Abstract
Purpose
Partial ossicular replacement (PORP) and total ossicular replacement prostheses (TORP) are used to restore ossicular chain function. Despite favorable auditory outcomes, these interventions have associated risks and complications. This study examines the FDA MAUDE database for ossicular chain prosthesis adverse events to highlight complications, interventions, and root cause analysis (RCA) findings.
Materials and methods
The MAUDE database was searched for Medical Device Reports (MDRs) relating to PORPs and TORPs from 2010 to 2020. MDR event descriptions were reviewed, and adverse events were identified as a device issue, patient issue, and/or packaging issue that occurred intraoperatively or postoperatively.
Results
Our search identified 70 MDRs which included 110 reported adverse events. Events consisted of 63 device issues, often due to device breaks and displacements, 39 patient issues, including common complaints of hearing loss and erosion, and 8 packaging issues. When comparing PORPs and TORPs, TORPs had more reported device issues whereas PORPs had more packaging issues. Intraoperative device issues were commonly resolved by completing the procedure with a backup device and most postoperative device issues required additional surgery. For devices returned to the manufacturer, RCA determined that most breaks were caused by modification and/or mishandling or that the product met specifications with an undetermined cause for the break.
Conclusion
Device issues were the most common adverse events and frequently required subsequent intervention. Displacement occurred more often with TORPs and was associated with changes in hearing or erosion. The findings of this study are purely descriptive and may not have direct clinical relevance.
Keywords: PORP, TORP, MAUDE, Ossicular chain reconstruction, Medical devices
Abbreviations: OCR, Ossicular chain replacement; PORP, Partial ossicular replacement prosthesis; TORP, Total ossicular replacement prosthesis; RCA, Root cause analysis; FDA, U.S. Food and Drug Administration; MAUDE, Manufacturer and User Facility Device Experience; MDR, Medical device report
1. Introduction
Ossicular chain reconstruction (OCR) has evolved since its introduction in the 1950's both regarding surgical technique and prosthesis design. Although allograft, autograft, and homograft prostheses have all been utilized and studied, autografts and allografts remain more commonly used, with the literature demonstrating comparable effectiveness (Kumar et al., 2018; O'Connell et al., 2016; Roth et al., 2009; Wood et al., 2019; Zhao et al., 2018). Since John Shea established the use of the Teflon (DuPont, Wilmington, Delaware) stapes piston in 1956, various allograft materials have been developed in pursuit of the ideal material for middle ear prosthesis. These synthetic materials have included plastics (polyethylene tubing, Proplast, Plastipore), biomaterials (aluminum oxide, ceramic hybrids, hydroxyapatite), and metals (stainless steel, platinum, nickel, gold) (Walker and Babu, 2020). In the 1990s, however, titanium emerged as a favored alloplastic prosthesis due to its lightweight, rigidity, and biocompatibility.
Although there have been numerous advances in prosthesis development, the underlying objective of ossiculoplasty has persisted over time. Surgical intervention must concurrently eliminate underlying disease and restore function of the middle ear ossicular chain by emulating the natural mechanism of sound energy transfer. Per the classification by the FDA MAUDE database, a partial ossicular replacement prosthesis (PORP) is a device that transmits sound in the inner ear by forming a connection to an intact stapes with mobile footplate while a total ossicular replacement prosthesis (TORP) attaches directly to the footplate without need for an intact stapes (Larson and Wong, 2009). OCR can treat various pathologies affecting the middle ear, including ossicular erosion due to chronic otitis media or cholesteatoma, fixation of the ossicles, and ossicular disruption as a result of trauma. The surgical goal is to restore or improve the patient's pathologic conductive hearing loss, and success is often measured by an air-bone gap below 20 dB on audiometry (Young and Ng, 2020).
Despite the favorable auditory outcomes of OCR, these interventions have associated risks and complications depending on the type of prosthesis used. Intraoperatively, these include further disruption and displacement of the ossicles, stapedial footplate fracture, annular ligament tear, perilymphatic fistula, or possibly sensorineural hearing loss (Bance, 2018). Postoperative complications range from failure to restore hearing, vertigo, tinnitus, erosion of the prosthesis, or extrusion of the prosthesis (Bance, 2018). These adverse events can be related to surgical technique, including positioning and stabilizing the prostheses, host factors, such as inflammation, ventilation, and scarring, and notably, inherent properties of the prosthesis.
The U.S. Food and Drug Administration (FDA) developed the Manufacturer and User Facility Device Experience (MAUDE) database to collect and organize complications related to medical devices. The MAUDE database is the largest and most widely used public database for device-related adverse events. There are numerous reports in the literature of authors using the MAUDE database to report device malfunctions, associated injuries, and related consequences (Bestourous et al., 2020; Doran et al., 2018; Hur et al., 2020; Lee et al., 2021; Tong et al., 2020a). To date, a formal database study has not been conducted to determine the failure rates and complications of ossicular replacement prostheses. Thus, this study examines MAUDE reported ossicular prosthesis devices associated with adverse events from 2010 to 2020. It aims to inform providers of documented complications that have led to adverse events, reported interventions, and manufacturer root cause analysis (RCA) findings.
2. Materials and Methods
The FDA MAUDE database compiles information on reported adverse effects of medical devices. Hundreds of thousands of medical device reports (MDRs) are sent to the FDA each year to report alleged device malfunctioning, device associated deaths, and patient injuries. MDRs are submitted either on a mandatory basis by manufacturers, importers, and device user facilities, or voluntarily by healthcare professionals and patients. MAUDE data is available publicly and updated monthly. Although the database is a valuable resource, it is a passive surveillance system and carries a potential for biased data. As such, the incidence or prevalence of an event cannot be determined since reported information may be incomplete or inaccurate. Information from the MAUDE database, however, can be useful to determine common patient or device related problems, reported outcomes, and analysis results from manufacturers.
The MAUDE database was searched for MDRs between January 1st, 2010 and January 1st, 2020. The codes “LBM” for Porous Polyethylene Ossicular Replacement, “ETB” for PORPs, “LBP” for Stapes Prosthesis Using Absorbable Gelatin Material, “ETA” for TORPs, and “LBN” for Total Ossicular Replacement Prosthesis Porous, Polyethylene were searched. MDRs for LBP were excluded, as the search produced results for cardiac ablation catheters. Other indications for exclusion included duplicate reports, including both follow-up and multiple MDRs for a single event, the discovery that an incorrect implant was almost used on a patient, and lack of information regarding the event. MDRs based on publications were also included in our dataset; however, the outcomes from publications were not reported in a standardized manner. Each adverse event within a publication was either reported as its own MDR or submitted with all adverse events as a single MDR.
The event description in each report was reviewed and each adverse event was identified as a device issue, patient issue, and/or packaging issue that occurred either intraoperatively or postoperatively. For MDRs that included several device and patient adverse events, each event that occurred was included in our dataset. The manufacturer, brand name, year of report, subsequent intervention, and RCA by the manufacturer were also recorded.
3. Results
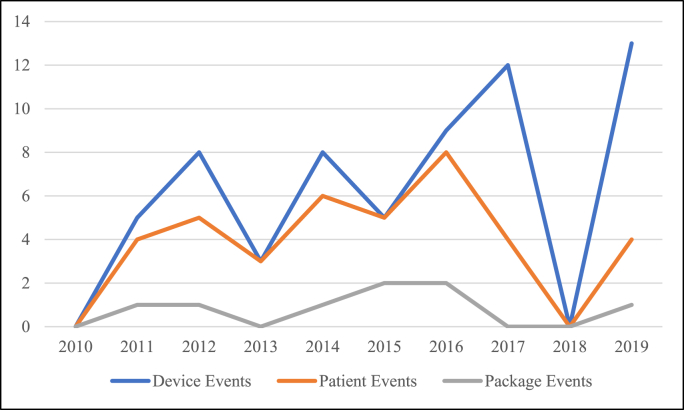
A total of 83 MDRs were identified after meeting inclusion criteria. Of those MDRs, 13 were duplicates and used to supplement additional information, if necessary. Of the 70 remaining MDRs, there were 110 total adverse events reported (Table 1). Most events occurred during the year 2016 and no events occurred in 2018 (Fig. 1). Adverse events consisted of 63 device issues (57.3%), 39 patient issues (35.5%), and 8 package issues (7.3%). There was nearly an equal number of events occurring intra-operatively (53) and post-operatively (57). For PORPs, 50% of adverse events were device issues, 35.7% were patient issues, and 14.3% were package issues (Table 2). For TORPs, 61.8% were device issues, 35.3% were patient issues, and 2.9% were package issues (Table 3).
Of the 63 adverse events reported as device issues, 52.4% were classified as a device break. Most device breaks occurred intraoperatively (81.8%). Eleven out of the 33 device breaks reported that the device broke inside the patient while implanting the prosthesis. In 7 of these cases, all pieces were retrieved; however, a foreign body was left inside the patient for the other 4 cases. Another common device issue was displacement (27%) which occurred postoperatively in all events. Twenty-one PORP device issues were reported, including: 10 breaks (47.6%), 3 displacements (14.3%), 2 with incorrect size or shape (9.5%), 4 that failed to crimp (19.0%), and 2 found with the loop of the stapes open (9.5%). There were 42 TORP device issues reported, including: 23 breaks (54.8%), 14 displacements (33.3%), and 5 that were the incorrect size or shape (11.9%).
Of the 39 events associated with patient complaints, 69.2% were reported at some point after the prosthesis was implanted. Hearing loss was the most common complaint, most often occurring post-operatively (91.7%). The 12 patient complaints that were reported as intra-operative were from eight MDRs. In half of these reports, the device broke during implantation and a foreign body was left inside the patient, as discussed. Two of these eight MDRs implicated possible surgeon error. One record reported that the surgeon accidently burned the chorda tympani while heating the piston, resulting in a burn and nerve damage. The other record reported that after the surgeon placed the implant, he decided to subsequently remove it. During removal, the device broke and the piston was stuck, resulting in excision of 2 mm of the vestibule to retrieve the piston, causing hearing loss and dizziness. Finally, the last 2 MDRs reported patient symptoms due to complications while explanting the prosthesis in which patients experienced vertigo, nystagmus, nausea, and/or pain. Of the patients who received PORP devices, 15 patient issues were reported, including: 4 hearing loss (26.7%), 2 with a foreign body left in the patient (13.3%), 1 distorted hearing (6.7%), 1 erosion (6.7%), 1 nerve damage (6.7%), 1 burn (6.7%), 1 pain (6.7%), 1 inner ear injury (6.7%), 1 dizziness (6.7%), 1 headache and pain during MRI (6.7%), and 1 nausea (6.7%). Twenty-four patient issues were reported from patients who received TORP devices. These issues included: 8 hearing loss (33.3%), 5 erosion (20.8%), 3 perforation (12.5%), 2 with a foreign body left in the patients (8.3%), 1 distorted hearing (4.2%), 1 fracture (4.2%), 1 pain (4.2%), 1 vertigo (4.2%), 1 drainage (4.2%), and 1 nystagmus (4.2%).
There were 29 device issues reported concordantly with patient issues in the same MDR. Reported events were due to displacement (44.8%), device break (37.9%), incorrect size or shape (10.3%), or failure to cramp (6.9%) (Table 4). There were 33 patient issues reported concordantly with device issues in the same MDR. Reported events were most commonly due to hearing loss (24.2%), erosion (18.2%), and a foreign body left in the patient (12.1%) (Table 5). Displacement was associated with hearing loss (33%), distorted hearing (13%), erosion (33%), perforation (13%), and fracture (6%). Six patient issues were reported without a device related event. One described a lawsuit in which a patient believed the ear implant caused permanent hearing loss, one reported a patient complaining with headache and ear pain during an MRI, and four were reported based on publications in the literature.
All eight packaging related events were identified intraoperatively before placing the prosthesis. There were no major trends found with reported packaging issues due to the variety of events submitted. Six packaging issues were from PORP devices, including: 2 that had a contaminate within the sterile packaging (33.3%), 1 device broken in the package (16.7%), 1 unsealed package (16.7%), 1 incorrect item within the package (16.7%), and 1 missing a component (16.7%). TORP devices had 2 packaging issues, including: 1 device broken in the package (50%) and 1 with improper size labeling (50%).
Subsequent intervention and RCA were described for device and packaging related adverse events. Of the 63 total device issues, 12 did not describe any intervention in the MDR (19%) (Table 6). Most intraoperative device issues resulted in completion of the procedure with a backup device (22 of 33 intraoperative device related events). In contrast, most postoperative device issues resulted in an additional surgery to replace the prosthesis (18 of 30 postoperative device related events). Subsequent intervention in the remaining device issues described explanting the device (9.5%), revision tympanoplasty (3.2%), other surgical intervention (1.6%), unspecified surgical intervention (1.6%) or aborting the procedure (1.6%). All eight packaging events were discovered intraoperatively. In half of these issues, the procedure was completed with a backup device whereas the other half did not describe intervention (Table 7).
For RCA of device issues, 27 out of 63 were not returned (42.9%) and 10 did not receive a response from the manufacturer (15.9%). Of the 26 (41.3%) reported device issues that were returned, RCA by the manufacturer determined breaks were caused by modification and/or mishandling (26.9%), the cause could not be determined (23.1%), the product met specifications and a cause was not discussed (23.1%), the device did not meet specifications (15.4%), or the device was confirmed for the alleged malfunction (7.7%) (Table 8). The RCA for one of the devices is still in progress. For the eight packaging issues, half did not receive a response from the manufacturer, and one was not returned. Of the three devices that were returned, the RCA could not determine a cause (33.3%) or the company declared the product was mispackaged or mislabeled (66.7%) (Table 9).
Only 29 of the 70 MDRs resulted in a return of the device to the manufacturer. In 35 of the MDRs, the manufacturer stated that they would provide an updated RCA if further information was acquired. For 13 records, there was no documented response from the manufacturer. In six MDRs, the response from the manufacturer included suggestions for safe prosthesis usage, including reminders that prostheses are very delicate, to inspect packaging prior to opening, or to take precaution with heat application.
4. Discussion
An estimated 48 million people in the United States suffer from hearing loss (Lin et al., 2011). To treat middle ear abnormalities, OCR was developed to improve ossicular sound transmission using autologous tissue or prostheses. To better understand the potential complications of total and partial ossicular replacement prostheses, we analyzed MDRs from the MAUDE database. From 2011 to 2020, our investigation found 70 adverse events of PORP or TORP OCRs which were attributed to device related issues (57.3%), adverse patient outcomes (35.5%), and device packaging issues (7.3%).
Device related issues were most commonly due to a break in the device or displacement of the prosthesis. Intraoperative breaks were almost always completed with a back-up device and postoperative breaks typically required subsequent intervention, such as removal or replacement of the prosthesis. More than half of the reported device issues occurred intraoperatively and thus required an additional prosthesis to complete the procedure. PORP and TORP devices are estimated to cost up to $390, not including the cost of services. This study alone found 33 intraoperative device issues, resulting in more than $12,000 worth of wasted prostheses due to adverse intraoperative events. Additionally, most postoperative device issues were resolved with additional surgery to replace the prosthesis, causing an even greater waste in time, finances, and resources for surgeons and patients. RCA conducted on returned devices determined that a majority (26.9%) of device related adverse outcomes occurred while handling the prosthesis. Furthermore, greater than 80% of device breaks were found to occur in the surgical field while implanting the device. For some of these patients, all device pieces could not be retrieved, and a foreign body was left inside of patient. Manufacturers should be encouraged to provide additional guidance on proper intraoperative handling to avoid damage to the prosthesis and potentially the patient. Additionally, adverse events may be prevented by strengthening wire or titanium pieces to account for intraoperative stresses. Small actions taken by manufacturers could result in thousands of dollars saved on wasted resources.
Device breaks remained the most common adverse outcome reported when PORPs and TORPs were considered separately. Interestingly, non-specific displacements were only reported in 3 PORP device events whereas TORP events included 14 displacements, 11 of which were due to extrusion. Yu et al. performed a meta-analysis of 4311 subjects and found that PORPs had a lower rate of extrusion with a risk ratio of 0.37 compared to TORPs after long-term follow up. They determined that the reason for this instability is the placement of TORPs on the foot plate or oval window, making them more vulnerable to displacement. Conversely, PORPs are secured to the bell-shaped capitulum of the stapes, making them more secure (Yu et al., 2013).
Luers et al. reported that prosthesis dislocation or displacement is a common cause of unsatisfactory postoperative hearing results (Luers et al., 2010). A study conducted by Govil et al. found that 72.7% of 120 pediatric OCR adverse outcomes required revision surgeries due to device related issues, with the most common being prosthesis extrusion or displacement (65.9%) (Govil et al., 2017). Our study found that displacement commonly resulted in patient complaints of changes in hearing or erosion. Surgeons should have a low threshold to evaluate postoperative patients for displaced prostheses to repair this common issue, with an even lower threshold if the patient complains of hearing loss or distorted hearing. According to Mocanu et al. problems with the design and function of these devices is a major factor involved in ossicular prosthesis failure (Mocanu et al., 2021). It is suggested that design changes may be necessary to improve long term fixation of prostheses to avoid extrusion. Recent design changes have been considered; for example, Luers et al. tested modified titanium implants that contained spikes extending into cartilaginous neotympanic membrane to improve stability of the prosthesis (Luers et al., 2010).
The remaining patient related adverse outcomes included perforation, erosion, and an intraoperative foreign body left in the patient. MDR descriptions frequently did not provide additional information, such as the timing of discovery or subsequent intervention of these outcomes. If this information was provided, the postoperative period with the highest risk of peroration or erosion could be determined. With this information, patients could be regularly evaluated during these postoperative periods to identify and address potential perforations and erosions.
Packaging related issues contributed to a minority of the adverse events related to PORP or TORP OCR. Any devices found to be broken, contaminated, or unsealed is a waste of time and resources. This study alone suggests that there have been at least $3000 of wasted resources due to unusable prostheses. To avoid further expenses and loss of time intraoperatively, manufacturers should consider implementing a more rigorous system to check packaging for contamination, errors, and/or defective implants.
There are multiple limitations for this study mostly attributed to the reporting structure of the MAUDE database. The database is made up of voluntarily reported adverse outcomes, which can result in underreporting and skewing of the data, contributing to reporting bias (Tong et al., 2020b). For example, when observing trends of adverse events from 2011 to 2020, there were no MDRs in 2018, while the highest number of adverse events were reported in 2016. Due to the likelihood of inconsistent reporting, these changes are presumably not associated with a change in PORP or TORP OCR trends. Furthermore, adverse outcomes may not be reported in patients with clinically silent deficits. Considering these limitations, the data from the current study cannot be used to infer prevalence or incidence of complications and we cannot report on the clinical significance of our findings. Finally, the RCA and follow-up based on adverse outcomes were not consistently described in the database as not all cases underwent RCA. This study found that the lack of RCA was often due to inadequate information or an unreturned device. As such, it is suggested that a detailed explanation of the event is provided to the manufacturer, and, if applicable, the device should also be returned. This information would provide manufacturers with the opportunity to perform a thorough RCA. Better documentation in future reports with specific reasons for adverse outcomes and future interventions would provide significant knowledge of common PORP or TORP complications. The overarching limitation, or the conglomerate of previously stated limitations, is the lack of clinical relevance of our findings. Given the reporting bias inherent to the database, the inability to infer prevalence or incidence, and that we redacted the manufacturer name associated with adverse events and analyses, we could not utilize our data to retrospectively report on superior or inferior prostheses. Additionally, given that we limited our search to titanium prosthesis adverse events, this study cannot compare findings to other materials, such as plastics or biomaterials, or other middle ear implant types. The findings of this study are not meant to change surgical management, but rather demonstrate commonly reported issues that can lead to adverse events. Future studies could potentially analyze PORP and TORP OCR outcomes in randomized patient populations to corroborate the findings in the current study and develop interventions to avoid harm in patients.
5. Conclusion
This study demonstrated commonly reported issues and highlights certain patterns of error with PORP and TORP OCR using the MAUDE database. We found that the most reported device issues were due to device breaks and displacements, most reported patient issues were due to hearing loss and erosion, and packing issues comprised a minority of reported adverse events. Displacement occurred more often with TORPs than PORPs and was associated with changes in hearing loss or distorted hearing and erosion. Device issues that occurred intraoperatively required an additional prosthesis to complete the procedure and postoperative device issues required subsequent surgical intervention. Quality improvement and guidance on OCR with prosthesis by the manufacturer could potentially results in less wasted time and resources. This study is limited by the voluntary reporting structure of the MAUDE database, inadequate details on the adverse events, and lack of RCA in several reports. The data gathered from this study cannot be used to analyze the incidence of the various errors, compare implant manufacturers, or compare implant types. The findings of this study are purely descriptive in nature and may not have direct clinical relevance. To better understand OCR complications, future submission to the MAUDE database is recommended to include a detailed explanation of the event with return of the device to the manufacturer.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Jaclyn Carey, Email: careyj1@amc.edu.
Sairisheel Gabbireddy, Email: gabbirs@amc.edu.
Luke Mammen, Email: mammenl@amc.edu.
Gianna Rosamilia, Email: rosamig@amc.edu.
Varun Patel, Email: vspatel116@gmail.com.
David Foyt, Email: foytd@amc.edu.
Steven Parnes, Email: parness@amc.edu.
Appendix.
Table 1.
Adverse events by category.
| Category |
n (%) |
Intra-Op (%) |
Post-Op (%) |
|---|---|---|---|
| Total Adverse Events | 110 | 53 (48.2) | 57 (51.8) |
| Total Device Issues | 63 (57.3) | 33 (52.4) | 30 (47.6) |
| Break | 33 (52.4) | 27 (81.8) | 6 (18.2) |
| Nonspecific break | 23 (69.7) | 19 (82.6) | 4 (17.4) |
| Shoe Broke Off | 4 (12.1) | 4 (100) | 0 (0) |
| Wire Broke from Piston | 1 (3) | 1 (100) | 0 (0) |
| Shaft Broke from Wire | 1 (3) | 0 (0) | 1 (100) |
| Shaft Broke Off | 2 (6.1) | 2 (100) | 0 (0) |
| Phalange Broke Off | 1 (3) | 1 (100) | 0 (0) |
| Disintegrated | 1 (3) | 0 (0) | 1 (100) |
| Displacement | 17 (27) | 0 (0) | 17 (100) |
| Nonspecific Displacement | 6 (35.3) | 0 (0) | 6 (100) |
| Extrusion | 11 (64.7) | 0 (0) | 11 (100) |
| Incorrect Size or Shape | 7 (11.1) | 3 (42.9) | 4 (57.1) |
| Failure to Crimp | 4 (6.3) | 3 (75) | 1 (25) |
| Loop of Stapes Open | 2 (3.2) | 0 (0) | 2 (100) |
| Total Patient Issues | 39 (35.5) | 12 (30.8) | 27 (69.2) |
| Hearing Loss | 12 (30.8) | 1 (8.3) | 11 (91.7) |
| Distorted Hearing | 2 (5.1) | 0 (0) | 2 (100) |
| Perforation | 3 (7.7) | 0 (0) | 3 (100) |
| Erosion | 6 (15.4) | 0 (0) | 6 (100) |
| Foreign Body in Patient | 4 (10.3) | 4 (100) | 0 (0) |
| Nerve damage | 1 (2.6) | 1 (100) | 0 (0) |
| Burns | 1 (2.6) | 1 (100) | 0 (0) |
| Fracture | 1 (2.6) | 0 (0) | 1 (100) |
| Pain | 2 (5.1) | 1 (50) | 1 (50) |
| Inner Ear Injury | 1 (2.6) | 0 (0) | 1 (100) |
| Vertigo | 1 (2.6) | 1 (100) | 0 (0) |
| Dizziness | 1 (2.6) | 1 (100) | 0 (0) |
| Headache and Pain During MRI | 1 (2.6) | 0 (0) | 1 (100) |
| Drainage | 1 (2.6) | 0 (0) | 1 (100) |
| Nausea | 1 (2.6) | 1 (100) | 0 (0) |
| Nystagmus | 1 (2.6) | 1 (100) | 0 (0) |
| Total Package Issues | 8 (7.3) | 8 (100) | 0 (0) |
| Device Broken in Package | 2 (25) | 2 (100) | 0 (0) |
| Contaminate Within Package | 2 (25) | 2 (100) | 0 (0) |
| Unsealed Package | 1 (12.5) | 1 (100) | 0 (0) |
| Improper Size Labeling | 1 (12.5) | 1 (100) | 0 (0) |
| Incorrect Item Within Package | 1 (12.5) | 1 (100) | 0 (0) |
| Missing Component | 1 (12.5) | 1 (100) | 0 (0) |
Fig. 1.
Adverse events by year.
Table 2.
ETB (PORP) adverse events by category.
| Category |
n (%) |
Intra-Op (%) |
Post-Op (%) |
|---|---|---|---|
| Total Adverse Events | 42 (100) | 26 (61.9) | 16 (38.1) |
| Total Device Issues | 21 (50) | 13 (61.9) | 8 (38.1) |
| Break | 10 (47.6) | 8 (80) | 2 (20) |
| Nonspecific break | 8 (80) | 7 (87.5) | 1 (12.5) |
| Shaft Broke from Wire | 1 (10) | 0 (0) | 1 (100) |
| Phalange Broke Off | 1 (10) | 1 (100) | 0 (0) |
| Displacement | 3 (14.3) | 0 (0) | 3 (100) |
| Nonspecific Displacement | 3 (100) | 0 (0) | 3 (100) |
| Incorrect Size or Shape | 2 (9.5) | 2 (100) | 0 (0) |
| Failure to Crimp | 4 (19) | 3 (75) | 1 (25) |
| Loop of Stapes Open | 2 (9.5) | 0 (0) | 2 (100) |
| Total Patient Issues | 15 (35.7) | 7 (46.7) | 8 (53.3) |
| Hearing Loss | 4 (26.7) | 1 (25) | 3 (75) |
| Distorted Hearing | 1 (6.7) | 0 (0) | 1 (100) |
| Erosion | 1 (6.7) | 0 (0) | 1 (100) |
| Foreign Body in Patient | 2 (13.3) | 2 (100) | 0 (0) |
| Nerve damage | 1 (6.7) | 1 (100) | 0 (0) |
| Burns | 1 (6.7) | 1 (100) | 0 (0) |
| Pain | 1 (6.7) | 0 (0) | 1 (100) |
| Inner Ear Injury | 1 (6.7) | 0 (0) | 1 (100) |
| Dizziness | 1 (6.7) | 1 (100) | 0 (0) |
| Headache and Pain During MRI | 1 (6.7) | 0 (0) | 1 (100) |
| Nausea | 1 (6.7) | 1 (100) | 0 (0) |
| Total Package Issues | 6 (14.3) | 6 (100) | 0 (0) |
| Device Broken in Package | 1 (16.7) | 1 (100) | 0 (0) |
| Contaminate Within Package | 2 (33.3) | 2 (100) | 0 (0) |
| Unsealed Package | 1 (16.7) | 1 (100) | 0 (0) |
| Incorrect Item Within Package | 1 (16.7) | 1 (100) | 0 (0) |
| Missing Component | 1 (16.7) | 1 (100) | 0 (0) |
Table 3.
ETA (TORP) adverse events by category.
| Category |
n (%) |
Intra-Op (%) |
Post-Op (%) |
|---|---|---|---|
| Total Adverse Events | 68 | 26 (38.2) | 42 (61.8) |
| Total Device Issues | 42 (61.8) | 20 (47.6) | 22 (52.4) |
| Break | 23 (54.8) | 19 (82.6) | 4 (17.4) |
| Nonspecific break | 15 (65.2) | 12 (80) | 3 (20) |
| Shoe Broke Off | 1 (4.3) | 1 (100) | 0 (0) |
| Wire Broke from Piston | 1 (4.3) | 1 (100) | 0 (0) |
| Shaft Broke Off | 5 (21.7) | 5 (100) | 0 (0) |
| Disintegrated | 1 (4.3) | 0 (0) | 1 (100) |
| Displacement | 14 (33.3) | 0 (0) | 14 (100) |
| Nonspecific Displacement | 3 (21.4) | 0 (0) | 3 (100) |
| Extrusion | 11 (78.6) | 0 (0) | 11 (100) |
| Incorrect Size or Shape | 5 (11.9) | 1 (20) | 4 (80) |
| Total Patient Issues | 24 (35.3) | 4 (16.7) | 20 (83.3) |
| Hearing Loss | 8 (33.3) | 0 (0) | 8 (100) |
| Distorted Hearing | 1 (4.2) | 0 (0) | 1 (100) |
| Perforation | 3 (12.5) | 0 (0) | 3 (100) |
| Erosion | 5 (20.8) | 0 (0) | 5 (100) |
| Foreign Body in Patient | 2 (8.3) | 2 (100) | 0 (0) |
| Fracture | 1 (4.2) | 0 (0) | 1 (100) |
| Pain | 1 (4.2) | 0 (0) | 1 (100) |
| Vertigo | 1 (4.2) | 1 (100) | 0 (0) |
| Drainage | 1 (4.2) | 0 (0) | 1 (100) |
| Nystagmus | 1 (4.2) | 1 (100) | 0 (0) |
| Total Package Issues | 2 (2.9) | 2 (100) | 0 (0) |
| Device Broken in Package | 1 (50) | 1 (100) | 0 (0) |
| Improper Size Labeling | 1 (50) | 1 (100) | 0 (0) |
Table 4.
Device issues reported with patient adverse events.
| Total Device Issues | n (%) |
|---|---|
| 29 | |
| Break | 11 (37.9) |
| Nonspecific Break | 8 (72.7) |
| Wire Broke from Piston | 1 (9.1) |
| Shaft Broke from Wire | 1 (9.1) |
| Disintegrated | 1 (9.1) |
| Displacement | 13 (44.8) |
| Nonspecific Displacement | 6 (46.2) |
| Extrusion | 7 (53.8) |
| Incorrect Size or Shape | 3 (10.3) |
| Failure to Crimp | 2 (6.9) |
Table 5.
Patient issues reported with device adverse events.
| Total Patient Issues | n (%) |
|---|---|
| 33 | |
| Hearing Loss | 8 (24.2) |
| Distorted Hearing | 2 (6.1) |
| Perforation | 2 (6.1) |
| Erosion | 6 (18.2) |
| Foreign Body in Patient | 4 (12.1) |
| Nerve damage | 1 (3) |
| Burns | 1 (3) |
| Fracture | 1 (3) |
| Pain | 2 (6.1) |
| Inner Ear Injury | 1 (3) |
| Vertigo | 1 (3) |
| Dizziness | 1 (3) |
| Drainage | 1 (3) |
| Nausea | 1 (3) |
| Nystagmus | 1 (3) |
Table 6.
Subsequent intervention for device related events.
| N/A | Completed Procedure with Backup Device | Prosthesis was Replaced | Explanted | Revision Tympanoplasty | Piston Removed by Laser Surgery | Unspecified Surgical Intervention | Procedure not Completed | |
|---|---|---|---|---|---|---|---|---|
| Total Device Issues (n = 63) | 12 (19) | 22 (34.9) | 18 (28.6) | 6 (9.5) | 2 (3.2) | 1 (1.6) | 1 (1.6) | 1 (1.6) |
| Break | 6 (50) | 17 (77.3) | 5 (27.8) | 3 (50) | 0 (0) | 1 (100) | 0 (0) | 1 (100) |
| Nonspecific break | 4 (66.7) | 12 (70.6) | 3 (60) | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Shoe Broke Off | 1 (16.7) | 3 (17.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Wire Broke from Piston | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Shaft Broke from Wire | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Shaft Broke Off | 0 (0) | 2 (11.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Phalange Broke Off | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Disintegrated | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Displacement | 4 (33.3) | 0 (0) | 9 (50) | 1 (16.7) | 2 (100) | 0 (0) | 1 (100) | 0 (0) |
| Nonspecific Displacement | 0 (0) | 0 (0) | 5 (55.6) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Extrusion | 4 (100) | 0 (0) | 4 (44.4) | 0 (0) | 2 (100) | 0 (0) | 1 (100) | 0 (0) |
| Incorrect Size or Shape | 1 (8.3) | 3 (13.6) | 3 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Failure to Crimp | 1 (8.3) | 2 (9.1) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Loop of Stapes Open | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Table 7.
Subsequent intervention for package related events.
| N/A | Completed Procedure with Backup Device | |
|---|---|---|
| Total Package Issues (n = 8) | 4 (50) | 4 (50) |
| Device Broken in Package | 1 (25) | 1 (25) |
| Contaminate Within Package | 1 (25) | 1 (25) |
| Unsealed Sterile Package | 0 | 1 (25) |
| Improper Size Labeling | 1 (25) | 0 |
| Incorrect Item Within Package | 0 | 1 (25) |
| Missing Component | 1 (25) | 0 |
Table 8.
RCA for returned devices with device related events.
| Cause Unknown | Modification/Sizing/Mishandling Causing Break | Met specifications | Confirmed for Alleged Malfunction | Product did not Meet Specifications | In Progress | |
|---|---|---|---|---|---|---|
| Total Device Issues (n = 29) | 6 (23.1) | 7 (26.9) | 6 (23.1) | 4 (15.4) | 2 (7.7) | 1 (3.8) |
| Break | 4 (66.7) | 7 (100) | 1 (16.7) | 4 (100) | 2 (100) | 0 (0) |
| Nonspecific Break | 4 (100) | 4 (57.1) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Shoe Broke Off | 0 (0) | 3 (42.9) | 0 (0) | 1 (25) | 0 (0) | 0 (0) |
| Wire Broke from Piston | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) |
| Shaft Broke from Wire | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| Shaft Broke Off | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 0 (0) |
| Displacement | 1 (16.7) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Nonspecific Displacement | 1 (100) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) |
| Extrusion | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) |
| Incorrect Size or Shape | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) |
| Failure to Crimp | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Loop of Stapes Open | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) |
Table 9.
RCA for returned devices with package related events.
| Cause Unknown | Mispackaging/Mislabeling | |
|---|---|---|
| Total Package Issues (n = 3) | 1 (33.3) | 2 (66.7) |
| Contaminate Within Package | 1 (100) | 0 (0) |
| Improper Size Labeling | 0 (0) | 1 (50) |
| Incorrect Item Within Package | 0 (0) | 1 (50) |
Funding sources
None.
References
- Bance M. Optimizing ossicular prosthesis design and placement. Adv. Oto-Rhino-Laryngol. 2018;81:14–23. doi: 10.1159/000485545. [DOI] [PubMed] [Google Scholar]
- Bestourous D.E., Pasick L.J., Benito D.A., Zapanta P.E. Adverse events associated with the Inspire implantable hypoglossal nerve stimulator: a MAUDE database review. Am. J. Otolaryngol. 2020;41(6):102616. doi: 10.1016/j.amjoto.2020.102616. [DOI] [PubMed] [Google Scholar]
- Doran J., Ward M., Ward B., Paskhover B., Umanoff M., Mammis A. Investigating complications associated with occipital nerve stimulation: a MAUDE study. Neuromodulation. 2018;21(3):296–301. doi: 10.1111/ner.12750. [DOI] [PubMed] [Google Scholar]
- Govil N., Kaffenberger T.M., Shaffer A.D., Chi D.H. Factors influencing hearing outcomes in pediatric patients undergoing ossicular chain reconstruction. Int. J. Pediatr. Otorhinolaryngol. 2017;99:60–65. doi: 10.1016/j.ijporl.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Hur K., Ge M., Kim J., Ference E.H. Adverse events associated with balloon sinuplasty: a MAUDE database analysis. Otolaryngol. Head Neck Surg. 2020;162(1):137–141. doi: 10.1177/0194599819884902. [DOI] [PubMed] [Google Scholar]
- Kumar S., Yadav K., Ojha T., Sharma A., Singhal A., Gakhar S. To evaluate and compare the result of ossiculoplasty using different types of graft materials and prosthesis in cases of ossicular discontinuity in chronic suppurative otitis media cases. Indian J. Otolaryngol. Head Neck Surg. : official publication of the Association of Otolaryngologists of India. 2018;70(1):15–21. doi: 10.1007/s12070-017-1184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T.L., Wong M.L. Imaging of the mastoid, middle ear, and internal auditory canal after surgery: what every radiologist should know. Neuroimaging Clin. 2009;19(3):307–320. doi: 10.1016/j.nic.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Lee E., Tong J.Y., Pasick L.J., Benito D.A., Joshi A., Goodman J.F., Thakkar P.G. Complications associated with PlasmaBlade TnA during tonsillectomy and adenoidectomy from 2010 to 2020: a MAUDE study. Am. J. Otolaryngol. 2021;42(1):102826. doi: 10.1016/j.amjoto.2020.102826. [DOI] [PubMed] [Google Scholar]
- Lin F.R., Niparko J.K., Ferrucci L. Hearing loss prevalence in the United States. Arch. Intern. Med. 2011;171(20):1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luers J.C., Huttenbrink K.B., Mickenhagen A., Beutner D. A modified prosthesis head for middle ear titanium implants--experimental and first clinical results. Otol. Neurotol. 2010;31(4):624–629. doi: 10.1097/MAO.0b013e3181d2f04e. [DOI] [PubMed] [Google Scholar]
- Mocanu H., Mocanu A.-I., Drăgoi A.M., Rădulescu M. Long-term histological results of ossicular chain reconstruction using bioceramic implants. Exp Ther Med. 2021;21(3):260. doi: 10.3892/etm.2021.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell B.P., Rizk H.G., Hutchinson T., Nguyen S.A., Lambert P.R. Long-term outcomes of titanium ossiculoplasty in chronic otitis media. Otolaryngol. Head Neck Surg. 2016;154(6):1084–1092. doi: 10.1177/0194599816633669. [DOI] [PubMed] [Google Scholar]
- Roth J.A., Pandit S.R., Soma M., Kertesz T.R. Ossicular chain reconstruction with a titanium prosthesis. J. Laryngol. Otol. 2009;123(10):1082–1086. doi: 10.1017/s0022215109990375. [DOI] [PubMed] [Google Scholar]
- Tong J.Y., Pasick L.J., Benito D.A., Sataloff R.T. Complications associated with tracheoesophageal voice prostheses from 2010 to 2020: a MAUDE study. Am. J. Otolaryngol. 2020;41(6):102652. doi: 10.1016/j.amjoto.2020.102652. [DOI] [PubMed] [Google Scholar]
- Tong J.Y., Pasick L.J., Benito D.A., Sataloff R.T. Complications associated with tracheoesophageal voice prostheses from 2010 to 2020: a MAUDE study. Am. J. Otolaryngol. 2020;41(6):102652. doi: 10.1016/j.amjoto.2020.102652. [DOI] [PubMed] [Google Scholar]
- Walker D.D., Babu S.C. History of ossicular chain reconstruction. Current Otorhinolaryngology Reports. 2020;8(1):61–64. doi: 10.1007/s40136-020-00259-w. [DOI] [Google Scholar]
- Wood C.B., Yawn R., Lowery A.S., O'Connell B.P., Haynes D., Wanna G.B. Long-term hearing outcomes following total ossicular reconstruction with titanium prostheses. Otolaryngol. Head Neck Surg. 2019;161(1):123–129. doi: 10.1177/0194599819831284. [DOI] [PubMed] [Google Scholar]
- Young A., Ng M. StatPearls. Treasure Island (FL) 2020. Ossiculoplasty. [Google Scholar]
- Yu H., He Y., Ni Y., Wang Y., Lu N., Li H. PORP vs. TORP: a meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2013;270(12):3005–3017. doi: 10.1007/s00405-013-2388-1. [DOI] [PubMed] [Google Scholar]
- Zhao L., Li J., Gong S. Comparison of the application of artificial ossicles and autologous ossicles in the reconstruction of a damaged ossicular chain. J. Laryngol. Otol. 2018;132(10):885–890. doi: 10.1017/s0022215118001627. [DOI] [PubMed] [Google Scholar]