode-of-care costs while improving quality of services.22,25,30
The current literature pertaining to safety and complications in outpatient TSA consists mainly of retrospective cohort studies or large database studies.2,3,18,21,23 Results have been inconsistent, as some have demonstrated equivalent rates of readmissions2,18 and postoperative complications,13,21 whereas others suggest that complications are less frequent in the outpatient setting.3,14 In addition, as evidence continues to validate outpatient total joint arthroplasty as a safe option, many surgeons have broadened their patient selection and have become more comfortable performing surgery on older patients with an increased comorbidity burden.31 With the heterogeneous findings of past studies and the evolving characteristics of patients undergoing outpatient arthroplasty, it is important to evaluate outpatient TSA complications utilizing up-to-date data sets.
The purpose of the present study was to use recent data from a large, multicenter, national database to investigate trends and compare rates of adverse events after outpatient and inpatient TSA over time. Given the improvement in TSA outcomes and expansion of outpatient procedures, we hypothesized that outpatient TSA would be associated with fewer adverse events than inpatient TSA in recent years, while having an older patient population with more comorbidities than an earlier outpatient cohort.
Methods
Study population
Patients undergoing primary TSA (both anatomic and reverse, as they share the same Current Procedural Terminology (CPT) code) during 2006-2019 were identified in the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database using the CPT code 23472. The ACS-NSQIP is a surgical database that prospectively collects data from over 700 participating hospitals regarding preoperative characteristics of included patients and postoperative complications within 30 days of surgical intervention.
Patients were included if they underwent primary elective TSA. Elective cases were identified by using the “emergency” and “elective” variables within ACS-NSQIP. As in previous studies using this database, outpatient surgery was defined as discharge on the day of surgery, whereas inpatient surgery was defined as discharge anytime after the day of surgery.5,6 Patient characteristics including age, gender, smoking status, and body mass index (BMI) were collected. Age was stratified into 3 cohorts (18-59, 60-69, and ≥70 years), and BMI was stratified into 4 groups (≤25, 25-30, 30-35, or ≥35 kg/m2). Preoperative comorbidities including hypertension, dyspnea on exertion, chronic obstructive pulmonary disease (COPD), and diabetes mellitus (no diabetes, insulin-dependent diabetes mellitus, or noninsulin-dependent diabetes mellitus) were recorded. Preoperative anemia was defined as a hematocrit <36% for women and <41% for men. Preoperative hypoalbuminemia was defined as serum albumin <3.5 g/dL. Anesthesia type was characterized as either general or regional anesthesia. Only one primary anesthesia technique could be recorded from the database, and thus, patients who may have had a combination of regional and general were not identified.
Outcomes
Adverse events within 30 days after surgical intervention recorded in ACS-NSQIP were used. The specific complications include death, surgical site infection (SSI), wound dehiscence, pneumonia, deep vein thrombosis, pulmonary embolism, unexpected intubation, acute kidney injury, urinary tract infection, stroke, myocardial infarction, blood transfusion, and systemic sepsis. An outcome variable termed “any complication” was created and was considered positive if any of the abovementioned complications occurred. Hospital readmission and return to the operating room were analyzed separately.
Patients were divided into two cohorts to analyze changes over time. Patients from 2006-2016 were included in the “early cohort”, and those from 2017-2019 were included in the “late cohort”. These cohorts were chosen to represent approximately 50% of the total number of included cases performed during the study period. Outcomes were compared between inpatient and outpatient procedures for these two cohorts. In addition, age and rate of comorbidities were compared between the early and late cohorts for both inpatient and outpatient TSA samples to assess how the population of patients undergoing inpatient and outpatient TSA has evolved over time.
Statistical analysis
Stata, version 16.1, (Statacorp LLP, College Station, TX, USA) was used to conduct statistical analyses. First, demographics, preoperative comorbidities, and laboratory characteristics were compared between inpatients and outpatients using Pearson’s chi-squared test. Second, the year of operation was tested for association with outpatient status using Poisson regression with robust error variance in which the outcome was outpatient (vs. inpatient) status and the independent variable was the year of procedure. The same method was then used to test the year of operation for association with occurrence of any complication. The occurrence of any complication between early and late cohorts was analyzed for both inpatient and outpatient groups using bivariate and multivariate Poisson regression with robust error variance. Adverse event rates were compared between inpatients and outpatients using bivariate and multivariate Poisson regression with robust error variance. Adjusted analyses of adverse events were controlled for age, sex, BMI, diabetes, dyspnea on exertion, hypertension, COPD, current smoking status, anemia, hypoalbuminemia, and anesthesia type. Analysis of adverse events was conducted separately among the early and late cohorts. Finally, age and comorbidity burden were compared between the early and late cohorts for both the inpatient and outpatient samples using Pearson’s chi-squared test. The level of significance was set at P < .05.
The ACS-NSQIP requires the following statement to be present in articles using its data: “The American College of Surgeons National Surgical Quality Improvement Program and hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”
Results
Study population
In total, 29,776 cases were identified. Of the 25,246 cases (84.8%) that met inclusion criteria, 1511 (6.0%) were performed as an outpatient. In total, 12,401 cases were performed from 2006 to 2016 (early cohort) and 12,845 cases were performed from 2017 to 2019 (late cohort). These cohorts were chosen to represent approximately 50% of the total number of included cases performed during the study period.
Percent of procedures in the entire cohort performed as an outpatient in accordance with demographic, comorbidity, and laboratory characteristics is displayed in Table I.
Table I.
Percent of procedures performed as outpatient, by demographic, comorbidity, and laboratory characteristics.
| Demographic, comorbidity, or laboratory characteristic | Percent performed as outpatient |
Statistical comparisons |
|||
|---|---|---|---|---|---|
| Percent | 95% CI | RR | 95% CI | P value | |
| Overall | 6.0 | 5.7-6.3 | |||
| Age | <.001 | ||||
| 18-59 | 8.4 | 7.5-9.4 | Ref. | ||
| 60-69 | 6.5 | 6.0-7.1 | 0.77 | 0.68-0.89 | |
| ≥70 | 5.0 | 4.6-5.3 | 0.59 | 0.52-0.67 | |
| Sex | <.001 | ||||
| Male | 7.3 | 6.9-7.8 | Ref. | ||
| Female | 4.9 | 4.6-5.3 | 0.67 | 0.61-0.74 | |
| Body mass index | |||||
| ≤25 | 7.0 | 6.2-7.8 | Ref. | ||
| 25-30 | 6.8 | 6.2-7.3 | 0.97 | 0.89-1.16 | .677 |
| 30-35 | 6.1 | 5.5-6.7 | 0.87 | 0.75-1.01 | .059 |
| ≥35 | 4.2 | 3.7-4.7 | 0.60 | 0.60-0.49 | <.001 |
| Diabetes mellitus | |||||
| No diabetes mellitus | 6.3 | 6.0-6.6 | Ref. | ||
| NIDDM | 4.7 | 4.0-5.5 | 0.75 | 0.64-0.89 | .001 |
| IDDM | 3.9 | 2.9-5.1 | 0.61 | 0.46-0.81 | .001 |
| Dyspnea on exertion | <.001 | ||||
| No | 6.3 | 6.0-6.6 | Ref. | ||
| Yes | 1.7 | 1.1-2.4 | 0.26 | 0.19-0.39 | |
| Hypertension | <.001 | ||||
| No | 7.8 | 7.2-8.4 | Ref. | ||
| Yes | 5.1 | 4.8-5.5 | 0.66 | 0.60-0.73 | |
| COPD | <.001 | ||||
| No | 6.2 | 5.9-6.5 | Ref. | ||
| Yes | 3.0 | 2.2-3.9 | 0.48 | 0.36-0.63 | |
| Current smoker | .053 | ||||
| No | 6.1 | 5.8-6.4 | Ref. | ||
| Yes | 5.1 | 4.3-6.0 | 0.84 | 0.71-1.00 | |
| Anemia∗ | <.001 | ||||
| No | 6.4 | 6.1-6.7 | Ref. | ||
| Yes | 4.6 | 4.1-5.2 | 0.72 | 0.63-0.82 | |
| Serum albumin | |||||
| Normal (≥3.5 g/dL) | 5.4 | 5.0-5.8 | Ref. | ||
| Hypoalbuminemia (<3.5 g/dL) | 3.1 | 2.0-4.6 | 0.58 | 0.39-0.87 | .007 |
| Albumin level not available | 6.7 | 6.3-7.2 | 1.26 | 1.14-1.39 | <.001 |
CI, confidence interval; RR, relative risk; Ref, reference; NIDDM, noninsulin-dependent diabetes mellitus; IDDM, insulin-dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Bold indicates statistical significance (P < .05).
Anemia was defined as preoperative hematocrit below 36 for women or 41 for men.
Within the full cohort, outpatients were less likely to be within the 60-69 or ≥70 age bracket (relative risk [RR] = 0.77, 95% confidence interval [CI] = 0.68-0.89; RR = 0.59, 95% CI = 0.52-0.67, P < .001 for both). Outpatients were also less likely to have BMI ≥35 and were less likely to have diabetes, dyspnea on exertion, hypertension, COPD, anemia, and hypoalbuminemia (Table I).
In the early cohort, dyspnea on exertion (P < .001), hypertension (P < .001), insulin-dependent diabetes mellitus (P = .035), and BMI ≥35 (P = .002) were more common among inpatients compared with outpatients (Table II). A greater proportion of inpatients were women, whereas a greater percentage of outpatients were men (P < .001). Regarding age, outpatient procedures were more likely to occur in the 18-59 and 60-69 year group, whereas the ≥70 year group was more likely to undergo inpatient procedures (P < .001).
Table II.
Comparisons of inpatients vs. outpatients by demographic, comorbidity, laboratory, and perioperative characteristics among the early cohort (2005-2016) and the late cohort (2017-2019).
| Demographic, comorbidity, or laboratory characteristic | Early |
Late |
||||
|---|---|---|---|---|---|---|
| Inpatient (n = 12,047) | Outpatient (n = 354) | P value | Inpatient (n = 11,688) | Outpatient (n = 1157) | P value | |
| Age | <.001 | <.001 | ||||
| 18-59 | 14.9% | 28.8% | 12.9% | 17.5% | ||
| 60-69 | 33.6% | 34.2% | 33.5% | 37.7% | ||
| ≥70 | 51.5% | 37.0% | 53.6% | 44.9% | ||
| Sex | <.001 | <.001 | ||||
| Male | 43.5% | 53.1% | 43.6% | 54.5% | ||
| Female | 56.6% | 46.9% | 56.5% | 45.6% | ||
| Body mass index | .002 | <.001 | ||||
| ≤25 | 17.2% | 20.6% | 16.4% | 19.5% | ||
| 25-30 | 32.4% | 37.0% | 31.3% | 36.2% | ||
| 30-35 | 26.1% | 26.6% | 26.7% | 26.9% | ||
| ≥35 | 24.2% | 15.8% | 25.7% | 17.4% | ||
| Diabetes mellitus | .035 | <.001 | ||||
| No diabetes mellitus | 82.8% | 87.3% | 81.7% | 86.8% | ||
| NIDDM | 12.2% | 10.5% | 13.2% | 9.8% | ||
| IDDM | 4.9% | 2.3% | 5.2% | 3.5% | ||
| Dyspnea on exertion | 7.0% | 1.1% | <.001 | 7.2% | 2.2% | <.001 |
| Hypertension | 67.6% | 55.7% | <.001 | 68.3% | 58.3% | <.001 |
| COPD | 6.3% | 4.2% | .109 | 7.2% | 2.9% | <.001 |
| Current smoker | 10.5% | 11.3% | .626 | 10.5% | 8.2% | .013 |
| Anemia∗ | 24.1% | 20.9% | .167 | 22.6% | 16.8% | <.001 |
| Serum albumin | .505 | <.001 | ||||
| Normal (≥3.5 g/dL) | 42.8% | 42.7% | 50.7% | 41.2% | ||
| Hypoalbuminemia (<3.5 g/dL) | 3.0% | 2.0% | 3.5% | 1.6% | ||
| Albumin level not available | 54.1% | 55.4% | 45.9% | 57.2% | ||
NIDDM, noninsulin-dependent diabetes mellitus; IDDM, insulin-dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Bold indicates statistical significance (P < .05).
Anemia was defined as preoperative hematocrit below 36 for women or 41 for men.
Trends regarding age, comorbidity, and sex in the late cohort were largely similar to the early cohort (Table II). However, compared with the early cohort, there was a lower comorbidity burden among outpatients in the late cohort, as they were less likely to have COPD (P < .001), be a current smoker (P = .013), or be anemic (P < .001). Outpatients were also less likely to have hypoalbuminemia (P < .001).
Trends over time
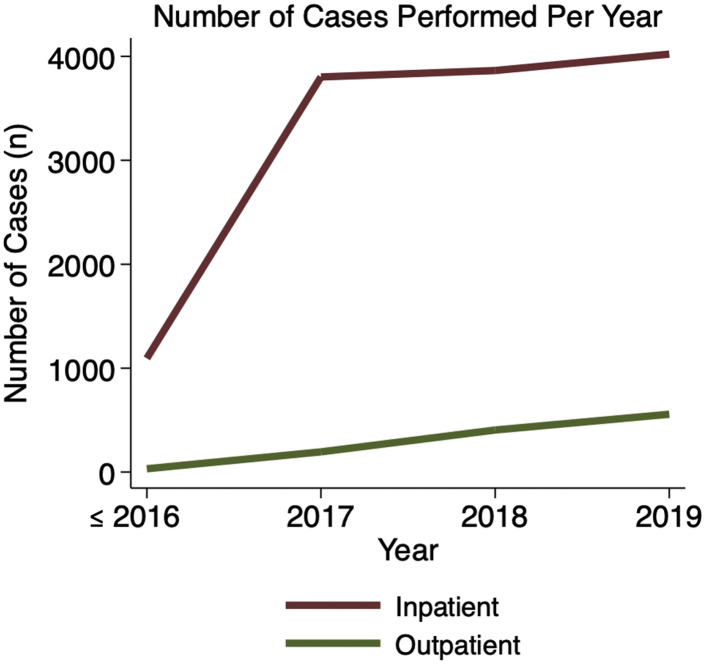
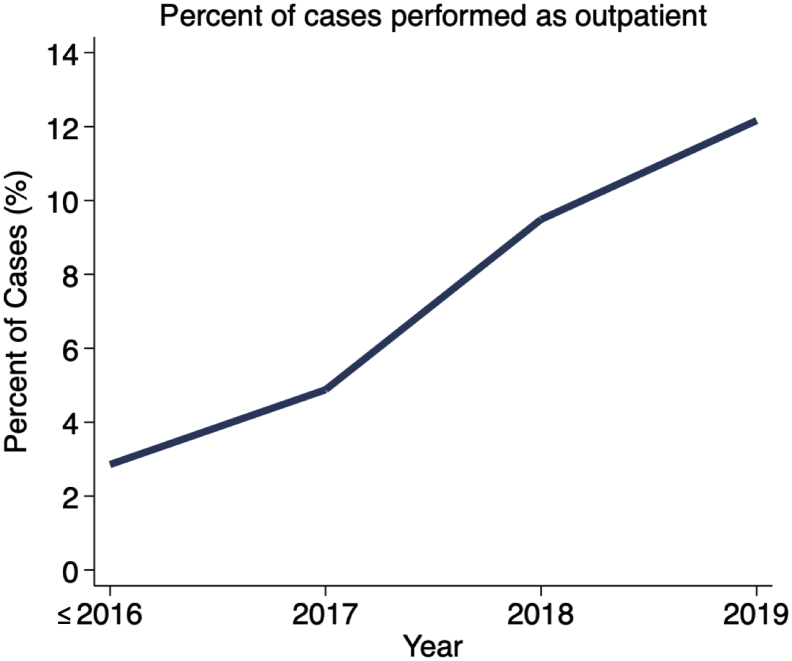
There was an increase in outpatient cases in the later study years, with cases being more likely to be performed on an outpatient basis as time went on (2.85% during 2006-2016 vs. 12.16% in 2019; RR of outpatient status with each increasing year = 1.32, 95% CI = 1.28-1.37, P < .001; Figs. 1 and 2).
Figure 1.
Number of inpatient (red) and outpatient (green) total shoulder arthroplasty procedures performed per year over the study period.
Figure 2.
Percentage of total shoulder arthroplasty cases performed as an outpatient procedure over time.
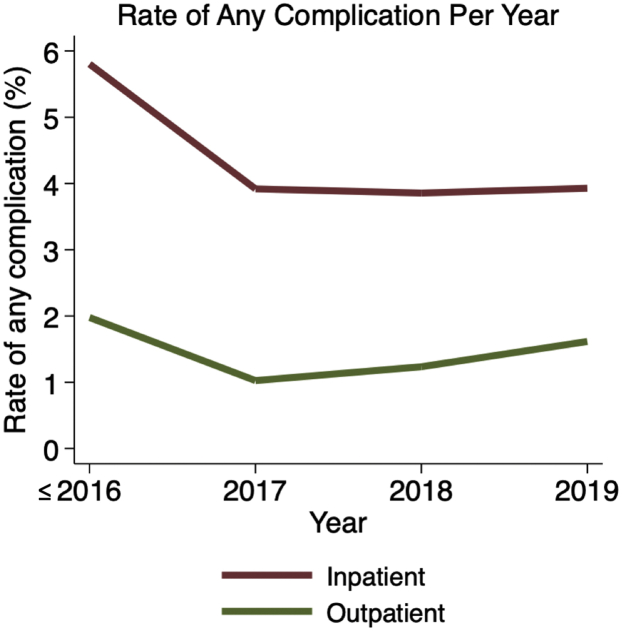
Cases were significantly less likely to have any complication during later study years (5.69% from 2006 to 2016 vs. 3.65% in 2019; RR of any complication for each increasing year = 0.91, 95% CI = 0.89-0.93, P < .001; Fig. 3). Similarly, complications were significantly less likely to occur among inpatients during later study years (5.80% from 2006 to 2016 vs. 3.93% in 2019, RR of any complication for each increasing year = 0.91, 95% CI = 0.90-0.93, P < .001). However, although complication rates dropped over time among outpatients, this result was not statistically significant (1.98% from 2006 to 2016 vs. 1.62% in 2019, RR of any complication for each increasing year = 0.93, 95% CI = 0.78-1.10, P = .375).
Figure 3.
Rate of any adverse event occurring within 30 days postoperatively after inpatient (red) and outpatient (green) total shoulder arthroplasty procedures over time.
When comparing early and late cohorts, there was a significant reduction in any complication over time when considering all cases (5.69% vs. 3.67%, adjusted RR = 0.65, 95% CI = 0.58-0.73, P < .001). The rate of any complication decreased over time among inpatients (5.80% vs. 3.90%, adjusted RR = 0.68, 95% CI = 0.60-0.76, P < .001). However, the rate of any complication among outpatients was not significant over time (1.98% vs. 1.38%, adjusted RR = 0.64, 95% CI = 0.28-1.47, P = .293).
TSA became more common in elderly patients over 70 years of age over time in both the inpatient and outpatient cohorts. A total of 53.6% of the inpatient late cohort was over 70 years of age compared with 51.5% in the early cohort (P = .001; Table IV). Similarly, 44.9% of outpatients in the late cohort and 37.0% in the early cohort were over 70 years of age (P = .009). Likewise, fewer young patients (aged 18-59 years) underwent TSA in the late cohorts than in the early cohorts for both the inpatient and outpatient samples (P < .001). A higher proportion of inpatients in the late cohort had COPD, noninsulin-dependent diabetes mellitus, and BMI ≥35 and were ASA class 3 or 4 (P = .007, P = .033, P = .011, and P < .001, respectively).
Table IV.
Comparisons of demographic, comorbidity, laboratory, and perioperative characteristics among inpatients and outpatients in the early cohort (2005-2016) vs. the late cohort (2017-2019).
| Demographic, comorbidity, or laboratory characteristic | Inpatient |
Outpatient |
||||
|---|---|---|---|---|---|---|
| Early (n = 12,047) | Late (n = 11,688) | P value | Early (n = 354) | Late (n = 1157) | P value | |
| Age | ||||||
| 18-59 | 14.9% | 12.9% | <.001 | 28.8% | 17.5% | <.001 |
| 60-69 | 33.6% | 33.5% | .788 | 34.2% | 37.7% | .232 |
| ≥70 | 51.5% | 53.6% | .001 | 37.0% | 44.9% | .009 |
| Sex | .884 | .657 | ||||
| Male | 43.5% | 43.6% | 53.1% | 54.5% | ||
| Female | 56.6% | 56.5% | 46.9% | 45.6% | ||
| Body mass index | ||||||
| ≤25 | 17.2% | 16.4% | .075 | 20.6% | 19.5% | .653 |
| 25-30 | 32.4% | 31.3% | .061 | 37.0% | 36.2% | .787 |
| 30-35 | 26.1% | 26.7% | .325 | 26.6% | 26.9% | .903 |
| ≥35 | 24.2% | 25.7% | .011 | 15.8% | 17.4% | .496 |
| Diabetes mellitus | ||||||
| No diabetes mellitus | 82.8% | 81.7% | .018 | 87.3% | 86.8% | .803 |
| NIDDM | 12.2% | 13.2% | .033 | 10.5% | 9.8% | .706 |
| IDDM | 4.9% | 5.2% | .372 | 2.3% | 3.5% | .261 |
| Dyspnea on exertion | 7.0% | 7.2% | .472 | 1.1% | 2.2% | .216 |
| Hypertension | 67.6% | 68.3% | .222 | 55.7% | 58.3% | .386 |
| COPD | 6.3% | 7.2% | .007 | 4.2% | 2.9% | .227 |
| Current smoker | 10.5% | 10.5% | .920 | 11.3% | 8.2% | .075 |
| Anemia∗ | 24.1% | 22.6% | .006 | 20.9% | 16.8% | .075 |
| ASA class 3 or 4 | 54.0% | 60.2% | <.001 | 39.0% | 42.7% | .215 |
| Serum albumin | ||||||
| Normal (≥3.5 g/dL) | 42.8% | 50.7% | <.001 | 42.7% | 41.2% | .633 |
| Hypoalbuminemia (<3.5 g/dL) | 3.0% | 3.5% | .045 | 2.0% | 1.6% | .586 |
| Albumin level not available | 54.1% | 45.9% | <.001 | 55.4% | 57.2% | .539 |
NIDDM, noninsulin-dependent diabetes mellitus; IDDM, insulin-dependent diabetes mellitus; COPD, chronic obstructive pulmonary disease; ASA, American Society of Anesthesiologists.
Bold indicates statistical significance (P < .05).
Anemia was defined as preoperative hematocrit below 36 for women or 41 for men.
Early cohort (2006-2016)
Among the 12,401 cases performed in the early cohort (12,047 inpatients and 354 outpatients), there were significantly more complications among inpatients than outpatients (5.80% vs. 1.98%, adjusted RR = 2.57, 95% CI = 1.24-5.34, P = .011). Regarding specific complications, inpatient American Society of Anesthesiologists was associated with a significantly higher rate of blood transfusion (3.34% vs. 0.86%, adjusted RR = 3.34, 95% CI = 1.09-10.28 P = .035). The rates of the remaining outcomes did not differ (P > .05 for each; Table III).
Table III.
Multivariate adjusted analysis∗ of adverse event rates, early cohort (2005-2016) and late cohort (2017-2019).
| Adverse event | Early cohort |
Late cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | RR | 95% CI | P value | Inpatient | Outpatient | RR | 95% CI | P value | |
| Any complication | 5.80% | 1.98% | 2.57 | 1.24-5.34 | .011 | 3.90% | 1.38% | 2.30 | 1.39-3.80 | .001 |
| Specific complications | ||||||||||
| Death | 0.16% | 0.28% | 0.39 | 0.05-3.13 | .372 | 0.15% | 0.09% | 1.31 | 0.17-10.12 | .794 |
| Surgical site infection | 0.45% | 0.28% | 2.08 | 0.28-15.61 | .476 | 0.54% | 0.26% | 2.23 | 0.68-7.36 | .188 |
| Wound dehiscence | 0.06% | 0.00% | - | - | - | 0.05% | 0.00% | - | - | - |
| Pneumonia | 0.50% | 0.28% | 1.32 | 0.18-9.74 | .786 | 0.49% | 0.09% | 5.12 | 0.80-32.74 | .085 |
| Deep vein thrombosis | 0.35% | 0.28% | 1.14 | 0.14-9.04 | .900 | 0.34% | 0.09% | 3.38 | 0.48-23.56 | .219 |
| Pulmonary embolism | 0.32% | 0.28% | 0.90 | 0.12-6.92 | .917 | 0.27% | 0.00% | - | - | - |
| Unexpected intubation | 0.18% | 0.00% | - | - | - | 0.23% | 0.00% | - | - | - |
| Acute kidney injury | 0.14% | 0.00% | - | - | - | 0.15% | 0.17% | 0.64 | 0.14-2.98 | .570 |
| Urinary tract infection | 0.77% | 0.28% | 2.08 | 0.28-15.24 | .472 | 0.20% | 0.41% | 0.71 | 0.34-1.46 | .531 |
| Stroke | 0.12% | 0.00% | - | - | - | 0.08% | 0.00% | - | - | - |
| Myocardial infarction | 0.22% | 0.00% | - | - | - | 0.27% | 0.17% | 1.63 | 0.41-6.52 | .492 |
| Blood transfusion | 3.34% | 0.86% | 3.34 | 1.09-10.28 | .035 | 1.21% | 0.00% | - | - | - |
| Sepsis | 0.27% | 0.00% | - | - | - | 0.15% | 0.00% | - | - | - |
| Return to the operating room | 1.04% | 0.28% | 3.63 | 0.50-26.33 | .202 | 1.44% | 1.04% | 1.37 | 0.74-2.53 | .322 |
| Readmission | 2.79% | 1.36% | 1.85 | 0.69-4.96 | .220 | 2.89% | 1.82% | 1.38 | 0.89-2.16 | .146 |
RR, relative risk; CI, confidence interval.
Bold indicates statistical significance (P < .05).
Adjusted for each of the baseline characteristics listed in Table I.
Late cohort (2017-2019)
Among the 12,845 cases performed in the early cohort (11,688 inpatients and 1157 outpatients), there were significantly more complications among inpatients than outpatients (3.90% vs. 1.38%, adjusted RR = 2.28, 95% CI = 1.39-3.74, P = .001). There were no specific outcomes that differed between the groups (P > .05 for each; Table III). Of note, readmissions were significantly higher in inpatients before adjustment (2.89% vs. 1.82%, unadjusted RR = 1.59, 95% CI = 1.03-2.47, P = .037), but this difference was not statistically significant after adjustment (adjusted RR = 1.38, 95% CI = 0.89-2.16, P = .146).
Discussion
Consistent with our hypothesis, the primary finding of this study was that the overall complication rate was lower in patients undergoing outpatient TSA than that in inpatient TSA, despite a growing number of elderly patients undergoing outpatient TSA in the more recent cohort. This finding remained statistically significant after controlling for baseline patient characteristics and comorbidities. These results suggest that outpatient TSA remains a safe and possibly advantageous alternative to the inpatient setting, even in appropriately selected patients over 70 years old. In addition, we found that the complication rate in the inpatient cohort decreased over time despite the more recent sample showing a greater comorbidity burden and advanced age. This finding suggests that recent advances in clinical care pathways, technique, and perioperative care have continued to improve the safety of TSA.
In the present study, patients had significantly fewer complications in the outpatient setting than inpatients when adjusted for comorbidities. The rate of any complication among inpatients in the early cohort was 5.8% compared with 1.98% in outpatients. Likewise, 3.9% of inpatients in the late cohort experienced a complication compared with 1.38% of outpatients. These findings are consistent with the results of several previous studies using older data from institutional and nationwide registries.
Basques et al3 used a Medicare database to evaluate adverse events in 123,347 patients who underwent anatomic or reverse TSA. The authors found that inpatients had higher rates of acute kidney injury, thromboembolic events, SSIs, urinary tract infections, hematoma, transfusion, capsulitis, dislocation, and 30-day and 90-day readmission when controlling for differences in comorbidities.3 Similarly, Arshi et al2 found that outpatient TSA had decreased rates of stiffness and higher rates of SSI at one year when adjusting for baseline characteristics. Erickson et al12 showed lower overall complication rates in patients undergoing outpatient reverse TSA than in inpatients on unadjusted analysis, although the inpatient cohort was older and had higher rates of obesity and diabetes. In contrast, Leroux et al20 demonstrated that inpatients and outpatients undergoing TSA showed similar rates of adverse events and readmissions when controlling for a higher comorbidity burden among inpatients. Several authors have replicated this finding, showing a favorable postoperative complication profile in outpatient TSA, although no advantage over the inpatient setting when comparing similar patient populations.7,10,18 Indeed, a recent meta-analysis found no significant differences between matched inpatient and outpatient TSA groups in regard to medical complications, surgical complications, or hospital readmissions.1 Compared with the aforementioned database studies, the present study benefits from the addition of more recent ACS-NSQIP data to include a greater number of outpatient procedures. We were also able to observe trends over time by dividing patients between an early and late cohort.
Unsurprisingly, inpatients in the present study had more comorbidities than outpatients in both the early and later cohorts, which reflects that younger, healthier patients are more commonly selected for outpatient surgery. In the early 2000s, a greater proportion of patients underwent inpatient TSA, largely due to historical precedent and a paucity of published literature supporting safe outpatient arthroplasty. However, as evidence grew that validated outpatient TSA as a viable option, the percentage of patients undergoing same-day TSA grew from 2.85% in 2006-2016 to 12.16% in 2017-2019. Appropriate patient selection for outpatient surgery has been thoroughly investigated in the total hip and knee literature,31 and a number of risk calculators and more well-defined patient selection criteria have aided decision-making for outpatient TSA.4,28 The results of the present study show that outpatient TSA has become more common in elderly patients, as the proportion of patients over the age of 70 undergoing outpatient TSA grew from 37% in 2006-2016 to 44.9% in 2017-2019. Despite these changing demographics, the rate of complications has remained low and suggests that outpatient TSA remains a safe option as patient selection criteria have evolved. As evidence accumulates supporting safe outpatient surgery in more medically complex patients, patient selection criteria will continue to be refined.
Of note, inpatients undergoing TSA from 2017 to 2019 had more comorbidities compared with 2006-2016, which may reflect a higher proportion of healthy patients being preferentially chosen for outpatient TSA in the later study period or simply expanding indications for TSA in elderly, sicker patients in recent years. Further research on the evolving patient characteristics of inpatient TSA in light of the growing popularity of outpatient TSA is warranted, particularly considering the implications for episode-of-care reimbursement. Recent data in the hip and knee literature demonstrate that patients with more comorbidities accrue significantly greater costs to the hospital that are not adequately accounted for in bundled payment models.15 For example, diagnoses of diabetes and congestive heart failure among Medicare patients increased the total episode-of-care cost by $3937 and $1,368, respectively.15 As the population undergoing inpatient TSA continues to increase in complexity and healthier patients are preferentially chosen for same-day surgery in ambulatory surgery centers, changes in reimbursement are warranted to abate financial risk.
Interestingly, despite the more recent cohort of inpatients having a greater number of higher-risk patients, we observed an overall decrease in complication rates over time. The favorable outcomes seen in the later cohort may reflect improvements in analgesia techniques that may facilitate more rapid discharge24 or a growing popularity of medical co-management to prevent postoperative medical complications.16 The overall low rate of complications in the present study further confirms that TSA is a safe procedure in patients of varying medical complexity.
There are several limitations to the present study. First, adverse events are collected for only 30 days postoperatively in the ACS-NSQIP database, which does not represent longer-term complications related to TSA. Adverse events are limited to medical complications and do not include surgery-specific complications such as stiffness, stress fracture, implant loosening, instability, chronic prosthetic joint infection, and others, unless they required a return to the operating room. In addition, more granular outcomes such as functional scores, strength, range of motion, and patient satisfaction are not collected. However, much of the focus regarding safety of the surgery setting is related to associated medical complications. Second, selection bias is inherent in this study design and may confound the rate of adverse events. A multivariable regression including all relevant patient characteristics was performed in this study in an effort to reduce such bias. However, the goal of preoperative indication protocols is to select patients with a risk profile most suitable for an outpatient setting. Thus, the findings of this study indicate that outpatient TSA can be performed safely when patient selection protocols are followed. Third, adverse events that occurred outside of the ACS-NSQIP participating hospitals were not captured and may under-represent complication rates. Fourth, because anatomic TSA and reverse TSA share the same CPT code, these procedures were not able to be distinguished in this study. Although consistent with prior findings,5 it is possible that the dramatic rise in cases in recent years (ie a similar number of cases performed in the first 11 years of available data as in the last 3 years) is due to the rapid expansion in the utilization of reverse TSA over time.5 Similarly, there may also have been greater variability in technique and perioperative protocols during the early cohort than the late cohort, which could limit our conclusions. Finally, because of the data utilized, these results are specific to the health care system in the United States and may not be generalizable to other nations.
Conclusion
The overall complication rate of TSA has decreased over time as outpatient TSA has become increasingly common. Despite the changing demographics of patients undergoing TSA, the complication rate of outpatient procedures remained constant over time, whereas that of inpatient procedures decreased. This indicates that outpatient TSA remains a safe procedure as patient selection criteria have evolved, while the safety of inpatient TSA continues to improve.
Disclaimers
Funding: No funding was disclosed by the authors.
Conflicts of interest: Dr. Bohl discloses the following: research support: OpEd Medical. Member: AOFAS Research Committee. These conflicts are all unrelated to the subject of this article. Dr. Nicholson discloses the following: royalties: Wright Medical Technology, Inc., Innomed, and Arthrosurface; consultancy: Wright Medical Technology, Inc.; research support: Arthrex, Inc. and Wright Medical Inc.; fellowship education support: Wright Medical Inc., and Smith & Nephew; paid presenter: Arthrosurface; board membership: American Shoulder and Elbow Surgeons. Dr. Garrigues discloses the following: board membership: ASES; Editorial Board: JSES, Techniques in Orthopaedics. Consultancy: Bioventus, Tornier, Mitek, and DJ Orthopaedics. Royalties: Tornier and DJ Orthopaedics; other financial support: Arthrex, Inc, Zimmer, SouthTech. Stock: Genesys, ROM 3. Dr. Verma discloses the following: board membership: AOSSM, ASES, AANA, Knee, SLACK Incorporated. Consultancy: Minivasive and OrthoSpace. Research support: Arthrex Inc., Breg, Ossur, Smith & Nephew, and Wright Medical Technology. Royalties: Arthroscopy, Smith & Nephew, and Vindico Medical-Orthopedics Hyperguide. Stock: Cymedica, Minivasive, and Omeros. The other authors, their immediate families, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was not required for this study.
References
- 1.Ahmed A.F., Hantouly A., Toubasi A., Alzobi O., Mahmoud S., Qaimkhani S., et al. The safety of outpatient total shoulder arthroplasty: a systematic review and meta-analysis. Int Orthop. 2021;45:697–710. doi: 10.1007/s00264-021-04940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arshi A., Leong N.L., Wang C., Buser Z., Wang J.C., Vezeridis P.S., et al. Relative complications and trends of outpatient total shoulder arthroplasty. Orthopedics. 2018;41:e400–e409. doi: 10.3928/01477447-20180409-01. [DOI] [PubMed] [Google Scholar]
- 3.Basques B.A., Erickson B.J., Leroux T., Griffin J.W., Frank R.M., Verma N.N., et al. Comparative outcomes of outpatient and inpatient total shoulder arthroplasty: an analysis of the Medicare dataset. Bone Joint J. 2017;99-B:934–938. doi: 10.1302/0301-620X.99B7.BJJ-2016-0976.R1. [DOI] [PubMed] [Google Scholar]
- 4.Biron D.R., Sinha I., Kleiner J.E., Aluthge D.P., Goodman A.D., Sarkar I.N., et al. A Novel Machine Learning model developed to assist in patient selection for outpatient total shoulder arthroplasty. J Am Acad Orthop Surg. 2020;28:e580–e585. doi: 10.5435/jaaos-d-19-00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bixby E.C., Boddapati V., Anderson M.J.J., Mueller J.D., Jobin C.M., Levine W.N. Trends in total shoulder arthroplasty from 2005 to 2018: lower complications rates and shorter lengths of stay despite patients with more comorbidities. JSES Int. 2020;4:657–661. doi: 10.1016/j.jseint.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovonratwet P., Ondeck N.T., Nelson S.J., Cui J.J., Webb M.L., Grauer J.N. Comparison of outpatient vs inpatient total knee arthroplasty: an ACS-NSQIP analysis. J arthroplasty. 2017;32:1773–1778. doi: 10.1016/j.arth.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Brolin T.J., Mulligan R.P., Azar F.M., Throckmorton T.W. Neer Award 2016: outpatient total shoulder arthroplasty in an ambulatory surgery center is a safe alternative to inpatient total shoulder arthroplasty in a hospital: a matched cohort study. J Shoulder Elbow Surg. 2017;26:204–208. doi: 10.1016/j.jse.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Bryant D., Litchfield R., Sandow M., Gartsman G.M., Guyatt G., Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:1947–1956. doi: 10.2106/JBJS.D.02854. [DOI] [PubMed] [Google Scholar]
- 9.Cancienne J.M., Brockmeier S.F., Gulotta L.V., Dines D.M., Werner B.C. Ambulatory total shoulder arthroplasty: a Comprehensive analysis of current trends, complications, readmissions, and costs. J Bone Joint Surg Am. 2017;99:629–637. doi: 10.2106/JBJS.16.00287. [DOI] [PubMed] [Google Scholar]
- 10.Charles M.D., Cvetanovich G., Sumner-Parilla S., Nicholson G.P., Verma N., Romeo A.A. Outpatient shoulder arthroplasty: outcomes, complications, and readmissions in 2 outpatient settings. J Shoulder Elbow Surg. 2019;28:S118–S123. doi: 10.1016/j.jse.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Chin P.Y., Sperling J.W., Cofield R.H., Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15:19–22. doi: 10.1016/j.jse.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Erickson B.J., Bohl D.D., Cole B.J., Verma N.N., Nicholson G., Romeo A.A., et al. Reverse total shoulder arthroplasty: indications and techniques across the World. Am J Orthop (Belle Mead Nj) 2018;47 doi: 10.12788/ajo.2018.0079. [DOI] [PubMed] [Google Scholar]
- 13.Erickson B.J., Shishani Y., Jones S., Sinclair T., Bishop M.E., Romeo A.A., et al. Outpatient versus inpatient anatomic total shoulder arthroplasty: outcomes and complications. JSES Int. 2020;4:919–922. doi: 10.1016/j.jseint.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson B.J., Shishani Y., Jones S., Sinclair T., Griffin J., Romeo A.A., et al. Outpatient vs. inpatient reverse total shoulder arthroplasty: outcomes and complications. J Shoulder Elbow Surg. 2020;29:1115–1120. doi: 10.1016/j.jse.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Fillingham Y.A., Zachwieja E., Rondon A.J., Vannello C., Austin M.S., Courtney P.M. Risk adjustment for episode-of-care costs after total joint arthroplasty: what is the additional cost of Individual comorbidities and demographics? J Am Acad Orthop Surg. 2020;29:345–352. doi: 10.5435/jaaos-d-19-00889. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald S.J., Palmer T.C., Kraay M.J. Improved perioperative care of elective joint Replacement patients: the Impact of an Orthopedic perioperative hospitalist. J Arthroplasty. 2018;33:2387–2391. doi: 10.1016/j.arth.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Gregory J.M., Wetzig A.M., Wayne C.D., Bailey L., Warth R.J. Quantification of patient-level costs in outpatient total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28:1066–1073. doi: 10.1016/j.jse.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Kramer J.D., Chan P.H., Prentice H.A., Hatch J., Dillon M.T., Navarro R.A. Same-day discharge is not inferior to longer length of in-hospital stay for 90-day readmissions following shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29:898–905. doi: 10.1016/j.jse.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Lapner P.L.C., Rollins M.D., Netting C., Tuna M., Bader Eddeen A., van Walraven C. A population-based comparison of joint survival of hemiarthroplasty versus total shoulder arthroplasty in osteoarthritis and rheumatoid arthritis. Bone Joint J. 2019;101-B:454–460. doi: 10.1302/0301-620X.101B4.BJR-2018-0620.R1. [DOI] [PubMed] [Google Scholar]
- 20.Leroux T.S., Basques B.A., Frank R.M., Griffin J.W., Nicholson G.P., Cole B.J., et al. Outpatient total shoulder arthroplasty: a population-based study comparing adverse event and readmission rates to inpatient total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:1780–1786. doi: 10.1016/j.jse.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Leroux T.S., Zuke W.A., Saltzman B.M., Go B., Verma N.N., Romeo A.A., et al. Safety and patient satisfaction of outpatient shoulder arthroplasty. JSES Open Access. 2018;2:13–17. doi: 10.1016/j.jses.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez C.D., Boddapati V., Anderson M.J.J., Ahmad C.S., Levine W.N., Jobin C.M. Recent trends in Medicare utilization and surgeon reimbursement for shoulder arthroplasty. J Shoulder Elbow Surg. 2021;30:120–126. doi: 10.1016/j.jse.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Ode G.E., Odum S., Connor P.M., Hamid N. Ambulatory versus inpatient shoulder arthroplasty: a population-based analysis of trends, outcomes, and charges. JSES Int. 2020;4:127–132. doi: 10.1016/j.jses.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel M.S., Abboud J.A., Sethi P.M. Perioperative pain management for shoulder surgery: evolving techniques. J Shoulder Elbow Surg. 2020;29:e416–e433. doi: 10.1016/j.jse.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 25.Rana A.J., Bozic K.J. Bundled payments in orthopaedics. Clin Orthop Relat Res. 2015;473:422–425. doi: 10.1007/s11999-014-3520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh J.A., Ramachandran R. Age-related differences in the use of total shoulder arthroplasty over time: use and outcomes. Bone Joint J. 2015;97-B:1385–1389. doi: 10.1302/0301-620X.97B10.35696. [DOI] [PubMed] [Google Scholar]
- 27.Sperling J.W., Cofield R.H., Schleck C.D., Harmsen W.S. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16:683–690. doi: 10.1016/j.jse.2007.02.135. [DOI] [PubMed] [Google Scholar]
- 28.Steinhaus M.E., Liu J.N., Gowd A.K., Chang B., Gruskay J.A., Rauck R.C., et al. The Feasibility of outpatient shoulder arthroplasty: risk Stratification and Predictive Probability modeling. Orthopedics. 2020:1–8. doi: 10.3928/01477447-20201216-01. [DOI] [PubMed] [Google Scholar]
- 29.Wagner E.R., Farley K.X., Higgins I., Wilson J.M., Daly C.A., Gottschalk M.B. The incidence of shoulder arthroplasty: rise and future projections compared with hip and knee arthroplasty. J Shoulder Elbow Surg. 2020;29:2601–2609. doi: 10.1016/j.jse.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Walters J.D., Walsh R.N., Smith R.A., Brolin T.J., Azar F.M., Throckmorton T.W. Bundled payment Plans are associated with notable cost savings for ambulatory outpatient total shoulder arthroplasty. J Am Acad Orthop Surg. 2020;28:795–801. doi: 10.5435/JAAOS-D-19-00441. [DOI] [PubMed] [Google Scholar]
- 31.Ziemba-Davis M., Caccavallo P., Meneghini R.M. Outpatient joint arthroplasty-patient selection: Update on the outpatient Arthroplasty risk Assessment score. J Arthroplasty. 2019;34:S40–S43. doi: 10.1016/j.arth.2019.01.007. [DOI] [PubMed] [Google Scholar]