Abstract
Objective
To compare disease characteristics and outcomes between patients with axial spondyloarthritis with non-radiographic disease (nr-axSpA), bilateral grade 2 sacroiliitis (r22axSpA) and unilateral/bilateral grade 3–4 sacroiliitis (r3+axSpA) according to the modified New York criteria.
Methods
We included patients with axial spondyloarthritis with available pelvic radiographs from the Swiss Clinical Quality Management Cohort. Retention of a first tumour necrosis factor inhibitor (TNFi) was investigated with multiple adjusted Cox proportional hazards models. The proportion of patients reaching 50% reduction in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50) at 1 year was assessed with multiple adjusted logistic regression analyses. Spinal radiographic progression, defined as an increase in ≥2 mSASSS units in 2 years, was assessed in generalised estimating equation models.
Results
From 2080 patients, those with nr-axSpA (n=485) and r22axSpA (n=443) presented with lower C reactive protein levels and less severe clinical spinal involvement compared with patients with r3+axSpA (n=1152). While TNFi retention was similar in r22axSpA and nr-axSpA, the risk of discontinuation was significantly lower in r3+axSpA (HR 0.60, 95% CI 0.44 to 0.82 vs nr-axSpA). BASDAI50 responses at 1 year were comparable in r22axSpA and nr-axSpA, with a better response associated with r3+axSpA (OR 2.05, 95% CI 1.09 to 3.91 vs nr-axSpA). Spinal radiographic progression was similar in r22axSpA and nr-axSpA and significantly higher in r3 +axSpA.
Conclusion
Patients with r22axSpA are comparable to nr-axSpA patients but differ from patients with more severe sacroiliac damage with regard to treatment effectiveness and spinal radiographic progression. Therefore, current differentiation between nr-axSpA and radiographic disease seems of limited use for outcome prediction.
Keywords: spondylitis, ankylosing, tumor necrosis factor inhibitors, epidemiology
Key messages.
What is already known about this subject?
Using the current cut-off of bilateral grade 2 radiographic sacroiliitis, patients with non-radiographic versus radiographic axial spondyloarthritis (axSpA) present with a comparable disease burden and respond similarly well to treatment with biologics in the context of objective signs of inflammation.
What does this study add?
In contrast to patients with at least unilateral grade 3 (moderate to severe) radiographic sacroiliitis patients with bilateral grade 2 sacroiliitis have a lower response to biologics, comparable to patients with axSpA with non-radiographic disease, while also having a very low risk of spinal radiographic progression.
How might this impact on clinical practice or further developments?
As current radiographic differentiation between disease states proves arbitrary, expanding the use of MRI for diagnostic purposes and including contextual evaluation of both active and structural lesions might improve detection of patients with early axSpA and potentially improve treatment responses.
Introduction
The spectrum of axial spondyloarthritis (axSpA) encompasses both a non-radiographic and a radiographic disease forms (non-radiographic axial spondyloarthritis(nr-axSpA) and radiographic axial spondyloarthritis (r-axSpA), respectively).1–5 Structural damage at the level of the sacroiliac joints (SIJs) on conventional radiographs differentiates between the two disease states: up to unilateral grade 2 sacroiliitis for nr-axSpA and bilateral grade 2 sacroiliitis (r22axSpA) or grade 3–4 unilaterally for r-axSpA.6–8 The cut-off of r22axSpA corresponds to the radiological item of the 1984 modified New York (mNY) classification criteria for ankylosing spondylitis9 and was originally proposed in 1966.10 Regulatory agencies such as the US Food and Drug Administration or the European Medicines Agency require randomised controlled trials (RCTs) to be performed in both axSpA disease forms for approval of new drugs.11 However, the difference between grade 1 and grade 2 sacroiliitis on radiographs is poorly defined (suspicious vs mild structural changes). This leads to an important inter-rater and intrarater variability of SIJ scoring,12 13 which cannot be significantly improved with training.14 Consequently, it has been suggested to abandon the distinction between nr-axSpA and r-axSpA and that RCTs should be performed across the whole spectrum of axSpA.15 The latter seems of particular relevance as patients with nr-axSpA and r-axSpA have been shown to present with a comparable disease burden16 and to respond similarly well to treatment with biologics when objective signs of inflammation are taken into consideration.17–23 Moreover, significant progress has been made over the past decade to improve identification of both inflammatory and structural SIJ changes on MRI, allowing not only for earlier diagnosis, but also for improved differentiation from alternative reasons of back pain.24–27 However, MRI is currently used in RCTs only for purposes of disease classification.28 29 To investigate the supposed arbitrariness of the current distinction between the two disease states based on conventional radiography, we took advantage of a large observational axSpA cohort in Switzerland19 to assess prediction of important outcomes. Baseline characteristics, spinal radiographic progression and response to tumour necrosis factor inhibitors (TNFis) were compared in three groups of patients: (1) patients with nr-axSpA, (2) patients with r22axSpA and (3) patients with at least unilateral grade 3 sacroiliitis.
Patients and methods
Study population
Patients with axSpA diagnosed by a certified rheumatologist in the Swiss Clinical Quality Management (SCQM) cohort19 were included if they fulfilled the Assessment of Spondyloarthritis International Society classification criteria6 and had an available pelvic radiograph at baseline. The population was divided in three groups according to central SIJ scoring of radiographs (see further): (1) patients with suspicious radiographic sacroiliitis (up to unilateral grade 2 sacroiliitis, nr-axSpA); (2) patients with definitive mild sacroiliitis (r22axSpA); and (3) patients with moderate to severe sacroiliitis (unilateral or bilateral grade 3–4 sacroiliitis, r3+axSpA). Clinical assessments were performed with validated tools30 at baseline, annual and intermediate visits as previously described.19 Enthesitis was assessed with the Maastricht Ankylosing Spondylitis Enthesitis Score, modified to include the proximal insertion of the plantar fascia bilaterally.31 The presence of enthesitis at a specific location was indicated in the online database by the treating rheumatologist clicking on the respective location on a homunculus shown both from front and back. A note adjacent to the homunculus indicates the intensity of the pressure to test for enthesitis: ‘thumb pressure of circa 4 kg, corresponding to the pressure leading to discolouration of a third of the thumbnail’. Start and stop dates of treatment with biologics are indicated by the rheumatologist in the SCQM database. Treatment with non-steroidal anti-inflammatory drugs (NSAIDs) is recorded as a dichotomous variable (yes/no) at every visit.
Effectiveness of treatment with TNFi was analysed in patients starting a first agent after inclusion in SCQM if baseline disease activity information was available. Spinal radiographic progression was assessed in patients with at least two sets of spinal radiographs at 2-year intervals. Research was carried out in compliance with the Helsinki declaration.
SIJ scoring of radiographs
Central reading of sacroiliitis on pelvic radiographs was conducted according to the mNY radiographic criterion: 0=no abnormalities; 1=suspicious changes (no specific abnormalities); 2=minimal sacroiliitis in the form of loss of definition of SIJ, small localised areas of sclerosis or erosion, without alteration of joint width; 3=moderate sacroiliitis (unequivocal abnormalities with one or more of sclerosis, erosive changes, widening, narrowing and partial ankylosis); 4=complete ankylosis of the SIJ.9 It was performed in the SCQM database by two readers out of a pool of eight trained and calibrated readers when the individual X-rays were received. The classification status as radiographic or non-radiographic disease is visible to the treating rheumatologist to potentially inform subsequent treatment decisions. In case of disagreement with regard to radiographic status, a third reader served as an adjudicator. At least two readers had to score a grade 3–4 sacroiliitis in at least one SIJ for a patient to be included in the r3+axSpA group.
Effectiveness of TNFi
With the exception of certolizumab-pegol, used in <1% of patients as a first TNFi, the Swiss label of anti-TNF agents does not differentiate between the radiographic and the non-radiographic disease form, and there is no requirement for the presence of objective signs of inflammation (positive MRI or elevated C reactive protein (CRP)).
Primary outcome was drug retention and observations were censored at the last SCQM visit. In an exploratory analysis in patients with available disease activity measurements at 1 year (±6 months), we also assessed the proportion of patients with a 50% reduction in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50), independently on whether patients had stopped or changed treatment (intention-to-treat analysis).
Spinal radiographic progression
Spinal radiographs were assessed according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS).32 Analyses were performed as previously described (two trained readers blinded to all other data, all available radiographs per patient scored at the same time with known chronology, use of averaged scores per vertebral corner and of an adaptation algorithm for individual missing corners).33 An independent adjudicator scored all radiographs from patients with an absolute difference in mSASSS status scores between the primary readers of ≥5 units in at least one radiographic set. Averaged scores per vertebral corner were used. The score of the primary reader closest to the adjudicator was used in case of adjudication. Syndesmophytes were only counted if both readers agreed on their presence. Radiographic progression was defined as an increase in mSASSS of at least 2 units in 2 years.34 Alternatively, it was defined as the formation of at least one new syndesmophyte and/or new bridges over 2 years.35
Statistical analyses
Baseline characteristics were compared overall between the three groups using Fisher’s exact test for categorical variables and the Kruskal test for continuous variables. The respective two-group comparisons were performed using Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous variables. Significance levels of two-group comparisons were Bonferroni-corrected. TNFi retention was assessed with Kaplan-Meier curves and log-rank test. A multiple adjusted Cox proportional hazards model was used to estimate a covariate-adjusted effect of the r22/r3+/nr-axSpA grouping on drug retention. The following baseline parameters were included in this model: age, sex, diagnostic delay, human leucocyte antigen B27 (HLA-B27), elevated CRP, Bath Ankylosing Spondylitis Mobility Index (BASMI) and Bath Ankylosing Spondylitis Functional Index (BASFI), presence of peripheral arthritis and enthesitis, body mass index group, type of TNFi (etanercept vs anti-TNF antibody). Differences in crude response at 1 year were assessed using the Fisher’s exact test, while logistic regression analysis was used to estimate an adjusted OR for BASDAI50 with the same covariates included as in the retention analysis. The relationship between the r22/3+/nr-axSpA grouping and spinal radiographic progression over time was analysed with binomial generalised estimating equations (GEE).33 An ‘exchangeable’ correlation structure was chosen, as we assumed that each patient had an individual constant level of radiographic progression probability for all time time points, given all covariates. Other correlation structures (‘independence’, ‘unstructured’ and ‘autoregressive’) were investigated in sensitivity analyses, and the resulting coefficients and CIs varied only slightly. The choice of covariates (baseline radiographic damage, sex, ASDAS, treatment with TNFi before the radiographic interval, treatment with NSAIDs at the start of the radiographic interval, current smoking and the duration of the radiographic interval) was informed by our previous investigations of spinal radiographic progression in SCQM.33 36 ASDAS, treatment with NSAIDs and current smoking were missing in 17%, 19% and 14% of the observations, respectively, and imputed using multiple missing value imputation by chained equations (MICE), assuming a missing-at-random data pattern. The algorithm was run for 30 imputations and 30 iterations. Diagnostic measures were used to evaluate the convergence of the MICE algorithm and the distribution of imputed values. The GEE analysis was performed for both complete-case and imputed datasets. R statistical software (R development Core Team, 2011) was used for all analyses.
Results
Baseline characteristic at inclusion
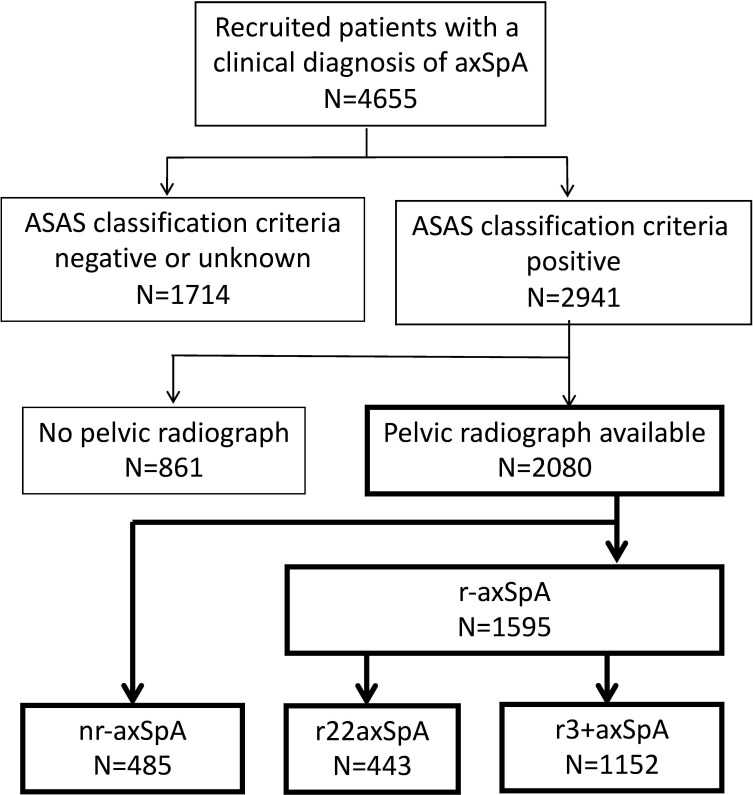
Patient disposition in the SCQM axSpA cohort is depicted in figure 1. From a total of 2080 patients with axSpA fulfilling the inclusion criteria of the current study, 485 (23.3%) were classified as having nr-axSpA, while 1595 had r-axSpA, with 7.9% of patients requiring adjudication of SIJ scoring with regard to classification. Within the r-axSpA group, 443 patients had r22axSpA and 1152 patients had at least a unilateral grade 3 sacroiliitis. Variation of SIJ scoring by the primary readers is shown in the online supplemental figure S1. Characteristics of patients in the three groups at inclusion in the cohort are presented in table 1 with significance levels shown for the overall population as well as for two-group comparisons. The proportion of male patients was higher in groups with more severe sacroiliac structural damage (46%, 57% and 72%, for nr-axSpA, r22axSpA and r3 +axSpA, respectively). A gradual increase was also observed with regard to age and disease duration. However, for several important disease characteristics related to systemic disease activity and more severe axial disease (HLA-B27 positivity, proportion of patients with elevated CRP and height of CRP and ESR elevations, ASDAS, BASFI, hip arthritis and smoking), patients in the r22axSpA group were comparable to patients with nr-axSpA, while both groups differed significantly from the r3+axSpA group. Peripheral disease, either arthritis or enthesitis, was associated with less sacroiliac damage.
Figure 1.
Patient disposition at inclusion in the Swiss Clinical Quality Management cohort. ASAS, Assessment of Spondyloarthritis International Society; axSpA, axial spondyloarthritis; nr-axSpA, non-radiographic axial spondyloarthritis; r22axSpA, bilateral grade 2 sacroiliitis; r3+axSpA, unilateral or bilateral grade 3–4 sacroiliitis.
Table 1.
Characteristics of patients with axial spondyloarthritis at inclusion in the cohort
| Parameter | nr-axSpA N=485 (23.3%) |
r22axSpA N=443 (21.3%) |
r3+axSpA N=1152 (55.4%) |
All N=2080 |
Overall P value |
| Male sex, N (%) | 225 (46.4) | 253 (57.1)† | 829 (72.0)*† | 1307 (62.8) | <0.001 |
| Age (years) | 37.0 (10.9) | 39.3 (11.0)† | 40.8 (11.3)*† | 39.6 (11.2) | <0.001 |
| Age at first symptoms (years) | 28.2 (8.6) | 27.2 (8.0) | 25.4 (8.4)*† | 26.4 (8.4) | <0.001 |
| Disease duration (years) | 8.8 (9.4) | 12.1 (10.7)† | 15.5 (11.1)*† | 13.2 (11.0) | <0.001 |
| Body mass index | 24.9 (4.2) | 25.8 (4.6)† | 25.5 (4.5)† | 25.4 (4.5) | 0.006 |
| Current smoker, N (%) | 131 (31.3) | 120 (31.7) | 417 (42.1)*† | 668 (37.4) | <0.001 |
| Family history of SpA, N (%) | 255 (62.2) | 245 (64.6) | 602 (64.2) | 1102 (63.8) | 0.72 |
| HLA-B27 positivity, N (%) | 334 (74.5) | 286 (71.3) | 853 (82.5)*† | 1472 (78.2) | 0.002 |
| BASFI | 3.0 (2.4) | 3.2 (2.5) | 3.6 (2.6)† | 3.4 (2.6) | <0.001 |
| BASMI | 1.2 (1.2) | 1.6 (1.6)† | 2.6 (2.2)*† | 2.1 (2.0) | <0.001 |
| BASDAI | 5.0 (2.2) | 4.7 (2.3) | 4.6 (2.3) | 4.7 (2.3) | 0.07 |
| ASDAS | 2.9 (0.9) | 2.9 (1.0) | 3.1 (1.1)*† | 3.0 (1.1) | 0.001 |
| Elevated CRP, N (%) | 122 (27.1) | 130 (32.1) | 552 (51.3)*† | 804 (41.6) | <0.001 |
| Elevated CRP >15 mg/L, N (%) | 52 (11.5) | 61 (15.0) | 312 (28.8)*† | 425 (21.9) | <0.001 |
| Elevated CRP >30 mg/L, N (%) | 22 (4.9) | 22 (5.4) | 137 (12.7)*† | 181 (9.3) | <0.001 |
| Elevated ESR >20 mm/hour, N (%) | 76 (17.0) | 92 (23.3) | 381 (35.6)*† | 549 (28.7) | <0.001 |
| Elevated ESR >30 mm/hour, N (%) | 35 (7.8) | 46 (11.7) | 236 (22.1)*† | 317 (16.6) | <0.001 |
| CRP (mg/L), median (IQR) | 3.5 (1; 8) | 5 (2; 9) | 8 (3; 16)*† | 6 (2; 13) | <0.001 |
| Current hip arthritis, N (%) | 36 (7.6) | 29 (6.8) | 204 (18.5)*† | 269 (13.4) | <0.001 |
| Current peripheral arthritis, N (%) | 182 (37.8) | 108 (24.7)† | 353 (31.2)*† | 643 (31.4) | <0.001 |
| Current enthesitis, N (%) | 343 (71.9) | 293 (68.0) | 665 (59.4)*† | 1301 (64.2) | <0.001 |
| Uveitis ever, N (%) | 64 (15.0) | 80 (20.0) | 280 (27.2)*† | 424 (22.8) | <0.001 |
| Psoriasis ever, N (%) | 32 (8.7) | 35 (10.3) | 102 (12.4) | 169 (11.1) | 0.17 |
| Inflammatory bowel disease ever, N (%) | 27 (6.4) | 42 (10.8) | 113 (11.3)† | 182 (10.0) | 0.01 |
| Current tumour necrosis factor inhibitor use, N (%) | 77 (15.9) | 118 (26.6)† | 285 (24.8)† | 480 (23.1) | <0.001 |
| csDMARD use ever, N (%) | 151 (31.1) | 137 (30.9) | 370 (32.2) | 658 (31.7) | 0.84 |
Except where indicated otherwise, values are the mean with corresponding SD in brackets. Significance levels of two-group comparisons are Bonferroni-corrected.
*P<0.15 compared with 22axSpA.
†P<0.15 compared with nr-axSpA.
ASDAS, Ankylosing Spondylitis Disease Activity Score; 22axSpA, axial spondyloarthritis with bilateral grade sacroiliitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Mobility Index; CRP, C reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; HLA-B27, human leucocyte antigen B27; nr-axSpA, non-radiographic axial spondyloarthritis; r3+axSpA, unilateral/bilateral grade 3–4 radiographic sacroiliitis; r22axSpA, bilateral grade 2 sacroiliitis.;
rmdopen-2021-002067supp001.pdf (540.2KB, pdf)
Retention of TNFi
A total of 781 patients started a first TNFi after inclusion in SCQM. The proportion of patients classified as having nr-axSpA, r22axSpA and r3+axSpA and patients characteristics in the three groups were comparable to the whole study population (table 2). After exclusion of patients immediately lost to follow-up, 723 patients (92.6%) were considered in drug retention analyses (online supplemental table S1). The reasons for drug discontinuation were evenly distributed between the three groups. Kaplan-Meier plots of the three groups are depicted in figure 2. Median values for TNFi retention was 1.79 (95% CI 1.09 to 2.89), 2.28 (95% CI 1.72 to 2.82) and 4.67 (95% CI 3.86 to 6.39) years for nr-axSpA, r22axSpA and r3+axSpA, respectively (logrank test p=0.35 for nr-axSpA vs r22axSpA, p<0.001 for nr-axSpA vs r3+axSpA, p<0.001 for r22axSpA vs r3+axSpA). A multiple adjusted Cox proportional model in 476 patients (282 discontinuation events) showed similar differences as in the unadjusted analyses (table 3 and for the full model, online supplemental table S2). The HR for discontinuing the first TNFi was lower in r3+axSpA in comparison to nr-axSpA and to r22axSpA. No evidence for a difference could be detected between nr-axSpA and r22axSpA.
Table 2.
Characteristics of all patients with axial spondyloarthritis at the start of the first tumour necrosis factor inhibitor
| Characteristics | nr-axSpA N=200 |
r22axSpA N=138 |
r3+axSpA N=443 |
All N=781 |
Overall P value |
| Male sex, N (%) | 89 (44.5) | 69 (50.0) | 305 (68.8) | 463 (59.3) | <0.001 |
| Age (years) | 37.3 (11.0) | 38.7 (10.9) | 41.1 (11.8) | 39.7 (11.5) | 0.002 |
| Symptom duration (years) | 9.0 (9.5) | 11.8 (10.5) | 15.1 (11.0) | 12.9 (10.9) | <0.001 |
| HLA-B27 positivity, N (%) | 127 (69.0) | 90 (72.0) | 319 (79.8) | 536 (75.6) | 0.008 |
| Elevated CRP, N (%) | 71 (38.0) | 61 (48.8) | 260 (61.6) | 392 (53.4) | <0.001 |
| BASDAI | 5.9 (1.9) | 5.5 (1.9) | 5.5 (2.1) | 5.6 (2.0) | 0.08 |
| ASDAS | 3.3 (0.8) | 3.3 (0.9) | 3.5 (1.0) | 3.4 (0.9) | 0.02 |
| BASFI | 3.8 (3.5) | 3.9 (2.3) | 4.3 (2.6) | 4.1 (2.5) | 0.04 |
| BASMI | 1.3 (1.2) | 1.7 (1.6) | 2.7 (2.2) | 2.2 (2.0) | <0.001 |
| Current peripheral arthritis, N (%) | 87 (44.6) | 45 (34.1) | 143 (32.8) | 275 (36.0) | 0.02 |
| Current hip arthritis, N (%) | 18 (10.2) | 7 (5.8) | 66 (16.2) | 91 (12.9) | <0.001 |
| Current enthesitis, N (%) | 159 (82.0) | 104 (77.6) | 285 (65.5) | 548 (71.8) | <0.001 |
| Current smokers, N (%) | 50 (29.6) | 41 (34.2) | 162 (42.4) | 253 (37.7) | 0.01 |
| Body mass index | 24.9 (4.4) | 25.7 (4.9) | 25.6 (4.5) | 25.4 (4.5) | 0.10 |
Except where indicated otherwise, values are the mean with corresponding SD in brackets.
ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Mobility Index; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HLA-B27, human leucocyte antigen B27; nr-axSpA, non-radiographic axial spondyloarthritis; r3+axSpA, unilateral grade 3–4 radiographic sacroiliitis; r22axSpA, bilateral grade 2 sacroiliitis.
Figure 2.
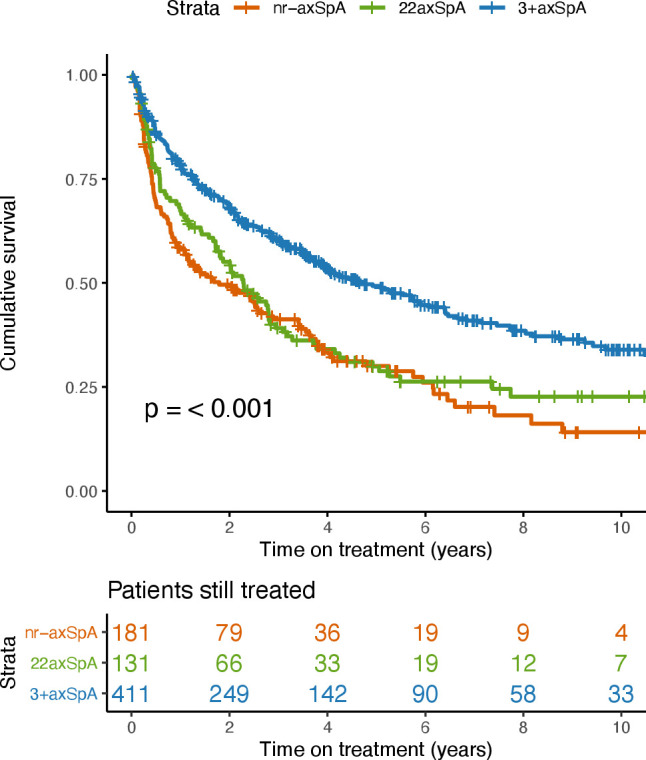
Drug survival of the first TNFi, stratified by classification as nr-axSpA (red), 22axSpA (green) and 3+axSpA (blue). 22axSpA, axial spondyloarthritis with bilateral grade sacroiliitis; 3+axSpA, axial spondyloarthritis with grade 3–4 unilateral sacroiliitis; nr-axSpA, non-radiographic axial spondyloarthritis; TNFi, tumour necrosis factor inhibitor.
Table 3.
Multivariable Cox proportional hazards model for analysis of retention of a first tumour necrosis factor inhibitor in r22axSpA and in patients with r3+axSpA versus nr-axSpA
| Key variable | HR | 95% CI | P value |
| r22axSpA versus nr-axSpA | 0.82 | 0.57 to 1.17 | 0.27 |
| r3+axSpA versus nr-axSpA | 0.60 | 0.44 to 0.82 | 0.001 |
| r3+axSpA versus r22axSpA | 0.73 | 0.53 to 1.02 | 0.06 |
Analysis performed in 476 patients (282 events). Variables included in the model: age, diagnostic delay, sex, human leucocyte antigen-B27, presence of elevated C reactive protein level, presence of peripheral arthritis and of enthesitis, current smoking, Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Mobility Index, treatment with etanercept versus treatment with monoclonal antitumour necrosis factor inhibitor antibodies, body mass index (>30 vs <25 and 25–30 vs <25, respectively). The full model is presented in online supplemental table S2.
nr-axSpA, non-radiographic axial spondyloarthritis; r3+axSpA, unilateral/bilateral grade 3–4 radiographic sacroiliitis; r22axSpA, bilateral grade 2 sacroiliitis.
Treatment response at 1 year
A follow-up consultation with a BASDAI value to assess BASDAI50 responses was available in 595 patients (76.2%) at 1 year. Baseline characteristics of these patients were comparable to those of all patients starting a TNFi and shown in the online supplemental table S3. In crude analyses, no evidence for a difference in BASDAI50 responses could be detected between patients with r22axSpA and nr-axSpA (table 4 and for the full model: online supplemental table S4). In contrast, the BASDAI50 response was significantly higher in r3+axSpA in comparison to nr-axSpA. The results were confirmed in a multiple adjusted BASDAI50 analysis (OR 2.05, 95% CI 1.09 to 3.91, p=0.03 for r3+axSpA vs nr-axSpA and OR 1.28, 95% CI: 0.59 to 2.77, p=0.54 for r22axSpA vs nr-axSpA; table 3). A trend for a better BASDAI50 response was found in r3+axSpA vs r22axSpA (OR 1.61, 95% CI: 0.83 to 3.15, p=0.16).
Table 4.
Association of r22axSpA or r3+axSpA with BASDAI50 response after 1 year of treatment with a first tumour necrosis factor inhibitor in univariable models and a multivariable analysis
| Model | Type of analysis | N | Key variable | OR | 95% CI | P value |
| Model 1 | Univariable | 203 | r22axSpA versus nr-axSpA | 1.22 | 0.67 to 2.21 | 0.57 |
| Model 2 | Univariable | 394 | r3+axSpA versus nr-axSpA | 1.69 | 1.06 to 2.70 | 0.03 |
| Model 3 | Multivariable* | 351 | r22axSpA versus nr-axSpA | 1.28 | 0.59 to 2.77 | 0.54 |
| r3+axSpA versus nr-axSpA | 2.05 | 1.09 to 3.91 | 0.03 |
*Model including the variables age, diagnostic delay, sex, human leucocyte antigen-B27, presence of elevated C reactive protein level, presence of peripheral arthritis, presence of enthesitis, current smoking, Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Mobility Index, respectively, treatment with etanercept versus treatment with monoclonal antitumour necrosis factor antibodies, body mass index (>30 vs <25 and 25–30 vs <25, respectively). The full model is presented in online supplemental table S4.
r3+axSpA, unilateral/bilateral grade 3–4 radiographic sacroiliitis; r22axSpA, bilateral grade 2 sacroiliitis.
Radiographic spinal progression
A total of 505 patients had follow-up sets of radiographs at 2-year intervals to assess spinal radiographic progression (725 radiographic intervals with a maximum of 5 intervals per patient, although the majority of patients (69.7%) presented with only one radiographic interval). Baseline characteristics of this population are shown in online supplemental table S5. The proportion of patients with presence of at least one syndesmophyte was 9.3%, 18.8% and 30.7% for nr-axSpA, r22axSpA and r3+axSpA, respectively (overall p<0.001). Interobserver agreement with regard to change in mSASSS (intraclass correlation coefficient (ICC) 0.85) and the smallest detectable change of radiographic progression over 2 years (1.85 mSASSS units) have been presented previously for the mSASSS of this population.31 Two-year progression of mSASSS in nr-axSpA, r22axSpA and r3+axSpA is depicted in a cumulative probability plot in figure 3. Radiographic progression as defined by at least 2 mSASSS units per 2 year radiographic intervals was observed in a higher proportion of patients with r3+axSpA in comparison to r22axSpA and nr-axSpA. Progression was found in only 5.5% (n=7) of radiographic intervals of patients with 22axSpA (figure 3). In an analysis adjusted for known prognostic factors of radiographic progression, including the baseline spinal damage, higher odds for mSASSS progression were observed for r3+axSpA versus nr-axSpA (OR 2.81, 95% CI 1.00 to 7.89, p=0.05), while no evidence for a difference was found in r22axSpA versus nr-axSpA (OR 1.76, 95% CI 0.54 to 5.69, p=0.35) (table 5 and for the full model: online supplemental table S6). Similar trends were observed with the use of an alternative definition of radiographic progression (development of at least one new syndesmophyte over 2 years) (table 5 and online supplemental table S6).
Figure 3.
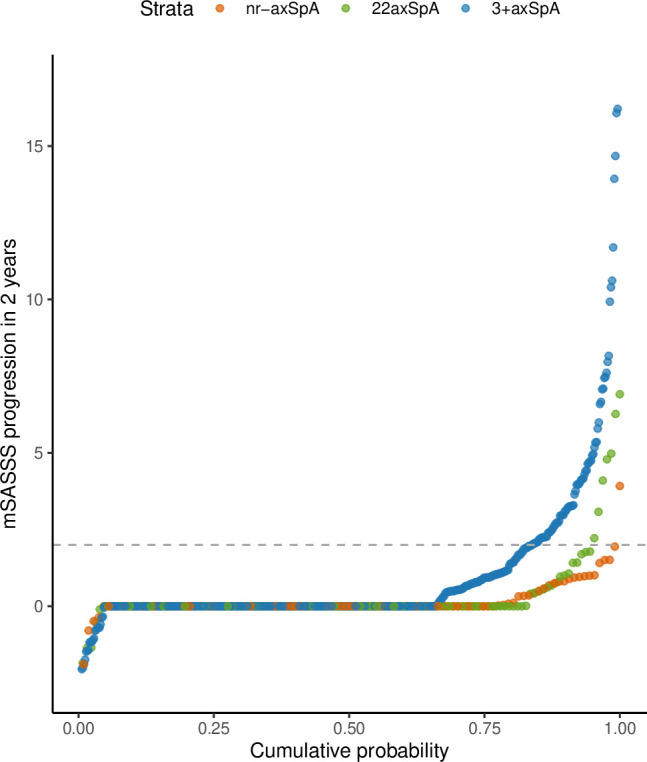
Two-year progression in the mSASSS depicted in a cumulative probability plot. The change in mSASSS values from start to end of individual 2-year radiographic intervals is change for patients with nr-axSpA (red; 86 patients, 107 intervals), for patients with bilateral grade 2 radiographic sacroiliitis (green; 101 patients, 127 intervals) and for patients with unilateral/bilateral grade 3–4 radiographic sacroiliitis (blue; 318 patients, 490 intervals). The horizontal broken line represents the cut-off value of 2 mSASSS points for the definition of progression. 22axSpA, axial spondyloarthritis with bilateral grade sacroiliitis; 3+axSpA, axial spondyloarthritis with grade 3–4 unilateral sacroiliitis; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; nr-axSpA, non-radiographic axial spondyloarthritis.
Table 5.
Association of either r22axSpA or r3+axSpA with spinal radiographic progression
| Type of analysis | Key variable* | Progression defined as ≥2 mSASSS units in 2 years | Progression defined as ≥1 new syndesmophyte in 2 years |
||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Based on multiple imputation | r22axSpA versus nr-axSpA | 1.76 | 0.54 to 5.69 | 0.35 | 0.88 | 0.30 to 2.55 | 0.81 |
| r3+axSpA versus nr-axSpA | 2.81 | 1.00 to 7.89 | 0.05 | 1.60 | 0.68 to 3.75 | 0.28 | |
| Complete case analysis | r22axSpA versus nr-axSpA | 2.67 | 0.56 to 12.7 | 0.22 | 1.31 | 0.33 to 5.12 | 0.70 |
| r3+axSpA versus nr-axSpA | 4.72 | 1.16 to 19.2 | 0.03 | 3.05 | 0.97 to 9.63 | 0.06 | |
Analyses performed in 724 radiographic intervals from 505 patients after multiple imputation of missing data and in 532 intervals from 394 patients in the complete case analysis. The full model is presented in online supplemental table S6.
*Adjustment for Ankylosing Spondylitis Disease Activity Score, sex, tumour necrosis factor inhibitor use prior to the radiographic interval, non-steroidal anti-inflammatory drug use at start of the radiographic interval, current smoking, length of the radiographic interval, as well as structural spinal damage at start of each radiographic interval (either mSASSS or presence of syndesmophytes).
mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; nr-axSpA, nonradiographic axial spondyloarthritis; r3+axSpA, unilateral/bilateral grade 3–4 radiographic sacroiliitis; r22axSpA, bilateral grade 2 radiographic sacroiliitis; r22axSpA, bilateral grade 2 sacroiliitis.
Discussion
According to current consensus, nr-axSpA and r-axSpA represent stages of a same disease.1–3 Treatment recommendations for both entities differ only with regard to the requirement for objective signs of inflammation on SIJ MRI and/or elevated CRP to be present in patients with nr-axSpA for consideration of treatment with biologics,37 38 as no significant response to these agents has been observed in nr-axSpA in the absence of objective inflammatory activity.39 40 Therefore, RCTs of new drugs have to performed in both axSpA disease states. Clinical responses to biologics in RCTs in nr-axSpA are generally lower than for the same therapeutic in RCTs in r-axSpA,41–46 a phenomenon that we have also observed for TNFi in a real-life setting in a previous analysis of the SCQM cohort.19
We demonstrate here that patients with r22axSpA—a subgroup of r-axSpA -perform similarly to patients with nr-axSpA and not like the remainder of patients with r-axSpA with regard to treatment effectiveness and spinal radiographic progression, emphasising the arbitrariness of the current differentiation between the two disease states.15
It is not our intention to suggest that shifting the cut-off between disease states to at least a moderate to severe (grade 3) radiographic sacroiliitis unilaterally would solve the issue, as it would further delay its diagnostic and predictive capacity. A more promising approach for RCTs as well as for day-to-day clinical practice would be to consider the important progress made in the assessment of MRI for diagnostic purposes, which entails simultaneous contextual evaluation of both active and structural SIJ lesions.24 25 The use of MRI in clinical trials is currently mainly restricted to classification purposes, and ‘positivity’ refers to the presence of at least two SIJ subchondral bone marrow oedema (BME) lesions on one slice or a single lesion on two consecutive slices.28 29 While an additional requirement stipulates that these lesions should be ‘highly suggestive’ of axSpA, the latter was not specifically defined. It can reasonably be hypothesised that a more detailed SIJ MRI evaluation with regard to the amount and intensity of inflammation as well as to the presence of different structural lesions would increase its diagnostic capacity and allow a more accurate detection of patients with early axSpA. Indeed, posthoc subgroup analyses in nr-axSpA patients treated with secukinumab have demonstrated that patients with a higher level of SIJ inflammation on baseline MRI experienced better efficacy in comparison to those with more limited SIJ inflammatory lesions.47 The opportunity to accurately detect patients with axSpA with short disease duration and marginal functional impairment bears the potential to select patients with nr-axSpA with early disease that hopefully will demonstrate better treatment responses than those with r-axSpA.48
Mechanical SIJ strain induced during recreational and athletic activities or during pregnancy may mimic inflammation,26 27 thus challenging the diagnosis and classification of axSpA in an unknown proportion of patients, potentially contributing to the lower treatment responses in nr-axSpA. While calibrated expert MRI readers in clinical trials certainly are expected to be able to correctly differentiate between BME lesions related to axSpA and false-positive BME, this might be more difficult to achieve by local radiologists and rheumatologists in a real-life setting.49 Reinforcing ongoing efforts to implement advances in MRI assessment into clinical practice seems of critical relevance.
While, as a major limitation of our analyses, we cannot provide information on the load of inflammation or of structural damage on MRI, patients with r3+axSpA differed from patients with nr-axSpA and r22axSpA when assessing the presence of elevated acute phase reactants as well as the height of the elevations and to factors associated with severity of axial disease and SIJ inflammation (HLA-B27 positivity, smoking and presence of hip arthritis).
Our study should be interpreted in a national context not requiring the presence of objective signs of inflammation to initiate treatment with the majority of TNF inhibitors. The respective drug labels were not formally extended to nr-axSpA with the rationale that Bechterew’s disease is a diagnosis and encompasses both the radiographic and the non-radiographic disease form, while the differentiation between the disease forms relates to classification. This provided the unique opportunity to compare groups of patients with different stages of radiographic structural SIJ damage with regard to several outcomes and confirming the arbitrariness of the current distinction between nr-axSpA and r-axSpA, based on conventional radiography.
Strengths of our study include the use of a large real-life axSpA cohort with available radiographs, as well as the central scoring of the images. The choice to perform a central scoring of pelvic radiographs as soon as each individual radiograph was available is in line with the aim of our study to assess its predictive value and also with the intention to assist rheumatologists to consider patients for treatment with biologics according to international recommendations. The important number of patients included in the individual analyses allowed the adjustment for a multitude of parameters known to potentially affect the different outcomes. The relatively high proportion of patients with r22axSpA (21.3%) in relation to the proportion of patients with nr-axSpA (from bilateral grade 0 to unilateral grade 2 sacroiliitis, 23.3%) might be explained by several factors: on one hand, it cannot be assumed that progression at the SIJ level is linear; on the other hand, patients eligible for biologics are preferentially enrolled in SCQM, as regulatory authorities have called for monitoring patients on expensive drugs. As data on TNF inhibition were not available for nr-axSpA at start of enrolment in SCQM in 2005, more patients with r-axSpA were included in the beginning. Furthermore, our cohort is not an inception cohort and mean disease duration is >10 years, biasing patients towards more important sacroiliac damage.
The observational design of our analyses represents its major limitation, as residual confounding cannot be definitively ruled out.
In conclusion, our data suggest that patients with r22axSpA perform comparably to patients with nr-axSpA, while patients with more severe radiographic sacroiliitis have a better response to treatment yet a higher risk of spinal ankylosis. The data supports the notion that the differentiation between nr-axSpA and r-axSpA is arbitrary and of limited use for clinical decision making. The study also highlights the need to expand the use of MRI in clinical trials to include more detailed information about both active and structural SIJ lesions, instead of relying on disease states based on conventional radiography.
Acknowledgments
We thank all patients and their rheumatologists for participation and the entire Swiss Clinical Quality Management (SCQM) staff for data management and support. A list of rheumatology private practices and hospitals that are contributing to the SCQM registries can be found online (http://wwwscqmch/institutions).
Footnotes
Twitter: @ramicheroli
Contributors: Study conception and design: AC, AS, RB and RM. Acquisition of data: AC, KB, XB, MdH, PE, RB, MJN, BM, PZ, DK, MA, OD and RM. Statistical analysis: SK, ME, EP and AS. All authors contributed to the interpretation of the data. AC wrote the manuscript. All authors critically revised the manuscript and approved the final manuscript to be published. AC accepts full responsibility for the finished work, had access to the data, and controlled the decision to publish.
Funding: The SCQM Foundation is supported by the Swiss Society of Rheumatology and by AbbVie, Amgen, Gilead, iQone Healthcare, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Sanofi Genzyme and UCB.
Competing interests: AC received consulting and/or speaking fees from AbbVie, Eli Lilly, Merck Sharp & Dohme, Novartis and Pfizer. AS received consulting fees from Pfizer and support for attending meetings from Gilead. BM received speaking fees from Jansen and Novartis and support for attending meetings from Pfizer (payments to institution). DK received consulting and/or speaking fees from Abbvie, Gilead, Eli Lilly, Novartis and Pfizer. MJN received consulting and/or speaking fees from Abbvie, Celgene, Eli Lilly, Novartis and Pfizer. OD received consulting and/or speaking fees from Abbvie, Amgen, Eli Lilly and Pfizer. RM received consulting and/or speaking fees from Abbvie, Eli Lilly, Gilead and Pfizer.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethics committee of the Canton of Zurich (KEK-ZH 2014-0439, amendment BASEC PB_2017-00215). Participants gave informed consent to participate in the Swiss Clinical Quality Management cohort before taking part.
References
- 1.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 2.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. 10.1056/NEJMra1406182 [DOI] [PubMed] [Google Scholar]
- 3.van Tubergen A. The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol 2015;11:110–8. 10.1038/nrrheum.2014.181 [DOI] [PubMed] [Google Scholar]
- 4.Baraliakos X, Weisman MH. Spondyloarthritis: the changing Landscape-Are we there yet? Rheum Dis Clin North Am 2020;46:xiii–xiv. 10.1016/j.rdc.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Robinson PC, van der Linden S, Khan MA, et al. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol 2021;17:109–18. 10.1038/s41584-020-00552-4 [DOI] [PubMed] [Google Scholar]
- 6.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis International Society classification criteria for axial spondyloarthritis (Part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 7.Sieper J, van der Heijde D. Review: Nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum 2013;65:543–51. 10.1002/art.37803 [DOI] [PubMed] [Google Scholar]
- 8.Boel A, Molto A, van der Heijde D, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. 10.1136/annrheumdis-2019-215707 [DOI] [PubMed] [Google Scholar]
- 9.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the new York criteria. Arthritis Rheum 1984;27:361–8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 10.Bennett PH, Burch TA. Population studies of the rheumatic diseases. Amsterdam. Excerpta MEDICA Foundation. International congress series 1966;148:456–7. [Google Scholar]
- 11.Deodhar A, Reveille JD, van den Bosch F, et al. The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the assessment of spondyloarthritis International Society in response to the US food and drug administration's comments and concerns. Arthritis Rheumatol 2014;66:2649–56. 10.1002/art.38776 [DOI] [PubMed] [Google Scholar]
- 12.Hollingsworth PN, Cheah PS, Dawkins RL, et al. Observer variation in grading sacroiliac radiographs in HLA-B27 positive individuals. J Rheumatol 1983;10:247–54. [PubMed] [Google Scholar]
- 13.Yazici H, Turunç M, Ozdoğan H, et al. Observer variation in grading sacroiliac radiographs might be a cause of 'sacroiliitis' reported in certain disease states. Ann Rheum Dis 1987;46:139–45. 10.1136/ard.46.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tubergen A, Heuft-Dorenbosch L, Schulpen G, et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis 2003;62:519–25. 10.1136/ard.62.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelena X, Marzo-Ortega H. Axial spondyloarthritis: time to stop the split 10 years on. Nat Rev Rheumatol 2020;16:5–6. 10.1038/s41584-019-0331-6 [DOI] [PubMed] [Google Scholar]
- 16.López-Medina C, Ramiro S, van der Heijde D, et al. Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 2019;5:e001108. 10.1136/rmdopen-2019-001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song I-H, Weiß A, Hermann K-GA, et al. Similar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trial. Ann Rheum Dis 2013;72:823–5. 10.1136/annrheumdis-2012-202389 [DOI] [PubMed] [Google Scholar]
- 19.Ciurea A, Scherer A, Exer P, et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. 10.1002/art.38140 [DOI] [PubMed] [Google Scholar]
- 20.Wallman JK, Kapetanovic MC, Petersson IF, et al. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients--baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res Ther 2015;17:378. 10.1186/s13075-015-0897-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corli J, Flipo R-M, Philippe P, et al. Tumor necrosis factor-α inhibition in ankylosing spondylitis and Nonradiographic axial spondyloarthritis: treatment response, drug survival, and patient outcome. J Rheumatol 2015;42:2376–82. 10.3899/jrheum.150372 [DOI] [PubMed] [Google Scholar]
- 22.Glintborg B, Sørensen IJ, Østergaard M, et al. Ankylosing spondylitis versus nonradiographic axial spondyloarthritis: comparison of tumor necrosis factor inhibitor effectiveness and effect of HLA-B27 status. An observational cohort study from the nationwide DANBIO registry. J Rheumatol 2017;44:59–69. 10.3899/jrheum.160958 [DOI] [PubMed] [Google Scholar]
- 23.Michelena X, Zhao SS, Dubash S, et al. Similar biologic drug response regardless of radiographic status in axial spondyloarthritis: data from the British Society for rheumatology biologics register in ankylosing spondylitis registry. Rheumatology 2021;60:5795–800. 10.1093/rheumatology/keab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maksymowych WP, Lambert RG, Østergaard M, et al. Mri lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI Working group. Ann Rheum Dis 2019;78:1550–8. 10.1136/annrheumdis-2019-215589 [DOI] [PubMed] [Google Scholar]
- 25.Maksymowych WP, Lambert RG, Baraliakos X, et al. Data-Driven definitions for active and structural MRI lesions in the sacroiliac joint in spondyloarthritis and their predictive utility. Rheumatology 2021;60:4778–89. 10.1093/rheumatology/keab099 [DOI] [PubMed] [Google Scholar]
- 26.Weber U, Jurik AG, Zejden A, et al. Frequency and Anatomic Distribution of Magnetic Resonance Imaging Features in the Sacroiliac Joints of Young Athletes: Exploring "Background Noise" Toward a Data-Driven Definition of Sacroiliitis in Early Spondyloarthritis. Arthritis Rheumatol 2018;70:736–45. 10.1002/art.40429 [DOI] [PubMed] [Google Scholar]
- 27.Renson T, Depicker A, De Craemer A-S, et al. High prevalence of spondyloarthritis-like MRI lesions in postpartum women: a prospective analysis in relation to maternal, child and birth characteristics. Ann Rheum Dis 2020;79:929–34. 10.1136/annrheumdis-2020-217095 [DOI] [PubMed] [Google Scholar]
- 28.Rudwaleit M, Jurik AG, Hermann K-GA, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. 10.1136/ard.2009.110767 [DOI] [PubMed] [Google Scholar]
- 29.Lambert RGW, Bakker PAC, van der Heijde D, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI Working group. Ann Rheum Dis 2016;75:1958–63. 10.1136/annrheumdis-2015-208642 [DOI] [PubMed] [Google Scholar]
- 30.Sieper J, Rudwaleit M, Baraliakos X, et al. The assessment of spondyloarthritis International Society (ASAS) Handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68 Suppl 2:ii1–44. 10.1136/ard.2008.104018 [DOI] [PubMed] [Google Scholar]
- 31.Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. 10.1136/ard.62.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creemers MCW, Franssen MJAM, van't Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. 10.1136/ard.2004.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molnar C, Scherer A, Baraliakos X, et al. Tnf blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss clinical quality management cohort. Ann Rheum Dis 2018;77:63–9. 10.1136/annrheumdis-2017-211544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramiro S, van Tubergen A, Stolwijk C, et al. Scoring radiographic progression in ankylosing spondylitis: should we use the modified Stoke ankylosing spondylitis spine score (mSASSS) or the radiographic ankylosing spondylitis spinal score (RASSS)? Arthritis Res Ther 2013;15:R14. 10.1186/ar4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Tubergen A, Ramiro S, van der Heijde D, et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 2012;71:518–23. 10.1136/annrheumdis-2011-200411 [DOI] [PubMed] [Google Scholar]
- 36.Hebeisen M, Micheroli R, Scherer A, et al. Spinal radiographic progression in axial spondyloarthritis and the impact of classification as nonradiographic versus radiographic disease: data from the Swiss clinical quality management cohort. PLoS One 2020;15:e0230268. 10.1371/journal.pone.0230268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 38.Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis association of America/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:1599–613. 10.1002/art.41042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haibel H, Rudwaleit M, Listing J, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91. 10.1002/art.23606 [DOI] [PubMed] [Google Scholar]
- 40.Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015;67:2702–12. 10.1002/art.39257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis JC, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230–6. 10.1002/art.11325 [DOI] [PubMed] [Google Scholar]
- 42.Dougados M, van der Heijde D, Sieper J, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014;66:2091–102. 10.1002/art.38721 [DOI] [PubMed] [Google Scholar]
- 43.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. 10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 44.Deodhar A, Blanco R, Dokoupilova E. Secukinumab improves signs and symptoms of nonradiographic axial spondyloarthritis: primary results of a randomized controlled phase III study. Arthritis Rheumatol 2021;73:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018;392:2441–51. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- 46.Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020;395:53–64. 10.1016/S0140-6736(19)32971-X [DOI] [PubMed] [Google Scholar]
- 47.Braun J, Blanco R, Marzo-Ortega H, et al. Secukinumab in non-radiographic axial spondyloarthritis: subgroup analysis based on key baseline characteristics from a randomized phase III study, prevent. Arthritis Res Ther 2021;23:231. 10.1186/s13075-021-02613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baraliakos X, Koenig AS, Jones H, et al. Predictors of clinical remission under anti-tumor necrosis factor treatment in patients with ankylosing spondylitis: pooled analysis from large randomized clinical trials. J Rheumatol 2015;42:1418–26. 10.3899/jrheum.141278 [DOI] [PubMed] [Google Scholar]
- 49.Maksymowych WP, Pedersen SJ, Weber U, et al. Central reader evaluation of MRI scans of the sacroiliac joints from the ASAS classification cohort: discrepancies with local readers and impact on the performance of the ASAS criteria. Ann Rheum Dis 2020;79:935–42. 10.1136/annrheumdis-2020-217232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002067supp001.pdf (540.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.