Abstract
Purpose: Cyclin D1 has been identified as a proto-oncogene associated with the uncontrolled proliferation of tumor cells. This systematic review and meta-analysis aims to estimate the prognostic significance of cyclin D1 in multiple myeloma (MM) patients. Method: We searched for qualified data in PubMed, Embase, and Web of Science up to February 2020. Data quality was assessed by the Newcastle-Ottawa scale (NOS). Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were used to evaluate the relationship between cyclin D1 expression and overall survival (OS), progression-free survival (PFS)/event-free survival (EFS) in patients with MM. Result: A total of 13 studies involving 961 patients were included. Overall, pooled analysis revealed significant heterogeneity between cyclin D1 expression and the prognosis of MM (OS, HR = 1.08, 95% CI: 0.71-1.64, I2 = 67.9%; PFS/EFS, HR = 0.97, 95% CI: 0.49-1.93, I2 = 85.8%). Subgroup analysis revealed that the prolongation of OS was relevant to increased expression of cyclin D1 in MM patients in the relapsed and refractory group (OS, HR = 0.46, 95% CI: 0.24-0.90). Another subgroup assessment of OS established that MM patients with CCND1 overexpression in the bortezomib group had longer survival time (HR = 0.30, 95% CI: 0.11-0.82), whereas, those overexpressing CCND1 in the conventional chemotherapy group had poor prognosis (HR = 2.19, 95% CI: 1.18-4.08). We also found that increased cyclin D1 expression correlated favorably with PFS in the autologous stem cell transplantation (ASCT) (HR = 0.45, 95% CI: 0.28-0.73) or reverse transcription-polymerase chain reaction (RT-PCR) group (HR = 0.41, 95% CI: 0.26-0.64). Conclusion: The result of this meta-analysis suggested that CCND1 overexpression might be a predictive biomarker for MM patients when treated with bortezomib, receiving ASCT, or in relapsed and refractory period.
Keywords: multiple myeloma, prognosis, bortezomib, cyclin D1, meta-analysis
Introduction
Multiple myeloma (MM) is the second most widespread tumor in the hematological system, and its main feature is the clonal proliferation of malignant plasma cells in the bone marrow. 1 In recent years, with the advent of proteasome inhibitors (PIs), immunomodulatory drugs (ImiDs), and monoclonal antibodies (Mab), the 5-year survival rate of MM patients has increased to 40%. 2 However, overall, their long-term prognosis remains unsatisfactory. The soluble IL-6 receptor (sIL-6R), β2-microglobulin (β2-MG), and TP53 mutation have all been shown to be valuable to predict survival of MM patients.3-5 Hematologists remain committed to discovering new prognostic indicators to guide clinical individualized treatment more accurately.
The double allele named CCND1, mostly hyperdiploid, is expressed in 40% of MM patients. 6 Cyclin D1, which is expressed by the CCND1 gene, belongs to the G1 cyclin family that promotes progression from the G1 phase to the S phase by regulating transcription factor E2F-1 and participates in tumorigenesis and development. 7 CCND1 is located at the second core of the 11q13q14 amplicon and acts as the “promotor” of 11q13q14 amplification. The frequent co-amplification of the 11q13 gene in cancer suggests the presence of a group of genes in the amplicons associated with tumor growth and metastasis dominance, which may be related to double-strand DNA breaks in fragile chromosomal sites.8,9 The most common chromosomal translocation involving CCND1 is t(11;14)(q13;q32), which juxtaposes CCND1 with immunoglobulin heavy chain enhancer elements, resulting in cyclin D1 protein expression. 10 These reactions and interactions suggest that cyclin D1 may play a major role in predicting prognosis and making a clinical decision.
So far, there has been evidence from meta-analyses that CCND1 overexpression has prognostic value in renal cancer, bladder cancer, oral squamous cell carcinoma, breast cancer, and melanoma.11-15 However, the relationship between CCND1 overexpression and the prognosis of MM patients remains controversial. While Sewify et al 16 and Tasidou et al 17 found that expression of CCND1 increased with the progression of MM, suggesting that MM patients overexpressing CCND1 have a poor prognosis, Soverini et al 18 and Cook et al 19 discovered that cyclin D1 overexpression was linked to better prognosis of MM patients. Due to the complex nature of the regulatory mechanism of cyclin D1 in the cell cycle, there are insufficient evidence-based analyses on the prognostic significance of cyclin D1 expression in MM. Therefore, in this systematic review and meta-analysis, we have analyzed the survival data extracted from publications to provide a basis for finding new targets for MM treatment.
Materials and Methods
The systematic review and meta-analysis has been registered at INPLASY (ID: INPLASY 202190019). Our study protocol was designed and implemented in accordance with PRISMA reporting guidelines 20 and Cochrane Collaboration criterion. 21
Retrieval Strategy
We searched for studies on the relationship between cyclin D1 and the prognosis of MM published up to February 2020 in PubMed, Web of Science, and EMBASE. We used the method of subject word + free word search to search the words “myeloma” or “multiple myeloma” and “cyclin D1 or CCND1” and “result” or “prognosis” or “survival” without geographical and linguistic restrictions. To further expand the scope of retrieval, we also manually searched unretrieved original literature and relevant references.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) studies that detected cyclin D1 expression/CCND1 amplification in MM cells via immunohistochemistry (IHC), reverse transcription-polymerase chain reaction (RT-PCR) or fluorescence in situ hybridization (FISH); (2) studies that reported the relationship between cyclin D1 expression and survival parameters, such as overall survival (OS), progression-free survival (PFS), and event-free survival (EFS); (3) studies that divided CCND1 expression into positive and negative groups; (4) studies that designed a prospective or retrospective cohort study; (5) studies that provided available hazard ratios (HRs) and 95% confidence intervals (95% CIs) for survival analysis.
Exclusion criteria: (1) reviews, case reports, and animal studies; (2) studies with no available data or no full text related to the topic; (3) repeated articles. If the selected study overlapped with data from other published literature, we selected the latest and largest sample study.
Data Extraction
Two researchers independently extracted the data from predetermined included studies: the first author, mean age, year of publication, sample size, country, detection methods, treatment methods, clinical-stage, disease phase, follow-up period, prognostic indicators (OS, PFS, EFS, HRs, and 95% CIs) and study design. If the data information in any study was inadequate, we requested the full data we needed from the corresponding author. Any differences of opinion between the two independent researchers were settled by consulting a neutral researcher.
Quality Evaluation
The quality of selected studies was assessed by the Newcastle-Ottawa scale (NOS). We graded the selected articles based on the choice of the study, comparability between groups, and determination of the results. Scoring ranged from 0 to 9, with 0 being the minimum score, and 9 being the maximum score. Studies with NOS ≥ 6 considered to be of high quality and low biased risk. All differences between the two observers involved in the process were settled through dialogue.
Statistical Analysis
The meta-analysis was conducted via the STATA software (version 16.0). OS and PFS/EFS were used to determine the prognosis. HRs and 95% CIs were used to evaluate the relationship between CCND1 expression and the prognosis of MM. For articles that did not directly report HRs and 95% CIs but provided Kaplan-Meier (KM) curves, we used Engauge Digitizer4.1 to extract survival data from the KM curves and then calculated the HRs and 95% CIs. An HR > 1 signified a negative result for CCND1 expression, which is associated with a poor prognosis. Conversely, an HR < 1 denoted a positive result. Heterogeneity among included studies was assessed by I2 statistics. For I2 ≥50%, a random-effects model was applied through inverse variance according to the Cochrane Collaboration guidelines. 21 Otherwise, a fixed-effect model was adopted. We used Begg's and Egger's tests to find the symmetry on the funnel chart to evaluate the publication bias on CCND1 expression. 22 Results were considered statistically significant if the 95% CI did not include 1. All the P values exploited in this paper are bilateral, with P < 0.05 considered statistically significant. In the event of heterogeneity, we used the subgroup analysis method to analyze the potential factors.
Result
Search Result
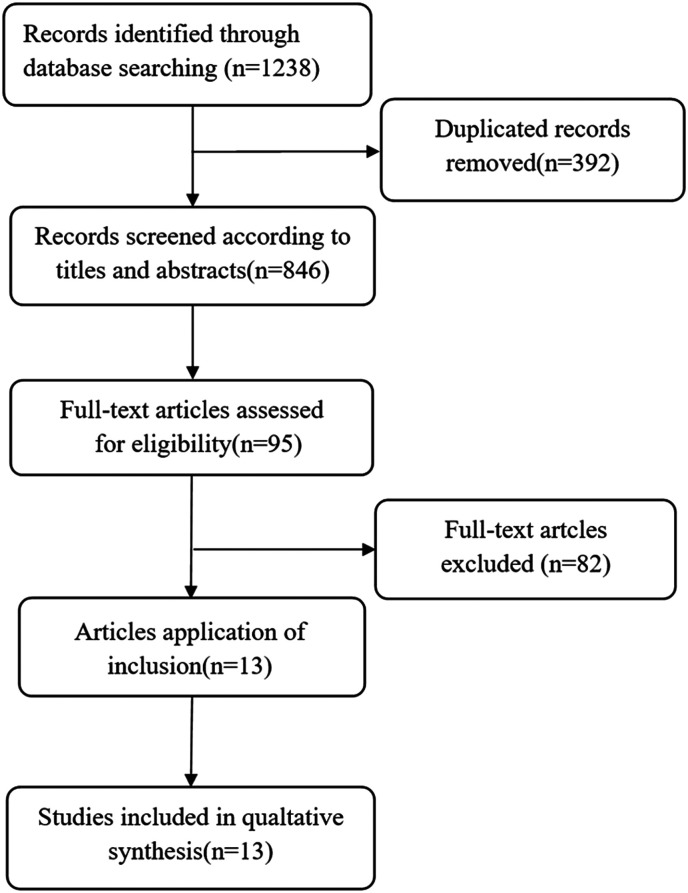
Based on a previously determined retrieval strategy, the researchers retrieved 1238 records from the database of which 392 duplicate articles were excluded, and 95 full-text articles were retrieved by screening titles and abstracts. Of these 95 full-text articles, 82 were subsequently rejected because of insufficient data. In the end, 13 cohort studies that reported the relationship between cyclin D1 expression and survival of MM patients were included in our meta-analysis.16-19,23-31 The flowchart of the specific literature search strategy is shown in Figure 1.
Figure 1.
Flowchart of database search and study inclusion.
Research Characteristics and Quality Evaluation
The literature included in this meta-analysis was published from 2000 to 2014, with average follow-up periods between 11 and 36 months. Of the 13 studies evaluated, 10 were prospective cohort studies and 3 were retrospective cohort studies. Four studies directly provided HRs and 95% CIs; for the other 9 studies, we derived these values from the given survival curve. Most of the MM patients were newly diagnosed, and a few of them were in refractory and recurrent conditions. The clinical features of the selected studies are shown in Table 1. The quality of the articles was evaluated per the NOS standard: 3 were of high quality (8-9 points), and 10 were of medium quality (6-7 points). Overall, the quality of this meta-analysis was good.
Table 1.
Detailed Characteristics of Included Studies.
| Author | Year | Country | Mean Age(year) | Sample Size | Follow-up period(months) | ND/RR | Stage | Detection methods | Outcome | NOS | Study design |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Markovic O | 2004 | Serbia | 63 | 59 | 29-36 | 59/0 | DS I-III | immunohistochemistry | OS | 6 | Prospective cohort study |
| Kelley TW | 2008 | USA | 59 | 94 | NR | 49/45 | SWOG stage l-IV | immunohistochemistry | OS/PFS | 7 | Prospective cohort study |
| Soverini S | 2003 | Italy | 53 | 74 | 9-64,34 | 74/0 | DS I-III | RT-PCR | EFS | 7 | Prospective cohort study |
| Cook JR | 2006 | USA | 58 | 44 | NR | 44/0 | NR | immunohistochemistry | OS/PFS | 9 | Prospective cohort study |
| Ngo BT | 2010 | Germany | 67 | 20 | NR | 0/20 | DS I-III | RT-PCR | OS/PFS | 7 | Retrospective cohort study |
| Pruneri G | 2008 | Italy | 64 | 128 | 27 | NR | DS I-III | immunohistochemistry | OS/PFS | 7 | Retrospective cohort study |
| Dawson MA | 2009 | Australia | 61 | 89 | 1-20,11 | 0/89 | NR | immunohistochemistry | OS | 8 | Prospective cohort study |
| Maillet D | 2012 | Mexico | 59 | 24 | 1-54 | 24/0 | NR | immunohistochemistry | OS | 6 | Prospective cohort study |
| Tasidou A | 2012 | Greece | 68 | 130 | 22 | 130/0 | ISS I-III | immunohistochemistry | OS | 8 | Prospective cohort study |
| Sewify EM | 2014 | Egypt | 59 | 50 | NR | NR | ISS I-III | FISH | OS/PFS | 7 | Retrospective cohort study |
| Rasmussen T | 2001 | Denmark | 62 | 76 | NR | 76/0 | DS I-III | RT-PCR | OS | 6 | Prospective cohort study |
| Hoechtlen-Vollmar | 2000 | Germany | 63 | 50 | 14 | 50/0 | DS I-III | immunohistochemistry | OS | 7 | Prospective cohort study |
| Inagaki A | 2013 | Japan | 68 | 123 | 24 | NR | DS I-III | RT-PCR | OS/PFS | 7 | Prospective cohort study |
Abbreviations: OS, overall survival; PFS, progression-free survival; EFS, event-free survival; NR, not report; ND, newly diagnosed; RR, relapsed/refractory; NOS, Newcastle-Ottawa scale.
The Relationship Between Expression of Cyclin D1 and Prognosis
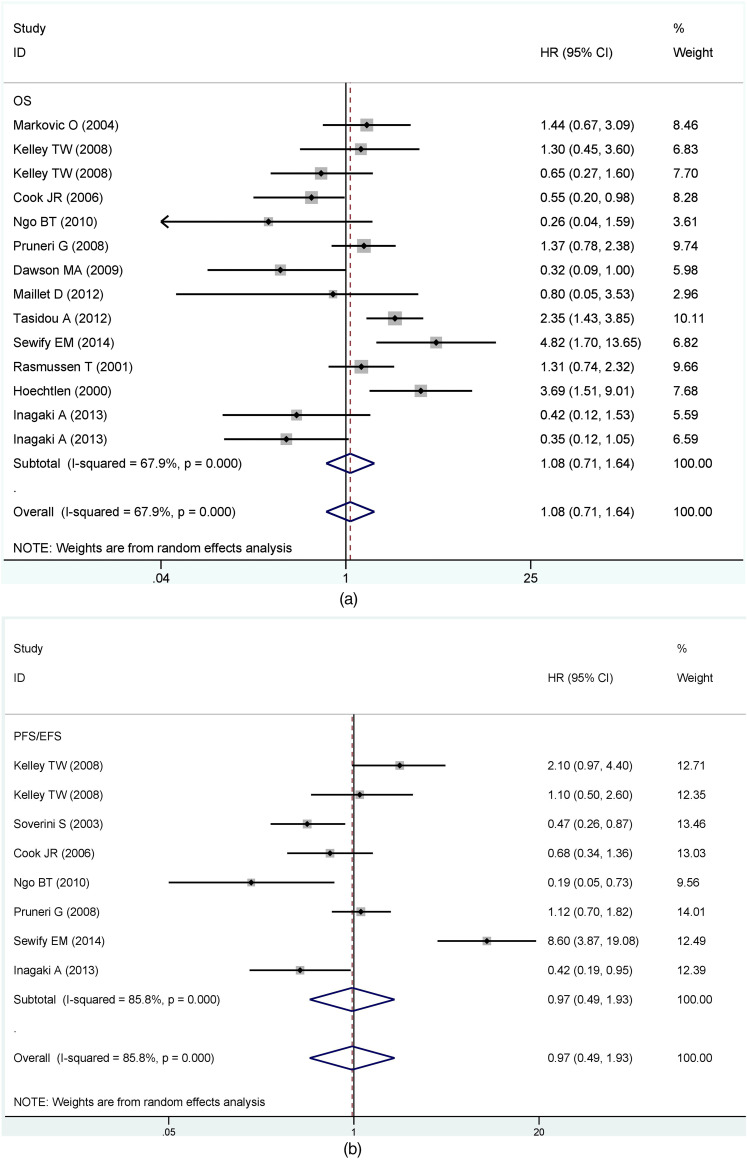
The main results of pooled HRs in the meta-analysis were presented in Table 2. In general, there was a significant degree of heterogeneity between CCND1 expression and prognosis (OS, I2 = 67.9%, P = 0.000; PFS/EFS, I2 = 85.8%, P = 0.000). Under the premise, the joint HRs of CCND1 expression were calculated using the random effect. A comprehensive analysis revealed no significant correlation between CCND1 expression and survival (OS, HR = 1.08, 95% CI: 0.71-1.64; PFS/EFS, HR = 0.97, 95% CI: 0.49-1.93) (Figure 2a and b), but the heterogeneity in two groups was significant.
Table 2.
Main Results of Pooled HRs in the Meta-Analysis.
| Comparisons | HRs | 95% CIs | Heterogeneity, I2, % | P-value |
|---|---|---|---|---|
| OS | 1.08 | 0.71-1.64 | 67.9 | 0.000 |
| Detection method | ||||
| IHC | 1.19 | 0.74-1.90 | 65.1 | 0.003 |
| RT-PCR | 0.57 | 0.24-1.35 | 59.3 | 0.061 |
| Cut-off value | ||||
| 10% | 0.73 | 0.17-3.17 | 76.6 | 0.039 |
| 20% | 1.69 | 0.61-4.68 | 83.5 | 0.002 |
| 30% | 1.32 | 0.77-2.27 | 0 | 0.629 |
| 50% | 0.87 | 0.44-1.71 | 0 | 0.321 |
| Cohort studies | ||||
| Prospective | 0.98 | 0.61-1.58 | 67.9 | 0.001 |
| Retrospective | 1.45 | 0.42-5.04 | 76 | 0.015 |
| Disease phase | ||||
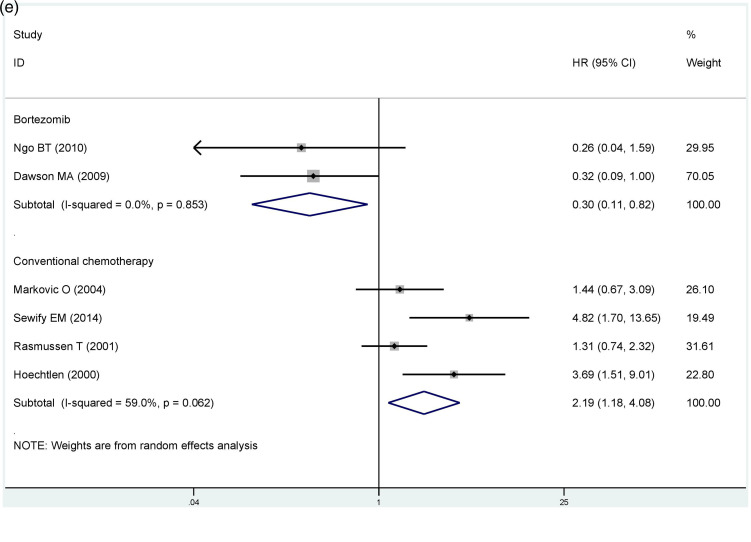
| Relapsed and refractory | 0.46* | 0.24-0.9 | 0 | 0.523 |
| Newly diagnosed | 1.24 | 0.73-2.09 | 73.5 | 0.000 |
| Treatment | ||||
| Bortezomib | 0.3* | 0.11-0.82 | 0 | 0.853 |
| Coventional chemotherapy | 2.19* | 1.18-4.08 | 59 | 0.062 |
| PFS | 0.97 | 0.49-1.93 | 85.8 | 0.000 |
| Detection method | ||||
| IHC | 1.13 | 0.75-1.72 | 35.5 | 0.199 |
| RT-PCR | 0.41* | 0.26-0.64 | 0 | 0.481 |
| Whether or not ASCT | ||||
| Non-ASCT | 1.28 | 0.43-3.83 | 88.2 | 0.000 |
| ASCT | 0.45* | 0.28-0.73 | 0 | 0.825 |
| Cohort studies | ||||
| Prospective studies | 0.78 | 0.44-1.39 | 67.4 | 0.015 |
| Retrospective studies | 1.31 | 0.22-7.72 | 93.1 | 0.000 |
Abbreviations: OS, overall survival; PFS, progression-free survival; ASCT, autologous stem cell transplantation; RT-PCR, transcription-polymerase chain reaction; IHC, immunohistochemistry. * means results were statistically significant.
Figure 2.
Forest plots for the association between cyclin D1 and (a)OS and (b) PFS/EFS of MM patients.
Abbreviations: OS, overall survival; PFS/EFS, progression-free survival/event-free survival.
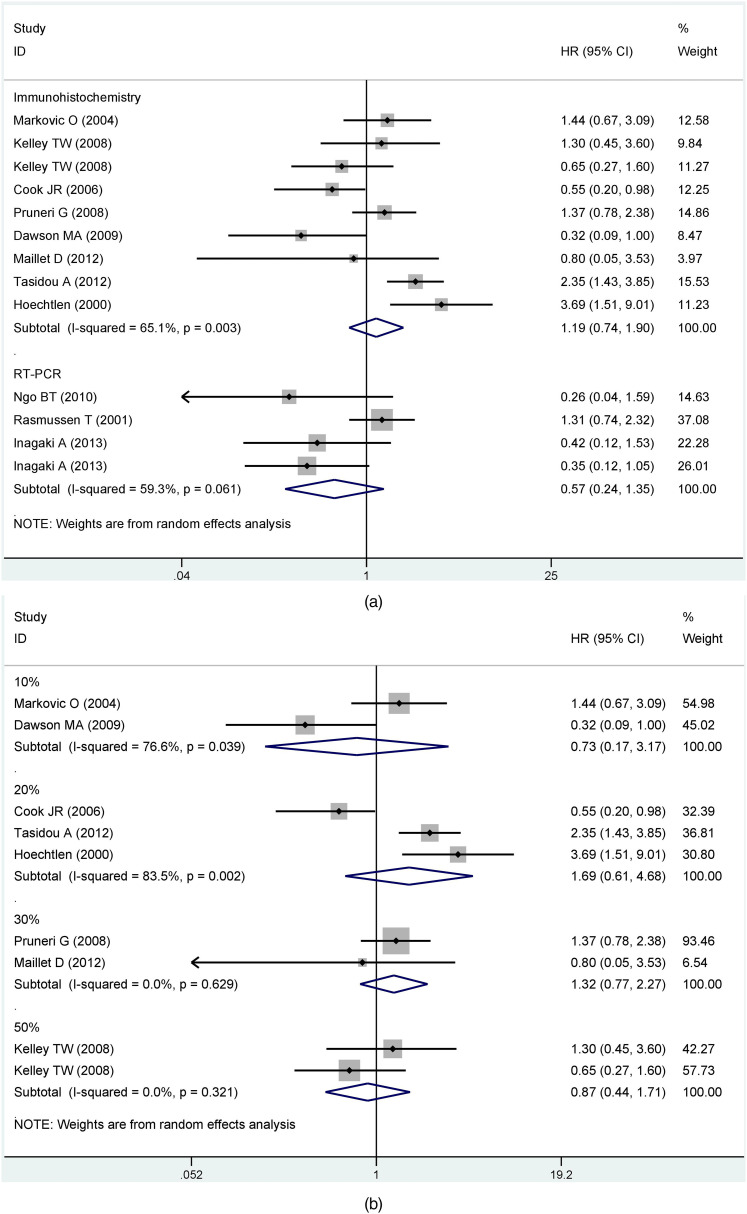
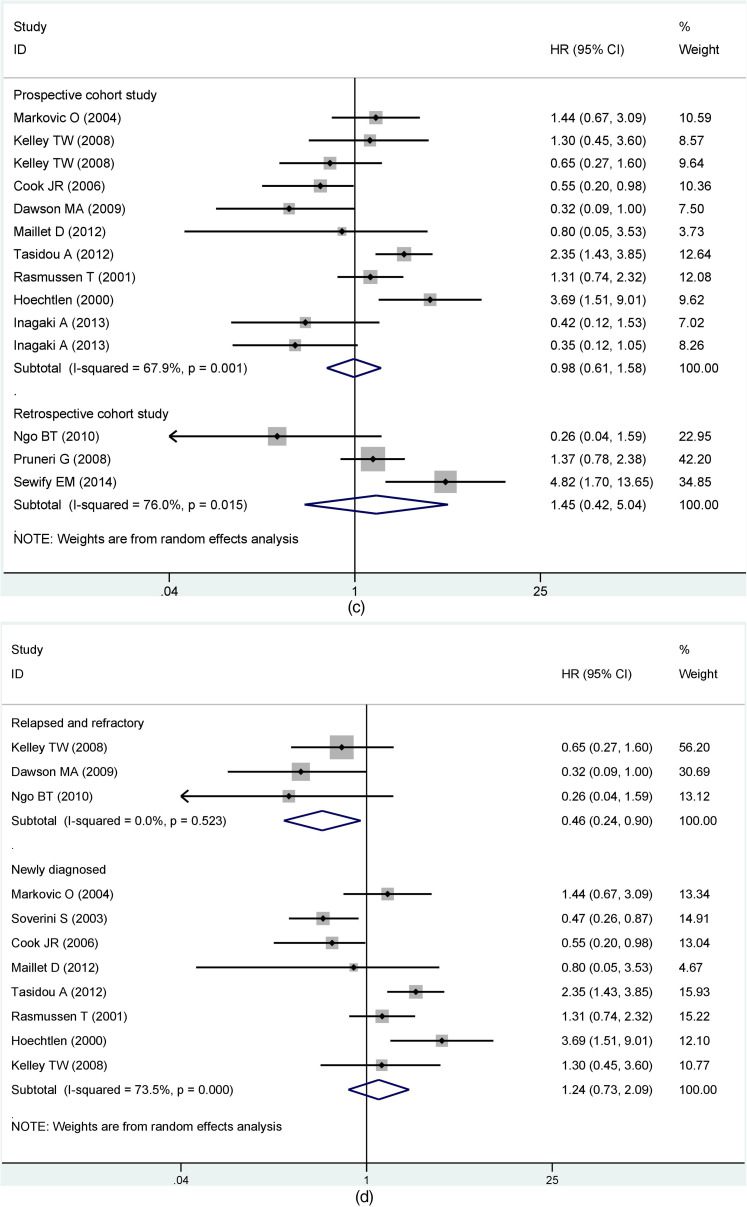
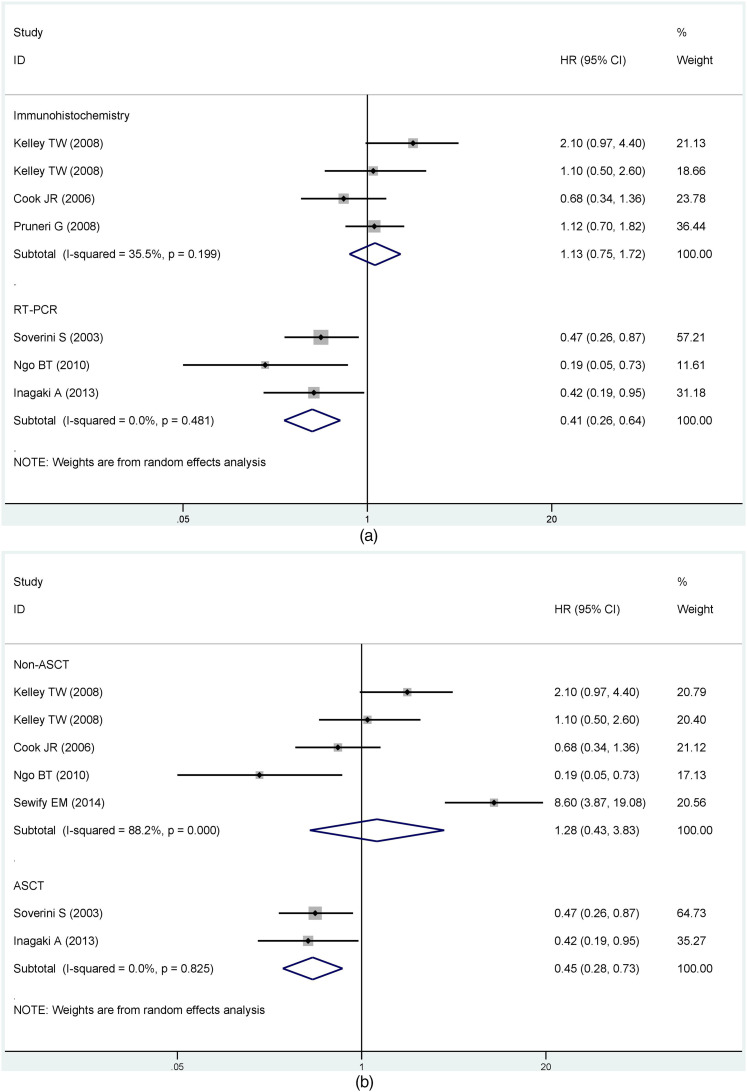
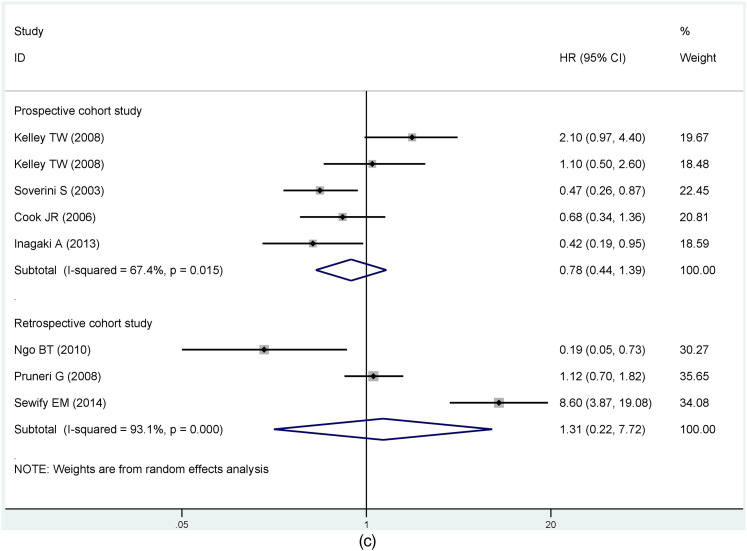
Based on results with great heterogeneity, we categorized the OS group with sufficient sample data into subgroups and analyzed them. First, we performed subgroup analysis by detection of cyclin D1/CCND1. The results showed that differences between detection methods had no statistically significant effect on the prognosis (Immunohistochemistry, HR = 1.19, 95% CI: 0.74-1.90; RT-PCR, HR = 0.57, 95% CI: 0.24-1.35) (Figure 3a). Grouped immunohistochemistry analyses established no correlation between cyclin D1 expression and the prognosis of MM in difference of the critical value (10%, HR = 0.73, 95% CI: 0.17-3.17; 20%, HR = 1.69, 95% CI: 0.61-4.68; 30%, HR = 1.32, 95% CI: 0.77-2.27; 50%, HR = 0.87, 95% CI: 0.44-1.71). Obviously, there was no clear heterogeneity for cut-off values between 30% and 50% (30%, I2 = 0%, P = 0.629; 50%, I2 = 0%, P =0 .321). Conversely, heterogeneity was substantial for critical values between 10% and 20% (10%, I2 = 76.6%, P = 0.039; 20%, I2 = 83.5%, P = 0.002) (Figure 3b). Another subgroup assessment revealed less heterogeneity in prospective cohort studies than in retrospective studies (prospective cohort study, I2 = 67.9%, P = 0.001; retrospective cohort study, I2 = 76%, P = 0.015) although there was no significant association between CCND1 expression and prognosis in both groups (prospective cohort study, HR = 0.98, 95% CI: 0.61-1.58; retrospective cohort study, HR = 1.45, 95% CI:0.42-5.04) (Figure 3c).
Figure 3.
Subgroup analysis of overall survival (OS) by (a) different detection methods; (b) cut-off value; (c) cohort studies; (d) disease phases; (e) treatment.
To further explore the source of heterogeneity in the OS group, we conducted subgroup analysis based on the disease phases and treatment. Our findings based on different disease phases suggested that CCND1 overexpression in the relapsed and refractory group was associated with longer survival periods for MM patients (OS, HR = 0.46, 95% CI: 0.24-0.90), and there was no significant heterogeneity in this group (I2 = 0.0%, P = 0.523). In the newly diagnosed group, cyclin D1 expression was not substantially linked to prognosis (OS, HR = 1.24, 95% CI: 0.73-2.09, I2 = 73.5%, P = 0.000) (Figure 3d). Per the results based on the mode of treatment, high cyclin D1 expression in the traditional chemotherapy group was linked to poor prognosis (OS, HR = 2.19, 95% CI: 1.18-4.08), and the heterogeneity in this group was considerably smaller than before (I2 = 59%, P = 0.062). In contrast, CCND1 overexpression in the bortezomib group was related to better prognosis with no heterogeneity (OS, HR = 0.30, 95% CI: 0.11-0.82, I2 = 0.0%, P =0 .853) (Figure 3e).
Similarly, the PFS/EFS group was subdivided by different CCND1 assays. The forest plot displayed that heterogeneity of the result was smaller (immunohistochemistry, I2 = 35.5%, P = 0.199; RT-PCR, I2 = 0.0%, P = 0.481) and MM patients with CCND1 overexpression had a better prognosis in the RT-PCR group (immunohistochemistry, HR = 1.13, 95% CI: 0.75-1.72; RT-PCR, HR = 0.41, 95% CI: 0.26-0.64) (Figure 4a). Then we conducted subgroup analyses of the PFS/EFS group based on whether autologous stem cell transplantation (ASCT) was performed. We found that the prognosis of MM patients with CCND1 overexpression in the ASCT group was better (HR = 0.45, 95% CI: 0.28-0.73) and there was no observable heterogeneity (I2 = 0%, P = 0.825). The non-ASCT group had no reference meaning because of its remarkable degree of heterogeneity (HR = 1.28, 95% CI: 0.43-3.83, I2 = 88.2%, P = 0.000) (Figure 4b). Results were moderately heterogeneous in the prospective cohort study and highly heterogeneous in the retrospective cohort study (prospective cohort study, I2 = 67.4%, P = 0.015; retrospective cohort study, I2 = 93.1%, P = 0.000), although no statistical significance existed in the results of both study types (Figure 4c).
Figure 4.
Subgroup analysis of PFS/EFS based on (a) different detection methods; (b) whether to perform ASCT; (c) cohort studies.
Abbreviations: PFS/EFS, progression-free survival/event-free survival; ASCT, autologous stem cell transplantation.
Sensitivity Analysis and Publication Bias
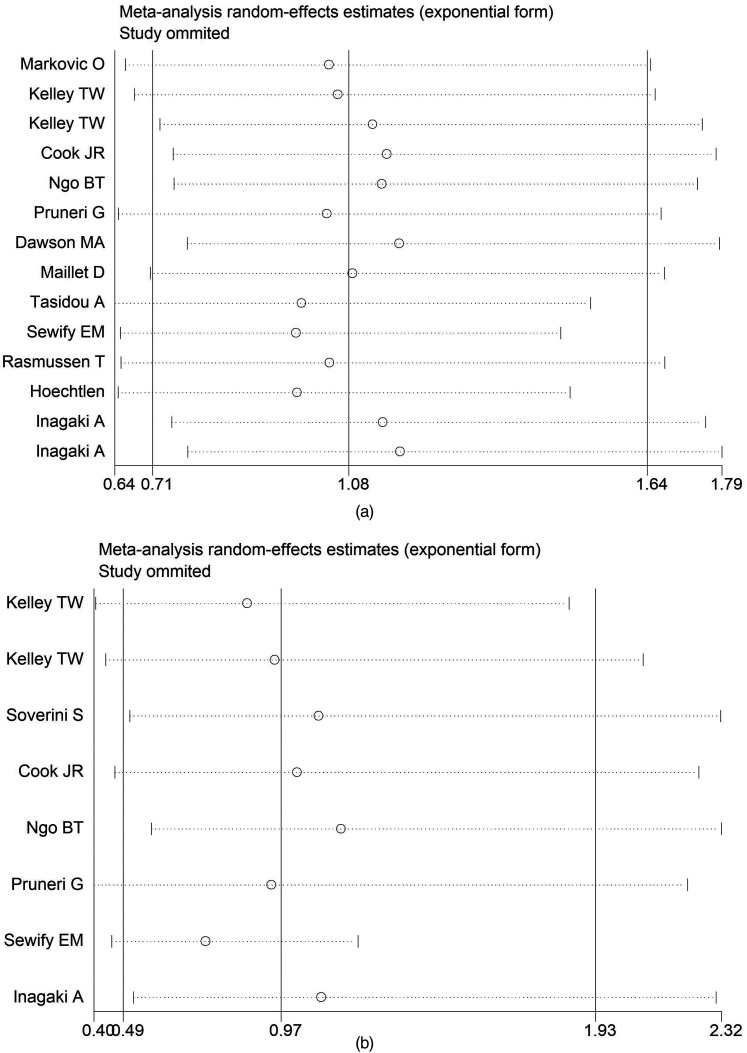
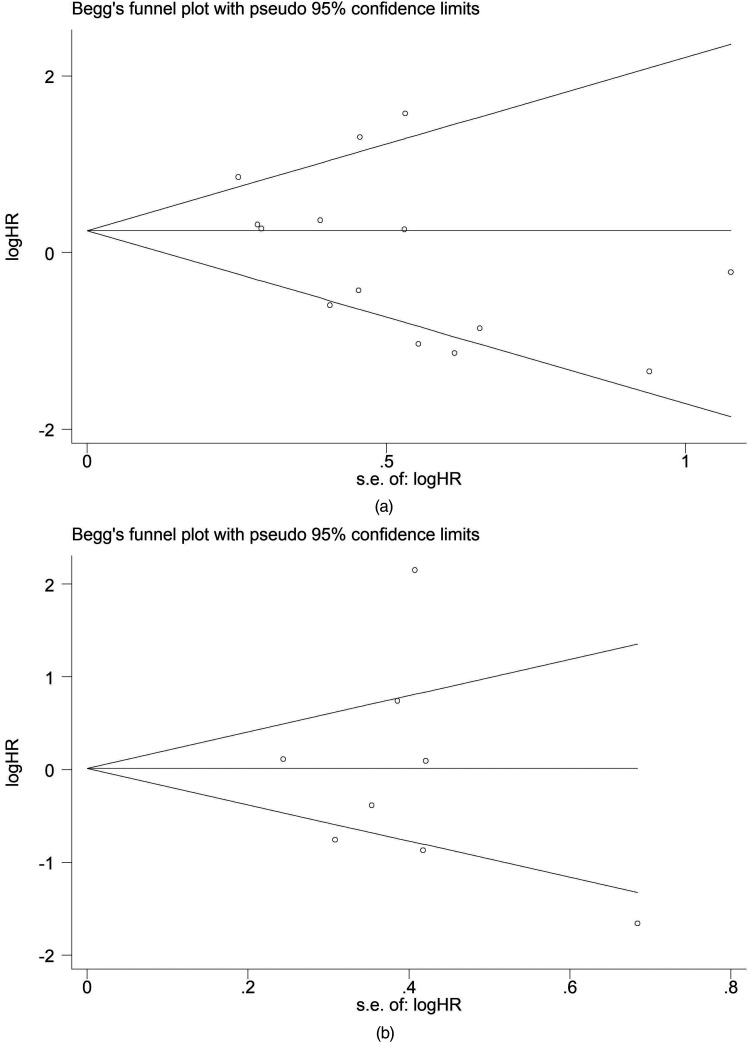
In our sensitivity analysis, we eliminated the included studies one after another and merged HRs to examine the degree of difference between re-merged HRs and original changes (Figure 5). We found that re-evaluated HRs did not differ from those obtained from the first evaluation. As a result of the enormous heterogeneity among our meta-analyses, a random-effects model was chosen for statistical analysis. We used Egger's test and Begg's test in the STATA 16.0 software to evaluate publication bias. The assessment of publication bias in the study displayed that shape of the figure obtained was symmetrical. Egger's test and Begg's test revealed no publication bias on the OS and PFS/EFS of patients, a nod to the robustness of our findings (Figure 6).
Figure 5.
(a) Sensitivity analysis of OS; (b) Sensitivity analysis of PFS/EFS.
Abbreviations: OS, overall survival; PFS/EFS, progression-free survival/event-free survival.
Figure 6.
Begg's funnel plots for the meta-analyses of the association between cyclin D1 and (a) OS or (b) PFS/EFS of MM.
Abbreviations: OS, overall survival; PFS/EFS, progression-free survival/event-free survival, MM, multiple myeloma.
Discussion
Cyclin D1 is a member of the protein kinase family that regulates cell cycle progression, binding to cyclin-dependent kinases (CDK4 or 6) to form a complex that produces activity. 32 Cyclin D1 plays a key role in healthy cells’processes through a series of phosphorylation and inactivation of tumor proteins in retinoblastoma. 33 It has been shown that the uncontrolled expression of cyclin D1 is an early and unified event in MM. Besides, CCND1 overexpression of MM patients is possibly associated with t(11;14)(q13;q32) and polyploidy on chromosome 11.34,35 These findings suggested that hematologists could use cyclin D1 as a new prognostic indicator.
Recently, Tasidou et al 17 followed up 130 patients with newly diagnosed MM for 22 months and noted that CCND1 overexpression was associated with poor survival in MM patients. Relevant discoveries in other previous studies seemed to support the point Tasidou made. First, it was demonstrated that increased expression of cyclin D1 promotes tumor cell proliferation and interferes with the synergistic effect of tumor suppressor genes on cell transformation. 36 Casimiro et al 37 then established that overexpression of the cyclin protein, which was linked to the proliferation and metastasis of MM cells, also affected the signal transduction process and expression of TSP-1. However, this conclusion has been questioned in recent years. Cook et al 19 suggested CCND1 overexpression benefited the prognosis of MM and increased the OS. Meanwhile, Pruneri et al 29 negated the above 2 inferences through a small sample study, revealing rather that there was no significant correlation between the CCND1 expression and survival time of MM patients. In short, opinions on the relationship between CCND1 expression and the prognosis of MM differ among investigators. Now CCND1 expression in the prognosis of MM remains uncertain, so we conducted a detailed meta-analysis to provide a statistical basis for the prognostic value of cyclin D1 expression in MM patients.
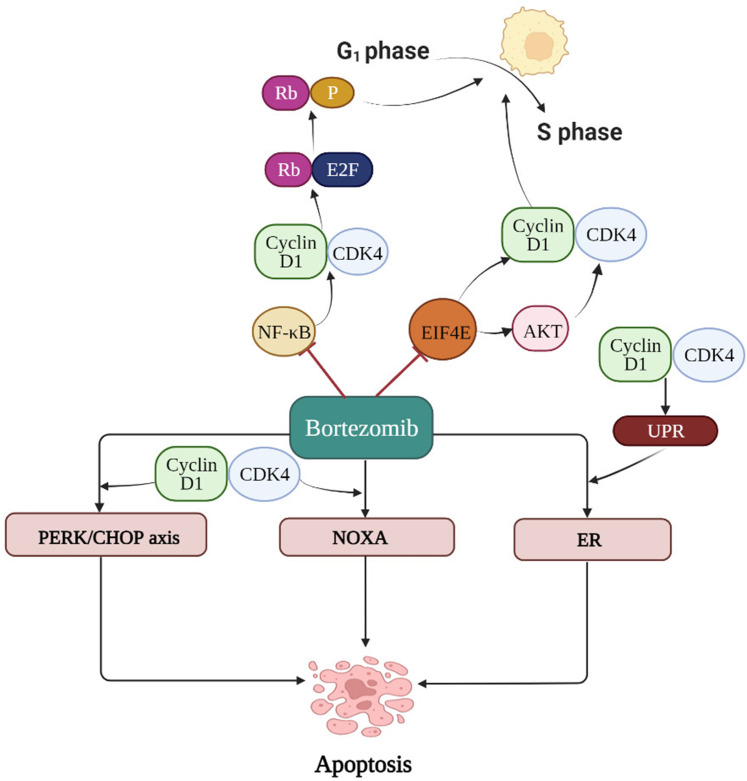
In general, our meta-analysis established no significant correlation between CCND1 overexpression and the prognosis of MM. However, bearing in mind the heterogeneity of results, we performed subgroup analyses of the OS group and found that MM patients with CCND1 overexpression tend to have a poor prognosis when treated with traditional chemotherapy, which validates the findings of Sewify et al. 16 In contrast, high expression of cyclin D1 acted as a favorable prognostic factor for MM patients treated with bortezomib. The possible mechanisms supporting our results are as follows: Combining CCND1 with cyclin kinase CDK4 phosphorylates the Rb protein. The phosphorylated Rb protein is dissociated from the E2F transcription factor, and the E2F transcription factor starts to transcribe the genes that activate the cell cycle, driving the cell cycle from the G1 phase to the S phase. 38 NF-κB, an important nuclear factor in the pathogenesis of MM, tends to promote CCND1 expression. Bortezomib, now widely used to treat MM, can regulate CCND1 expression by inhibiting the activity of NF-κB, subsequently obstructing cell proliferation. 39 Furthermore, studies have shown that bortezomib down-regulates EIF4E mRNA expression to reduce CCND1 mRNA levels, resulting in the arrest of MM cells at the G1/S phase. Therefore, MM patients with CCND1 overexpression could be more sensitive to bortezomib and have a better prognosis. 40 The proteasome inhibitor, bortezomib, mediates cell death by facilitating the stability of the NOXA protein, one of the pro-apoptotic members of the Bcl-2 family. The NOXA protein requires the CCND1/CDK4 activity for effective accumulation, with this accumulation increasing the sensitivity of tumor cells to bortezomib. There is evidence that inhibiting the activity of CCND1/CDK4 reduces the degree of induction of the NOXA protein by bortezomib. 41 Remarkably, bortezomib can mediate antitumor effects via CHOP transcription or by increasing the PERK activity, whereas CCND1 expression can enhance the effect of bortezomib on the PERK/CHOP axis. At the same time, CCND1 can also activate the unfolded protein response (UPR) to induce bortezomib's apoptosis pathway mediated by endoplasmic reticulum (ER) stress. 42 The mechanisms that reflect the relationship between bortezomib and cyclin D1 are shown in Figure 7.
Figure 7.
Mechanism diagram for interaction between bortezomib and cyclin D1 is displayed in the figure below. Bortezomib can regulate expression of cyclin D1 by inhibiting the activity of NF- κB and down-regulating expression of EIF4EmRNA to reduce expression of CCND1mRNA. Cyclin D1/CDK4 activity plays an important role in the induction of NOXA protein, thus directly enhancing the anti-tumor effect of bortezomib. Besides, cyclin D1 can enhance the effect of bortezomib on PERK/CHOP axis and activate unfolded protein response (UPR) to induce the apoptosis pathway of bortezomib through endoplasmic reticulum (ER) stress.
Abbreviations: NF-κB, nuclear factor kappa-B; EIF4E, eukaryotic initiation factor 4E; NOXA, A BH3-only member of Bcl-2; AKT, protein kinase B; PERK, PKR-like ER kinase; CHOP, C/EBP-homologous protein; UPR, unfolded protein response; ER, endoplasmic reticulum; Rb, retinoblastoma gene; E2F, the E2F family of DNA-binding transcription factors; P, phosphoric acid.
Remarkably, MM patients with CCND1 overexpression had better OS in the subgroup with relapsed and refractory recurrence, possibly owing to the high utilization rate of bortezomib. We also found that MM patients with high CCND1 levels in the ASCT group was more likely to have a longer PFS than individuals in the non-ASCT group. Based on Soverini's research, this better survival tended to be associated with transplantation itself and had no obvious correlation with treatment before and after ASCT. 18
In particular, our subgroup analysis showed that results were less heterogeneous when CCND1 was detected by RT-PCR, the immunohistochemical threshold above 30%, or prospective cohort study methods were used. Therefore, a more than 30% immunohistochemical threshold can be selected as a positive result to reduce heterogeneity. Additionally, more samples and higher-quality prospective studies can be performed to further elucidate the relationship between cyclin D1 expression and MM prognosis.
Despite our endeavor to avoid errors and improve the credibility of our statistical results, this investigation still had limitations: (1) The total number of subjects included in the study was not large enough, meaning that the assessment of MM patients was not representative enough. (2) There was no single defined method for determining CCND1 expression, including IHC, RT-PCR, and FISH. (3) A subset of data such as follow-up time and mode of treatment, were not mentioned in certain cases, resulting in the incompleteness of the subgroups. (4) The patients' age, nationality and research methods differed and resulted in the heterogeneity of results.
Nevertheless, this is the first meta-analysis to assess the prognostic value of cyclin D1 expression in MM. As a potential prognostic factor, cyclin D1 provides a new direction to evaluate the survival status of MM patients. On the basis of the qualitative analysis of the included articles, we offer the following recommendations for future cohort studies. First, attention should be paid to the combined impact of genetics and environmental factors on prognosis. Second, more standardized and automated procedures could be developed for specimen collection and detection. Furthermore, prospective studies with greater sample sizes and longer follow-up periods must be scrutinized.
Conclusion
Our meta-analysis showed that cyclin D1 may deserve as a valuable prognostic biomarker for MM. MM patients overexpressing CCND1 treated with bortezomib or receiving ASCT are more likely to have better prognoses. Besides, the increased expression of CCND1 in relapsed and refractory MM patients probably be associated with survival benefits.
Acknowledgments
We thanked the financial support provided by Nantong Science and Technology Project (grant nos. JCZ19029 and MS22019003).
Glossary
Abbreviations
- OS
Overall survival
- PFS
Progression-free survival
- EFS
Event-free survival
- NR
Not report
- ND
Newly diagnosed
- RR
Relapsed/refractory
- NOS
Newcastle-Ottawa Scale
- NF-κB
Nuclear factor κ-B
- EIF4E
Eukaryotic initiation factor 4E
- NOXA
A BH3-only member of Bcl-2
- AKT
Protein kinase B
- PERK
PKR-like ER kinase
- CHOP
C/EBP-homologous protein
- UPR
Unfolded protein response
- ER
Endoplasmic reticulum
- Rb
Retinoblastoma gene
- E2F
The E2F family of DNA-binding transcription factors
- P
Phosphoric acid.
Footnotes
Authors’ Contributions: YWJ designed the research study. YWJ, CLZ, and LL collected data and performed data analysis. YWJ drafted the manuscript and designed the figures. HYL, YJJ, and LMH contributed to the editing of the final manuscript. HMH and DG reviewed the manuscript. All authors checked the full text carefully and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Nantong Science and Technology Project (grant number JCZ19029, MS22019003).
ORCID iD: Hongming Huang https://orcid.org/0000-0001-9695-4674
References
- 1.Packard E, Shahid Z, Groff A, Patel R, Jain R. Multiple myeloma in an agricultural worker exposed to pesticides. Cureus. 2019;11(5):e4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabata M, Tsubaki M, Takeda T, et al. Inhibition of HSP90 overcomes melphalan resistance through downregulation of Src in multiple myeloma cells. Clin Exp Med. 2020;20(1):63‐71. [DOI] [PubMed] [Google Scholar]
- 3.Kamińska J, Koper OM, Dymicka-Piekarska V, Motybel-Iwańczuk E, Ołdziej A, Kemona H. Serum soluble CD40L concentration depending on the stage of multiple myeloma and its correlation with selected angiogenic cytokines. Pol Arch Med Wewn. 2016;126(5):321‐329. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty R, Muchtar E, Kumar SK, et al. Serial measurements of circulating plasma cells before and after induction therapy have an independent prognostic impact in patients with multiple myeloma undergoing upfront autologous transplantation. Haematologica. 2017;102(8):1439‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynt E, Bisht K, Sridharan V, Ortiz M, Towfic F, Thakurta A. Prognosis, biology, and targeting of TP53 dysregulation in multiple myeloma. Cells. 2020;9(2):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah V, Sherborne AL, Walker BA, et al. Prediction of outcome in newly diagnosed myeloma: a meta-analysis of the molecular profiles of 1905 trial patients. Leukemia. 2018;32(1):102‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He S, Yang S, Niu M, et al. HMG-box transcription factor 1: a positive regulator of the G1/S transition through the cyclin-CDK-CDKI molecular network in nasopharyngeal carcinoma. Cell Death Dis. 2018;9(2):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkerson PM, Reis-Filho JS. The 11q13-q14 amplicon: clinicopathological correlations and potential drivers. Genes Chromosomes Cancer. 2013;52(4):333‐355. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-García P, Ruiz-Ávila I, Gil-Montoya JA, et al. Relevance of chromosomal band 11q13 in oral carcinogenesis: an update of current knowledge. Oral Oncol. 2017;72(2017):7‐16. [DOI] [PubMed] [Google Scholar]
- 10.Tsuyama N, Abe Y, Yanagi A, et al. Induction of t(11;14) IgH enhancer/promoter-cyclin D1 gene translocation using CRISPR/Cas9. Oncol Lett. 2019;18(1):275‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Liu J, Zhang X, et al. Prognostic significance of cyclin D1 expression in renal cell carcinoma: a systematic review and meta-analysis. Pathol Oncol Res. 2020;26(3):1401‐1409. [DOI] [PubMed] [Google Scholar]
- 12.Ren B, Li W, Yang Y, Wu S. The impact of cyclin D1 overexpression on the prognosis of bladder cancer: a meta-analysis. World J Surg Oncol. 2014;12(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos-García P, González-Moles M, González-Ruiz L, Ruiz-Ávila I, Ayén Á, Gil-Montoya JA. Prognostic and clinicopathological significance of cyclin D1 expression in oral squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2018;83(2018):96‐106. [DOI] [PubMed] [Google Scholar]
- 14.He Q, Wu J, Liu XL, et al. Clinicopathological and prognostic significance of cyclin D1 amplification in patients with breast cancer: a meta-analysis. J Buon. 2017;22(5):1209‐1216. [PubMed] [Google Scholar]
- 15.González-Ruiz L, González-Moles M, González-Ruiz I, Ruiz-Ávila I, Ramos-García P. Prognostic and clinicopathological significance of CCND1/cyclin D1 upregulation in melanomas: a systematic review and comprehensive meta-analysis. Cancers (Basel). 2021;13(6):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sewify EM, Afifi OA, Mosad E, Zaki AH, El Gammal SA. Cyclin D1 amplification in multiple myeloma is associated with multidrug resistance expression. Clin Lymphoma Myeloma Leuk. 2014;14(3):215‐222. [DOI] [PubMed] [Google Scholar]
- 17.Tasidou A, Roussou M, Terpos E, et al. Increased expression of cyclin-D1 on trephine bone marrow biopsies independently predicts for shorter overall survival in patients with multiple myeloma treated with novel agents. Am J Hematol. 2012;87(7):734‐736. [DOI] [PubMed] [Google Scholar]
- 18.Soverini S, Cavo M, Cellini C, et al. Cyclin D1 overexpression is a favorable prognostic variable for newly diagnosed multiple myeloma patients treated with high-dose chemotherapy and single or double autologous transplantation. Blood. 2003;102(5):1588‐1594. [DOI] [PubMed] [Google Scholar]
- 19.Cook JR, Hsi ED, Worley S, Tubbs RR, Hussein M. Immunohistochemical analysis identifies two cyclin D1 + subsets of plasma cell myeloma, each associated with favorable survival. Am J Clin Pathol. 2006;125(4):615‐624. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone JC, Glass K, Munn Z, Tugwell P, Doi SAR. Comparison of bias adjustment methods in meta-analysis suggests that quality effects modeling may have less limitations than other approaches. J Clin Epidemiol. 2020;117(2020):36‐45. [DOI] [PubMed] [Google Scholar]
- 23.Ngo BT, Felthaus J, Hein M, et al. Monitoring bortezomib therapy in multiple myeloma: screening of cyclin D1, D2, and D3 via reliable real-time polymerase chain reaction and association with clinico-pathological features and outcome. Leuk Lymphoma. 2010;51(9):1632‐1642. [DOI] [PubMed] [Google Scholar]
- 24.Dawson MA, Opat SS, Taouk Y, et al. Clinical and immunohistochemical features associated with a response to bortezomib in patients with multiple myeloma. Clin Cancer Res. 2009;15(2):714‐722. [DOI] [PubMed] [Google Scholar]
- 25.Hoechtlen-Vollmar W, Menzel G, Bartl R, Lamerz R, Wick M, Seidel D. Amplification of cyclin D1 gene in multiple myeloma: clinical and prognostic relevance. Br J Haematol. 2000;109(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 26.Kelley TW, Baz R, Hussein M, Karafa M, Cook JR. Clinical significance of cyclin D1, fibroblast growth factor receptor 3, and p53 immunohistochemistry in plasma cell myeloma treated with a thalidomide-based regimen. Hum Pathol. 2009;40(3):405‐412. [DOI] [PubMed] [Google Scholar]
- 27.Maillet D, Montiel-Cervantes L, Padilla-González Y, et al. Serum calcium is an independent prognostic factor of overall survival in Mexican patients with multiple myeloma. Rev Invest Clin. 2012;64(1):17‐24. [PubMed] [Google Scholar]
- 28.Markovic O, Marisavljevic D, Cemerikic V, Suvajdzic N, Milic N, Colovic M. Immunohistochemical analysis of cyclin D1 and p53 in multiple myeloma: relationship to proliferative activity and prognostic significance. Med Oncol. 2004;21(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 29.Pruneri G, Alietti A, Agnelli L, et al. Immunoreactivity for cyclin D1 is a reliable marker of gene aberration in plasma cell myeloma but does not specify patients prognosis. Leuk Res. 2008;32(10):1628‐1632. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen T, Knudsen LM, Johnsen HE. Frequency and prognostic relevance of cyclin D1 dysregulation in multiple myeloma. Eur J Haematol. 2001;67(5-6):296‐301. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki A, Tajima E, Uranishi M, et al. Global real-time quantitative reverse transcription-polymerase chain reaction detecting proto-oncogenes associated with 14q32 chromosomal translocation as a valuable marker for predicting survival in multiple myeloma. Leuk Res. 2013;37(12):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 32.Guiley KZ, Stevenson JW, Lou K, et al. P27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science. 2019;366(6471):eaaw2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao X, Hong Y, Mao Y, et al. A novel cyclin-dependent kinase 4/6 inhibitor with good anticancer efficacy and strong blood-brain barrier permeability. Cancer Sci. 2020;111(5):1761‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J, Jr. Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106(1):296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vodicka P, Musak L, Vodickova L, et al. Genetic variation of acquired structural chromosomal aberrations. Mutat Res Genet Toxicol Environ Mutagen. 2018;836(Pt A):13‐21. [DOI] [PubMed] [Google Scholar]
- 36.Borello U, Berarducci B, Delahaye E, Price DJ, Dehay C. SP8 Transcriptional regulation of cyclin D1 during mouse early corticogenesis. Front Neurosci. 2018;12(2018):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casimiro MC, Velasco-Velázquez M, Aguirre-Alvarado C, Pestell RG. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present. Expert Opin Investig Drugs. 2014;23(3):295‐304. [DOI] [PubMed] [Google Scholar]
- 38.Schaal C, Pillai S, Chellappan SP. The Rb-E2F transcriptional regulatory pathway in tumor angiogenesis and metastasis. Adv Cancer Res. 2014;121:147‐182. [DOI] [PubMed] [Google Scholar]
- 39.Anwer F, Gee KM, Iftikhar A, et al. Future of personalized therapy targeting aberrant signaling pathways in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19(7):397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutter G, Rieken M, Pastore A, et al. The proteasome inhibitor bortezomib targets cell cycle and apoptosis and acts synergistically in a sequence-dependent way with chemotherapeutic agents in mantle cell lymphoma. Ann Hematol. 2012;91(6):847‐856. [DOI] [PubMed] [Google Scholar]
- 41.Heine S, Kleih M, Giménez N, et al. Cyclin D1-CDK4 activity drives sensitivity to bortezomib in mantle cell lymphoma by blocking autophagy-mediated proteolysis of NOXA. J Hematol Oncol. 2018;11(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bustany S, Cahu J, Guardiola P, Sola B. Cyclin D1 sensitizes myeloma cells to endoplasmic reticulum stress-mediated apoptosis by activating the unfolded protein response pathway. BMC Cancer. 2015;15(2015):262. [DOI] [PMC free article] [PubMed] [Google Scholar]