Abstract
Our patient was admitted to hospital with a 1-week history of an upper respiratory tract infection and a rapidly progressive encephalopathy dominated by brainstem features and widespread areflexia. Her antiganglioside antibodies and electroencephalography were consistent with Bickerstaff brainstem encephalitis (BBE), and her postmortem examination revealed a predominantly florid brainstem encephalitis and myelitis. Her sputum and throat swabs isolated Haemophilus influenzae and Fusobacterium, respectively, the former being the most probable trigger of BBE. Our patient’s death, despite the otherwise good prognosis of the disorder, may reflect the severity of the pathological changes at postmortem or the association of comorbid disorders such as sepsis-associated encephalopathy. Her poor outcome may also be an indication to treat rapidly progressive cases of BBE with more than one immune modulating drug.
Keywords: brain stem/cerebellum, clinical neurophysiology
Background
Bickerstaff brainstem encephalitis (BBE) is an autoimmune disorder which usually presents with impaired consciousness, flaccid quadriparesis, ptosis and ophthalmoplegia. It may also manifest with ataxia, bulbar palsy, sensory disturbances, hypersomnolence and positive GQ1b antiganglioside antibodies in a third of cases.1 2 The prognosis of BBE is usually good especially in those who have anti-GQ1b antibodies.3 4 Our patient presented with typical features of BBE with positive anti-GQ1b antibodies but, despite the expected good outcome, she progressed rapidly and died soon after admission to hospital. In this case report, we will review her presentation and post-mortem findings, and discuss the possible contributory factors to her death.
Case presentation
Our patient was a 26-year-old woman who presented with a 1-week history of malaise, sore throat, hoarseness and cough. On the day of admission to hospital, she had developed fluctuating double vision and drooping of the left eyelid along with transient paraesthesias and numbness of the gums and teeth. She did not have a history of allergies and there was no history of recent travel. She had not had any skin rash and did not have haematuria. She smoked five cigarettes a day but did not drink alcohol. Her medical history was of febrile convulsion at the age of 22 months. She was not on any regular medications and there was no relevant family history. On admission she was coughing and was making wheezing noises. Her tonsils were injected. Her lungs were clear and her heart sounds were normal. She had a slight dysarthria and bilateral fluctuating ptosis which was worse on the left. She had diplopia in the primary position and this was worse on left gaze. Her pupils were equal and reactive and her fundi were normal. She had an alternating esotropia with restriction of horizontal gaze bilaterally. Facial power and sensation were normal. There was no neck flexion or extension weakness. She had normal limb tone. There was hip flexion weakness of grade 4 on the Medical Research Council Scale; power in her other muscle groups was normal. She had widespread areflexia and marked lower limb ataxia. Pain and joint position sensations were normal.
Investigations
Her significant laboratory findings were a raised C reactive protein (CRP) of 158; a raised white cell count of 20 cells/mm3 (reference range 3.6–11); a raised neutrophil count of 16 cells/mm3 (reference range 1.8–1.5) and positive anti-GQ1b antibody with a titre of 6400 (reference range 0–25). Her cerebrospinal fluid (CSF) protein was mildly elevated at 0.67 g/L (reference range 0.2–0.5) and her CSF glucose was 4 mmol/L (reference range 2.2–3.9) with contemporaneous serum glucose of 5.7 mmol/L. CSF cytology showed 11 cells which were immunophenotypically normal lymphocytes. CSF PCR was negative for adenovirus, enterovirus, herpes simplex virus 1 and 2 and varicella zoster. Her serology was negative for HIV and chlamydia, and her urine antigen tests were negative for Legionella and pneumococcus. In this case report, we will review her presentation and post-mortem findings, and discuss the possible contributory factors to her death. Nasal swab nucleic acid detection with PCR was negative for adenovirus, parainfluenza types 1–4, human metapneumovirus and rhinovirus. Her sputum however tested positive to Haemophilus influenzae, and her throat swab was positive for Fusobacterium. Her X-ray of the chest, CT angiography of the brain and MRI of the brain were normal. Her antinuclear antibodies, antineutrophil cytoplasmic antibodies, extractable nuclear antigen, anti-acetylcholine receptor antibodies, anti-muscle-specific kinase antibodies anti-N-Methyl-D-aspartate antibodies, anti-voltage-gated potassium channel antibodies and anti-GM1 antibodies were negative.
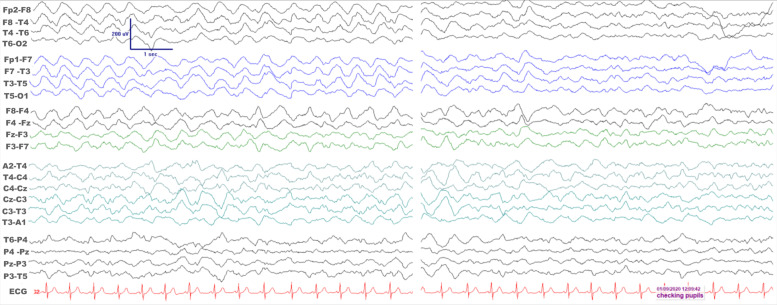
Electroencephalogram (EEG) was performed 9 days after admission to the intensive care unit (ICU) while the patient was comatose and 24 hours after all sedative anaesthetic agents were stopped (figure 1). It showed high amplitude mainly delta slow wave activity maximal anteriorly, with remarkable synchrony between the two hemispheres. There was some reactivity to verbal and tactile stimulation. Overall, there was evidence of non-specific widespread cerebral dysfunction; the synchronous bihemispheric involvement was in keeping with a subcortical involvement to include a brainstem lesion. Only a limited examination with nerve conduction studies was possible 4 days after admission. No response could be elicited from the superficial peroneal nerve, while the sural nerve potential was mildly attenuated in amplitude with normal associated conduction velocity. The compound muscle action potentials from the median nerve were borderline in amplitude with normal distal latency and conduction velocity in the elbow-to-wrist segment of the nerve; there was however extreme paucity (2/15) of very ill-formed F waves. The findings from the abductor hallucis were similar, with a borderline amplitude compound muscle action potential, normal distal latency and tibial conduction velocity, but with relative paucity (7/15) of ill-formed F waves. Although no specific features of demyelination were seen, the findings would be consistent with a disorder within the Guillain-Barre syndrome (GBS) spectrum.
Figure 1.
Electroencephalogram (EEG) recordings in the intensive care unit while the patient was comatose. A bipolar montage is shown while the recording was undertaken according to the 10–20 international system of electrode placement. There is remarkable bilateral synchrony of anteriorly maximal rather monorhythmic high amplitude delta frequency activity on the left EEG epoch. On the right, there is significant EEG reactivity as the patient’s pupils were examined.
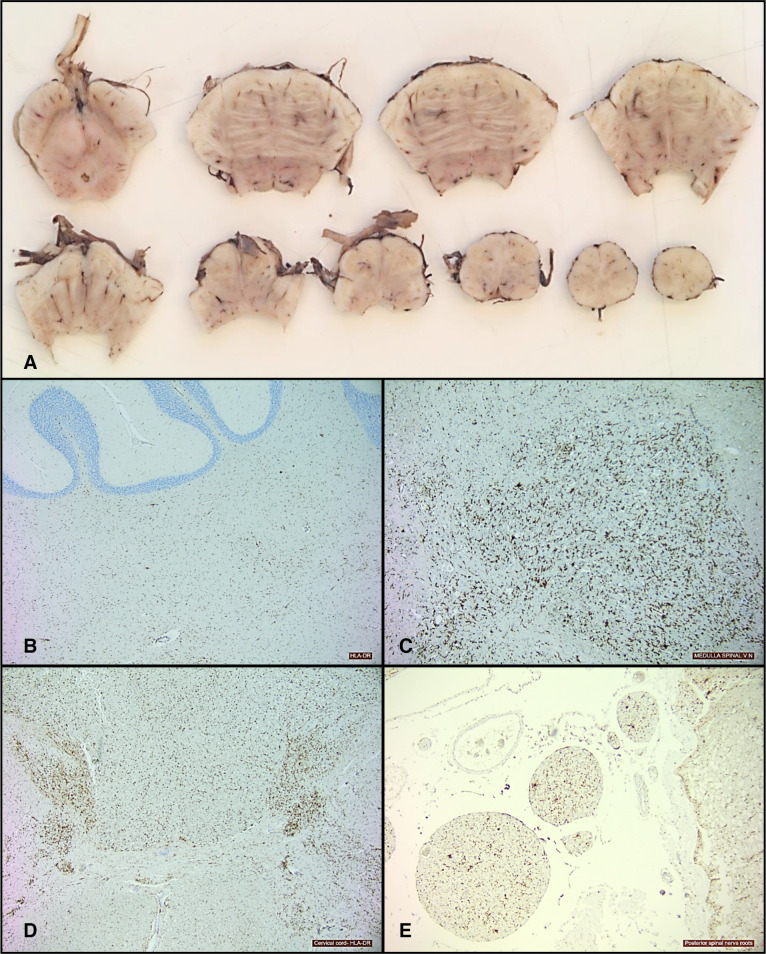
At postmortem, gross examination of her brain showed moderate congestion of the leptomeninges with mild flattening of the cerebral gyri and narrowing of the sulci, suggesting cerebral oedema. The cranial nerves and basal blood vessels were normal and there was no evidence of cerebral herniation. On sectioning the brain, there was distinct grey-white matter differentiation. No focal abnormality was present within the cerebrum. The brainstem showed mild congestion but no other focal lesions (figure 2A). The spinal cord also appeared normal on macroscopic examination. Histology showed mild focal reactive meningothelial hyperplasia and sparse chronic inflammatory cells comprising small lymphocytes and macrophages limited to the temporal leptomeninges. There were occasional perivascular lymphocytes. Immunohistochemistry showed diffuse upregulation of HLA-DR-positive macrophages and microglia which were not only marked throughout the brainstem (figure 2B) but were also present in the cerebellar white matter (figure 2C), corpus callosum, spinal cord grey matter, posterior columns (figure 2D) and spinal nerve roots (figure 2E). The heart showed no significant abnormality. There was pulmonary congestion and oedema. The right lobe of the thyroid was mildly enlarged. The rest of her visceral organs were normal.
Figure 2.
Sections from brainstem showing mild congestion only and no other focal abnormality (A).HLA-DR stain showing upregulation of macrophages/microglia within cerebellar white matter (B), medulla (C), cervical cord (D) and posterior spinal nerve roots (E).
Differential diagnosis
The presumptive admission diagnosis was of the pharyngo–cervical–brachial variant of GBS triggered by upper respiratory tract infection. Her subsequent progression and nerve conduction studies were however not consistent with this diagnosis. The presence of anti-GQ1b antibodies also suggested the possibility of Miller Fisher syndrome (MFS), but the profound impairment of brainstem function is not a feature of MFS. Viral encephalitis and bacterial meningoencephalitis were also differential diagnoses in our patient but her CSF infection screen and PCR were negative for bacteria and viruses.
Treatment
She was initially treated with intravenous immunoglobulins (IVIg) and variously with acyclovir, penicillin V, amoxicillin, meropenem, vancomycin, gentamicin and metronidazole. In view of her poor treatment response, she was subsequently treated with intravenous methylprednisolone.
Outcome and follow-up
By the second day of admission, she became drowsy and was unable to adequately clear her secretions; her forced vital capacity at this stage was 1.4 L and she was therefore admitted to the ICU. She was sedated and intubated but her condition became complicated by hypotension and sinus tachycardia which did not respond to fluids and norepinephrine. She died 12 days after admission from a cardiac arrest.
Discussion
Our patient’s clinical, laboratory, neurophysiological and postmortem findings are consistent with BBE. Our patient’s relatively normal CSF finding is consistent with the presentation of two-thirds of patients with this disorder. The brain MRI in BBE is normal in two-thirds of patients, and this was the case with our patient. While non-specific, the EEG changes in our patient were compatible with BBE.
Brain biopsy in BBE often reveals a perivascular lymphocytic inflammation, as was seen in the postmortem findings in our patient in which the HLA-DR-positive cells were predominantly in the brainstem. In the only autopsy case in the largest series of BBE in the literature, the subject’s brainstem showed perivascular lymphocytic infiltration with perivascular oedema and glial nodules, chromatolytic changes in the trigeminal motor and the spinal anterior horn cells and focal lymphocytic infiltration of the dorsal root ganglia.1 Two previous autopsy cases also showed brainstem oedema and perivascular lymphocytic inflammation, astrocytic proliferation, microglial activation and slight Purkinje cell loss.5 6 Our case expands on the previously documented neuropathological features of BBE by demonstrating the presence of patchy leptomeningeal involvement and widespread involvement of the spinal cord.
The best treatment strategy for BBE is not clear. Treatment is usually with IVIg or plasma exchange, but steroids have been used along with IVIg.3 4 6 Our patient had both IVIg and methylprednisolone, but it is not clear if instituting methylprednisolone earlier would have altered her outcome.
The outcome of BBE is usually favourable especially for subjects who are positive for anti-GQ1b antibodies; they have less frequent abnormalities in their CSF and MRI scans, and they tend to recover more rapidly and have fewer residual deficits than those without the antibodies.3 4 There are very few reports of mortality from BBE in the literature; two of these were associated with pulmonary embolism, and another two were attributed to recurrence of BBE.6–9 The poor outcome in our patient, who had positive anti-GQ1b antibodies and did not have pulmonary embolism or recurrent BBE, is therefore unexpected. While her poor outcome may be attributed to the florid pathological changes at postmortem, it is also possible that sepsis-associated encephalopathy (SAE) contributed to her death. This is because SAE is associated with a high morbidity and mortality, and her raised CRP and elevated white cell counts may be reflections of underlying sepsis.10
Learning points.
Our case emphasises the need to consider Bickerstaff brainstem encephalitis (BBE) as a differential diagnosis in patients presenting with Guillain-Barre syndrome, Miller Fisher syndrome, viral encephalitis, infective meningoencephalitis or sepsis-associated encephalopathy (SAE).
Our patient’s poor outcome, despite the otherwise good prognosis in most cases of BBE, suggests the need to consider comorbidities such as SAE in cases presenting with severe and rapidly progressive symptoms.
The early use of adjunctive steroids may be considered in patients presenting with severe and rapidly progressive BBE.
Footnotes
Contributors: II managed the patient, wrote the draft and approved the final version. PGS provided the neurophysiological report, contributed to the case report and approved the final version. AS provided the neuropathological report, contributed to the case report and approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Odaka M, Yuki N, Yamada M, et al. Bickerstaff’s brainstem encephalitis: clinical features of 62 cases and a subgroup associated with Guillain–Barré syndrome. Brain 2003;126:2279–90. 10.1093/brain/awg233 [DOI] [PubMed] [Google Scholar]
- 2.Wakerley BR, Soon D, Chan Y-C, et al. Atypical Bickerstaff brainstem encephalitis: ataxic hypersomnolence without ophthalmoplegia. J Neurol Neurosurg Psychiatry 2013;84:1206–7. 10.1136/jnnp-2013-304993 [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa K, Kuwahara M, Morikawa M, et al. Bickerstaff brainstem encephalitis with or without anti-GQ1b antibody. Neurol Neuroimmunol Neuroinflamm 2020;7:e889. 10.1212/NXI.0000000000000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koga M, Kusunoki S, Kaida K, et al. Nationwide survey of patients in Japan with Bickerstaff brainstem encephalitis: epidemiological and clinical characteristics. J Neurol Neurosurg Psychiatry 2012;83:1210–5. 10.1136/jnnp-2012-303060 [DOI] [PubMed] [Google Scholar]
- 5.Bickerstaff ER. Brain-Stem encephalitis: further observations on a Grave syndrome with benign prognosis. Br Med J 1957;1:1384–7. 10.1136/bmj.1.5032.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Din AN, Anderson M, Bickerstaff ER, et al. Brainstem encephalitis and the syndrome of Miller Fisher a clinical study. Brain 1982;105:481–95. 10.1093/brain/105.3.481 [DOI] [PubMed] [Google Scholar]
- 7.Hunter G, Young GB, Ang LC. Bickerstaff’s Brainstem Encephalitis Presenting to the ICU. Neurocrit Care 2012;17:102–6. 10.1007/s12028-012-9691-3 [DOI] [PubMed] [Google Scholar]
- 8.Tyrakowska Z, Jakubowicz-Lachowska D, Kułakowska A, et al. Relapsing-Remitting Severe Bickerstaff’s Brainstem Encephalitis – Case Report and Literature Review. Pol J Radiol 2016;81:622–8. 10.12659/PJR.898647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamsi Basha A, Kolko N, Santoro JD, et al. A probable case of recurrent Bickerstaff brainstem encephalitis with fulminant course in a pediatric patient. Neurologist 2020;25:14–16. 10.1097/NRL.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 10.Chung H-Y, Wickel J, Brunkhorst FM, et al. Sepsis-Associated encephalopathy: from delirium to dementia? JCM 2020;9:703. 10.3390/jcm9030703 [DOI] [PMC free article] [PubMed] [Google Scholar]