Abstract
Introduction
For many people with type 1 diabetes who struggle to achieve glycaemic control with multiple daily injections of insulin (MDI) plus self-monitoring of blood glucose, MDI plus intermittently scanned continuous glucose monitoring (IS-CGM) or real-time continuous glucose monitoring (RT-CGM), or insulin administration using insulin pump therapy represent optimised care in many regions. Through technological advances an advanced hybrid closed loop (AHCL) system has been developed; studies of incremental effects relative to MDI plus IS-CGM are lacking.
Methods and analysis
The Advanced Hybrid Closed Loop study in Adult Population with Type 1 Diabetes (ADAPT) study is a multinational, prospective, open-label, confirmatory and exploratory randomised controlled trial to examine outcomes with the MiniMed 670G version 4.0 AHCL system (with an equivalent algorithm and commercialised as the MiniMed 780G system, referred to as AHCL) relative to MDI plus IS-CGM in adults with baseline HbA1c≥8.0%. An exploratory cohort will compare AHCL with MDI plus RT-CGM. The study will be conducted in approximately 124 adults on MDI plus either IS-CGM or RT-CGM for at least 3 months prior to screening. The primary endpoint will be the difference in mean HbA1c change from baseline to 6 months between the AHCL and the MDI plus IS-CGM arms. Secondary endpoints will include proportion of time spent in hypoglycaemic, euglycaemic and hyperglycaemic ranges.
Ethics and dissemination
The ADAPT study will be conducted in accordance with the requirements of the Declaration of Helsinki and local laws and regulations, and has been approved by ethics committees. The trial will provide valuable information on the incremental benefits that may be provided by AHCL for patients failing to achieve glycaemic targets on MDI plus IS-CGM or RT-CGM and form a basis for health economic evaluations to support market access.
Trial registration number
NCT04235504; Pre-results.
Keywords: general diabetes, diabetes & endocrinology, diabetes & endocrinology
Strengths and limitations of this study.
To date, long-term, head-to-head studies of advanced hybrid closed loop versus multiple daily injection plus intermittently scanned continuous glucose monitoring (or real-time continuous glucose monitoring) are lacking and the Advanced Hybrid Closed Loop study in Adult Population with Type 1 Diabetes (ADAPT) study has been designed to directly address this need.
The inclusion criteria limit trial enrolment to subjects with a baseline HbA1c of ≥8.0% (64 mmol/mol), that is subjects failing to achieve good glycaemic control as stipulated by HbA1c targets recommended in major guidelines, in line with the patient population using insulin pumps and CGM in many settings.
Many previous studies of hybrid closed loop systems have been of a duration of 12 weeks or less but the ADAPT study will evaluate the durability of outcomes over a study phase of 6 months, with a further 6-month follow-up continuation phase in a home setting.
A limitation of the study is that the comparator arms represent the current standard of care for patients with type 1 diabetes and as a result it may not fully quantify the benefits of advanced hybrid closed loop compared with the frequent, stepwise changes in treatment and/or addition of supplementary technologies in patients failing to achieve glycaemic targets or experiencing problematic hypoglycaemia in routine clinical practice.
The ADAPT study will assess patient-reported outcomes, including fear of hypoglycaemia, quality of life and treatment satisfaction, and provide valuable input data for future health economic analyses, allowing better informed decision making among healthcare payers, for whom the acquisition costs of new technologies can represent a barrier to uptake or reimbursement.
Introduction
Type 1 diabetes (T1D) is a chronic lifelong condition that is associated with a risk of long-term complications including cardiovascular disease, renal disease and ophthalmic complications. The standard of care for people with T1D has evolved greatly over time, with each advance offering stepwise incremental improvements in glycaemic control and/or reductions in the risk of hypoglycaemic events. Improvements in disease management include both drug treatments and advances in technology, such as the development of real-time continuous glucose monitoring (RT-CGM), intermittently scanned continuous glucose monitoring (IS-CGM) and continuous subcutaneous insulin infusion (CSII). Each generation of insulin pumps has become progressively more sophisticated, with advanced hybrid closed loop (AHCL) systems representing the latest and most advanced generation of insulin pumps.1–4
Despite improvements in the standard of care increasing life expectancy for people with T1D over the last two decades, life expectancy for young people with T1D remains around 8–13 years below that of the general population, suggesting there is still much to be achieved in terms of improving long-term outcomes.5–7 In an increasing number of countries, multiple daily injections of insulin (MDI) plus either RT-CGM or IS-CGM are emerging as the standard of care for many patients, particularly for those struggling with either glycaemic control or hypoglycaemia.8 9 Moreover, recently published national and international guidelines are increasingly moving towards advocating the use of CGM in people with T1D, particularly those with a history of severe hypoglycaemic events or unawareness of hypoglycaemia.10 11 Both CGM methods use a sensor placed subcutaneously but whereas with RT-CGM sensor readings are transmitted to the receiver every 5 min, with IS-CGM the receiver must be scanned directly over the sensor. Real-world studies have shown that IS-CGM use can lead to improved glycaemic control measures for some patients, with improvements linked to a higher frequency of scanning.12 13 In parallel, insulin pumps are also becoming more widely used.14 One of the most recently developed and commercialised insulin pumps is the MiniMed 780G, which is an AHCL system approved for use in Europe in individuals with T1D aged 7–80 years, has been shown to significantly improve time in range relative to previous generation systems.3
The Advanced Hybrid Closed Loop study in Adult Population with Type 1 Diabetes (ADAPT) study will examine potential improvements associated with the use of the AHCL system in people with T1D with sub-optimal glycaemic control on a non-automated system. Previous studies of insulin pumps, including hybrid closed loop (HCL) systems, have largely used a comparator arm of MDI plus self-monitoring of blood glucose (SMBG). However, uptake of RT-CGM and IS-CGM, particularly among patients struggling with disease management, is increasing and this now represents the standard of care for some patients with suboptimally controlled T1D. The ADAPT study has been designed to provide insights into the potential incremental improvement in outcomes that could be achieved with the use of an AHCL system.
Methods and analysis
Study design
The ADAPT study will be a prospective open-label, multicentre, adaptive, confirmatory and randomised controlled trial in adults with T1D. The study will be conducted at multiple sites with experience in CSII use in adults with T1D in France, Germany, and the UK, with a study start date of 13 July 2020. The estimated primary completion date is 15 December 2021 and estimated study closure is 30 July 2022. The primary objective is to compare the mean change in glycosylated hemoglobin (HbA1c) from baseline to 6 months between the active intervention arm (MiniMed 670G V.4.0 AHCL) and the control arm (MDI plus IS-CGM). There will also be an additional exploratory part of the study, with a separate cohort, comparing the same AHCL system with MDI plus RT-CGM to look for potential similarities in trends. The study will comprise three phases: a 2-week run-in phase, a 6-month study phase and a 6-month continuation phase (figure 1 and online supplemental material 1). In the run-in phase, subjects will continue on their current baseline therapy of MDI plus blinded CGM (using the Guardian Link 3 attached to the Guardian Sensor 3) to collect baseline CGM data and determine subject’s ability to tolerate wearing the sensor and transmitter continuously. Patients who successfully complete blinded CGM during the run-in phase, including wearing and acceptable tolerance to the sensor plus at least two fingerstick blood glucose measurements per day and compliance with study procedures will undergo randomisation. Blinded CGM will be performed at baseline for all patients and at two additional timepoints for patients in the control MDI plus CGM (IS-CGM or RT-CGM) arm (at month 3 and month 6 of the study phase). The same CGM system will be used in both arms to allow for comparisons of CGM data.
Figure 1.
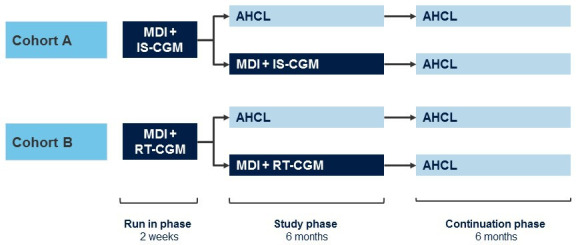
Study design. AHCL, advanced hybrid closed loop; IS-CGM, intermittently scanned continuous glucose monitoring; MDI, multiple daily injections; RT-CGM, real-time continuous glucose monitoring.
bmjopen-2021-050635supp001.pdf (387.9KB, pdf)
At the start of the 6-month study phase, subjects will be randomly allocated to either the AHCL arm or the control arm. The study will consist of two cohorts (Cohort A: confirmatory part of study and Cohort B: exploratory part of study) as follows:
Cohort A
Treatment arm—begin treatment with AHCL.
Control arm—continue treatment with MDI plus IS-CGM.
Cohort B
Treatment arm—begin treatment with AHCL.
Control arm—continue treatment with MDI plus RT-CGM.
Participants using IS-CGM will be randomised in cohort A, and those using RT-CGM will be randomised in cohort B. In each cohort, participants will be randomly allocated to treatment in a 1:1 ratio using an investigator-blinded block randomisation procedure with blocks of different sizes. The order of the block sizes will be selected randomly at a country level. Participants who are allocated to AHCL will receive training on how to use the pump and will be expected to use the device in closed loop with Auto Basal and Auto Correction at all times as well as regularly upload pump and sensor glucose data into CareLink therapy management software.
The AHCL used in this study incorporates a HCL algorithm. In closed loop, basal insulin is delivered every 5 min, with the basal insulin delivery rate calculated and adjusted as required based on CGM, users in the ADAPT study are also able to customise their target glucose level to either 120 mg/dL (6.7 mmol/L) or 100 mg/dL (5.6 mmol/L). During the ADAPT study, the recommended settings are a target glucose level of 100 mg/dL (5.6 mmol/L) and an active insulin time of 2 hours. The AHCL also delivers automatic correction boluses based on CGM data, with this feature designed to increase the proportion of time spent in the euglycaemic range. In closed loop, the user is still required to record pre-meal carbohydrates. When used in open loop, SmartGuard features such as suspend before low (which temporarily suspends basal insulin delivery if sensor glucose levels go below, or are predicted to go below, a predefined threshold level) can be used. Subjects in the MDI plus IS-CGM arm (cohort A) will use an Abbott FreeStyle Libre IS-CGM device. With this device, the sensor is placed on the arm subcutaneously and glucose levels are obtained by manually scanning the reader over the sensor. While several commercially available glucose sensors are available, in the ADAPT trial the comparator arm will use Abbott FreeStyle Libre IS-CGM device for the primary analysis. Participants will use the IS-CGM device according to the specific model and to the current best clinical practice. Subjects in the MDI plus RT-CGM arm (cohort B) will use any RT-CGM model available at the study site, in line with standard of care.
The duration of the study phase will be 6 months. Following completion of the study phase subjects will enter a 6-month continuation phase, during which all subjects will use the 670G V.4.0 AHCL system (figure 1). The overall duration of the study from initiation to completion of all patients is anticipated to be a maximum of 13 months.
Study eligibility and key inclusion/exclusion criteria
For inclusion in the ADAPT study, subjects will be required to be aged ≥18 years with a diagnosis of T1D made at least 2 years prior to screening, on MDI therapy, using IS-CGM or RT-CGM for ≥3 months (with daily average of ≥5 scans for IS-CGM) and sensor readings >70% of time in the month prior to screening to ensure the proper utilisation of the CGM device and have a HbA1c ≥8.0% (64 mmol/mol). Measurement of HbA1c will be performed in accordance with the National Glycohemoglobin Standardisation Programme at a centralised laboratory. Full details of inclusion and exclusion criteria are provided in table 1.
Table 1.
Inclusion and exclusion criteria for the ADAPT study
| Inclusion criteria | Exclusion criteria |
|
|
*Defined as ≥3 insulin injections per day and/or a basal/bolus regimen.
ADAPT, Advanced Hybrid Closed Loop study in Adult Population with Type 1 Diabetes; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; IS-CGM, intermittently scanned; MDI, multiple daily injection; RT-CGM, real-time continuous glucose monitoring; SGLT-2, sodium-glucose co-transporter-2; T1D, type 1 diabetes.
Patient involvement
Patients were not involved in the development of research question, outcome measures and design of the study. The participants will be informed once the trial results are published.
Study endpoints
The primary and confirmatory analyses will be performed in cohort A and the primary endpoint of the study will be the difference in the mean HbA1c change (baseline vs 6 months) between the AHCL arm and the MDI plus IS-CGM arm. Secondary endpoints will include the proportion of time spent in hyperglycaemic range with sensor glucose (SG) >250 mg/dL (13.9 mmol/L) and SG >180 mg/dL (>10.0 mmol/L), proportion of time spent within range with sensor glucose (SG) between 70 and 180 mg/dL (3.9–10.0 mmol/L) and the proportion of time spent in hypoglycaemic range with SG <54 mg/dL (3.0 mmol/L) and <70 mg/dL (3.9 mmol/L) (box 1). Safety endpoints will include the number of severe hypoglycaemic events (defined as an event requiring assistance due to altered consciousness), the number of diabetic ketoacidosis events, number of serious adverse events, number of serious adverse device effects, number of unanticipated serious adverse device effects and the number of device deficiencies. Ancillary endpoints will include the proportion of time spent in closed loop and open loop in the AHCL arm and number of days lost from work or school, the coefficient of variation of SG values, change in total daily dose of insulin from baseline to end of study, change in weight, change in body mass index, and mean change in HbA1c from baseline to 12 months (box 2). The primary, secondary and ancillary endpoints will be assessed in cohort B in an exploratory fashion. Several patient-reported outcomes (PROs) will also be evaluated including quality of life, assessed using the Diabetes Quality of Life Questionnaire,15 16 treatment satisfaction, assessed using the Diabetes Treatment Satisfaction Questionnaire17 18 and fear of hypoglycaemic (FoH), assessed using the Hypoglycaemic Fear Survey.19
Box 1. Secondary endpoints to be assessed in cohort A.
Secondary endpoints
Percentage time spent in hyperglycaemic range with SG >250 mg/dL (13.9 mmol/L mmol/L).
Percentage time spent in hyperglycaemic range with SG >180 mg/dL (>10.0 mmol/L),
Percentage time spent within range with SG between 70 and 180 mg/dL (3.9–10.0 mmol/L).
Percentage time spent in hypoglycaemic range with SG <54 mg/dL (3.0 mmol/L mmol/L).
Percentage time spent in hypoglycaemic range with SG<70 mg/dL (3.9 mmol/L mmol/L)
SG, sensor glucose.
Box 2. Ancillary endpoints.
Endpoint
Percentage time spent in 70–140 mg/dL (3.9–7.8 mmol/L) range
AUC in hypoglycaemic range with SG <54 mg/dL (3.0 mmol/L mmol/L), <70 mg/dL (3.9 mmol/L mmol/L)
Percentage time and AUC in hyperglycaemic range with SG >140 mg/dL (7.8 mmol/L mmol/L), >350 mg/dL (19.4 mmol/L mmol/L) and AUC in hyperglycaemic range with SG >180 mg/dL (10 mmol/L mmol/L), >250 mg/dL (13.9 mmol/L mmol/L)
Number of biochemical hypoglycaemic events with SG <54 mg/dL (3.0 mmol/L mmol/L), <70 mg/dL (3.9 mmol/L mmol/L) (defined as sensor values below the threshold per 15 and 20 consecutive minutes, respectively)
Mean of SG values (mg/dL)
Percentage time spent in closed loop and open loop
All above endpoints will be categorised by daytime (06:01–23:59 hours) and night-time (00:00–06:00 hours) and overall (24h hours).
Percentage time spent in hyperglycaemic range with SG >250 mg/dL (13.9 mmol/L mmol/L)
Percentage time spent in hyperglycaemic range with SG >180 mg/dL (>10.0 mmol/L)
Percentage time spent within range with SG between 70 and 180 mg/dL (3.9–10.0 mmol/L)
Percentage time spent in hypoglycaemic range with SG <54 mg/dL (3.0 mmol/L mmol/L)
Percentage time spent in hypoglycaemic range with SG <70 mg/dL (3.9 mmol/L mmol/L)
The above five endpoints will be categorised by daytime (06:01–23:59 hours) and night-time (00:00–06:00).
Number of scans and percentage of sensor readings for MDI plus IS-CGM control arm
Percentage of sensor readings for MDI plus RT-CGM control arm only
Number of SMBG tests in the AHCL arm
Percentage of sensor use
Excursion amplitudes of the glucose values measured by MAGE
Coefficient of variation of SG values
Change in total daily dose of insulin from baseline to EOS
Change in weight from baseline to EOS
Change in BMI from baseline to EOS
Mean HbA1c change (from baseline to 12 months)
Mean HbA1c change (baseline to 6 months) by age groups and duration of diabetes
Diabetes-related number and mean duration of hospitalisations, number and mean duration intensive care unit care, number of emergency room admissions, number of events requiring ambulance assistance, categorised by reason of diagnosis
Number of lost days from school or work
Hypoglycaemic Fear Survey score
Diabetes Treatment Satisfaction Questionnaire score
Diabetes Quality of Life questionnaire score
AHCL, advanced hybrid closed loop; AUC, area under the curve; BMI, body mass index; EOS, end of study; IS-CGM, intermittently scanned continuous glucose monitoring; MAGE, mean amplitude of glycaemic excursions; MDI, multiple daily injections; RT-CGM, real time continuous glucose monitoring; SG, sensor glucose; SMBG, self-monitoring of blood glucose.
Sample size
For cohort A (670G V.4.0 AHCL vs MDI plus IS-CGM), it is anticipated that a total enrollment of 84 subjects will be required. It is also assumed that, based on a drop-out rate of 10% at screening, 5% following the run-in phase and 7.5% during the 6-month study phase, approximately 70 subjects will undergo randomisation and 64 will complete the 6-month study phase. The sample size calculation also assumes an alpha of 0.05, a power of 80% and a minimum difference in mean (SD) reduction of 0.5 (0.7)% in HbA1c in the treatment arm versus the control arm. The value of 0.5% in terms of HbA1c change also constitutes the minimum clinically meaningful difference, and is based on the findings of a 2011 study by Hermanides et al.20 Due to uncertainty about the magnitude of the SD and the effect of treatment, the study has been designed to allow for a reassessment of sample size based on an interim analysis to be performed by an independent data monitoring committee (DMC) after at least 30 patients have completed the 6-month study phase in cohort A. The interim analysis for sample size reassessment with one interim look, protecting the overall two-sided type 1 error of 0.05, is based on the conditional power approach of Li et al21 and Chen et al22 as extended by Mehta and Pocock.23 On the basis of this interim analysis, the DMC will recommend termination or completion of the study, and if appropriate an increase in the sample size. Drop-out rates will also be reassessed. For Cohort B (670G V.4.0 AHCL vs MDI plus RT-CGM) a total enrolment of 40 subjects will be required to achieve approximately 34 subjects undergoing randomisation and 30 subjects completing the 6-month study phase for exploratory analysis.
Statistical analysis
HbA1c measurements will be performed at baseline, the end of month 3 and the end of month 6. The primary endpoint (change in HbA1c from baseline to 6 months) will be analysed using a repeated measures random effects model that accounts for subjects who drop-out of the study and for possible missing at random data. All analyses will be performed using the intention-to-treat population, which will consist of all randomised patients. To preserve the overall type I error and claim significance, a hierarchical test procedure will be performed for the predefined secondary endpoints (box 1). The study statistician analysing the data will be masked to group assignment until final database lock. Patient baseline demographics and characteristics will be collected and presented using descriptive statistics for continuous variables and counts or percentages for categorical variables.
Ethics and dissemination
The ADAPT study will be conducted in accordance with the requirements of the Declaration of Helsinki as well as local laws and regulations of the countries in which the study will be conducted. The study will also be conducted in compliance with the principles of good clinical practice, which includes review and approval by an independent ethics committee or institutional review board in France (Comité de Protection des Personnes Ile de France IV), Germany (Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster), and the UK (London-Dulwich Research Ethics Committee), and is aligned with the Standard Protocol Items: Recommendations for Interventional Trials 2013 Statement (online supplemental materials 2, 3).24 Each participating centre will not commence any patient-related study activities until approval by the relevant ethics committee or institutional review board has been received and the study centre has received clearance from the sponsor to commence the study.
bmjopen-2021-050635supp002.pdf (82.2KB, pdf)
bmjopen-2021-050635supp003.pdf (99.2KB, pdf)
Discussion
The aim of the ADAPT study will be to determine the change in HbA1c from baseline to 6 months for adults with T1D using the AHCL system relative to those using MDI plus IS-CGM. The clinical benefits as well as the convenience of technologies such as CGM and insulin pumps are increasingly recognised by payers and policy makers as well as treating physicians. International and national level guidelines also frequently recommend the use of CGM and/or insulin pumps in people with T1D struggling to achieve good glycaemic control. For example, the French national guidelines recommend the use of IS-CGM as an alternative or replacement for SMBG in patients with T1D or type 2 diabetes on intensified insulin therapy.25 Similarly, the current ADA guidelines note that the use of technology should be individualised based on a combination of need, desire, skill level and availability.11
The inclusion criteria limit trial enrolment to subjects with a baseline HbA1c of ≥8.0% (64 mmol/mol), that is, subjects failing to achieve good glycaemic control as stipulated by HbA1c targets recommended in major guidelines.26 This aligns with the patient population using insulin pumps and CGM in many settings, where reimbursement of medical devices such as CGM is often limited to those with poor glycaemic control or frequent severe hypoglycaemic events.27 The use of MDI plus IS-CGM as the comparator/standard of care arm in the ADAPT study has both clinical and economic implications. Clinical studies have consistently shown that both IS-CGM, RT-CGM and SAP or AHCL can improve glycaemic control and increase the proportion of patients obtaining these goals, while reducing the proportion of time spent in the hypoglycaemic range relative to SMBG.28 However, to date, long-term, head-to-head studies of AHCL versus MDI plus IS-CGM (or RT-CGM) are lacking.
Given the continued evolution of medical devices in the management of people with T1D payers and policy-makers must determine whether the incremental clinical benefits provided by the latest advances in technology represent good value for money relative to the standard of care. It is, therefore, important that cost-effectiveness analyses use clinical input data that reflects contemporary clinical practice to avoid overestimating or underestimating long-term clinical or economic outcomes. ADAPT will provide valuable data in this regard by providing head-to-head data for future economic evaluations of AHCL vs MDI plus IS-CGM. Additionally, the ADAPT study will include days of work/school lost as an ancillary endpoint, which will provide valuable input data for health economic analyses performed from the societal perspective. The ADAPT study will also assess several PROs including FoH, QoL and treatment satisfaction. The inclusion of PROs is important to give an accurate measure of the patient experience in both treatment arms. Moreover, health economic analyses have shown that factors such as reduced FoH can be a key driver of the cost-effectiveness of HCL systems.29
For many people with T1D there is frequently a stepwise change in treatment or addition of supplementary technologies such as CGM or insulin pump therapy only when people fail to achieve glycaemic targets or experience problematic hypoglycaemic.30 Alongside this, a degree of therapeutic inertia has been reported in some settings, resulting in delays in intensification of treatment or addition of technology, which may potentially have implications in terms of the risk for long-term complications.31 There is evidence of a legacy effect in T1D with good glycaemic control early in the course of the disease reducing or delaying the incidence of serious long-term complications.32 This may, in turn, have economic implications in terms of the medical costs associated with long-term complications. The importance of optimising treatment for patients with T1D is clear and it is hoped that the ADAPT study will provide valuable information regarding the use of AHCL systems in adult with T1D.
The ADAPT study will address the issue of whether the AHCL system can provide incremental benefits over a period of 6 months in terms of glycaemic control relative to MDI plus IS-CGM in adults with T1D. The study will also provide an important evidence base for future cost-effectiveness analyses of the one of the most advanced AHCL systems currently available to support market access.
Supplementary Material
Footnotes
Contributors: SdP, LV, RR, JS, JC, AH and OC contributed to the design of the study and critically reviewed the present manuscript. SdP, LV, RR, JS, JC, AH and OC approved the final version of the manuscript, and are accountable for all aspect of the work. The authors are grateful to Jayne Smith-Palmer at Ossian Health Economics and Communications for medical writing support in the preparation of the manuscript.
Funding: The ADAPT study is sponsored and funded by Medtronic International Trading Sàrl. Award/Grant number is not applicable.
Competing interests: SdP, LV, RR, JS, AH, JC and OC are employees of Medtronic.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-Month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–17. 10.1056/NEJMoa1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leelarathna L, Choudhary P, Wilmot EG, et al. Hybrid closed-loop therapy: where are we in 2021? Diabetes Obes Metab 2021;23:655–60. 10.1111/dom.14273 [DOI] [PubMed] [Google Scholar]
- 3.Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose Suspend in people with type 1 diabetes. Diabetes Care 2021;44:dc202250. 10.2337/dc20-2250 [DOI] [PubMed] [Google Scholar]
- 4.McAuley SA, Lee MH, Paldus B, et al. Six months of hybrid closed-loop versus manual insulin delivery with Fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care 2020;43:3024–33. 10.2337/dc20-1447 [DOI] [PubMed] [Google Scholar]
- 5.Huo L, Harding JL, Peeters A, et al. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia 2016;59:1177–85. 10.1007/s00125-015-3857-4 [DOI] [PubMed] [Google Scholar]
- 6.Petrie D, Lung TWC, Rawshani A, et al. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia 2016;59:1167–76. 10.1007/s00125-016-3914-7 [DOI] [PubMed] [Google Scholar]
- 7.Bjornstad P, Donaghue KC, Maahs DM. Macrovascular disease and risk factors in youth with type 1 diabetes: time to be more attentive to treatment? Lancet Diabetes Endocrinol 2018;6:809–20. 10.1016/S2213-8587(18)30035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72. 10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS England . Flash glucose monitoring: national arrangements for funding of relevant diabetes patients, 2019. Available: https://www.england.nhs.uk/wp-content/uploads/2020/02/national-arrangements-for-funding-of-relevant-diabetes-patients-v1.2.pdf [Accessed 11 Aug 2020].
- 10.Grunberger G, Handelsman Y, Bloomgarden ZT, et al. American association of clinical endocrinologists and American College of endocrinology 2018 position statement on integration of insulin pumps and continuous glucose monitoring in patients with diabetes mellitus. Endocr Pract 2018;24:302–8. 10.4158/PS-2017-0155 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association . 7. Diabetes Technology: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S71–80. 10.2337/dc19-S007 [DOI] [PubMed] [Google Scholar]
- 12.Jangam S, Dunn T, Xu Y, et al. Flash glucose monitoring improves glycemia in higher risk patients: a longitudinal, observational study under real-life settings. BMJ Open Diabetes Res Care 2019;7:e000611. 10.1136/bmjdrc-2018-000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landau Z, Abiri S, Gruber N, et al. Use of flash glucose-sensing technology (FreeStyle Libre) in youth with type 1 diabetes: Awesome Study Group real-life observational experience. Acta Diabetol 2018;55:1303–10. 10.1007/s00592-018-1218-8 [DOI] [PubMed] [Google Scholar]
- 14.van den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care 2019;42:2050–6. 10.2337/dc19-0345 [DOI] [PubMed] [Google Scholar]
- 15.Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care 1988;11:725–32. 10.2337/diacare.11.9.725 [DOI] [PubMed] [Google Scholar]
- 16.Renard E, Vague V, et al. , Bringer pour le groupe Evadiac . Validation psychométrique d’une version française du questionnaire d’évaluation de la qualité de vie du diabétique élaboré par le DCCT Research Group (DQOL). Diabetes Metab 1999;25:22.10746008 [Google Scholar]
- 17.Bradley . The diabetes treatment satisfaction questionnaire: DTSQ. In: Handbook of psychology and diabetes, 1994. [Google Scholar]
- 18.Health psychology research. Available: https://www.healthpsychologyresearch.com/ [Accessed 14 Sep 2021].
- 19.Cox DJ, Irvine A, Gonder-Frederick L, et al. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10)::617–21. Sep-Oct;. 10.2337/diacare.10.5.617 [DOI] [PubMed] [Google Scholar]
- 20.Hermanides J, Nørgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med 2011;28:1158–67. 10.1111/j.1464-5491.2011.03256.x [DOI] [PubMed] [Google Scholar]
- 21.Li G, Shih WJ, Xie T, et al. A sample size adjustment procedure for clinical trials based on conditional power. Biostatistics 2002;3:277–87. 10.1093/biostatistics/3.2.277 [DOI] [PubMed] [Google Scholar]
- 22.Chen YHJ, DeMets DL, Lan KKG. Increasing the sample size when the unblinded interim result is promising. Stat Med 2004;23:1023–38. 10.1002/sim.1688 [DOI] [PubMed] [Google Scholar]
- 23.Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med 2011;30:3267–84. 10.1002/sim.4102 [DOI] [PubMed] [Google Scholar]
- 24.SPIRIT Statement Website . Available: https://www.spirit-statement.org [Accessed 29 Mar 2021].
- 25.Borot S, Benhamou PY, Atlan C. SFD), Société française d’endocrinologie (SFE); Évaluation dans le diabète des implants actifs Group (EVADIAC). Practical implementation, education and interpretation guidelines for continuous glucose monitoring: A French position statement. Diabetes Metab 2018;44:61–72. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association . 6. Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S61–70. 10.2337/dc19-S006 [DOI] [PubMed] [Google Scholar]
- 27.Graham C, Monitoring CG. And global reimbursement: an update. Diabetes Technol Ther 2017;19:S60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Ridder F, den Brinker M, De Block C. The road from intermittently scanned continuous glucose monitoring to hybrid closed-loop systems. Part B: results from randomized controlled trials. Ther Adv Endocrinol Metab 2019;10:2042018819871903. 10.1177/2042018819871903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jendle J, Pöhlmann J, de Portu S, et al. Cost-Effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol Ther 2019;21:110–8. 10.1089/dia.2018.0328 [DOI] [PubMed] [Google Scholar]
- 30.Brunton S. Therapeutic inertia is a problem for all of US. Clin Diabetes 2019;37:105–6. 10.2337/cd19-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brož J, Janíčková Žďárská D, Urbanová J, et al. Current level of glycemic control and clinical inertia in subjects using insulin for the treatment of type 1 and type 2 diabetes in the Czech Republic and the Slovak Republic: results of a multinational, multicenter, observational survey (DIAINFORM). Diabetes Ther 2018;9:1897–906. 10.1007/s13300-018-0485-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the epidemiology of diabetes interventions and complications (EDIC) study. JAMA 2003;290:2159–67. 10.1001/jama.290.16.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050635supp001.pdf (387.9KB, pdf)
bmjopen-2021-050635supp002.pdf (82.2KB, pdf)
bmjopen-2021-050635supp003.pdf (99.2KB, pdf)


