Abstract
Despite advancements in our understanding of anterior cruciate ligament (ACL) injury prevention and nonsurgical management, ACL reconstruction continues to occur at an alarming rate. Among athletic patients, individuals participating in basketball, soccer, and football have the highest incidence of ACL injury, often requiring surgical intervention. To ensure the optimal treatment strategy for return to sport and prevention of secondary graft re-tear, it is important to tailor to the specific demands of the injured athlete and apply evidence-based best practices and rehabilitation principles. The purpose of this review is to provide readers with a brief background regarding ACL injuries, a focused review of clinical outcome studies after ACL reconstruction, and an updated framework with expert-guided recommendations for postoperative rehabilitation and return to sporting activity. Currently, there is no gold standard for rehabilitation after ACL reconstruction, highlighting the need for robust studies evaluating the best modalities for athlete rehabilitation, as well as determining the efficacy of new tools for improving therapy including blood flow restriction therapy and neuromuscular electrical stimulation. Based on clinical experience, a renewed focus on objective, criteria-based milestones may maximize the ability of return to preinjury levels of athletic function.
At all participation levels, the risk of anterior cruciate ligament (ACL) injury is exceedingly high among athletes involved in jumping, pivoting, and rapid change of direction. Previous literature has demonstrated that soccer, football, basketball, and lacrosse are among the sports with highest incidence of ACL tears, with females having higher rates of injury per exposure.1,2 Additionally, the large majority of ACL injuries result in surgical intervention, resulting in lost participation in sport and high risk of inability to return to baseline performance.2,3 Although a number of studies in the literature have attempted to evaluate appropriate screening tests and prevention programs for ACL injuries in athletes, the frequency of ACL injury remains high, particularly among athletes after primary ACL reconstruction (ACLR), with estimates as high as 29% among those returning to pivoting/cutting sport.4, 5, 6 To add to the complexity of managing ACL injuries, there are a plethora of studies describing outcomes at short-, mid-, and long-term follow-up for a number of different reconstruction modalities, with little consensus on a gold-standard for surgical treatment of ACL injuries in athletes.7 Additionally, guidance on appropriate postoperative rehabilitation in the athlete population remains inconsistent. As such, the purpose of this review is to summarize the literature on clinical and functional outcomes after ACLR, with a targeted emphasis on postoperative rehabilitation, and to provide data-driven guidelines for return to sporting activity after ACLR.
Review of ACL Anatomy and Biomechanics
Reconstruction of the anterior cruciate ligament (ACL) aims to reestablish knee function by restoring its native anatomy. To best achieve this, a foundational understanding of ACL anatomy and function is required to optimize the surgical approach, tunnel preparation, graft selection, and placement of the grafts. The ACL extends from the lateral femoral condyle to the anteromedial tibial plateau and is primarily composed of two bundles: the anteromedial (AM) and posterolateral (PL) bundles, named for their tibial insertion sites8 (Fig 1). Previous studies have shown that ACL length ranges from 31 to 38 mm and its width ranges from 10 to 12 mm. The widths of the AM and PL bundles range from 6 to 7 mm and 5 to 6 mm, respectively.9,10 A third bundle of the ACL, the intermediate (IM) bundle, has also been described.11,12 Whether the IM bundle is an anatomic variant or simply a universal component that is difficult to visualize arthroscopically is a matter of debate in the current literature. However, cadaveric studies have demonstrated that the IM bundle functions most similarly to the AM bundle,13,14 and thus for the purposes of this review, the 2-bundle system will be discussed.
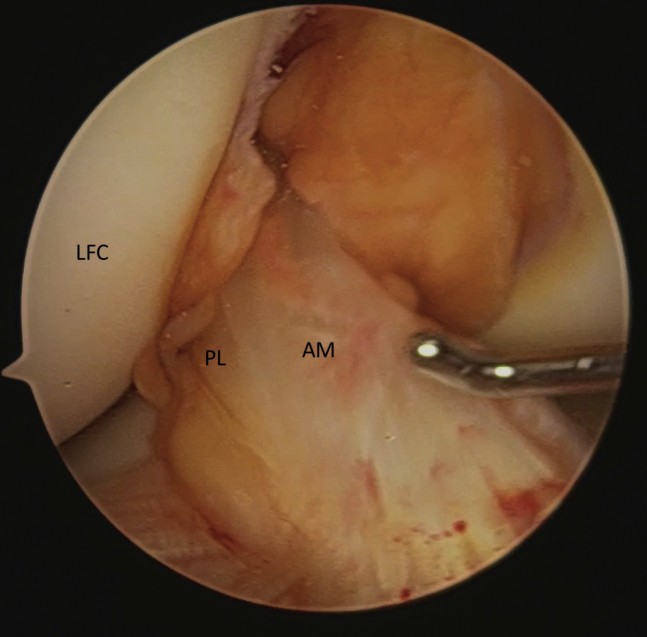
Fig. 1.
Arthroscopic view of a right knee demonstrates both the anteromedial (AM) and posterolateral (PL) bundles of the ACL in proximity to the lateral femoral condyle (LFC).
The precise attachment points on the lateral femoral condyle can be identified using the lateral intercondylar ridge (resident’s ridge) and the lateral bifurcate ridge (cruciate ridge).15 Within this area, the AM bundle occupies a position located on the proximal portion of the medial wall of the lateral femoral condyle, and the PL bundle occupies a more distal position near the anterior articular cartilage surface of the lateral femoral condyle. In general, the tibial attachment is located anterolaterally to the medial intercondylar tubercle (Fig 2). Its lateral border is adjacent to the anterior horn, and its posterior border is in close approximation to the posterior root of the lateral meniscus, in some individuals attaching to the meniscus itself.16,17 Interestingly, although the femoral footprint of both bundles is consistent, there is larger variation in the tibial footprint location.18,19 With respect to ACLR, the optimal tibial insertion site that best reduces knee laxity is an area of debate,20, 21, 22 but it is likely affected by the patient’s native anatomy23 and relationship with the femoral insertion.
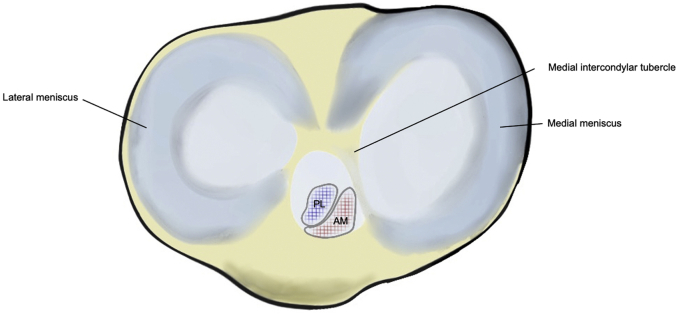
Fig. 2.
Illustration of a right knee demonstrating the AM and PL bundles attachment sites anterolaterally to the medial intercondylar tubercle.
The primary role of the ACL is to resist anterior tibial translation.11,16 This increase in anterior translation laxity is maximal at 20° to 40° of knee flexion, and thus ACL tears are best diagnosed near knee extension.24 The ACL also serves as a secondary restraint to external and internal tibial rotation, in conjunction with the medial collateral ligament and surrounding posteromedial structures.15,25,26 The bundles serve unique roles throughout the entirety of the knee range of motion (ROM)—the AM bundle is tight in flexion and is primarily responsible for restraining anterior tibial translation, whereas the PL bundle is tight in extension and more responsible for rotational stability.27,28 The bundles change alignment as the knee moves from extension to flexion. In full extension (0°), the 2 bundles are oriented vertically/in parallel (Fig 4a). As the knee flexes to 90°, the femoral AM bundle insertion site rotates posteroinferiorly while the PL bundle site rotates anterosuperiorly.28 Consequently, the 2 bundles cross around each other into a horizontal orientation.
Evidence in the literature has indicated that the tensile strength of the ACL is affected by age, stress, and overall strain.15 In a cadaveric study, Woo et al.29 reported that the tensile strength of the ACL in patients ages 22 to 35 was approximately 2160 N, with tensile strength reducing by a factor of 2.5 as age increases. Several studies have shown that the AM bundle has a higher maximum stress and strain (change in length under a certain load) compared to the PL bundle.30,31 Biomechanical and kinematic studies have also evaluated the effect of surrounding muscle activity on knee mechanics and the impact on the ACL. Quadriceps activation can result in up to 4% ACL strain in near-extension, with reduced strain with increasing flexion.32,33 Bach and Hull34 found that ACL strain was significantly decreased with simultaneous hamstring and quadricep activation compared to quadricep activation alone, suggesting that hamstring activation provides a protective effect on the ACL.35,36 Thus clinicians should be mindful of not overloading the graft and its fixation. Closed-chain exercises with hamstring activation are commonly used to avoid overload, but there is emerging evidence that open kinetic chain exercises, when done appropriately and within a limited range, can maximize quadriceps gains while maintaining graft safety.37,38
Lower Extremity Biomechanics and Neuromuscular Function After ACLR
The literature has demonstrated that patients who underwent ACLR may demonstrate aberrations in biomechanics associated with decrease in muscle strength and altered knee kinematics.39,40 Slater and colleagues40 performed a meta-analysis evaluating lower extremity kinematics and kinetics of walking among ACL-deficient patients, those who underwent ACLR, and healthy controls. The authors observed reductions in peak knee-flexion angle, external knee-flexion moment, and external knee-adduction moment among ACL-deficient and ACLR patients, which have been linked to knee-cartilage degeneration.40,41 Studies investigating asymmetry in lower extremity kinetics and kinematics reported significant intralimb compensation in ACLR patients.42,43 Multiple studies have identified that individuals after ACLR develop a hip strategy (increase in hip extensor torque and activation), potentially to compensate for poor quadriceps function.44 Although limb asymmetry may improve by 24 months,45 understanding the biomechanics and neuromuscular function of the lower extremities after ACLR will help clinicians better design efficient postoperative rehabilitation protocols that address asymmetry. This is especially critical as most rehabilitation protocols are limited (i.e., 6 months) and athletes usually aim to return to sport at 1 year.45,46 Concurrent assessments of functional ability and symmetry are warranted for patients with goals to return both quickly and safely to sport.
Impact of Rehabilitation on Functional Outcomes After ACLR
Postoperative rehabilitation programs after ACL reconstruction aim to address knee neuromuscular function, sport-specific training, and eventual return-to-activity/sport.46,47 Emphasis is placed on early weight-bearing, pain management, restoring normal ROM, decreasing joint effusion, and improving quadriceps and hamstring muscle and strength. Some postoperative programs are based on the time since surgery. In athletic populations, many have tried to accelerate the rehabilitation process by reducing protocols from 12 to 6 months to return to sport. Randomized clinical trials have demonstrated that accelerated programs show similar or even superior results in patient satisfaction, functional performance, knee laxity, and biomarkers of articular cartilage metabolism.48, 49, 50 Despite the success in improvement of functional outcomes associated with accelerated rehabilitation protocols, some athletes still report persistent neuromuscular deficits at 9 to 12 months.51,52 Other studies have reported even longer-lasting deficits and suggest not returning to sport until 24 months after ACLR.53,54 Although the cause of poor outcomes after ACLR is multifactorial, high variability of rehabilitation protocols may contribute to inconsistent rates of returning to baseline sports function. In a survey of more than 1000 physical therapists, Greenberg et al.46 reported that 58% of therapists aimed to achieve jogging at 3 to 4 months, 50% aimed for modified sports activity at 4 to 5 months, and 40% worked on unrestricted sports participation at 12 months. This study showed a wide variety in performance criteria with fewer than half of therapists using patient-reported outcome measures to gauge patient progress.
There may be more benefit to criterion-based rehabilitation protocols that can individualize progressions based on time and an athlete’s progress and current function. These protocols can address the sequencing of activity and allow clinicians to make decisions based on movement quality and other data, including patient-reported outcome measures. In a recent case series, Joreitz and colleagues55 evaluated the effect of return to sport after ACL reconstruction with subsequent postoperative rehabilitation that focused on a criterion-based rehabilitation protocol. This protocol, according to authors, emphasized criterion-based progression rather than a rigid timeline after surgery, which is often reported. At 2-year follow-up, the authors observed that 84% of patients were able to return to preinjury level of sports competition.55 While extremely limited to size and loss of follow-up, this study indicated that most participants included in the study were able to return to baseline performance with an average return to sport of 10.6 months after surgery. Although this is a significant time away from sport for most athletes, particularly professional and semiprofessional athletes, the rates of returning to the preinjury level is promising. However, more robust studies are warranted to determine whether similar findings are reproducible and whether a longer rehabilitation process may lead to lower reinjury rates or the slower progression of degenerative changes in the knee.
The progression of ACL rehabilitation should not be limited to physical factors. Cognitive and psychological factors, including kinesiophobia or pain catastrophizing, have been evaluated in the literature as milestone limitations during postoperative rehabilitation.56,57 Lack of psychological readiness, specifically fear of reinjury or contralateral injury, has been cited as a major obstacle hindering the athlete’s return to sport.58, 59, 60 This is a valid challenge, particularly in athletes who participate in pivoting sports, such as basketball, football, and soccer. However, most research in this area identifies psychosocial factors associated with positive outcomes, rather than studying their tangible impact when addressed in the rehabilitation setting.61,62 Furthermore, of the limited evidence on addressing psychosocial factors, there is inconsistency of techniques used (guided imagery, coping modeling, positive self-talk, goal setting), making comparison difficult. Future research that overcomes these limitations may aid in educating patients on postoperative expectations assist in safely returning to their optimal sports performance.
Return to Preinjury Level of Sport
One of the main goals among athletes undergoing ACLR is to return to preinjury level of sports performance. Despite substantial improvements in functional scores with rehabilitation, their return-to-sport outcomes are poor. In a cohort of more than 600 patients, Webster et al.63 reported that despite 84% of patients expecting to return to preinjury sport level, fewer than a quarter had achieved that goal at 1-year follow-up. Muller et al.64 report similar findings in a multi-institutional study that showed that fewer than half of patients returned to preinjury sport level at 3.4-year follow-up. Importantly, they found that younger age, higher competition level, reduced time until surgery, and absence of cartilage injury were associated with return to preinjury sports participation. The influence of sport type and competition level is well documented in the literature.4,5,23,65, 66, 67 From a rehabilitation perspective, it is important to provide evidence-based interventions that reduce the barriers that are preventing athletes from returning to competitive sport-specific performance levels and causing high rates of reinjury.
Rehabilitation Recommendations
The Role of Prehabilitation
The role of muscle strengthening and rehabilitation before surgery, known as “prehabilitation” or “prehab,” is a growing topic in the literature. Patients with ACL injuries that display severe deficits in quadriceps strength and gait symmetry have worse outcomes after ACLR.68 Prehab aims to buffer against this loss. However, the evidence supporting the utility of prehab is conflicting.69 A preoperative focus on quadriceps strength or neuromuscular perturbation response does not appear to significantly improve gait biomechanics after ACLR when compared to protocols without prehab.70,71 Meanwhile, Giesche et al.72 and Failla et al.73 reported improved functional scores and return-to-sport rates in prehab patients at 2-year follow-up. These results suggest that although prehab may not significantly alter postoperative biomechanics, it may provide improvements in outcomes that are more clinically relevant for the patients.
Sequencing and Progression of Rehabilitation
In the athletic population, early and progressive rehabilitation is imperative. We advocate that clinicians initiate rehabilitation on athletes within the first week after their procedure. Initial goals of rehab revolve around reducing joint effusion, restoring volitional quadriceps contraction, progressing ROM, and restoring basic lower extremity function required for transfers, ambulation, and light activities of daily living performance, all while protecting the surgical repair. Care is taken early on to restore knee extension ROM as quickly as possible, both passively and actively, because altered joint loading and common functional limitations can occur because of even mild flexion contractures that develop in the surgical limb.
Unless specific restrictions are applied, ROM and basic weightbearing function should be progressed to patient tolerance. The goal of the first phase of ACLR rehabilitation in the athletic population is to restore the strength and function required to complete activities of daily living, restore neuromuscular control of the lower extremity through basic lower body movement patterns, and tolerate weight room exercise prescription for a gradual return to team activities.
Important milestones during the rehabilitation process after ACLR include the introduction and progression of running, plyometric training, and sport-specific change-of-direction activities. It has been advised that the return to sport process be separated into 3 phases; return to participation, return to sport, and return to performance.74 The progression of these activities is not exclusive, and muscular performance variables should be progressed along a continuum, oftentimes returning to previous activities that may inherently include less complexity and less neuromotor demand based on day-to-day presentation. Functional progression will also greatly depend on the individual case characteristics and the haste at which return-to-sport is being sought, although generally 9 to 12 months of rehabilitation is recommended before full unrestricted participation. For truly effective return to sport rehabilitation, the entire force-velocity curve must be addressed with a shift from more general to more specific variables based on the sport demands. For instance, athletes need to be exposed to and possess the ability to produce high amounts of force in environments that include low-velocity joint movements with high load to high-velocity, low-load movement patterns.
As stated previously, functional progression should depend on clinical assessment and the achievement of milestones. Current suggestions include pain <2 on a visual analogue scale, 95% of knee flexion ROM, full knee extension ROM, and no effusion/trace effusion on examination.75 The most frequent recommendations when it comes to electronic dynamometry suggest >80% limb symmetry index (LSI) for quadriceps force production with isometric assessment and >70% with isokinetic assessment.75 In addition, the commenting therapist suggests using a Y-balance assessment for assessment of balance and neuromuscular coordination, achievement of step and holds with good control, good control with single-leg squatting to 45° of knee flexion, tolerance to 15 heel-elevated bridges with the knee flexed to 30°, and the ability to achieve 75 small ROM hops on 2 and 1 limb before initiation of an interval running protocol (Fig 3). Additional assessment is left to the discretion of the treating physical therapist, and the Delaware Interval Running protocol76 is one example of a graded progression of straight-line running.
Fig. 3.
Images demonstrating tests for returning to sport including Y-balance assessment (top left), single-leg squatting to 45° of knee flexion (top right), and heel-elevated bridges with the knee flexed to 30° (bottom).
In a typical scenario, the athlete progresses through at least 4 weeks of straight-line running. For track, field, and team sport athletes who demonstrate good tolerance to this progression, a return to sprinting program is warranted. Additional progression criteria to begin sprinting has been recommended, including demonstration of 70% LSI on hop testing.77 For multidirectional athletes specifically, plyometric and change-of-direction training should be commenced. Suggested criteria would include maintenance of full ROM with no effusion, the completion of a straight-line running program, >85% LSI on isokinetic dynamometry, and a 100% composite score on Lower Extremity Y-Balance Assessment to begin plyometric training. Including a requirement of achieving 75% LSI on hop testing is suggested before multidirectional change-of-direction work. Additional testing at the discretion of the treating physical therapist is recommended. Multidirectional training selection will depend on several factors, including the sports biomechanical demands, the athlete position with their sport, the surface of the field of play, as well as the prior and current fitness level of the athlete. It is recommended that the athlete demonstrates ∼85% LSI via strength assessment before the initiation of more advanced plyometric activities. Careful monitoring of the athlete’s response is critical during late-stage rehabilitation to ensure proper progression toward full unrestricted participation.
Localized Interventions to Improve Quadriceps Function
Quadriceps function can be a major limiting factor during rehabilitation after ACLR. Evidence-based interventions such as blood flow restriction training (BFRT) and neuromuscular electrical stimulation (NMES) can augment traditional rehabilitation practices to enhance quadriceps function.
Volitional force production is limited by many factors during acute rehabilitation. This results in exercise intensities that may be appropriate for the current functional abilities but are relatively low compared to the amount of structural muscle mass in the athletic patient. As a result, muscle atrophy occurs in the early stages, and interventions such as BFRT may be appropriate to attenuate muscle atrophy and force production loss. BFRT can be useful in situations that involve limited weightbearing and limited ROM. It has been shown that there are benefits to low-load exercise plus BFRT when compared to low-load exercise alone, serving as an important variable to consider when progressing an athlete back to higher load resistance exercise.78, 79, 80, 81 Through a number of different physiological processes, applying a pneumatic cuff to the lower extremity to reduce blood flow to the limb during low-intensity resistance training has been shown to increase thigh muscle cross-sectional area and blood vessel compliance in healthy volunteers.37,38 As such, it is possible that BFRT may limit muscle atrophy and resulting altered biomechanics during the postsurgical rehabilitation period. Only a few studies have evaluated the effect of BFRT as a tool during post-ACLR rehabilitation, showing early promising results. In a randomized control trial, Hughes and colleagues82 observed superior self-reported function, y-balance performance, range of motion, as well as reduction in knee joint pain and effusion among patients undergoing rehabilitation with BFRT versus those undergoing rehabilitation with heavy-load resistance training. However, other studies have demonstrated that similar results are subjective to duration of rehabilitation.83 Authors have pointed out that the specific benefits of BFRT are difficult to elucidate without consistent protocols, standardized outcomes, longer-term follow-up, and increased sample sizes.84 Only by overcoming these limitations can more definitive conclusions be made.
NMES is commonly used alongside postoperative rehabilitation programs to promote neural activation after ACL injury and reconstruction.85 Several studies have shown that NMES prevents muscle atrophy in orthopaedic populations, but the overall strength of patients who receive increased NMES therapy has not been reported to be superior.86, 87, 88 This is clinically important because residual muscle weakness after ACLR is associated with patient dissatisfaction, pathologically alters knee biomechanics, and contributes to the development of osteoarthritis.89 Toth et al.86 showed that muscle biopsies of patients undergoing an NMES protocol 5 times/week in the immediate 3 weeks after surgery reduces atrophy of myosin heavy chain II fibers while maintaining myosin heavy chain I fibers. While this study showed cellular advantages of NMES, 6-month measures of muscle strength did not differ between NMES and non-NMES groups. Like BFR, investigation of NMES treatment duration/frequency, standardization of outcome measures, and relevance to return-to-sport is warranted to make better evidence-based conclusions regarding its use.
NMES is typically used within the first 2 weeks as early as the patient can tolerate its application. Frequent use of NMES has been shown to be beneficial. Critical parameters include intensity level, frequency of stimulation, waveform, and duty cycle. High volt, Russian stimulation to maximum tolerable intensity is recommended in addition to progressive intensity escalation throughout the treatment as intensity level and quadriceps strength appear to share a linear relationship. Higher frequencies (50 and 100 Hz) and electrodes with a large surface area can be helpful in improving patient tolerance. Waveform shape seems to be dependent on patient preference, so modification of the waveform may be indicated when the clinician feels that the maximum tolerable intensity can be improved upon. A 10 seconds on/20 seconds off duty cycle for a 15-minute intervention period to begin treatment sessions has been reported as an effective paradigm.90
Position of application is another factor to consider. Recommendations are to begin NMES application in an extended position adding a prop for the heel to facilitate hyperextension ROM as tolerated. Once slight hyperextension (0°-5°) has been achieved and maintained actively, applying NMES in a position of 60° to 90° of knee flexion is advised because 60° has been shown to produce the largest voluntary knee extension torque during exercise.91 Progression toward isotonic contractions may be used in conjunction with NMES application as the patient improves in function. Principles of progressive overload may be followed while using NMES through the arc of motion that the patient can tolerate, improving motor unit recruitment and muscle fiber activation at every joint angle.
The patient should be educated on the importance of producing an active contraction during electrical stimulation, because it appears that there are benefits when following neuroplastic principles. There is little consensus when it comes to the remaining treatment parameters, with studies describing treatment sessions that range between 15 and 40 minutes per session, 1 to 3 times per day for 2 to 12 weeks. It should be noted that this frequency of application may not be feasible in the outpatient setting.90 We suggest using a goal of achieving an LSI of 80% on isometric or 70% on isokinetic dynamometry as a stopping point for the use of NMES.
Other considerations
It is worth noting that often in the case of ACL rupture, other structures in the knee are injured during the mechanism of injury. This may include the meniscus, cartilage, bone, or other ligamentous structures which require modification of the rehab program.92 Although protocols differ, some surgeons prefer a period of non- or limited weightbearing after meniscal repair, particularly in the case of compromised tissue quality or a bucket handle, radial, or root tears. A 4-week restriction to 90° of knee flexion may also be recommended, as well as caution with hamstring contractions in the case of medial meniscal repair.93 Alternatively, others argue that allowing weightbearing in full extension in the case of a bucket-handle or longitudinal repair may help to reduce the meniscus and stabilize the tear.94,95 In the setting of multiligamentous knee injuries, the rehabilitation protocol will be unique to each individual case. For athletes with ACL and concomitant medial or lateral collateral ligament injuries, a restriction to valgus or varus forces may be advised. Associated posterior collateral ligament and posterolateral corner of the knee injuries may benefit from a delay in active hamstring contractions and caution when pushing hyperextension range-of-motion, being careful to avoid tibial sag.
Conclusions
Currently, there is no gold standard for rehabilitation after ACL reconstruction, highlighting the need for robust studies evaluating the best modalities for athlete rehabilitation, as well as determining the efficacy of new tools for improving therapy including blood flow restriction therapy and neuromuscular electrical stimulation. Based on clinical experience, a renewed focus on objective, criteria-based milestones may maximize the ability of return to preinjury levels of athletic function.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.R.W. reports other from the American Academy of Orthopaedic Surgeons, American Journal of Orthopedics, ARTHROSCOPY, the Arthroscopy Association of North America, the Society of Military Orthopaedic Surgeons, Encore Medical, Midwest Orthopedics, Wright Medical Technologies, Depuy Synthes, and Desert Mountain Medical; and personal fees from Elsevier and Genzyme. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Gornitzky A.L., Lott A., Yellin J.L., Fabricant P.D., Lawrence J.T., Ganley T.J. Sport-specific yearly risk and incidence of anterior cruciate ligament tears in high school athletes: A systematic review and meta-analysis. Am J Sports Med. 2016;44:2716–2723. doi: 10.1177/0363546515617742. [DOI] [PubMed] [Google Scholar]
- 2.Joseph A.M., Collins C.L., Henke N.M., Yard E.E., Fields S.K., Comstock R.D. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48:810–817. doi: 10.4085/1062-6050-48.6.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beischer S., Gustavsson L., Senorski E.H., et al. Young athletes who return to sport before 9 months after anterior cruciate ligament reconstruction have a rate of new injury 7 times that of those who delay return. J Orthop Sports Phys Ther. 2020;50:83–90. doi: 10.2519/jospt.2020.9071. [DOI] [PubMed] [Google Scholar]
- 4.Wiggins A.J., Grandhi R.K., Schneider D.K., Stanfield D., Webster K.E., Myer G.D. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Am J Sports Med. 2016;44:1861–1876. doi: 10.1177/0363546515621554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster K.E., Feller J.A. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44:2827–2832. doi: 10.1177/0363546516651845. [DOI] [PubMed] [Google Scholar]
- 6.Paterno M.V., Rauh M.J., Schmitt L.C., Ford K.R., Hewett T.E. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42:1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widner M., Dunleavy M., Lynch S. Outcomes following ACL reconstruction based on graft type: Are all grafts equivalent? Curr Rev Musculoskelet Med. 2019;12:460–465. doi: 10.1007/s12178-019-09588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraeutler M.J., Wolsky R.M., Vidal A.F., Bravman J.T. Anatomy and biomechanics of the native and reconstructed anterior cruciate ligament: Surgical implications. J Bone Joint Surg Am. 2017;99:438–445. doi: 10.2106/JBJS.16.00754. [DOI] [PubMed] [Google Scholar]
- 9.Siegel L., Vandenakker-Albanese C., Siegel D. Anterior cruciate ligament injuries: Anatomy, physiology, biomechanics, and management. Clin J Sport Med. 2012;22:349–355. doi: 10.1097/JSM.0b013e3182580cd0. [DOI] [PubMed] [Google Scholar]
- 10.Scheffler S. The cruciate ligaments: Anatomy, biology, and biomechanics. Knee Joint. 2012:11–21. [Google Scholar]
- 11.Amis A.A., Dawkins G.P. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br. 1991;73:260–267. doi: 10.1302/0301-620X.73B2.2005151. [DOI] [PubMed] [Google Scholar]
- 12.Norwood L.A., Cross M.J. Anterior cruciate ligament: Functional anatomy of its bundles in rotatory instabilities. Am J Sports Med. 1979;7:23–26. doi: 10.1177/036354657900700106. [DOI] [PubMed] [Google Scholar]
- 13.Kato Y., Ingham S.J., Maeyama A., et al. Biomechanics of the human triple-bundle anterior cruciate ligament. Arthroscopy. 2012;28:247–254. doi: 10.1016/j.arthro.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Otsubo H., Shino K., Suzuki D., et al. The arrangement and the attachment areas of three ACL bundles. Knee Surg Sports Traumatol Arthrosc. 2012;20:127–134. doi: 10.1007/s00167-011-1576-z. [DOI] [PubMed] [Google Scholar]
- 15.Hassebrock J.D., Gulbrandsen M.T., Asprey W.L., Makovicka J.L., Chhabra A. Knee ligament anatomy and biomechanics. Sports Med Arthrosc Rev. 2020;28:80–86. doi: 10.1097/JSA.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M., Doi M., Abe M., Suzuki D., Nagano A. Anatomical study of the femoral and tibial insertions of the anteromedial and posterolateral bundles of human anterior cruciate ligament. Am J Sports Med. 2006;34:787–792. doi: 10.1177/0363546505282625. [DOI] [PubMed] [Google Scholar]
- 17.Fujishiro H., Tsukada S., Nakamura T., Nimura A., Mochizuki T., Akita K. Attachment area of fibres from the horns of lateral meniscus: Anatomic study with special reference to the positional relationship of anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2017;25:368–373. doi: 10.1007/s00167-015-3813-3. [DOI] [PubMed] [Google Scholar]
- 18.Hwang M.D., Piefer J.W., Lubowitz J.H. Anterior cruciate ligament tibial footprint anatomy: Systematic review of the 21st century literature. Arthroscopy. 2012;28:728–734. doi: 10.1016/j.arthro.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Colombet P., Robinson J., Christel P., et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: A cadaveric dissection and radiographic study. Arthroscopy. 2006;22:984–992. doi: 10.1016/j.arthro.2006.04.102. [DOI] [PubMed] [Google Scholar]
- 20.Chiba D., Yamamoto Y., Kimura Y., et al. Laterally shifted tibial tunnel can be the risk of residual knee laxity for double-bundle anterior cruciate ligament reconstruction [published online April 30, 2021]. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-021-06546-3 [DOI] [PubMed]
- 21.Asai S., Kim D., Hoshino Y., et al. Coronal tibial anteromedial tunnel location has minimal effect on knee biomechanics. Knee Surg Sports Traumatol Arthrosc. 2018;26:2960–2965. doi: 10.1007/s00167-018-4844-3. [DOI] [PubMed] [Google Scholar]
- 22.Tank S., Dutt S., Sehrawat R., Kumar V., Sabat D. 3D CT evaluation of femoral and tibial tunnels in anatomic double bundle anterior cruciate ligament reconstruction. J Clin Orthop Trauma. 2021;15:22–26. doi: 10.1016/j.jcot.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansdown D.A., Pedoia V., Zaid M., et al. Variations in knee kinematics after ACL injury and after reconstruction are correlated with bone shape differences. Clin Orthop Relat Res. 2017;475:2427–2435. doi: 10.1007/s11999-017-5368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amis A.A. Anterolateral knee biomechanics. Knee Surg Sports Traumatol Arthrosc. 2017;25:1015–1023. doi: 10.1007/s00167-017-4494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiapour A.M., Murray M.M. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20–31. doi: 10.1302/2046-3758.32.2000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quatman C.E., Kiapour A.M., Demetropoulos C.K., et al. Preferential loading of the ACL compared with the MCL during landing: A novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med. 2014;42:177–186. doi: 10.1177/0363546513506558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabriel M.T., Wong E.K., Woo S.L.Y., Yagi M., Debski R.E. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22:85–89. doi: 10.1016/S0736-0266(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 28.Petersen W., Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res. 2007;454:35–47. doi: 10.1097/BLO.0b013e31802b4a59. [DOI] [PubMed] [Google Scholar]
- 29.Woo S.L., Hollis J.M., Adams D.J., Lyon R.M., Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 30.Skelley N.W., Castile R.M., York T.E., Gruev V., Lake S.P., Brophy R.H. Differences in the microstructural properties of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Am J Sports Med. 2015;43:928–936. doi: 10.1177/0363546514566192. [DOI] [PubMed] [Google Scholar]
- 31.Castile R.M., Skelley N.W., Babaei B., Brophy R.H., Lake S.P. Microstructural properties and mechanics vary between bundles of the human anterior cruciate ligament during stress-relaxation. J Biomech. 2016;49:87–93. doi: 10.1016/j.jbiomech.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Beynnon B.D., Fleming B.C., Johnson R.J., Nichols C.E., Renstrom P.A., Pope M.H. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- 33.Luque-Seron J.A., Medina-Porqueres I. Anterior cruciate ligament strain in vivo: A systematic review. Sports Health. 2016;8:451–455. doi: 10.1177/1941738116658006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bach J.M., Hull M.L. Strain inhomogeneity in the anterior cruciate ligament under application of external and muscular loads. J Biomech Eng. 1998;120:497–503. doi: 10.1115/1.2798020. [DOI] [PubMed] [Google Scholar]
- 35.Li G., Rudy T.W., Sakane M., Kanamori A., Ma C.B., Woo S.L.Y. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 36.Markolf K.L., O'Neill G., Jackson S.R., McAllister D.R. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32:1144–1149. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- 37.Glass R., Waddell J., Hoogenboom B. The effects of open versus closed kinetic chain exercises on patients with acl deficient or reconstructed knees: A systematic review. N Am J Sports Phys Ther. 2010;5:74–84. [PMC free article] [PubMed] [Google Scholar]
- 38.Wilk K.E., Arrigo C.A., Andrews J.R. Teamwork is Essential to Success: Sports Physical Therapy is No Exception. Int J Sports Phys Ther. 2021;16:19041. doi: 10.26603/001c.19041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart H.F., Culvenor A.G., Collins N.J., et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Br J Sports Med. 2016;50:597–612. doi: 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 40.Slater L.V., Hart J.M., Kelly A.R., Kuenze C.M. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: A review and meta-analysis. J Athl Train. 2017;52:847–860. doi: 10.4085/1062-6050-52.6.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantashloo Z., Letafatkar A., Moradi M. Vertical ground reaction force and knee muscle activation asymmetries in patients with ACL reconstruction compared to healthy individuals. Knee Surg Sports Traumatol Arthrosc. 2020;28:2009–2014. doi: 10.1007/s00167-019-05743-5. [DOI] [PubMed] [Google Scholar]
- 42.Di Stasi S.L., Logerstedt D., Gardinier E.S., Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med. 2013;41:1310–1318. doi: 10.1177/0363546513482718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paterno M.V., Ford K.R., Myer G.D., Heyl R., Hewett T.E. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17:258–262. doi: 10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- 44.Taylor J.B., Westbrook A.E., Head P.L., Glover K.M., Paquette M.R., Ford K.R. The single-leg vertical hop provides unique asymmetry information in individuals after anterior cruciate ligament reconstruction. Clin Biomech. 2020;80:105107. doi: 10.1016/j.clinbiomech.2020.105107. [DOI] [PubMed] [Google Scholar]
- 45.Sharafoddin-Shirazi F., Letafatkar A., Hogg J., Saatchian V. Biomechanical asymmetries persist after ACL reconstruction: Results of a 2-year study. J Exp Orthop. 2020;7:86. doi: 10.1186/s40634-020-00301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg E.M., Greenberg E.T., Albaugh J., Storey E., Ganley T.J. Rehabilitation practice patterns following anterior cruciate ligament reconstruction: A survey of physical therapists. J Orthop Sports Phys Ther. 2018;48:801–811. doi: 10.2519/jospt.2018.8264. [DOI] [PubMed] [Google Scholar]
- 47.van Melick N., van Cingel R.E., Brooijmans F., et al. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br J Sports Med. 2016;50:1506–1515. doi: 10.1136/bjsports-2015-095898. [DOI] [PubMed] [Google Scholar]
- 48.Beynnon B.D., Johnson R.J., Naud S., et al. Accelerated versus nonaccelerated rehabilitation after anterior cruciate ligament reconstruction: A prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. Am J Sports Med. 2011;39:2536–2548. doi: 10.1177/0363546511422349. [DOI] [PubMed] [Google Scholar]
- 49.Beynnon B.D., Uh B.S., Johnson R.J., et al. Rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind comparison of programs administered over 2 different time intervals. Am J Sports Med. 2005;33:347–359. doi: 10.1177/0363546504268406. [DOI] [PubMed] [Google Scholar]
- 50.Gerber J.P., Marcus R.L., Dibble L.E., Greis P.E., Burks R.T., LaStayo P.C. Effects of early progressive eccentric exercise on muscle size and function after anterior cruciate ligament reconstruction: A 1-year follow-up study of a randomized clinical trial. Phys Ther. 2009;89:51–59. doi: 10.2522/ptj.20070189. [DOI] [PubMed] [Google Scholar]
- 51.Eitzen I., Holm I., Risberg M.A. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43:371–376. doi: 10.1136/bjsm.2008.057059. [DOI] [PubMed] [Google Scholar]
- 52.Xergia S.A., McClelland J.A., Kvist J., Vasiliadis H.S., Georgoulis A.D. The influence of graft choice on isokinetic muscle strength 4-24 months after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:768–780. doi: 10.1007/s00167-010-1357-0. [DOI] [PubMed] [Google Scholar]
- 53.Tayfur B., Charuphongsa C., Morrissey D., Miller S.C. Neuromuscular function of the knee joint following knee injuries: does it ever get back to normal? a systematic review with meta-analyses. Sports Med. 2021;51:321–338. doi: 10.1007/s40279-020-01386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagelli C.V., Hewett T.E. Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47:221–232. doi: 10.1007/s40279-016-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joreitz R., Lynch A., Popchak A., Irrgang J. Criterion-based rehabilitation program with return to sport testing following ACL reconstruction: A case series. Int J Sports Phys Ther. 2020;15:1151–1173. doi: 10.26603/ijspt20201151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartigan E.H., Lynch A.D., Logerstedt D.S., Chmielewski T.L., Snyder-Mackler L. Kinesiophobia after anterior cruciate ligament rupture and reconstruction: Noncopers versus potential copers. J Orthop Sports Phys Ther. 2013;43:821–832. doi: 10.2519/jospt.2013.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jochimsen K.N., Pelton M.R., Mattacola C.G., et al. Relationship between pain catastrophizing and 6-month outcomes following anterior cruciate ligament reconstruction. J Sport Rehabil. 2020;29:808–812. doi: 10.1123/jsr.2018-0431. [DOI] [PubMed] [Google Scholar]
- 58.Christino M.A., Fantry A.J., Vopat B.G. Psychological aspects of recovery following anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2015;23:501–509. doi: 10.5435/JAAOS-D-14-00173. [DOI] [PubMed] [Google Scholar]
- 59.Everhart J.S., Best T.M., Flanigan D.C. Psychological predictors of anterior cruciate ligament reconstruction outcomes: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;23:752–762. doi: 10.1007/s00167-013-2699-1. [DOI] [PubMed] [Google Scholar]
- 60.Beischer S., Hamrin Senorski E., Thomee C., Samuelsson K., Thomee R. How is psychological outcome related to knee function and return to sport among adolescent athletes after anterior cruciate ligament reconstruction? Am J Sports Med. 2019;47:1567–1575. doi: 10.1177/0363546519843073. [DOI] [PubMed] [Google Scholar]
- 61.Gennarelli S.M., Brown S.M., Mulcahey M.K. Psychosocial interventions help facilitate recovery following musculoskeletal sports injuries: A systematic review. Phys Sportsmed. 2020;48:370–377. doi: 10.1080/00913847.2020.1744486. [DOI] [PubMed] [Google Scholar]
- 62.Coronado R.A., Bird M.L., Van Hoy E.E., Huston L.J., Spindler K.P., Archer K.R. Do psychosocial interventions improve rehabilitation outcomes after anterior cruciate ligament reconstruction? A systematic review. Clin Rehabil. 2018;32:287–298. doi: 10.1177/0269215517728562. [DOI] [PubMed] [Google Scholar]
- 63.Webster K.E., Feller J.A. Expectations for return to preinjury sport before and after anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47:578–583. doi: 10.1177/0363546518819454. [DOI] [PubMed] [Google Scholar]
- 64.Muller B., Yabroudi M.A., Lynch A., et al. Return to preinjury sports after anterior cruciate ligament reconstruction is predicted by five independent factors [published online April 22, 2021]. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-021-06558-z [DOI] [PubMed]
- 65.Mohtadi N.G., Chan D.S. A randomized clinical trial comparing patellar tendon, hamstring tendon, and double-bundle ACL reconstructions: Patient-reported and clinical outcomes at 5-year follow-up. J Bone Joint Surg Am. 2019;101:949–960. doi: 10.2106/JBJS.18.01322. [DOI] [PubMed] [Google Scholar]
- 66.Lai C.C.H., Ardern C.L., Feller J.A., Webster K.E. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: A systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med. 2018;52:128–138. doi: 10.1136/bjsports-2016-096836. [DOI] [PubMed] [Google Scholar]
- 67.Mai H.T., Chun D.S., Schneider A.D., et al. Performance-based outcomes after anterior cruciate ligament reconstruction in professional athletes differ between sports. Am J Sports Med. 2017;45:2226–2232. doi: 10.1177/0363546517704834. [DOI] [PubMed] [Google Scholar]
- 68.Ithurburn M.P., Altenburger A.R., Thomas S., Hewett T.E., Paterno M.V., Schmitt L.C. Young athletes after ACL reconstruction with quadriceps strength asymmetry at the time of return-to-sport demonstrate decreased knee function 1 year later. Knee Surg Sports Traumatol Arthrosc. 2018;26:426–433. doi: 10.1007/s00167-017-4678-4. [DOI] [PubMed] [Google Scholar]
- 69.Carter H.M., Littlewood C., Webster K.E., Smith B.E. The effectiveness of preoperative rehabilitation programmes on postoperative outcomes following anterior cruciate ligament (ACL) reconstruction: A systematic review. BMC Musculoskel Disord. 2020;21:647. doi: 10.1186/s12891-020-03676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arhos E.K., Capin J.J., Buchanan T.S., Snyder-Mackler L. Quadriceps strength symmetry does not modify gait mechanics after anterior cruciate ligament reconstruction, rehabilitation, and return-to-sport training. Am J Sports Med. 2021;49:417–425. doi: 10.1177/0363546520980079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Capin J.J., Zarzycki R., Arundale A., Cummer K., Snyder-Mackler L. Report of the primary outcomes for gait mechanics in men of the ACL-SPORTS Trial: Secondary prevention with and without perturbation training does not restore gait symmetry in men 1 or 2 years after ACL reconstruction. Clin Orthop Relat Res. 2017;475:2513–2522. doi: 10.1007/s11999-017-5279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giesche F., Niederer D., Banzer W., Vogt L. Evidence for the effects of prehabilitation before ACL-reconstruction on return to sport-related and self-reported knee function: A systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Failla M.J., Logerstedt D.S., Grindem H., et al. Does extended preoperative rehabilitation influence outcomes 2 years after ACL reconstruction? A comparative effectiveness study between the MOON and Delaware-Oslo ACL Cohorts. Am J Sports Med. 2016;44:2608–2614. doi: 10.1177/0363546516652594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ardern C.L., Glasgow P., Schneiders A., et al. 2016 Consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50:853–864. doi: 10.1136/bjsports-2016-096278. [DOI] [PubMed] [Google Scholar]
- 75.Rambaud A.J.M., Ardern C.L., Thoreux P., Regnaux J.P., Edouard P. Criteria for return to running after anterior cruciate ligament reconstruction: A scoping review. Br J Sports Med. 2018;52:1437–1444. doi: 10.1136/bjsports-2017-098602. [DOI] [PubMed] [Google Scholar]
- 76.Manal T.J., Snyder-Mackler L. Practice guidelines for anterior cruciate ligament rehabilitation: A criterion-based rehabilitation progression. Operative Tech Orthop. 1996;6:190–196. [Google Scholar]
- 77.Lorenz D., Domzalski S. Criteria-based return to sprinting progression following lower extremity injury. Int J Sports Phys Ther. 2020;15:326–332. [PMC free article] [PubMed] [Google Scholar]
- 78.Lorenz D., Bailey L., Wilk K., et al. Current clinical concepts: Blood flow restriction training. J Athl Train. 2021;56:937–944. doi: 10.4085/418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spranger M.D. Commentary: Blood flow restriction exercise: Considerations of methodology, application, and safety. Front Physiol. 2020;11:599592. doi: 10.3389/fphys.2020.599592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wortman R.J., Brown S.M., Savage-Elliott I., Finley Z.J., Mulcahey M.K. Blood flow restriction training for athletes: A systematic review. Am J Sports Med. 2021;49:1938–1944. doi: 10.1177/0363546520964454. [DOI] [PubMed] [Google Scholar]
- 81.Scott B.R., Loenneke J.P., Slattery K.M., Dascombe B.J. Exercise with blood flow restriction: An updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313–325. doi: 10.1007/s40279-014-0288-1. [DOI] [PubMed] [Google Scholar]
- 82.Hughes L., Rosenblatt B., Haddad F., et al. Comparing the effectiveness of blood flow restriction and traditional heavy load resistance training in the post-surgery rehabilitation of anterior cruciate ligament reconstruction patients: A UK National Health Service randomised controlled trial. Sports Med. 2019;49:1787–1805. doi: 10.1007/s40279-019-01137-2. [DOI] [PubMed] [Google Scholar]
- 83.Lipker L.A., Persinger C.R., Michalko B.S., Durall C.J. Blood flow restriction therapy versus standard care for reducing quadriceps atrophy after anterior cruciate ligament reconstruction. J Sport Rehabil. 2019;28:897–901. doi: 10.1123/jsr.2018-0062. [DOI] [PubMed] [Google Scholar]
- 84.Lu Y., Patel B.H., Kym C., et al. Perioperative blood flow restriction rehabilitation in patients undergoing ACL reconstruction: A systematic review. Orthop J Sports Med. 2020;8 doi: 10.1177/2325967120906822. 2325967120906822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonnery-Cottet B., Saithna A., Quelard B., et al. Arthrogenic muscle inhibition after ACL reconstruction: A scoping review of the efficacy of interventions. Br J Sports Med. 2019;53:289–298. doi: 10.1136/bjsports-2017-098401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toth M.J., Tourville T.W., Voigt T.B., et al. Utility of neuromuscular electrical stimulation to preserve quadriceps muscle fiber size and contractility after anterior cruciate ligament injuries and reconstruction: A randomized, sham-controlled, blinded trial. Am J Sports Med. 2020;48:2429–2437. doi: 10.1177/0363546520933622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauger A.V., Reiman M.P., Bjordal J.M., Sheets C., Ledbetter L., Goode A.P. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee Surg Sports Traumatol Arthrosc. 2018;26:399–410. doi: 10.1007/s00167-017-4669-5. [DOI] [PubMed] [Google Scholar]
- 88.Moran U., Gottlieb U., Gam A., Springer S. Functional electrical stimulation following anterior cruciate ligament reconstruction: A randomized controlled pilot study. J Neuroeng Rehabil. 2019;16:89. doi: 10.1186/s12984-019-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hart J.M., Turman K.A., Diduch D.R., Hart J.A., Miller M.D. Quadriceps muscle activation and radiographic osteoarthritis following ACL revision. Knee Surg Sports Traumatol Arthrosc. 2011;19:634–640. doi: 10.1007/s00167-010-1321-z. [DOI] [PubMed] [Google Scholar]
- 90.Conley C.E.W., Mattacola C.G., Jochimsen K.N., Dressler E.V., Lattermann C., Howard J.S. A comparison of neuromuscular electrical stimulation parameters for postoperative quadriceps strength in patients after knee surgery: A systematic review. Sports Health. 2021;13:116–127. doi: 10.1177/1941738120964817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Ruiter C.J., Kooistra R.D., Paalman M.I., de Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol. 2004;97:1693–1701. doi: 10.1152/japplphysiol.00230.2004. [DOI] [PubMed] [Google Scholar]
- 92.Andersson C., Gillquist J. Treatment of acute isolated and combined ruptures of the anterior cruciate ligament. A long-term follow-up study. Am J Sports Med. 1992;20:7–12. doi: 10.1177/036354659202000103. [DOI] [PubMed] [Google Scholar]
- 93.Wu I.T., Hevesi M., Desai V.S., et al. Comparative outcomes of radial and bucket-handle meniscal tear repair: A propensity-matched analysis. Am J Sports Med. 2018;46:2653–2660. doi: 10.1177/0363546518786035. [DOI] [PubMed] [Google Scholar]
- 94.Richards D.P., Barber F.A., Herbert M.A. Compressive loads in longitudinal lateral meniscus tears: A biomechanical study in porcine knees. Arthroscopy. 2005;21:1452–1456. doi: 10.1016/j.arthro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 95.McCulloch P.C., Jones H.L., Hamilton K., Hogen M.G., Gold J.E., Noble P.C. Does simulated walking cause gapping of meniscal repairs? J Exp Orthop. 2016;3:11. doi: 10.1186/s40634-016-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.