Abstract
Background:
Many studies have documented the use of platelet-rich plasma (PRP) alongside anterior cruciate ligament (ACL) reconstruction (ACLR) in the management of ACL injury, but evidence on the benefits of PRP in improving the clinical outcomes of ACLR is inconsistent.
Purpose:
To help in our understanding, we undertook a systematic review and meta-analysis of randomized controlled trials (RCTs) that evaluated the effects of PRP on patient-reported functional scores, the clinical assessments of knee function and structure, and complications.
Study Design:
Systematic review; Level of evidence, 1.
Methods:
We searched 9 online databases for RCTs published in English or Chinese that examined the effects of PRP on ACLR. The primary outcome measures were visual analog scale (VAS) for pain and International Knee Documentation Committee (IKDC) scores. The secondary outcomes included KT-1000 arthrometer, pivot-shift test, Lysholm and Tegner scores, tunnel widening, graft characterization, and complications. Subgroup analyses were performed according to time of assessments. Fixed- and random-effects models were selected for data analysis.
Results:
A total of 14 studies were included. When PRP was injected to graft tunnels, the pooled VAS scores of the 2 groups were similar (P = .31), and the subgroup analysis found that VAS and IKDC only improved at 3 months postoperatively (P = .0003 and P < .00001, respectively). When PRP was used at the bone–patellar tendon–bone harvest sites, VAS was decreased in the first 6 months postoperatively (P < .00001), whereas IKDC score was not remarkably different (P = .07). After PRP injection, Lysholm scores at 3 months postoperatively was different between the 2 groups (P < .00001), but the Tegner scores (P = .86), KT-1000 measurements (P = .12), the positive rate of pivot-shift test (P = .64), the enlargement of tunnels (femoral, P = .91; tibial, P = .80), and the characterization of grafts (P = .05) were not different. No difference in complications was found in either group.
Conclusion:
PRP applied alongside ACLR could reduce postoperative pain and improve knee function in the short and medium terms but is ineffective in the long term. PRP does not improve knee stability and the enlargement of tunnels and does not accelerate the healing of grafts. Further studies would be required.
Keywords: anterior cruciate ligament, anterior cruciate ligament reconstruction, platelet-rich plasma, meta-analysis
Anterior cruciate ligament (ACL) injury is one of the most common sports-related orthopaedic injuries that can potentially lead to the end of a career in pivoting sports. 17 The overall sex- and age-adjusted annual incidence of isolated ACL tears is about 0.07%, and the prevalence is about 3.45% and 2% in female and male athletes, respectively, in a window span of 1 season to 25 years. 21,26 After an injury, the ACL’s environment becomes poorly vascularized and produces synovial fluid with proteases that inhibit fibrin clot formation needed for initiating wound healing. 18
ACL reconstruction (ACLR) is the current clinical standard to treat ACL injuries for active individuals with knee instability. 23,32,34 Despite satisfactory knee function being reported after ACLR, only 53% of athletes return to their preinjury sports level, and athletes who return to a high activity level have a high risk of sustaining a second ACL injury. 15,27 In addition, the rehabilitation period is long. A review postulated that patients should delay returning to sports for nearly 2 years to reduce the incidence of second ACL injuries. 23 Therefore, demands for effective treatments to accelerate healing after ACLR have been increasing.
Platelet-rich plasma (PRP), a regenerative therapy that has gained popularity in musculoskeletal medicine for its potential to augment the repair of tissues with low healing ability, is a preparation of autologous blood enriched with a platelet concentration above that which is normally contained in whole blood and is isolated via differential centrifugation. 13,39 The efficacy and potential healing effects of PRP have been evaluated in the trauma of cartilage tissue and the restoration of impaired function, such as rotator cuff repair, osteoarthritis, and medial epicondylitis. 2,18 PRP could perform healing action via the enhanced adhesion, recruitment, proliferation, migration, and differentiation of stromal cells and participate in tissue remodeling, matrix production, and chondrogenic differentiation. 18
Many studies have documented the use of PRP in the treatment of ACL injuries. 5 –9,16,30,37 PRP has had great success in accelerating ACL healing in animal models. 3,10 However, clinical studies using human participants to investigate the effects of PRP on ACLR have shown inconsistent clinical outcomes. Mahdi and Hatem 16 reported that PRP was a safe procedure that enhanced graft-bone integration at 12 weeks’ follow-up after ACLR. de Almeida et al 7 also documented that PRP was beneficial to patellar tendon harvest-site healing at 6 months postoperatively. Walters et al 37 found that patients had similar levels of kneeling pain and patellar defect sizes after ACLR, whether they received PRP or not. Sözkesen et al 30 also did not recommend the routine use of PRP for the prevention of tunnel enlargement after ACLR.
The present study was a systematic review and meta-analysis of currently available randomized controlled trials (RCTs) that evaluated the effects of PRP on the clinical outcomes of ACLR to provide clinicians with a guide. We hypothesized that PRP would offer positive effects on the clinical outcomes of ACLR recovery.
Methods
This systematic review and meta-analysis was performed by following the guidance of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 20
Search Strategy
PubMed, EMBASE, Cochrane Library, Web of Science, Google Scholar, China National Knowledge Infrastructure, Chinese Biomedical Literature Database, WanFang database, and Chinese Scientific Journal Database were searched up to January 26, 2021. The following search terms and synonyms were entered in various combinations in the title, abstract, or keywords: (“Anterior Cruciate Ligament Reconstruction” OR “ACLR” OR “Anterior Cruciate Ligament Injury” OR “Anterior Cruciate Ligament” OR “ACL”) AND (“Platelet-Rich Plasma” OR “Platelet Rich Plasma” OR “PRP”).
Inclusion and Exclusion Criteria
All included studies met the following criteria: (1) published clinical RCT; (2) studies on ACLR with PRP on the injured side compared with a control group that included patients who underwent ACLR surgery without PRP; (3) reported measures for at least 1 of the following clinical outcomes after ACLR: visual analog scale (VAS) for pain, International Knee Documentation Committee (IKDC) score, Lysholm score, Tegner score, KT-1000 measurement, pivot-shift test, tunnel widening (assessed via computed tomography [CT]), graft characterization (assessed via magnetic resonance imaging [MRI]), and complications; and (4) articles written in English or Chinese. The exclusion criteria were (1) other article types than the original (eg, reviews, letters to editors, trial registrations, proposals for protocols, editorials, and book chapters), (2) laboratory studies, (3) papers not published in English or Chinese, and (4) conference or meeting abstracts with no full text available.
Data Extraction
Two investigators (T.Z. and X.X.) followed the inclusion and exclusion criteria for the preliminary and secondary screening of the literatures independently. The disagreements were resolved by a third reviewer. Duplicate manuscripts were excluded. A total of 14 studies were included after overall screening. The main data extracted in this meta-analysis included the basic characteristics of the studies, the details of PRP injections, and outcome indicators. The basic characteristics of studies included the name of the first author, year of publication, sample size, average age, sex ratio of patients, assessment time point, follow-up period, and writing language. The details of PRP injections included graft type, mean platelet concentration, centrifugation parameters, and intervention methods (such as the number and volume of injections). The extracted data were reviewed and checked by the third researcher to ensure accuracy. The primary outcomes included VAS pain and IKDC scores. The secondary outcomes were KT-1000 arthrometer laxity measurement, pivot-shift test, Lysholm score, Tegner score, tunnel widening, graft characterization, and complications.
Quality Assessment
The quality of the selected studies was assessed by 2 investigators (T.Z. and X.X.) independently according to the Cochrane Collaboration's tool for randomized controlled trials. 11 Each study was evaluated according to 7 characteristics (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias), which were rated as being of low, unclear, or high risk of bias. Studies were deemed to have an overall low risk of bias when every item was scored as “low risk,” a moderate risk of bias when 1 or 2 items were classified as “high risk” or “unclear risk,” and a high risk of bias if >2 items were scored as “high risk.”
Data Analysis
Data were recorded as means with SDs or as absolute values. Continuous variables were reported as mean difference (MD) and 95% CI, whereas dichotomous variables were reported as odds ratio (OR) and 95% CI. Considering the variables were presented by the same unit of measurement among all studies, raw MD and 95% CI were calculated for all outcomes. Statistical heterogeneity was judged via the combination of Q value statistics and I 2 statistics. 12 Fixed-effect models were selected for analyses when the heterogeneity was not statistically significant (I 2 < 50%), and random-effect models were selected for analyses when the heterogeneity was statistically significant (I 2 ≥ 50%). 12 Funnel plot was made to inspect the possibility of publication bias when the accumulated number of eligible studies for individual outcome was not <10. Meta-analyses of VAS and IKDC scores were performed on studies that applied PRP to graft tunnels as well as studies that injected PRP into patellar tendon harvest sites to obtain more accurate results. Subgroup analyses were performed to compare the different time points of assessments of VAS, IKDC, and Lysholm scores. Review Manager Version 5.4 was used for statistical analysis. Statistical significance was set at P < .05.
Results
Search Results
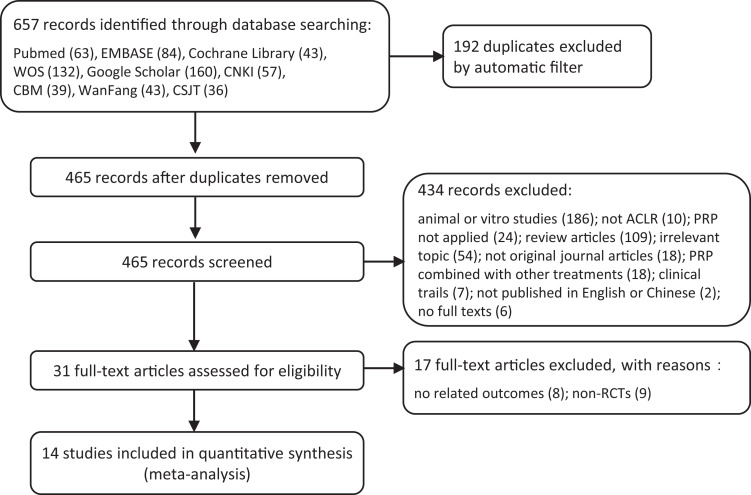
A total of 657 related studies were identified from the 9 electronic databases. Of these, we evaluated the full text of 31 RCTs. Only 14 clinical RCTs were included in the final comprehensive analysis. ¶ The flow diagram of the study selection is shown in Figure 1.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) study selection flow diagram. ACLR, anterior cruciate ligament reconstruction; CBM, Chinese Biomedical Literature Database; CNKI, China National Knowledge Infrastructure; CSJT, Chinese Scientific Journal Database; PRP, platelet-rich plasma; RCT, randomized controlled trial; WOS, Web of Science.
Study Characteristics
The characteristics of the included studies are shown in Table 1. The 14 studies had a total of 634 patients: 319 patients were in the PRP group, and 315 patients were in the control group; the enrolled patients consisted of 449 male and 185 female patients. The age range of the included patients was 18 to 65 years. The average assessment time points and follow-up periods were various among the included studies.
Table 1.
Characteristics of Studies Included a
| First Author (Year) | Patients, n | Sex, M/F, n | Age, y, mean ± SD (range) | Assessment Time (range) | Follow-up Period (range) | Risk of Bias |
|---|---|---|---|---|---|---|
| Vadalà (2013) 35 | PRP: 20 C: 20 |
All: 40/0 | All: 34.5 (18-48) | 14.7 mo (10-16 mo) b | 14.7 mo (10-16 mo) | High |
| Cervellin (2012) 5 | PRP: 20 C: 20 |
PRP: 20/0 C: 20/0 |
PRP: 22.9 ± 4.3 (18-29) C: 22.7 ± 3.5 (19-27) |
12 mo | PRP: 14.3 mo (12.4-16 mo) C: 13.7 mo (12.4-16 mo) |
Moderate |
| de Almeida (2012) 7 | PRP: 12 C: 15 |
PRP: 10/2 C: 14/1 |
PRP: 25.8 (18-44) C: 23.1 (15-34) |
1 d and 6 mo postop | 6 mo | High |
| Figueroa (2010) 9 | PRP: 30 C: 20 |
PRP: 18/12 C: 15/5 |
PRP: 26.8 (14-28) C: 23.6 (13-35) |
6 mo | 14 mo | High |
| Starantzis (2014) 31 | PRP: 25 C: 26 |
All: 38/13 | PRP: 29.4 ± 7.3 C: 31.3 ± 8.0 |
Preop and 1 and 12 mo postop | 12 mo | Moderate |
| Mahdi (2019) 16 | PRP: 14 C: 13 |
PRP: 14/0 C: 13/0 |
All: 25.77 | 12 wk postop | 3 mo postop | High |
| Mirzatolooei (2013) 19 | PRP: 23 C: 23 |
PRP: 20/3 C: 22/1 |
PRP: 26.4 (18-40) C: 26.9 (18-40) |
1 d and 3 mo postop | 3 mo postop | Moderate |
| Seijas (2016) 28 | PRP: 23 C: 20 |
PRP: 20/3 C: 17/3 |
All: range, 18-65 | Preop and 4, 8, 12, 16, 24, 36, 48, and 96 wk postop | 2 y | Low |
| Sözkesen (2018) 30 | PRP: 18 C: 26 |
PRP: 2/16 C: 1/25 |
All: 26 ± 6.96 | Preop and 1 d and 3 mo postop | 12 mo (4-16 mo) | High |
| Nin (2009) 24 | PRP: 50 C: 50 |
PRP: 40/10 C: 38/12 |
PRP: 26.1 (14-57) C: 26.6 (15-59) |
1 d and 24 mo postop | 24.3 mo (18-36 mo) | Moderate |
| Walters (2018) 37 | PRP: 27 C: 23 |
PRP: 10/17 C: 12/11 |
All: 30 ± 12 | Preop and 12 wk, 6 mo, 1 y, and 2 y postop | 2 y | Low |
| Chen (2020) 6 | PRP: 20 C: 20 |
PRP: 12/8 C: 14/6 |
PRP: 35.11 ± 14.23 C: 36.86 ± 12.02 |
Preop and 3, 6, and 12 mo postop | PRP: 12.57 mo C: 13.04 mo |
High |
| Dong (2014) 8 | PRP: 20 C: 20 |
PRP: 13/7 C: 11/9 |
PRP: 39.2 ± 16.3 C: 31.4 ± 16.2 |
1, 3, and 12 mo postop | 18 mo | High |
| Ji (2017) 14 | PRP: 17 C: 19 |
PRP: 8/9 C: 7/12 |
PRP: 31.59 (17-44) C: 33.68 (18-44) |
Preop and 3 and 12 mo postop | 9.83 mo (3-12 mo) | High |
A summary of the quality assessment is shown in Figure 2. Only 1 study had incomplete outcomes, 31 and all studies had no reporting bias or other biases. Therefore, 2 studies were rated as low risk, 28,37 4 studies were rated as moderate risk, 5,19,31,36 and 8 studies were rated as high risk of bias 6 –9,14,16,30,35 (Table 1).
Figure 2.
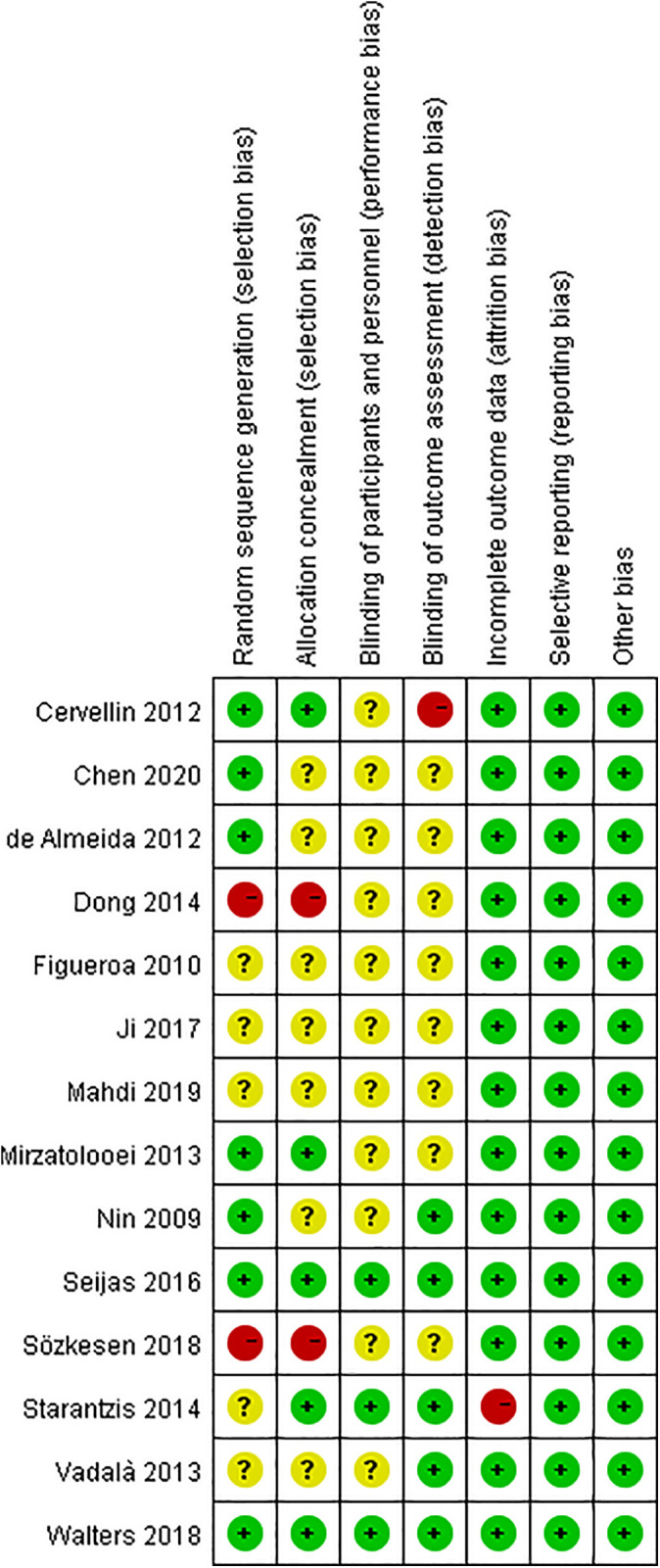
Details of the study quality assessment according to Cochrane Collaboration risk-of-bias tool.
The characteristics of the PRP injection are shown in Table 2. The preparation and intervention treatments of PRP varied among studies. Four RCTs added PRP to the patellar tendon harvest sites after ACLR with bone–patellar tendon–bone (BPTB), 5,7,28,37 and 10 RCTs added PRP to graft tunnels. 6,8,9,14,16,19,24,30,31,35 Only 1 study 14 performed 3 PRP injections (during ACLR and at 15 and 30 days postoperatively), and the rest performed a single PRP injection.
Table 2.
PRP Injection Characteristics of the Included Studies a
| First Author (Year) | Graft Type | Platelet Concentration | Centrifugation Parameters | PRP Interventions |
|---|---|---|---|---|
| Vadalà (2013) 35 | Hamstrings autograft | NR | NR | PRP: PRP (5 mL) added between the peripheral part of the graft and the tunnel wall before passing the graft through the femoral tunnel. Another 5 mL of PRP was added in a semisolid pattern above the graft before the graft was pulled down into the femoral tunnel. Another 5 mL of liquid and semisolid PRP was added before fixing the graft on the tibial side. C: ACLR with hamstrings. |
| Cervellin (2012) 5 | BPTB autograft | NR | 15 min at 3200 rpm | PRP: PRP gel was applied to the patellar and tendon bone plug’s harvest site. C: ACLR with BPTB. |
| de Almeida (2012) 7 | BPTB autograft | 1,185,166/mm 3 ± 404,472/mm3 | NR | PRP group: Patellar tendon defect was completely filled with 20-40 mL of PRP gel. C: Nothing was added. |
| Figueroa (2010) 9 | Hamstring tendon autograft | NR | 15 min at 3200 rpm | PRP: PRP was added in the tibial (3 mL) and femoral (3 mL) tunnels, and the remaining 4 mL was directly applied in the intra-articular portion of the graft. C: ACLR with hamstrings. |
| Starantzis (2014) 31 | Hamstring tendon (semitendinosus and gracilis) | NR | 15 min at 3200 rpm | PRP: PRP (3 mL) was added between the strands of the graft. Once the graft was fixed, the remaining 3 mL was injected into the femoral tunnel. C: ACLR with hamstrings. |
| Mahdi (2019) 16 | Semitendinosus and gracilis tendon | 5-7 × 107/L | NR | PRP: PRP (3 mL) was separately injected in the femoral tunnel and intra-articular portion. C: ACLR with semitendinosus and gracilis tendons. |
| Mirzatolooei (2013) 19 | Hamstrings autograft | NR | 5 min at 1500 rpm | PRP: The graft was immersed in PRP solution for approximately 5 min, and 2 and 1.5 mL of PRP were injected into the femoral tunnel and tibial tunnel, respectively. C: ACLR with hamstrings. |
| Seijas (2016) 28 | BPTB autograft | NR | 8 min at 1800 rpm | PRP: After skin closure, 1 mL each of PRGF was injected into the patellar bone gap, tibial bone gap, patellar tendon line, and harvest gap. C: PRGF injection was not given at the end of surgery. |
| Sözkesen (2018) 30 | Hamstring autograft | NR | Men: 5 min at 2500 rpm Women: 5 min at 2200 rpm |
PRP: The graft was soaked in 2 mL of PRP, and 2 mL of PRP was injected into the femoral and tibial tunnels. C: ACLR with hamstrings. |
| Nin (2009) 24 | BPTB allograft | 837 × 103/mm3 | 1st: 8 min at 3000 rpm 2nd: 6 min at 1000 rpm |
PRP: The ligament was covered with gel and sutured over itself with gel in its interior; the rest of the gel was introduced after the implantation of the graft inside the tibial tunnel. C: ACLR with patellar tendon allograft. |
| Walters (2018) 37 | BPTB autograft | NR | 5 min at 1500 rpm | PRP: The PRP was mixed with autologous cancellous bone chips and placed into the patellar donor site. C: Untreated bone chips were placed in the donor site as described for the other group. |
| Chen (2020) 6 | Semitendinosus and gracilis autograft | NR | NR | PRP: PRP (1 mL) was injected into the tibial and femoral ends of the graft; after the ACL graft was fixed, 3 mL of PRP was injected into the graft surface. C: The same amount of normal saline was injected, and the other operations were the same. |
| Dong (2014) 8 | Semitendinosus and gracilis autograft | NR | 12 min at 2400 rpm | PRP: The graft was soaked in 3 mL of PRP; after the ACL graft was fixed, 3 mL of PRP was injected into the graft, femoral tunnel, and tibial tunnels; then, 3 mL of PRP was injected into the joint cavity. C: The same amount of normal saline was injected, and the other operations were the same. |
| Ji (2017) 14 | Semitendinosus and gracilis autograft | 695 × 109/L | 10 min at 2000 rpm | PRP: The graft was soaked in 5 mL of PRP for 10 min, and 5 mL of PRP was injected into the joint cavity after graft fixation; PRP (5 mL) was injected at 15 and 30 d postoperatively. C: The graft was soaked in 5 mL of normal saline. |
a ACL, anterior cruciate ligament; ACLR, ACL reconstruction; BPTB, bone–patellar tendon–bone; C, control; NR, not reported in the original paper; PRGF, plasma rich in growth factors; PRP, platelet-rich plasma.
VAS Score
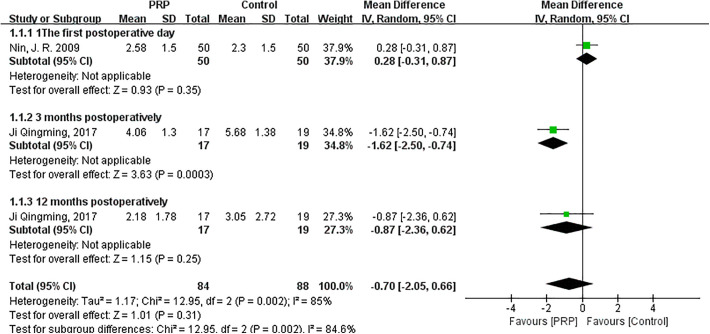
As shown in Figure 3, the random-effects meta-analysis (I 2 = 85%; P = .002) showed that VAS score was not different between the PRP and control groups when PRP was added to graft tunnels (MD = −0.70; 95% CI = −2.05 to 0.66; P = .31). The VAS score of the PRP group was significantly lower than that of the control group at 3 months after surgery (MD = −1.62; 95% CI = −2.50 to −0.74; P = .0003). The differences between the 2 groups at 1 day (MD = 0.28; 95% CI = −0.31 to 0.87; P = .35) and 12 months postoperatively (MD = −0.87; 95% CI = −2.36 to 0.62; P = .25) were not statistically significant.
Figure 3.
Forest plot of the effects of platelet-rich plasma (PRP) injection to graft tunnels on visual analog scale pain score. IV, inverse variance.
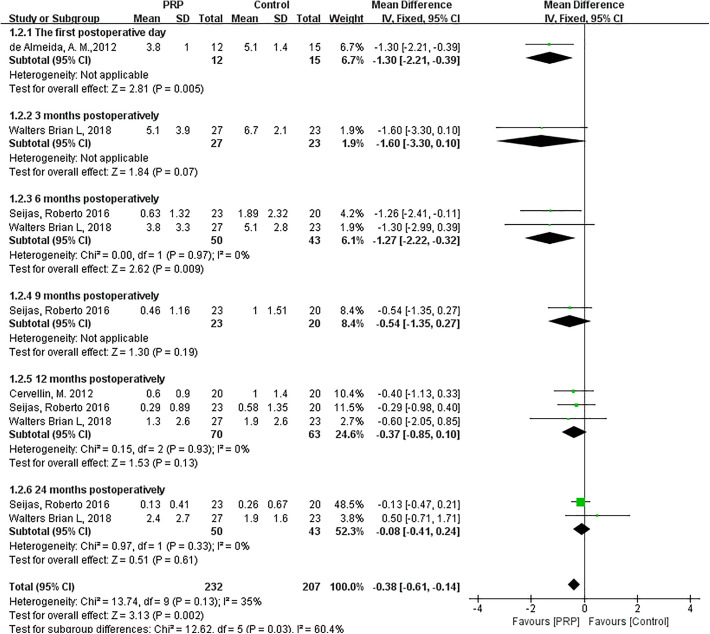
As shown in Figure 4, the fixed-effects meta-analysis (I 2 = 35%; P = .13) showed that VAS score was significantly lower post-PRP compared with the control group when PRP was added to patellar tendon harvest sites (MD = −0.38; 95% CI = −0.61 to −0.14; P = .002). The differences between the PRP and control groups at 1 day (MD = −1.30; 95% CI = −2.21 to −0.39; P = .005) and 6 months postoperatively (MD = −1.27; 95% CI = −2.22 to −0.32; P = .009) were statistically significant. VAS scores of the 2 groups at 3 months (MD = −1.60; 95% CI = −3.30 to 0.10; P = .07), 9 months (MD = −0.54; 95% CI = −1.35 to 0.27; P = .19), 12 months (MD = −0.37; 95% CI = −0.85 to 0.10; P = .13), and 24 months postoperatively (MD = −0.08; 95% CI = −0.41 to 0.24; P = .61) were similar.
Figure 4.
Forest plot of the effects of platelet-rich plasma (PRP) injection to harvest sites on visual analog scale. IV, inverse variance.
IKDC Score
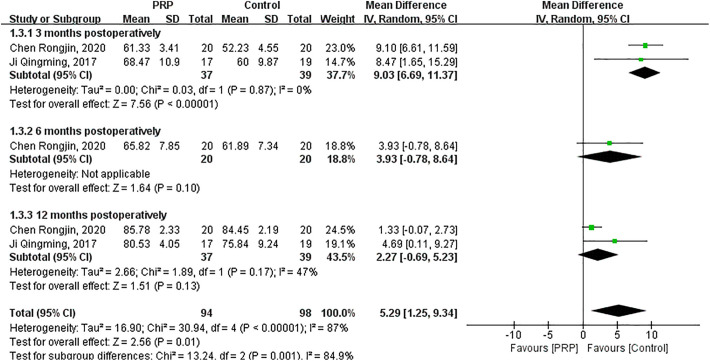
As shown in Figure 5, the random-effects meta-analysis (I 2 = 87%; P < .00001) showed that IKDC score was significantly increased after PRP injections to graft tunnels compared with the control group (MD = 5.29; 95% CI = 1.25 to 9.34; P = .01). IKDC score significantly increased after PRP intervention at 3 months postoperatively (MD = 9.03; 95% CI = 6.69 to 11.37; P < .00001). IKDC scores of the 2 groups had no significant difference at 6 months (MD = 3.93; 95% CI = −0.78 to 8.64; P = .10) and 12 months postoperatively (MD = 2.27; 95% CI = −0.69 to 5.23; P = .13).
Figure 5.
Forest plot of the effects of platelet-rich plasma (PRP) injection to graft tunnels on International Knee Documentation Committee score. IV, inverse variance.
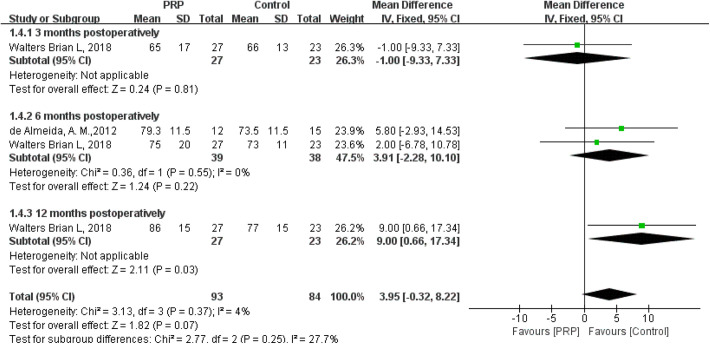
As shown in Figure 6, the fixed-effects meta-analysis (I 2 = 4%; P = .37) showed that IKDC score was not significantly different between the 2 groups (MD = 3.95; 95% CI = −0.32 to 8.22; P = .07) after PRP injections to the harvest sites. IKDC score significantly increased after PRP intervention at 12 months postoperatively (MD = 9.00; 95% CI = 0.66 to 17.34; P = .03). No significant difference in IKDC score was observed between the 2 groups at 3 months (MD = −1.00; 95% CI = −9.33 to 7.33; P = .81) or 6 months postoperatively (MD = 3.91; 95% CI = −2.28 to 10.10; P = .22).
Figure 6.
Forest plot of the effects of platelet-rich plasma (PRP) injection to harvest sites on International Knee Documentation Committee score. IV, inverse variance.
KT-1000 Measurement
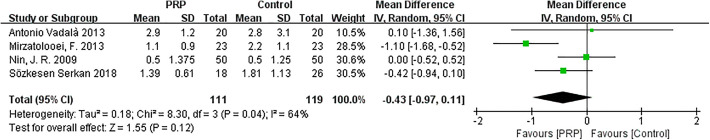
The random-effects meta-analysis (I 2 = 64%; P = .04) showed that the difference in KT-1000 measurements between the 2 groups was not statistically significant (MD = −0.43; 95% CI = −0.97 to 0.11; P = .12) (Figure 7).
Figure 7.
Forest plot of the effects of platelet-rich plasma (PRP) on KT-1000 arthrometer measurements. IV, inverse variance; PRP, platelet-rich plasma.
Pivot-Shift Test
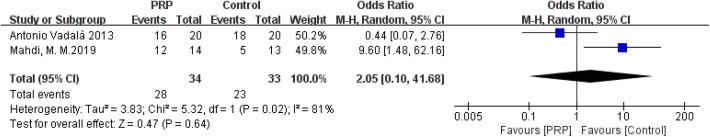
The random-effects meta-analysis (I 2 = 81%; P = .02) demonstrated that the positive rate in the pivot-shift test did not change after PRP injections (OR = 2.05; 95% CI = 0.10-41.68; P = .64) (Figure 8).
Figure 8.
Forest plot of the effects of platelet-rich plasma (PRP) on pivot-shift test. M-H, Mantel-Haenszel.
Lysholm Score
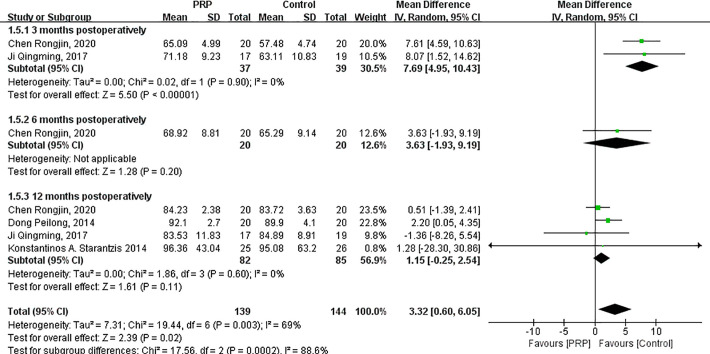
The random-effects meta-analysis (I 2 = 69%; P = .003) indicated that the Lysholm score significantly increased after PRP injections (MD = 3.32; 95% CI = 0.60 to 6.05; P = .02) (Appendix Figure A1). Lysholm score significantly increased after PRP intervention compared with the control group at 3 months postoperatively (MD = 7.69; 95% CI = 4.95 to 10.43; P < .00001). The differences between the 2 groups at 6 months (MD = 3.63; 95% CI = −1.93 to 9.19; P = .20) and 12 months postoperatively (MD = 1.15; 95% CI = −0.25 to 2.54; P = .11) were not statistically significant.
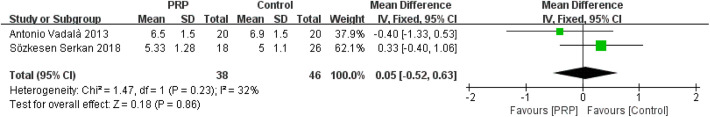
Tegner Score
The fixed-effects meta-analysis (I 2 = 32%; P = .23) showed that Tegner score was not significantly different between the 2 groups (MD = 0.05; 95% CI = −0.52 to 0.63; P = .86) (Appendix Figure A2).
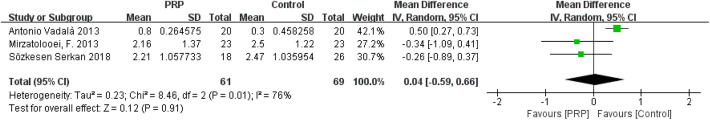
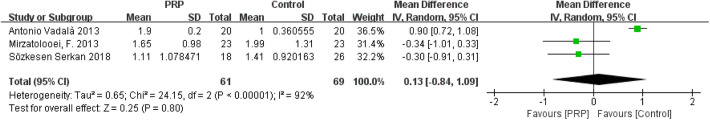
Tunnel Widening (Measured via CT)
The forest plots of the effects of PRP on tunnel widening (femoral and tibial tunnels) are shown in Appendix Figures A3 and A4, respectively. Heterogeneities were found in the studies on femoral tunnel width (I 2 = 76%; P = .01) and tibial tunnel width (I 2 = 92%; P < .00001). The comprehensive results showed that the differences in femoral tunnel width (MD = 0.04; 95% CI = −0.59 to 0.66; P = .91) and tibial tunnel width (MD = 0.13; 95% CI = −0.84 to 1.09; P = .80) between the 2 groups were not statistically significant.
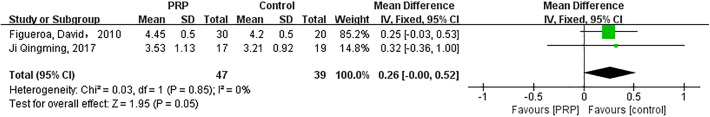
Graft Characterization (Measured via MRI)
The fixed-effects meta-analysis (I 2 = 0%; P = .85) illustrated that the characterization of grafts did not improve after PRP intervention (MD = 0.26; 95% CI = 0.00-0.52; P = .05) (Appendix Figure A5).
Complications
Most of the included studies reported no intra- or postoperative complications. Although 1 study 16 showed the presence of more complications in the PRP group (which may have been related to intense inflammatory reaction), most studies indicated no difference in complications between the 2 groups. 5 -9,14,19,24,28,30,31,35,37 When the results of this study were removed, 16 we found that among the patients in the PRP group (305 people), 2 had graft reruptures, 1 had knee stiffness, 1 had postoperative pain, and 1 had a superficial infection. In comparison, among the patients in the control group (302 people), 1 had graft rerupture, 2 had postoperative pain, 1 patient had superficial infection, and 1 patient had hemarthrosis. For patients who developed infection, infection was controlled by applying intravenous antibiotic therapy or saline.
Sensitivity Analysis
Subgroup analyses were employed to investigate the sources of high heterogeneity in VAS, IKDC, and Lysholm scores. For KT-1000 score (I 2 = 64%), femoral tunnel widening (I 2 = 76%), and tibial tunnel widening (I 2 = 92%), sensitivity was tested by eliminating studies 1 by 1 to investigate the possible sources of high heterogeneity.
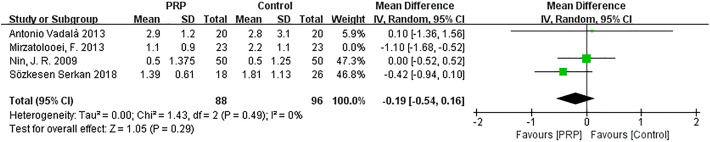
One study performed by Mirzatolooei et al 19 was responsible for the high heterogeneity in the KT-1000 score and was removed. A pooled analysis of data from the remaining studies indicated that the heterogeneity changed (I 2 = 0%; P = .49), and the differences between groups remained constant, with little fluctuation (MD = −0.19; 95% CI = −0.54 to 0.16; P = .29) (Appendix Figure A6).
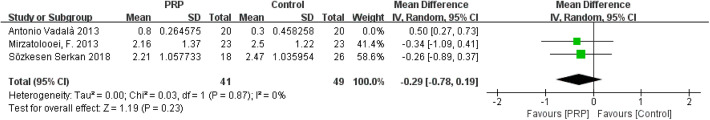
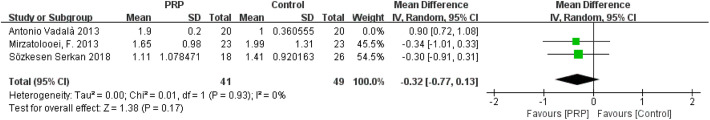
The study of Vadalà et al 35 was responsible for the high heterogeneity in the tunnel widening of femoral and tibial tunnels. Vadalà et al measured the tunnel width at the final follow-up (median, 14.7 months; range, 10-16 months), whereas Mirzatolooei et al 19 and Sözkesen et al 30 performed CT scanning at 3 months postoperatively. After the study of Vadalà et al35 was removed, a pooled analysis of data from the remaining studies indicated that the heterogeneities changed (femoral: I 2 = 0%, P = .87; tibial: I 2 = 0%, P = .93), and the differences between groups remained constant, with little fluctuation (femoral: MD = −0.29, 95% CI = −0.78 to 0.19, P = .23; tibial: MD = −0.32, 95% CI = −0.77 to 0.13, P = .17) (Appendix Figures A7 and A8).
Publication Bias
VAS score was one of the main clinical outcomes of PRP intervention. This outcome index was used to make a contour-enhanced funnel plot (10 studies 5,7,28,37 that applied PRP to harvest sites) using the metafor package in R to detect publication bias as demonstrated in Figure 9. A visual inspection of the funnel plot did not show substantial publication bias; thus, the publication bias was acceptable.
Figure 9.
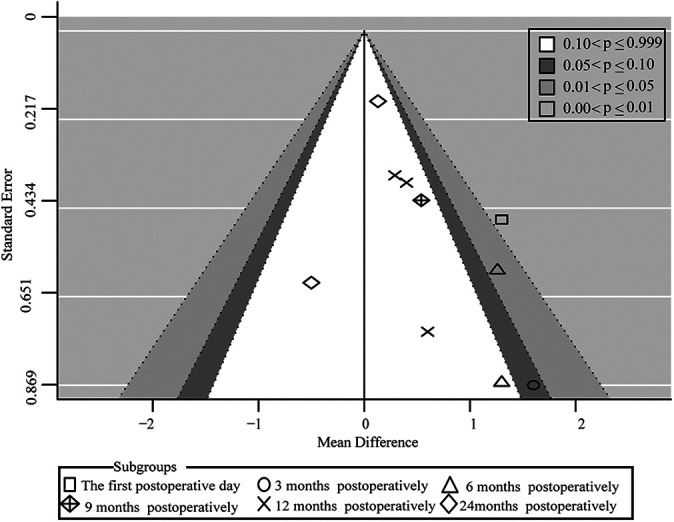
Contour-enhanced funnel plot of the publication bias of the included studies.
Discussion
The main finding of this study was that PRP applied alongside ACLR improved VAS score (graft tunnel injection at 3 months postoperatively: 95% CI = −2.50 to −0.74, P = .0003; BPTB injection before 6 months postoperatively: 95% CI = −1.94 to −0.72, P < .0001) and knee function (IKDC score: graft tunnel injection at 3 months postoperatively: 95% CI = 6.69 to 11.37, P < .00001; Lysholm score at 3 months postoperatively: 95% CI = 4.95 to 10.43; P < .00001) in the short and medium terms after surgery but did not improve clinical outcomes in the long term. PRP did not improve knee stability (KT-1000 measurements: 95% CI = −0.97 to 0.11, P = .12; pivot-shift test: 95% CI = 0.10 to 41.68, P = .64), tunnel enlargement (femoral: 95% CI = −0.59 to 0.66, P = .91; tibial: 95% CI = −0.84 to 1.09, P = .80), and graft characterization (95% CI = 0.00 to 0.52; P = .05) when injected into graft tunnels. PRP could be safely applied in ACLR because of the low incidence of complications.
This study demonstrated that PRP treatment reduced the knee pain of patients with ACLR in the short and medium terms after surgery but did not improve knee pain in the long term. Knee pain after ACLR is common. The biochemical mediators of inflammation and the inflammatory response are the link to any pain. 22 Inflammation and the production of proinflammatory cytokines, such as tumor necrosis factor alpha, and interleukin (IL)-6, IL-18, and IL-1β, may tend to the pathway of pain in anterior knee pain syndrome. 38,41 Harvesting patellar tendon grafts would also cause anterior knee pain after ACLR; this pain is related to the weakening of the extensor apparatus, patellofemoral strain, and pressure. 22
A subgroup analysis of VAS scores when PRP was injected into graft tunnels was performed because of the differences in assessment time points. The 2 groups had no remarkable difference in VAS score on the first day after surgery. Many confounding factors, such as pain caused by the wound and tunnel and capsular distension, might have come into play at early postoperative periods. The causes of changes in pain symptoms have been difficult to distinguish because of these factors. 5 For this reason, the VAS scores of the PRP group were not statistically different from those of the control group on the first day after surgery (MD = 0.28; 95% CI = −0.31 to 0.87; P = .35). VAS scores were lower in the PRP group than in the control group in the short-term postoperative period (3 months postoperatively). Platelets are rapidly deployed to injury sites and degraded to modulate inflammatory processes by interacting with leukocytes and secreting chemokines, cytokines, and other inflammatory mediators. 24 The long-term benefits of PRP injected into graft tunnels in reducing knee pain were lacking (12 months postoperatively). This result might be because the grafts were soaked with PRP and intra-articularly injected with PRP, PRP had a short duration of action in the knee joint and was lost as synovial fluid was metabolized, or detecting the clinical effect of PRP at 12 months postoperatively might have been too late. 37 However, each subgroup only consisted of 1 study; therefore, more RCTs are needed to draw firm conclusions.
Our research showed that when PRP was used at the sites of BPTB harvest, the VAS scores were lower in the PRP group than in the control group in the short- and medium-term postoperative periods (before 6 months) but not different in the long-term postoperative period (9-24 months). The 2 groups had no remarkable difference at 3 months postoperatively probably because only 1 study 37 was included in the subgroup analysis. The pooled result (I 2 = 0%; P = .99) in VAS scores before 6 months postoperatively (1 day, 3 months, and 6 months postoperatively) showed that the VAS score was significantly smaller in the PRP group than in the control group (MD = −1.33; 95% CI = −1.94 to −0.72; P < .0001). Therefore, the VAS scores were lower in the PRP group than in the control group in the short- and medium-term postoperative periods. This result may be due to the biological properties of PRP. 7,41,42 In the present study, the long-term benefits of PRP injected into BPTB harvest sites to reduce knee pain were lacking (9-24 months postoperatively). Patellar defect might be the source of postoperative kneeling pain in ACLR, and PRP injections to the harvest sites were not effective in providing any long-term relief against kneeling pain. 37
Our study revealed that PRP injections to graft tunnels improved the knee function of patients with ACLR in the short-term postoperative period but had no potential in improving knee function in the medium and long term. Knee function was evaluated using the IKDC, Lysholm, and Tegner scales. These scales are simple and reliable and validly measure symptoms such as pain and swelling, function, and sports activities for knee ligament-injured populations. 4,33 The IKDC and Lysholm scores in the PRP group at 3 months postoperatively were greater than those in the control group. Albu et al 1 also reported PRP therapy improving clinical scores, reducing pain and swelling at 3 months postoperatively, and speeding up the rehabilitation process. PRP improves joint homeostasis via the release of growth factors and bioactive molecules, reduces pain and knee swelling, and leads to improved knee function in the short- and medium-term postoperative periods. 1 The medium- and long-term (6 and 12 months postoperatively) benefits of PRP in improving knee function were lacking, as proven by the IKDC and Lysholm scores. This finding might indicate that PRP has a short duration of action in the knee joint. Most patients return to strenuous sports at 6 months after ACLR; hence, patients could almost have a good recovery of knee function at this period regardless of whether they received PRP intervention or not. 7 The present study indicated that PRP did not result in any improvement in Tegner scores. Only 2 studies measured Tegner scores, and the assignment points were different (14.7 and 3 months). 30,35 Therefore, more relevant studies are needed to make more reasonable conclusions.
In included studies, PRP was also injected into the BPTB harvest sites to improve IKDC scores. However, the pooled results showed that PRP treatments did not remarkably improve knee function (P = .07). Subgroup analysis demonstrated that IKDC scores were similar between the 2 groups before 6 months postoperatively and improved in the PRP group at 12 months postoperatively. Hence, the subsequent differences between the 2 groups might have been unrelated to PRP injection. 37
The KT-1000 arthrometer and pivot-shift tests were used to evaluate the stability of the knee joint. KT-1000 score and the negative ratio of pivot-shift test were not different between the 2 groups when PRP was injected into graft tunnels. However, basic laboratory studies showed conflicting outcomes. 19,29 Smith et al 29 reported that robust tendon grafts combined with a synthetic internal brace and PRP may provide superior durability for knee stability in a canine model and allow for more rapid and robust tendon-bone healing and graft ligamentization. PRP enhances tissue repair by releasing growth factors and improving the ligamentous anchorage of ACLR grafts. 19 In the present study, the heterogeneity in the KT-1000 score and pivot-shift test results were all >50%, and the heterogeneity in KT-1000 score decreased to 0% after removing the study of Mirzatolooei et al. 19 The difference between the 2 groups remained statistically unremarkable; therefore, PRP intervention alongside ACLR had no effects on knee stability for patients.
PRP had no positive effects on reducing tunnel widening when used at graft tunnels. Tunnel enlargement is the most common complication of ACLR, and mechanical and biological factors, such as the windshield wiper phenomenon and synovial bathing effect, are thought to potentially lead to tunnel enlargement. Several in vitro and animal studies have indicated that the intraligamentous application of PRP leads to an increase in the cellular density and neovascularization of the ACL and a faster and better integration process should lessen femoral and tibial enlargement because of the small duration for which these factors might have their effects. 13,39 However, our meta-analysis showed the opposite result. The heterogeneity of studies on tunnel widening was high (I 2 = 76% [femoral] and 92% [tibial]). Nonetheless, PRP was ineffective in decreasing tunnel enlargement regardless of whether the heterogeneity was eliminated or not (after elimination: I 2 = 0% [femoral] and 0% [tibial]). This result was consistent with the study performed by Orrego et al, 25 who indicated that the use of platelet concentrate does not have any remarkable effects on the osteoligamentous interface or tunnel-widening evolution.
This study indicated that no remarkable difference was noted in ligament grafts when PRP was used in graft tunnels or not used. In the 2 recruited studies, 9,14 graft characterization, including graft integration and ligamentization, was assessed via MRI score. 9 MRI is an accurate, noninvasive tool for diagnosing acute ACL injury and evaluating its ACLR; MRI has been used to evaluate the stages of ACL healing after different grafting techniques. 36 The tendon osseointegration and revascularization of the graft after ACLR are key factors ensuring the remodeling of the reconstructed graft ligament and maintaining the long-term stability of the knee joint. 9 Xie et al 40 treated ACL grafts with PRP in beagles; they demonstrated that PRP alters the expression of some target genes at certain times, particularly during the early stages of graft remodeling, and indicated that PRP could promote revascularization and reinnervation, which might explain the enhancing effects of PRP on ACL graft maturation. However, they did not find substantial differences between the 2 groups in terms of graft integration or maturation as evaluated via MRI scores. More studies are needed to detect the effects of PRP on graft characterization.
This study found that PRP applied during ACLR could reduce postoperative pain and improve knee function only in the short and medium terms (before 6 months postoperatively). The present study demonstrated that PRP does not provide any long-term effects in improving clinical outcomes after ACLR. Therefore, PRP does not seem to be an effective treatment for improvement of clinical outcomes. However, this study had a small sample size, and the included studies had some heterogeneity. Thus, more research would be necessary to confirm these results.
Study Limitations
This systematic review and meta-analysis had several limitations. First, the volume, concentration, intensity, and number of injections of PRP varied across the different studies. These factors might have led to variations in the results and conclusions. Zayni et al 43 demonstrated 2 consecutive PRP injections resulting in more improvement in clinical outcomes compared with a single injection in patients with chronic patellar tendinopathy. Second, the graft types (allografts and autografts) and fixation techniques in the included studies might also have affected the results. Third, only 2 studies (Seijas et al 28 and Walters et al 37 ) were deemed to have low risk of bias, and some heterogeneity existed in the included studies. These factors might have influenced the reliability of the results and conclusions.
Conclusion
PRP applied alongside ACLR might reduce postoperative pain and improve knee function in the short and medium terms after surgery but is ineffective in the long term. PRP does not improve knee stability and the enlargement of femoral and tibial tunnels. It also does not accelerate the healing of grafts. Nonetheless, more studies are required to confirm these results.
APPENDIX
Figure A1.
Forest plot of the effects of platelet-rich plasma (PRP) on Lysholm score. IV, inverse variance.
Figure A2.
Forest plot of the effects of platelet-rich plasma (PRP) on Tegner score. IV, inverse variance.
Figure A3.
Forest plot of the effects of platelet-rich plasma (PRP) on femoral tunnel widening. IV, inverse variance.
Figure A4.
Forest plot of the effects of platelet-rich plasma (PRP) on tibial tunnel widening. IV, inverse variance.
Figure A5.
Forest plot of the effects of platelet-rich plasma (PRP) on graft characterization. IV, inverse variance.
Figure A6.
Forest plot of the effects of platelet-rich plasma (PRP) on KT-1000 arthrometer measurements after sensitivity analyses. IV, inverse variance.
Figure A7.
Forest plot of the effects of platelet-rich plasma (PRP) on femoral tunnel widening after sensitivity analyses. IV, inverse variance.
Figure A8.
Forest plot of the effects of platelet-rich plasma (PRP) on tibial tunnel widening after sensitivity analyses. IV, inverse variance.
Footnotes
Final revision submitted August 5, 2021; accepted September 3, 2021.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
The protocol for this meta-analysis was registered on INPLASY (#202110110, https://inplasy.com/inplasy-2021-1-0110/).
References
- 1. Albu DE, Copotoiu M, Melinte R, BĂţagĂ T, Copotoiu S-M. Rehabilitation in patients with anterior cruciate ligament reconstruction using auxiliary platelet-rich plasma therapy. Acta Medica Marisiensis. 2016;62(2):167–172. [Google Scholar]
- 2. Bohlen HL, Schwartz ZE, Wu VJ, et al. Platelet-rich plasma is an equal alternative to surgery in the treatment of type 1 medial epicondylitis. Orthop J Sports Med. 2020;8(3):2325967120908952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozynski CC, Stannard JP, Smith P, et al. Acute management of anterior cruciate ligament injuries using novel canine models. J Knee Surg. 2016;29(7):594–603. [DOI] [PubMed] [Google Scholar]
- 4. Briggs KK, Lysholm J, Tegner Y, et al. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890–897. [DOI] [PubMed] [Google Scholar]
- 5. Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):114–120. [DOI] [PubMed] [Google Scholar]
- 6. Chen R, Wang W, Xiang X, et al. The maturity of grafts after application of platelet rich plasma in the anterior cruciate ligament reconstruction. Chin J Sports Med. 2020;39(4):257–262. [Google Scholar]
- 7. de Almeida AM, Demange MK, Sobrado MF, et al. Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. Am J Sports Med. 2012;40(6):1282–1288. [DOI] [PubMed] [Google Scholar]
- 8. Dong P, Tang X, Wang J, Zhu Z. The effect of platelet-rich plasma in anterior cruciate ligament reconstruction. Chin J Orthop. 2014;34(6):135–139. [Google Scholar]
- 9. Figueroa D, Melean P, Calvo R, et al. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy. 2010;26(10):1318–1325. [DOI] [PubMed] [Google Scholar]
- 10. Hexter AT, Sanghani-Kerai A, Heidari N, et al. Mesenchymal stromal cells and platelet-rich plasma promote tendon allograft healing in ovine anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3678–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 13. Hudgens JL, Sugg KB, Grekin JA, et al. Platelet-rich plasma activates proinflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. Am J Sports Med. 2016;44(8):1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji Q, Yang Y, Chen H, et al. Clinical evaluations of anterior cruciate ligament reconstruction with platelet rich plasma. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindanger L, Strand T, Mølster AO, Solheim E, Inderhaug E. Return to play and long-term participation in pivoting sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47(14):3339–3346. [DOI] [PubMed] [Google Scholar]
- 16. Mahdi M, Hatem HJ. Value of platelet-rich plasma in osteointegration of tendon graft in anterior cruciate ligament. Res J Med Sci. 2019;13(4):76–81. [Google Scholar]
- 17. McPherson AL, Feller JA, Hewett TE, Webster KE. Psychological readiness to return to sport is associated with second anterior cruciate ligament injuries. Am J Sports Med. 2019;47(4):857–862. [DOI] [PubMed] [Google Scholar]
- 18. Mehrabani D, Seghatchian J, Acker JP. Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus Apher Sci. 2019;58(6):102675. [DOI] [PubMed] [Google Scholar]
- 19. Mirzatolooei F, Alamdari MT, Khalkhali HR. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: a randomised clinical trial. Bone Joint J. 2013;95B(1):65–69. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:B2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montalvo AM, Schneider DK, Yut L, et al. “What’s my risk of sustaining an ACL injury while playing sports?” A systematic review with meta-analysis. Br J Sports Med. 2019;53(16):1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukhopadhyay R, Shah N, Vakta R, Bhatt J. Anterior knee pain following BPTB graft harvest. Reality or overhyped. J Clin Orthop Trauma. 2018;9(4):307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagelli CV, Hewett TE. Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47(2):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nin JR, Gasque GM, Azcárate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25(11):1206–1213. [DOI] [PubMed] [Google Scholar]
- 25. Orrego M, Larrain C, Rosales J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008;24(12):1373–1380. [DOI] [PubMed] [Google Scholar]
- 26. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502–1507. [DOI] [PubMed] [Google Scholar]
- 27. Schilaty ND, Bates NA, Sanders TL, et al. Incidence of second anterior cruciate ligament tears (1990-2000) and associated factors in a specific geographic locale. Am J Sports Med. 2017;45(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seijas R, Cusco X, Sallent A, et al. Pain in donor site after BTB-ACL reconstruction with PRGF: a randomized trial. Arch Orthop Trauma Surg. 2016;136(6):829–835. [DOI] [PubMed] [Google Scholar]
- 29. Smith PA, Stannard JP, Bozynski CC, et al. Patellar bone-tendon-bone autografts versus quadriceps tendon allograft with synthetic augmentation in a canine model. J Knee Surg. 2020;33(12):1256–1266. [DOI] [PubMed] [Google Scholar]
- 30. Sözkesen S, Karahan HG, Kurtulmus A, Kayali C, Altay T. PRP on prevention of tunnel enlargement in ACL reconstruction. Ortop Traumatol Rehabil. 2018;20(4):285–291. [DOI] [PubMed] [Google Scholar]
- 31. Starantzis KA, Mastrokalos D, Koulalis D, et al. The potentially positive role of PRPs in preventing femoral tunnel widening in ACL reconstruction surgery using hamstrings: a clinical study in 51 patients. J Sports Med. 2014;2014:789317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tay KS, Tan AHC. Clinical outcomes, return to sports, and patient satisfaction after anterior cruciate ligament reconstruction in young and middle-aged patients in an Asian population---a 2-year follow-up study. Arthroscopy. 2018;34(4):1054–1059. [DOI] [PubMed] [Google Scholar]
- 33. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 34. Toanen C, Demey G, Ntagiopoulos PG, Ferrua P, Dejour D. Is there any benefit in anterior cruciate ligament reconstruction in patients older than 60 years? Am J Sports Med. 2017;45(4):832–837. [DOI] [PubMed] [Google Scholar]
- 35. Vadalà A, Iorio R, Carli A, et al. Platelet-rich plasma: does it help reduce tunnel widening after ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2013;21(4):824–829. [DOI] [PubMed] [Google Scholar]
- 36. Vogrin M. The role of platelet derived growth factors on graft healing after ACL reconstruction. Arthroscopy. 2011;27:e125–e126. [Google Scholar]
- 37. Walters BL, Porter DA, Hobart SJ, et al. Effect of intraoperative platelet-rich plasma treatment on postoperative donor site knee pain in patellar tendon autograft anterior cruciate ligament reconstruction: a double-blind randomized controlled trial. Am J Sports Med. 2018;46(8):1827–1835. [DOI] [PubMed] [Google Scholar]
- 38. Witoński D, Wągrowska-Danilewicz M, Kęska R, Raczyńska-Witońska G, Stasikowska-Kanicka O. Increased interleukin 6 and tumour necrosis factor α expression in the infrapatellar fat pad of the knee joint with the anterior knee pain syndrome: a preliminary report. Pol J Pathol. 2010;61(4):213–218. [PubMed] [Google Scholar]
- 39. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825–853. [DOI] [PubMed] [Google Scholar]
- 40. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214–222. [DOI] [PubMed] [Google Scholar]
- 41. Xin F, Wang H, Yuan F, Ding Y. Platelet-rich plasma combined with alendronate reduces pain and inflammation in induced osteoarthritis in rats by inhibiting the nuclear factor-kappa B signaling pathway. Biomed Res Int. 2020;2020:8070295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xue Y, Su X, Jiang M, et al. Pure platelet-rich plasma facilitates the repair of damaged cartilage and synovium in a rabbit hemorrhagic arthritis knee model. Arthritis Res Ther. 2020;22(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zayni R, Thaunat M, Fayard JM, et al. Platelet-rich plasma as a treatment for chronic patellar tendinopathy: comparison of a single versus two consecutive injections. Muscles Ligaments Tendons J. 2015;5(2):92–98. [PMC free article] [PubMed] [Google Scholar]