Abstract
Background
Despite unprecedented benefit from immune checkpoint inhibitors (ICIs) in patients with mismatch repair deficient (dMMR)/microsatellite instability high (MSI-H) advanced gastrointestinal cancers, a relevant proportion of patients shows primary resistance or short-term disease control. Since malignant effusions represent an immune-suppressed niche, we investigated whether peritoneal involvement with or without ascites is a poor prognostic factor in patients with dMMR/MSI-H metastatic colorectal cancer (mCRC) and gastric cancer (mGC) receiving ICIs.
Methods
We conducted a global multicohort study at Tertiary Cancer Centers and collected clinic-pathological data from a cohort of patients with dMMR/MSI-H mCRC treated with anti-PD-(L)1 ±anti-CTLA-4 agents at 12 institutions (developing set). A cohort of patients with dMMR/MSI-high mGC treated with anti-PD-1 agents±chemotherapy at five institutions was used as validating dataset.
Results
The mCRC cohort included 502 patients. After a median follow-up of 31.2 months, patients without peritoneal metastases and those with peritoneal metastases and no ascites had similar outcomes (adjusted HR (aHR) 1.15, 95% CI 0.85 to 1.56 for progression-free survival (PFS); aHR 0.96, 95% CI 0.65 to 1.42 for overall survival (OS)), whereas inferior outcomes were observed in patients with peritoneal metastases and ascites (aHR 2.90, 95% CI 1.70 to 4.94; aHR 3.33, 95% CI 1.88 to 5.91) compared with patients without peritoneal involvement. The mGC cohort included 59 patients. After a median follow-up of 17.4 months, inferior PFS and OS were reported in patients with peritoneal metastases and ascites (aHR 3.83, 95% CI 1.68 to 8.72; aHR 3.44, 95% CI 1.39 to 8.53, respectively), but not in patients with only peritoneal metastases (aHR 1.87, 95% CI 0.64 to 5.46; aHR 2.15, 95% CI 0.64 to 7.27) when compared with patients without peritoneal involvement.
Conclusions
Patients with dMMR/MSI-H gastrointestinal cancers with peritoneal metastases and ascites should be considered as a peculiar subgroup with highly unfavorable outcomes to current ICI-based therapies. Novel strategies to target the immune-suppressive niche in malignant effusions should be investigated, as well as next-generation ICIs or intraperitoneal approaches.
Keywords: immunotherapy, gastrointestinal neoplasms, translational medical research, tumor biomarkers
Introduction
Although mismatch repair deficient (dMMR) and/or microsatellite instability high (MSI-H) advanced gastrointestinal cancers are relatively uncommon (about 4% of both metastatic colorectal cancer (mCRC) and gastric cancer (mGC)), immune checkpoint inhibitors (ICIs) have reliably demonstrated durable responses and unprecedented survival outcomes in this patients’ population independent from the tumor site of origin.1 ICIs have become a new standard of care for patients with dMMR/MSI-H cancers, and their use is nowadays supported by randomized clinical trials (RCTs).2–4 Nevertheless, about 30% of patients with MSI/dMMR advanced mCRC display intrinsic resistance to anti-PD(L)-1 monotherapy both in phase II proof-of-concept trials conducted in pretreated patients,5 6 and in the Keynote-177 phase III first-line trial.2 A similar percentage of primary resistance was reported in a non-colorectal cohort and in post hoc analyses of RCT in mGC.7–9
Of note, the occurrence of early progressive disease (PD) at first disease reassessment may be explained by the phenomenon of pseudoprogression,10 or by the misdiagnosis of dMMR/MSI-H status.11 However, several promising studies investigated the association of primary resistance with specific biomarkers, including a relatively lower tumor mutational burden,12 13 dMMR/MSI-H heterogeneity,13 and elevated systemic inflammation as assessed by blood-based parameters.14
Malignant ascites is a common clinical complication in patients with peritoneal metastases from gastrointestinal cancers. Despite the well-established poor prognostic effect of both peritoneal involvement and ascites in patients with several types of advanced cancers, malignant ascites is also characterized by a pervasive immunosuppressive microenvironment.15 16 Indeed, serous cavities are an immunologic niche due to a variety of immunosuppressive networks, such as those driven by cavity-resident macrophages with Tim-4 overexpression.17 Drawing from these considerations, we hypothesized that patients with MSI-H cancers and coexisting malignant ascites may have poorer outcomes and high rates of primary resistance to ICI treatment. To examine this hypothesis, we assembled a large multinational cohort of patients with dMMR/MSI-H mCRC or GC receiving ICIs to investigate the prognostic role of peritoneal involvement with or without ascites.
Methods
Patients’ population
This was a retrospective, multicentric, international study that included patients with advanced dMMR/MSI-H CRC or GC treated with ICI-based therapy from June 2014 to May 2021. We assembled two cohorts of dMMR/MSI-H gastrointestinal cancers consisting of mCRC (developing set or cohort 1) and mGC (validating set or cohort 2) taking advantage of the electronic medical records of the participating centers. Inclusion criteria were: (1) pathologically/cytologically confirmed diagnosis of dMMR/MSI-H CRC or GC, with dMMR/MSI-H status confirmed by local institutional testing per international guidelines18; (2) treatment with at least one cycle of ICI-based therapy for advanced disease within clinical trials or per clinical practice; (3) age ≥18 years. Cohort 1 contained patients with dMMR/MSI-H mCRC treated with anti-PD-(L)1±anti CTLA-4 agents at 12 institutions worldwide. Cohort 2 contained patients with dMMR/MSI-H mGC treated with anti-PD-1±chemotherapy at five institutions worldwide. Information about clinical outcomes of ICI-based treatment (ie, progression-free survival (PFS) and overall survival (OS)) as well as the following demographic and clinico-pathological data (baseline to the initiation of the ICI-based treatment) were retrieved for all the patients included: age, sex, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), primary tumor resection, presence of synchronous metastases, number and localization of metastases, presence of peritoneal involvement and ascites, information about prior systemic treatment for metastatic disease, type of ICI-based treatment. For patients with dMMR/MSI-H mCRC the following disease-specific data were also retrieved when available: primary tumor sidedness, RAS mutational status and BRAF mutational status. For patients with dMMR/MSI-H mGC the following disease-specific data were also retrieved when available: primary site of origin and histology.
The presence of peritoneal involvement and ascites was assessed by means of conventional imaging techniques (ie, abdominal CT scan or MRI) at each participating center. The presence of metastatic peritoneal involvement was coded as a polytomous categorical variable encompassing three values: no peritoneal metastases, peritoneal metastases without ascites, peritoneal metastases with ascites. Patients with evidence of peritoneal metastases with ascites were included in the ‘ascites’ group, whereas patients with no peritoneal metastases or peritoneal metastases without ascites were included in the ‘no ascites’ group.
Statistical analyses
PFS was defined as the time from the first dose of ICIs administration to PD or death from any cause, whichever occurred first. OS was defined as the time from the first dose of ICIs administration to death from any cause. To examine baseline differences between groups, Pearson’s χ2 test or Fisher’s exact test were used, as appropriate. To examine baseline differences according to the metastatic peritoneal involvement coded as a polytomous categorical variable, pairwise Fisher’s exact test was used. Survival analyses were performed using the Kaplan-Meier method and Cox proportional hazards regression. Univariable Cox regression analysis was used to assess the effect of different baseline factors on PFS and OS. Variables significantly associated with survival outcomes at the univariable analysis were then fitted in a multivariable Cox proportional hazards regression model to identify independent predictors of OS and PFS. HRs with the corresponding 95% CIs were provided for Cox’s proportional hazards regression models. All statistical tests were two tailed, and a p<0.05 was considered statistically significant. Statistical analyses were performed using R software (V.3.5.0) and R Studio (V.1.1.447).
Results
Patients’ characteristics
Cohort 1 included 502 patients with dMMR/MSI-H mCRC from 12 institutions (online supplemental table 1). About 60% of the patients included in cohort 1 received the ICI-based treatment within clinical trials. Clinico-pathological and treatment characteristics are reported in table 1, both overall and according to the presence or absence of ascites. Briefly, peritoneal involvement without ascites was present in 172 patients (34.3%), whereas ascites was present in 25 patients (5.0%). The presence of ascites was associated with poorer PS and RAS wild-type/BRAF mutated status. Online supplemental table 2 shows the pairwise analysis of all characteristics according to the three subgroups of patients with peritoneal involvement with or without ascites and those without peritoneal involvement.
Table 1.
Patients and disease characteristics in the developing set (Cohort 1: dMMR/MSI-high mCRC), overall and according to the presence or absence of ascites
| Characteristics | Total (N=502) N (%) |
No ascites (N=477) N (%) |
Ascites (N=25) N (%) |
P value |
| Sex | 0.511 | |||
| Female | 229 (45.6) | 216 (45.3) | 13 (52.0) | |
| Male | 273 (54.4) | 261 (54.7) | 12 (48.0) | |
| Age | 0.301 | |||
| <70 | 378 (75.3) | 357 (74.8) | 21 (84.0) | |
| ≥70 | 124 (24.7) | 120 (25.2) | 4 (16.0) | |
| ECOG PS | 0.005 | |||
| 0 | 237 (47.2) | 232 (48.6) | 5 (20.0) | |
| ≥1 | 265 (52.8) | 245 (51.4) | 20 (80.0) | |
| Primary tumor resection | >0.999 | |||
| No | 90 (17.9) | 86 (18.0) | 4 (16.0) | |
| Yes | 412 (82.1) | 391 (81.9) | 21 (84.0) | |
| Primary tumor sidedness | 0.267 | |||
| Left | 172 (34.3) | 166 (34.8) | 6 (24.0) | |
| Right | 330 (65.7) | 311 (65.2) | 19 (76.0) | |
| NA | ||||
| RAS mutational status | 0.048 | |||
| All wild type | 317 (63.1) | 296 (62.1) | 21 (84.0) | |
| RAS mutated | 165 (32.9) | 161 (33.7) | 4 (16.0) | |
| NA | 20 (4.0) | 20 (4.2) | 0 (0.0) | |
| BRAF mutational status | 0.054 | |||
| All wild-type | 351 (69.9) | 337 (70.6) | 14 (56.0) | |
| BRAF mutated | 132 (26.3) | 121 (25.4) | 11 (44.0) | |
| NA | 19 (3.8) | 19 (4.0) | 0 (0.0) | |
| Synchronous metastases | 0.203 | |||
| No | 242 (48.2) | 233 (48.9) | 9 (36) | |
| Yes | 258 (51.4) | 242 (50.7) | 16 (64) | |
| NA | 2 (0.4) | 2 (0.4) | 0 (0) | |
| Liver metastases | 0.246 | |||
| No | 306 (61.0) | 288 (60.4) | 18 (72.0) | |
| Yes | 196 (39.0) | 189 (39.6) | 7 (28.0) | |
| Lung metastases | 0.595 | |||
| No | 412 (82.1) | 390 (81.8) | 22 (88.0) | |
| Yes | 90 (17.9) | 87 (18.2) | 3 (12.0) | |
| Lymphnodal metastases | 0.521 | |||
| No | 210 (41.8) | 198 (41.5) | 12 (48.0) | |
| Yes | 292 (58.2) | 279 (58.5) | 13 (52.0) | |
| Bone metastases | 0.320 | |||
| No | 479 (95.4) | 456 (95.6) | 23 (92.0) | |
| Yes | 23 (4.6) | 21 (4.4) | 2 (8.0) | |
| No of metastatic sites | 0.076 | |||
| 1 | 227 (45.2) | 220 (46.1) | 7 (28.0) | |
| ≥2 | 275 (54.8) | 257 (53.9) | 18 (72.0) | |
| Prior treatment for metastatic disease | 0.195 | |||
| No | 96 (19.1) | 94 (19.7) | 2 (8.0) | |
| Yes | 406 (80.9) | 383 (80.3) | 23 (92.0) | |
| ICI regimen | 0.525 | |||
| a-PD(L)−1 | 332 (66.1) | 314 (65.8) | 18 (72.0) | |
| a-PD-1+a-CTLA-4 | 170 (33.9) | 163 (34.2) | 7 (8.0) |
P-values marked with bold indicate statistically significant p-values.
dMMR, mismatch repair deficient; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; mCRC, metastatic colorectal cancer; MSI-H, microsatellite instability high; NA, not available; PS, Performance Status.
jitc-2021-004001supp001.pdf (40.7KB, pdf)
jitc-2021-004001supp002.pdf (84.9KB, pdf)
Cohort 2 included 59 patients with dMMR/MSI-high mGC from 5 institutions (online supplemental table 3). About 30% of the patients included in cohort 2 received the ICI-based treatment within clinical trials. Clinico-pathological and treatment characteristics are reported in table 2, both overall and according to the presence or absence of ascites. Peritoneal involvement without ascites was present in 11 patients (18.6%), whereas ascites was present in 17 patients (28.8%). Of note, ascites was significantly associated with poorer PS, non-resected primary tumor, synchronous presentation of metastases and >1 sites of metastases.
Table 2.
Patients and disease characteristics in the validating set (Cohort 2: dMMR/MSI-high mGC), overall and according to the presence or absence of ascites
| Characteristics | Total (N=59) N (%) |
No ascites (N=42) N (%) |
Ascites (N=17) N (%) |
P value |
| Sex | 0.840 | |||
| Female | 22 (37.3) | 16 (38.1) | 6 (35.3) | |
| Male | 37 (62.7) | 26 (61.9) | 11 (64.7) | |
| Age | 0.304 | |||
| <70 | 32 (54.2) | 21 (50.0) | 11 (64.7) | |
| ≥70 | 27 (45.8) | 21 (50.0) | 6 (35.3) | |
| ECOG PS | 0.047 | |||
| 0 | 18 (30.5) | 16 (38.1) | 2 (11.8) | |
| ≥1 | 41 (69.5) | 26 (61.9) | 15 (88.2) | |
| Primary tumor resection | 0.029 | |||
| No | 32 (54.2) | 19 (45.2) | 13 (76.5) | |
| Yes | 27 (45.8) | 23 (54.8) | 4 (23.5) | |
| Primary site of origin | 0.662 | |||
| Gastroesophageal junction (GEJ) | 6 (10.2) | 5 (11.9) | 1 (5.9) | |
| Gastric | 53 (89.8) | 37 (88.1) | 16 (94.1) | |
| Histology | 0.195 | |||
| Intestinal | 43 (72.9) | 33 (78.6) | 10 (58.8) | |
| Diffuse/other | 16 (27.1) | 9 (21.4) | 7 (41.2) | |
| Synchronous metastases | 0.033 | |||
| No | 23 (39.0) | 20 (47.6) | 3 (17.6) | |
| Yes | 36 (61.0) | 22 (52.3) | 14 (82.4) | |
| Liver metastases | 0.310 | |||
| No | 45 (76.3) | 30 (71.4) | 15 (88.2) | |
| Yes | 14 (23.7) | 12 (28.6) | 2 (11.8) | |
| Lymphnodal metastases | >0.999 | |||
| No | 13 (22.0) | 9 (21.4) | 4 (23.5) | |
| Yes | 46 (78.0) | 33 (78.6) | 13 (76.5) | |
| Lung metastases | >0.999 | |||
| No | 49 (83.1) | 35 (83.3) | 14 (82.4) | |
| Yes | 10 (16.9) | 7 (16.7) | 3 (17.6) | |
| Bone metastases | 0.620 | |||
| No | 54 (91.5) | 39 (92.9) | 15 (88.2) | |
| Yes | 5 (8.5) | 3 (7.1) | 2 (11.8) | |
| No of metastatic sites | 0.013 | |||
| 1 | 17 (28.8) | 16 (38.1) | 1 (5.9) | |
| ≥2 | 42 (71.2) | 26 (61.9) | 16 (94.1) | |
| Prior treatment for metastatic disease | >0.999 | |||
| No | 7 (11.9) | 5 (11.9) | 2 (11.8) | |
| Yes | 52 (88.1) | 37 (88.1) | 15 (88.2) | |
| ICI regimen | 0.308 | |||
| a-PD-1 | 54 (91.5) | 37 (88.1) | 17 (100) | |
| a-PD-1+chemotherapy | 5 (8.5) | 5 (11.9) | 0 (0) |
P-values marked with bold indicate statistically significant p-values.
dMMR, mismatch repair deficient; ECOG, Eastern Cooperative Oncology Group; ICIs, immune checkpoint inhibitors; mGC, metastatic gastric cancer; MSI, microsatellite instability; PS, Performance Status.
jitc-2021-004001supp003.pdf (39.4KB, pdf)
Survival outcomes according to the presence of peritoneal involvement and ascites
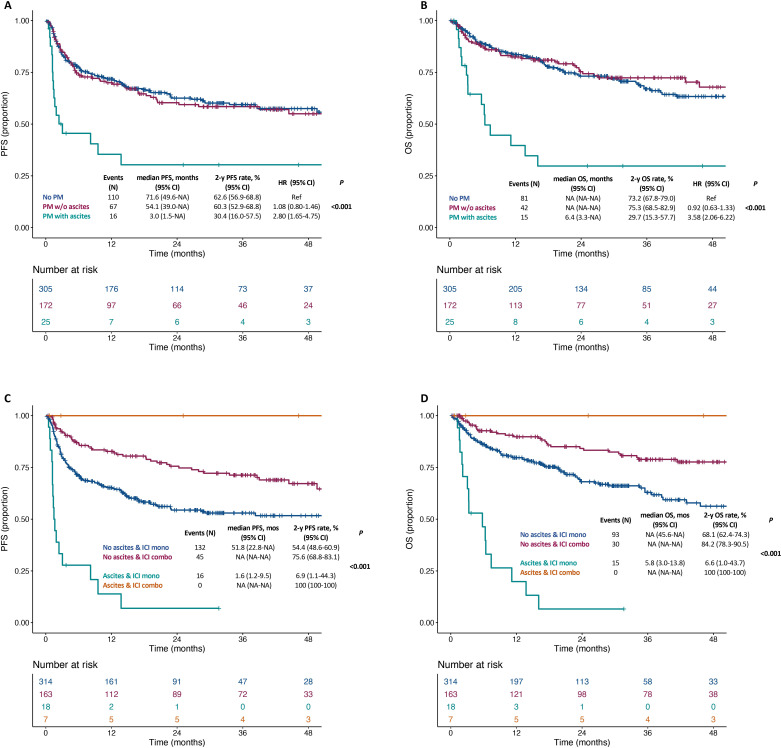
In cohort 1, the median follow-up time was 31.2 months (IQR 15.6–46.0). Online supplemental figure 1A, B shows PFS and OS in the overall population. The presence of ascites was significantly associated with shorter PFS and OS (both p<0.001; figure 1A, B). Patients without peritoneal metastases had similar PFS and OS to those with peritoneal metastases and no ascites (2-year PFS rate: 62.6% vs 60.3%; HR 1.08, 95% CI 0.80 to 1.46; 2 year OS rate: 73.2% vs 75.3%; HR 0.92, 95% CI 0.63 to 1.33), whereas poorer PFS and OS were restricted to patients with peritoneal metastases and ascites (2-year PFS rate: 30.4%; HR 2.80, 95% CI 1.65 to 4.75; 2-year OS rate: 29.7%, HR 3.58, 95% CI 2.06 to 6.22). table 3 shows the univariable and multivariable models for PFS and OS: presence of ascites had an independent effect on both survival outcomes (adjusted HR for PFS: 2.90 (95% CI 1.70 to 4.94); for OS: 3.33 (95% CI 1.88 to 5.91)), as well as ECOG PS, presence of lung metastases, prior treatment for advanced disease and ICI treatment type (anti-PD(L)-1 monotherapy or anti-CTLA-4 combination). We then investigated the impact of specific ICI approaches (combination with CTLA-4 vs PD-1 monotherapy) and observed that shorter PFS and OS outcomes were restricted to patients with ascites receiving anti-PD(L)-1 monotherapy (median and 2-year PFS: 1.6 months and 6.9%; median and 2-year OS: 5.8 months and 6.6%; figure 1C, D). Patients without peritoneal metastases had similar PFS and OS to those with peritoneal metastases and no ascites, regardless of the specific ICI approach (online supplemental figure 2).
Figure 1.
Kaplan-Meier curves for progression-free survival (A) and overall survival (B) in the subgroups of dMMR/MSI-H mCRC patients without peritoneal metastases, with peritoneal metastases and no ascites or with peritoneal metastases and ascites; Kaplan-Meier curves for progression-free survival (C) and overall survival (D) of patients dMMR/MSI-H mCRC according to the presence of ascites and the type of ICI regimen. dMMR, mismatch repair deficient; ICI, immune checkpoint inhibitors; mCRC, metastatic colorectal cancer; MSI-H, microsatellite instability high; NA, not available; OS, overall survival; PFS, progression-free survival.
Table 3.
Univariable and multivariable Cox proportional hazards regression models for PFS and OS in the developing set (Cohort 1: dMMR/MSI-high mCRC)
| Characteristics | PFS | OS | ||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | 0.673 | 0.644 | ||||||
| Female | Ref | Ref | ||||||
| Male | 1.06 (0.80 to 1.41) | 1.08 (0.77 to 1.52) | ||||||
| Age (years) | 0.723 | 0.588 | ||||||
| <70 | Ref | Ref | ||||||
| ≥70 | 1.061 (0.77 to 1.47) | 1.11 (0.76 to 1.63) | ||||||
| ECOG S | 0.001 | 0.003 | <0.001 | 0.001 | ||||
| 0 | Ref | Ref | Ref | Ref | ||||
| ≥1 | 1.608 (1.20 to 2.15) | 1.56 (1.16 to 2.08) | 1.79 (1.30 to 2.48) | 1.78 (1.25 to 2.55) | ||||
| Primary tumor resection | 0.802 | 0.317 | ||||||
| No | Ref | Ref | ||||||
| Yes | 1.05 (0.72 to 1.54) | 1.281 (0.79 to 2.08) | ||||||
| Primary tumor sidedness | 0.917 | 0.342 | ||||||
| Left | Ref | Ref | ||||||
| Right | 1.02 (0.75 to 1.37) | 0.85 (0.60 to 1.20) | ||||||
| RAS mutational status | 0.724 | 0.459 | ||||||
| All wild-type | Ref | Ref | ||||||
| RAS mutated | 0.95 (0.70 to 1.28) | 0.87 (0.61 to 1.25) | ||||||
| BRAF mutational status | 0.196 | 0.151 | ||||||
| All wild-type | Ref | Ref | ||||||
| BRAF mutated | 1.23 (0.90 to 1.67) | 1.303 (0.91 to 1.87) | ||||||
| Synchronous metastases | 0.737 | 0.460 | ||||||
| No | Ref | Ref | ||||||
| Yes | 1.05 (0.79 to 1.39) | 1.14 (0.81 to 1.59) | ||||||
| Liver metastases | 0.221 | 0.105 | ||||||
| No | Ref | Ref | ||||||
| Yes | 1.20 (0.90 to 1.59) | 1.32 (0.94 to 1.85) | ||||||
| Lung metastases | <0.001 | <0.001 | 0.001 | 0.023 | ||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.812 (1.31 to 2.50) | 1.79 (1.29 to 2.48) | 1.83 (1.26 to 2.66) | 1.60 (1.06 to 2.40) | ||||
| Lymphnodal metastases | 0.249 | 0.885 | ||||||
| No | Ref | Ref | ||||||
| Yes | 0.85 (0.64 to 1.13) | 1.03 (0.73 to 1.45) | ||||||
| Bone metastases | 0.085 | 0.011 | 0.078 | |||||
| No | Ref | Ref | Ref | |||||
| Yes | 1.67 (0.93 to 3.01) | 2.22 (1.20 to 4.12) | 1.77 (0.94 to 3.35) | |||||
| No of metastatic sites | 0.078 | 0.013 | 0.356 | |||||
| 1 | Ref | Ref | Ref | |||||
| ≥2 | 1.30 (0.97 to 1.74) | 1.56 (1.10 to 2.22) | 1.21 (0.81 to 1.80) | |||||
| Prior systemic treatment for metastatic disease | 0.001 | 0.015 | 0.007 | 0.0563 | ||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes | 2.061 (1.31 to 3.24) | 1.76 (1.12 to 2.79) | 2.13 (1.23 to 3.70) | 1.73 (0.99 to 3.03) | ||||
| ICI regimen | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| a-PD(L)−1 | Ref | Ref | Ref | Ref | ||||
| a-PD-1+a-CTLA-4 | 0.46 (0.33 to 0.64) | 0.46 (0.33 to 0.64) | 0.41 (0.27 to 0.62) | 0.41 (0.27 to 0.62) | ||||
| Peritoneal metastases | <0.001 | 0.002 | <0.001 | 0.001 | ||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes, without ascites | 1.08 (0.80 to 1.46) | 1.15 (0.85 to 1.56) | 0.92 (0.63 to 1.33) | 0.96 (0.65 to 1.42) | ||||
| Yes, with ascites | 2.80 (1.65 to 4.75) | 2.90 (1.70 to 4.94) | 3.58 (2.06 to 6.22) | 3.33 (1.88 to 5.91) | ||||
P-values marked with bold indicate statistically significant p-values.
dMMR, mismatch repair deficient; ECOG, Eastern Cooperative Oncology Group; ICIs, immune checkpoint inhibitors; mCRC, metastatic colorectal cancer; MSI, microsatellite instability; OS, overall survival; PFS, progression-free survival; PS, Performance Status.
jitc-2021-004001supp004.pdf (109.5KB, pdf)
jitc-2021-004001supp005.pdf (100.9KB, pdf)
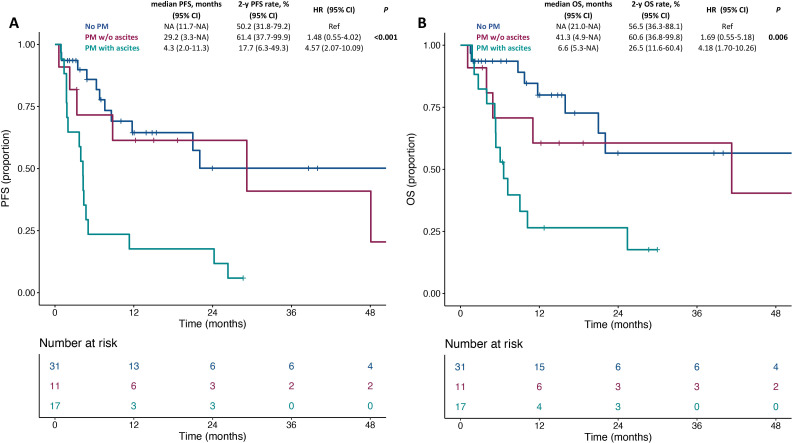
In cohort 2, the median follow-up was 17.4 month (IQR: 11.8–51.3). Online supplemental figure 3A, B shows PFS and OS in the overall population. The presence of ascites was significantly associated with shorter PFS and OS (p<0.001 and p=0.006; figure 2A, B). Patients without peritoneal metastases had similar PFS and OS to those with peritoneal metastases and no ascites (2-year PFS rate: 50.2% vs 61.4%; HR 1.48, 95% CI 0.55 to 4.02; 2-year OS rate: 56.5% vs 60.6%; HR 1.69, 95% CI 0.55 to 5.18), whereas worse PFS and OS were restricted to patients with peritoneal metastases and ascites (2-year PFS rate: 17.7%; HR 4.57, 95% CI 2.07 to 10.09; 2-year OS rate: 26.5%, HR 4.18, 95% CI 1.70 to 10.26). Table 4 shows the univariable and multivariable models for PFS and OS: presence of ascites had an independent effect on survival outcomes (adjusted HR for PFS: 3.83 (95% CI 1.68 to 8.72); for OS: 3.44 (95% CI 1.39 to 8.53)).
Figure 2.
Kaplan-Meier curves for progression-free survival (A) and overall survival (B) in the subgroups of dMMR/MSI-H mGC patients without peritoneal metastases, with peritoneal metastases and no ascites or with peritoneal metastases and ascites. dMMR, mismatch repair deficient; mGC, metastatic gastric cancer; MSI-H, microsatellite instability high; NA, not available; OS, overall survival; PFS, progression-free survival.
Table 4.
Univariable and multivariable Cox proportional hazards regression models for PFS and OS in the validating set (Cohort 2: dMMR/MSI-high mGC)
| Characteristics | PFS | OS | ||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | 0.643 | 0.870 | ||||||
| Female | Ref | Ref | ||||||
| Male | 0.84 (0.41 to 1.73) | 0.94 (0.41 to 2.11) | ||||||
| Age | 0.695 | 0.483 | ||||||
| <70 | Ref | Ref | ||||||
| ≥70 | 0.87 (0.44 to 1.73) | 0.76 (0.35 to 1.65) | ||||||
| Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) | 0.018 | 0.410 | 0.022 | 0.301 | ||||
| 0 | Ref | Ref | Ref | Ref | ||||
| ≥1 | 2.94 (1.20 to 7.18) | 1.54 (0.55 to 4.31) | 3.49 (1.20 to 10.2) | 1.84 (0.58 to 5.88) | ||||
| Primary tumor resection | 0.003 | 0.133 | 0.009 | 0.066 | ||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes | 0.30 (0.13 to 0.66) | 0.46 (0.17 to 1.27) | 0.30 (0.12 to 0.74) | 0.37 (0.13 to 1.07) | ||||
| Primary site of origin | 0.353 | 0.419 | ||||||
| Gastroesophageal junction (GEJ) | Ref | Ref | ||||||
| Gastric | 0.60 (0.21 to 1.75) | 0.60 (0.18 to 2.05) | ||||||
| Histology | 0.506 | 0.575 | ||||||
| Intestinal | Ref | Ref | ||||||
| Diffuse/other | 1.29 (0.61 to 2.71) | 1.27 (0.56 to 2.93) | ||||||
| Synchronous metastases | 0.460 | 0.855 | ||||||
| No | Ref | Ref | ||||||
| Yes | 1.33 (0.63 to 2.81) | 0.93 (0.42 to 2.06) | ||||||
| Liver metastases | 0.294 | 0.201 | ||||||
| No | Ref | Ref | ||||||
| Yes | 0.60 (0.23 to 1.56) | 0.46 (0.14 to 1.52) | ||||||
| Lymphnodal metastases | 0.767 | 0.947 | ||||||
| No | Ref | Ref | ||||||
| Yes | 1.14 (0.49 to 2.62) | 0.97 (0.39 to 2.42) | ||||||
| Lung metastases | 0.789 | 0.802 | ||||||
| No | Ref | Ref | ||||||
| Yes | 0.88 (0.34 to 2.28) | 0.87 (0.30 to 2.53) | ||||||
| Bone metastases | 0.039 | 0.065 | 0.057 | |||||
| No | Ref | Ref | Ref | |||||
| Yes | 3.13 (1.06 to 9.27) | 2.94 (0.94 to 9.20) | 3.36 (0.98 to 11.64) | |||||
| No of metastatic sites | 0.175 | 0.165 | ||||||
| 1 | Ref | Ref | ||||||
| ≥2 | 1.74 (0.78 to 3.87) | 1.92 (0.76 to 4.80) | ||||||
| Prior treatment for metastatic disease | 0.792 | 0.719 | ||||||
| No | Ref | Ref | ||||||
| Yes | 0.87 (0.30 to 2.48) | 0.80 (0.24 to 2.68) | ||||||
| ICI regimen | 0.722 | 0.908 | ||||||
| a-PD-1 | Ref | Ref | ||||||
| a-PD-1 +chemotherapy | 0.77 (0.18 to 3.24) | 1.10 (0.27 to 4.63) | ||||||
| Peritoneal metastases | <0.001 | 0.005 | 0.006 | 0.023 | ||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes, without ascites | 1.48 (0.55 to 4.02) | 1.87 (0.64 to 5.46) | 1.69 (0.55 to 5.18) | 2.15 (0.64 to 7.27) | ||||
| Yes, with ascites | 4.57 (2.07 to 10.09) | 3.83 (1.68 to 8.72) | 4.18 (1.70 to 10.26) | 3.44 (1.39 to 8.53) | ||||
P-values marked with bold indicate statistically significant p-values.
dMMR, mismatch repair deficient; ICIs, immune checkpoint inhibitors; mGC, metastatic gastric cancer; MSI, microsatellite instability; OS, overall survival; PFS, progression-free survival.
jitc-2021-004001supp006.pdf (105.1KB, pdf)
Discussion
In this study, the presence of ascites, but not peritoneal involvement without ascites, was associated with extremely poor survival outcomes in the largest reported cohort of patients with dMMR/MSI-H mCRC and mGC treated with ICIs. The results were consistent in both a very large primary analysis dataset of mCRC and an external validation set of patients with dMMR/MSI-H mGC. Despite the association of ascites with worse performance status and other unfavorable features (such as RAS wild-type/BRAF mutated status in mCRC, and synchronous presentation/non-resected primary tumor in mGC), the presence of ascites was independently associated with both PFS and OS, and notably demonstrated a similar HR >3.0 in both dMMR/MSI-H mCRC and dMMR/MSI mGC. We acknowledge that the lack of independent, central imaging revision is a limitation of our study, since mild amounts of ascites may not be reported systematically. However, the presence of peritoneal involvement and ascites was assessed at each center by expert radiologists, ensuring a high quality of the radiology reports. The reproducibility of our results confirms the agnostic role of MSI as a predictive biomarker for the efficacy of ICIs and establish the role of malignant ascites as a poor prognostic factor for dMMR/MSI-H mCRC treated with anti-PD(L)-1 agents or dMMR/MSI-H mGC treated with anti-PD-1 agents±chemotherapy independently from the anatomic tumor site of origin.
The frequency of malignant ascites was much higher in patients with mGC (28.8%), consistent with the higher frequency of peritoneal involvement in this tumor type, compared with those with mCRC (5%).19 Despite the potential generalizability of our results to all patients with MSI-high advanced solid tumors, caution should be adopted in specific tumor types with extremely low frequency of dMMR/MSI-H—such as cholangiocarcinoma and pancreatic cancers. In fact, non-randomized trial cohorts showed lower than expected response rates to PD-1 blockade in these specific cancers, consistent with a highly immune-suppressive tumor microenvironment that may counterbalance the positive effect of hypermutation.7
Our study cannot clarify if malignant ascites is just a poor prognostic factor (which is well established for patients with advanced GI cancers20 21) or if it is predictive of resistance to current ICI-based strategies. However, several works highlighted the immunosuppressive environment of serous human cavities and malignant effusions.15–17 22–25 Contrary to the more intuitive theory that peritoneum and pleura could represent an immune-excluded milieu because of the difficulty of immune system to penetrate the effusion fluids, recent studies supported the idea of serous cavities as an immune-enriched environment with high concentration of immune-suppressive cells such as macrophages, myeloid-derived suppressive cells and T regulatory cells.15–17 Also, a recent work showed that cavity-resident Tim-4+ macrophages populate the pleural and peritoneal microenvironment and can induce an immune-suppressed microenvironment by impairing CD8+ T cells proliferation.17 In this context, dual Tim-4/PD-1 blockade was synergic in murine models.17 In attempt to clinically validate these results, the authors analyzed a monocentre cohort of 61 patients with MSI-high mCRC treated with ICIs, reporting worse outcomes in those with peritoneal metastases, with unadjusted HRs for PFS and OS of 2.69 and 3.59, respectively. However, given the relatively low prevalence of ascites in their cohort of mCRC, it was not possible to investigate the prognostic impact of peritoneal metastases in a multivariable model and according to the presence or absence of malignant effusion.
Recently, the immune-suppressed microenvironment of ascites was demonstrated also in patients with mGC, irrespective of MMR status.22 Consistent with these findings, similar data were found in other immune-sensitive tumors.23 24 In a multicentre retrospective series, patients with metastatic non-small cell lung cancer and pleural effusion treated with anti-PD(L)-1 agents showed poorer survival outcomes and higher early death rates, even in the subgroup with high PD-L1 expression.25
Our work has several potential implications for clinical practice. First, a subset of patients may sometimes develop ascites as a late-stage complication of peritoneal metastases. Based on the recent results of the Keynote-177 first-line mCRC trial,2 and considering that our study showed that no prior treatment for advanced disease was independently associated with good outcomes, ICIs should be offered as early as possible in patients with peritoneal metastases and hopefully prior to the development of malignant ascites. Second, we showed that patients with ascites treated with anti-PD(L)-1 monotherapy had worse outcomes and almost all were dead by the 2-year time point, whereas no PFS and OS events were reported in patients treated with anti-CTLA-4 plus anti-PD-1 combination. The peculiar immune microenvironment of the serous cavities in the presence of ascites might confer a particular susceptibility to anti-CTLA-4-mediated priming of T cells, explaining (at least in part) why we observed extremely good outcomes in mCRC patients with ascites treated with the combination of anti-CTLA-4 plus anti-PD-1 combination. Despite the greater efficacy reported with dual immune checkpoint blockade in non-randomized studies and in our series, the potential benefit of adding anti-CTLA-4 agents to an anti-PD(L)-1 backbone in patients with dMMR/MSI-H mCRC and malignant ascites should be interpreted with caution considering the low number of patients included in this analysis. Notably our results are aligned with the observation that dual checkpoint inhibition yielded relatively better outcomes in patients with poor prognostic features such as those with elevated systemic inflammation and poorer PS.14 26–28 Based on these considerations, the use of more aggressive therapies such as dual CTLA-4/PD-1 inhibition and chemoimmunotherapy in patients with dMMR/MSI-H advanced GI cancers and ascites may be warranted. Additional novel strategies including intraperitoneal delivery of immunomodulatory agents and exploration of checkpoints enriched in peritoneal metastases (eg, VISTA, LAG3, and TIM3) are worthy of further exploration.29–31
Prospective validation of the potential predictive role of ascites with regard to the intensification of ICI-based regimens may be possible from post-hoc analyses of ongoing randomized trials, for instance, the ongoing COMMIT trial investigating FOLFOX/bevacizumab/atezolizumab vs atezolizumab (NCT02997228) or the ongoing CA209-8HW trial with ipilimumab–nivolumab versus nivolumab across multiple treatment lines (NCT04008030). The same concept may be applied to patients with dMMR/MSI-H mGC. In fact, although the outcomes of these subjects were not different when receiving anti-PD-1 alone or with chemotherapy,4 8 32 the addition of chemotherapy to ICI should be further investigated in the relevant subgroup of patients with dMMR/MSI-H mGC and malignant ascites.
In conclusion, patients with dMMR/MSI-H mCRC or mGC with peritoneal metastases and ascites have inferior outcomes with ICI therapy and should be viewed as a clinical subgroup requiring particular attention. Importantly, peritoneal involvement without ascites may not impart the same unfavorable outcomes and should not be regarded as a clinical factor associated with failure of ICIs.
Footnotes
Contributors: Conception and design: GF and FP. Acquisition of data: RC, SL, KS, MEE, MF, JC, SJK, ME, PJ, FB, MDG, LP, RC, MS, MA, GR, ATS, SM, EF, LS, KC, AK, VC, GC, FC, CC and MO. Analysis and interpretation of data: GF, SM, LP and FP. Manuscript drafting: GF, RC, LP, GM and FP. Manuscript revision: SL, KS, MEE, MF, JC, SJK, ME, PJ, FB, MDG, RC, MS, MA, GR, ATS, SM, EF, LS, KC, AK, VC, GC, FC, CC, MO and TA. Final approval: GF, RC, SL, KS, MEE, MF, JC, SJK, ME, PJ, FB, MDG, GM, LP, RC, MS, MA, GR, ATS, SM, EF, LS, KC, AK, VC, GC, FC, CC, MO, TA and FP. Author responsible for the overall content as the guarantor: FP.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: KS paid consulting or advisory roles for Astellas, Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono, MSD, Taiho, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Amgen, and Boehringer Ingelheim; honoraria from Novartis, AbbVie, and Yakult; and research funding from Astellas, Lilly, Ono, Sumoitomo Dainippon, Daiichi Sankyo, Taiho, Chugai, MSD, Medi Science and Eisai. JC reports grants and personal fees from Merck, Amgen, Macrogenics, Ono Pharmaceuticals, Foundation Medicine, Daiichi-Sankyo and Bristol Myers Squibb. AK reports honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Daiichi-Sankyo, Taiho Pharmaceutical, and Eli Lilly outside the submitted work. CC received honoraria from Amgen, Bayer, Merck, Roche, and Servier; has consulting or advisory role at Amgen, Bayer, MSD, and Roche; was a speakers bureau member at Servier; received research funding from Bayer, Merck, and Servier; and received travel and accommodation expenses from Roche and Servier. MO: consulting for Merck Sharp & Dohme, AbbVie, Agilvax, Acrotech Biopharma, and Novartis Pharmaceuticals Corp; and scientific/ advisory committee member of Takeda Pharmaceuticals (Japan). AK reports honoraria from from Ono Pharmaceutical, Bristol-Myers Squibb, Daiichi-Sankyo, Taiho Pharmaceutical, and Eli Lilly outside the submitted work. FP received honoraria from Amgen, Merck-Serono, Sanofi, Lilly, Bayer, Servier, Astrazeneca; research grants from Astrazeneca and BMS. All the remaining authors declared no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: TA and FP are joint last authors.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (INT 117/15).
References
- 1.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.André T, Shiu K-K, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 2020;383:2207–18. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 3.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40. 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrantonio F, Randon G, Di Bartolomeo M, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open 2021;6:100036. 10.1016/j.esmoop.2020.100036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020;38:11–19. 10.1200/JCO.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marabelle A, Cassier PA, Fakih M, et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: pooled results from the KEYNOTE-028 and KEYNOTE-158 studies. JCO 2020;38:4020. 10.1200/JCO.2020.38.15_suppl.4020 [DOI] [Google Scholar]
- 8.Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 2020;6:1571–80. 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shitara K, Özgüroğlu M, Bang Y-J, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123–33. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 10.Colle R, Radzik A, Cohen R, et al. Pseudoprogression in patients treated with immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Eur J Cancer 2021;144:9–16. 10.1016/j.ejca.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Cohen R, Hain E, Buhard O, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol 2019;5:551–5. 10.1001/jamaoncol.2018.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol 2019;30:1096–103. 10.1093/annonc/mdz134 [DOI] [PubMed] [Google Scholar]
- 13.Kwon M, An M, Klempner SJ, et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov 2021;11:1–18. 10.1158/2159-8290.CD-21-0219 [DOI] [PubMed] [Google Scholar]
- 14.Corti F, Lonardi S, Intini R, et al. The pan-immune-inflammation value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer 2021;150:155–67. 10.1016/j.ejca.2021.03.043 [DOI] [PubMed] [Google Scholar]
- 15.Donnenberg AD, Luketich JD, Dhupar R, et al. Treatment of malignant pleural effusions: the case for localized immunotherapy. J Immunother Cancer 2019;7:110. 10.1186/s40425-019-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buechler MB, Kim K-W, Onufer EJ, et al. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity 2019;51:119–30. 10.1016/j.immuni.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow A, Schad S, Green MD, et al. Tim-4+ cavity-resident macrophages impair anti-tumor CD8+ T cell immunity. Cancer Cell 2021;39:973–88. 10.1016/j.ccell.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–43. 10.1093/annonc/mdz116 [DOI] [PubMed] [Google Scholar]
- 19.Cortés-Guiral D, Hübner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers 2021;7:91. 10.1038/s41572-021-00326-6 [DOI] [PubMed] [Google Scholar]
- 20.Pietrantonio F, Miceli R, Rimassa L, et al. Estimating 12-week death probability in patients with refractory metastatic colorectal cancer: the colon life nomogram. Ann Oncol 2017;28:555–61. 10.1093/annonc/mdw627 [DOI] [PubMed] [Google Scholar]
- 21.Pietrantonio F, Barretta F, Fanotto V, et al. Estimating survival probabilities of advanced gastric cancer patients in the second-line setting: the gastric life nomogram. Oncology 2018;95:344–52. 10.1159/000491753 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Chiwaki F, Kojima S, et al. Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat Cancer 2021;2:962–77. 10.1038/s43018-021-00240-6 [DOI] [PubMed] [Google Scholar]
- 23.Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 2015;20:654–9. 10.1111/resp.12496 [DOI] [PubMed] [Google Scholar]
- 24.Aarnink A, Fumet JD, Favier L, et al. Role of pleural and peritoneal metastasis in immune checkpoint inhibitors efficacy patients with non-small cell lung cancer: real-world data from a large cohort in France. J Cancer Res Clin Oncol 2020;146:2699–707. 10.1007/s00432-020-03262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epaillard N, Benitez JC, Gorria T, et al. Pleural effusion is a negative prognostic factor for immunotherapy in patients with non-small cell lung cancer (NSCLC): the pluie study. Lung Cancer 2021;155:114–9. 10.1016/j.lungcan.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 26.Pietrantonio F, Loupakis F, Randon G, et al. Efficacy and safety of immune checkpoint inhibitors in patients with microsatellite instability-high end-stage cancers and poor performance status related to high disease burden. Oncologist 2020;25:803–9. 10.1634/theoncologist.2020-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fucà G, Corti F, Ambrosini M, et al. Prognostic impact of early tumor shrinkage and depth of response in patients with microsatellite instability-high metastatic colorectal cancer receiving immune checkpoint inhibitors. J Immunother Cancer 2021;9:e002501. 10.1136/jitc-2021-002501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrantonio F, Lonardi S, Corti F, et al. Nomogram to predict the outcomes of patients with microsatellite instability-high metastatic colorectal cancer receiving immune checkpoint inhibitors. J Immunother Cancer 2021;9:e003370. 10.1136/jitc-2021-003370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Principe N, Kidman J, Lake RA, et al. Malignant pleural effusions-A window into local anti-tumor T cell immunity? Front Oncol 2021;11:14–22. 10.3389/fonc.2021.672747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy P, Ekeke CN, Russell KL, et al. Making cold malignant pleural effusions hot: driving novel immunotherapies. Oncoimmunology 2019;8:e1554969. 10.1080/2162402X.2018.1554969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Song S, Harada K, et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 2020;69:18–31. 10.1136/gutjnl-2018-318070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite Instability-High gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol 2021;7:895–902. 10.1001/jamaoncol.2021.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-004001supp001.pdf (40.7KB, pdf)
jitc-2021-004001supp002.pdf (84.9KB, pdf)
jitc-2021-004001supp003.pdf (39.4KB, pdf)
jitc-2021-004001supp004.pdf (109.5KB, pdf)
jitc-2021-004001supp005.pdf (100.9KB, pdf)
jitc-2021-004001supp006.pdf (105.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.