Abstract
Background
The beneficial effects of vitamin D, together with the high prevalence of vitamin D deficiency, have led to an expanding use of vitamin D analogues. While inappropriate consumption is a recognized cause of harm, the determination of doses at which vitamin D becomes toxic remains elusive.
Case Presentation
A 56-year woman was admitted to our Hospital following a 3-week history of nausea, vomiting, and muscle weakness. The patient had been assuming a very high dose of cholecalciferol for 20 months (cumulative 78,000,000UI, mean daily 130,000UI), as indicated by a non-conventional protocol for multiple sclerosis. Before starting vitamin D integration, serum calcium and phosphorus levels were normal, while 25OH-vitamin D levels were very low (12.25 nmol/L). On admission, hypercalcemia (3.23 mmol/L) and acute kidney injury (eGFR 20 mL/min) were detected, associated with high concentrations of 25OH-vitamin D (920 nmol/L), confirming the suspicion of vitamin D intoxication. Vitamin D integration was stopped, and in a week, hypercalcemia normalized. It took about 6 months for renal function and 18 months for vitamin D values to go back to normal.
Conclusion
This case confirms that vitamin D intoxication is possible, albeit with a high dose. The doses used in clinical practice are far lower than these and, therefore, intoxication rarely occurs even in those individuals whose baseline vitamin D serum levels have never been assessed. Repeated measurements of vitamin D are not necessary for patients under standard integrative therapy. However, patients and clinicians should be aware of the potential dangers of vitamin D overdose.
Keywords: Vitamin D, intoxication, overdose, cholecalciferol, toxicity, hypercalcemia
1. BACKGROUND
Vitamin D exerts its physiologic effects on the gastrointestinal tract, bone, and kidney and is traditionally recognized as a master regulator of calcium homeostasis [1]. However, restricting the biological action of this hormone only to the regulation of calcium-phosphorus metabolism would be over-simplistic. Over the past two decades, the scientific interest regarding vitamin D has surged and many other, equally important, extra-skeletal functions of vitamin D have also been investigated [1, 2]. In this scenario, vitamin D has been linked with multiple health outcomes, such as overall mortality, cancer, cardiovascular diseases, metabolic and autoimmune disorders [2].
Multiple sclerosis is one of the most studied chronic disorders, which hasbeen associated with vitamin D deficiency. Hypovitaminosis D has been depicted as a potential contributor to the risk of developing multiple sclerosis [3, 4] in predisposed individuals owing to non-calcemic immunomodulatory effects of vitamin D, which involves complex and poorly elucidated environmental and genetic stimuli [4]. On these grounds, several clinical trials of cholecalciferol supplementation have been conducted. However, findings regarding disease-related outcomes are conflicting [5].
The suggested beneficial effects, along with the high global prevalence of vitamin D deficiency, have prompted the widespread increase in the use of vitamin D supplements. Pros and cons of high-dose vitamin D supplementation remain a controversial topic. Vitamin D integration is not free of risk, as toxicity is a potentially serious adverse effect, and several cases of vitamin D-related intoxication have been reported within a few months of treatment. The knowledge about long-term complications of high-dose intake is limited, since only few cases with a longer time frame of high-dose therapy have been reported, demonstrating high heterogeneity in individual responses to supra-physiological concentrations of vitamin D for extended periods of time [6-8]. Here, we report a patient affected by multiple sclerosis who developed hypercalcemia and acute severe renal failure after more than one year of ultra-high dose intake of cholecalciferol. Finally, we review the literature about adult patients who had received high doses of oral vitamin D to compare cumulative dosages of vitamin D, causing toxicity.
2. CASE PRESENTATION
A 56-year-old woman came to medical observation in our Hospital in September 2016 on the grounds of heartburn, nausea, vomiting, muscle weakness, and profuse fatigue for 3 weeks.
The patient’s medical history included iron deficiency anemia, hypertension, menopause for 4 years, and chronic autoimmune thyroiditis. Multiple sclerosis, first diagnosed in 1989, had been treated with Azathioprine, Betaferon, Natalizumab with disappointing results. Over time, her disease had progressively more frequent relapses, necessitating repeated courses of steroids. Owing to the poor efficacy of the treatment, our patient adhered to a non-conventional management schedule based on oral supra-physiological cholecalciferol dosages (the Coimbra protocol).
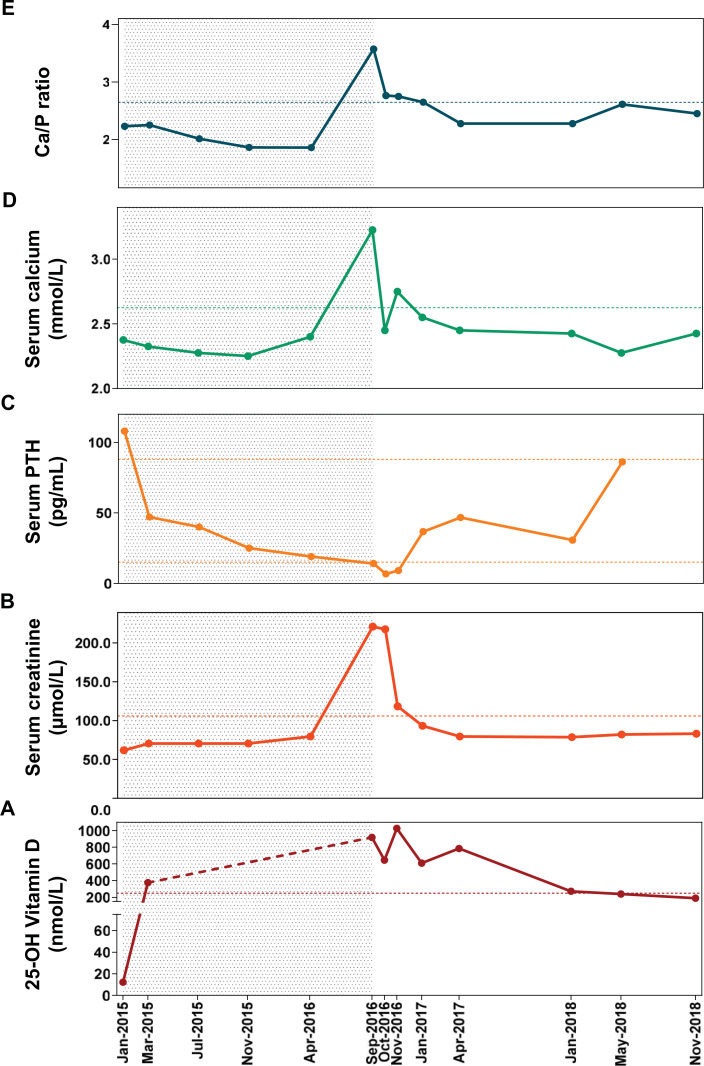
In January 2015, before starting vitamin D integration, serum levels of calcium (2.38 mmol/L) and phosphorus (1.06 mmol/L) were normal, while 25OH-vitamin D levels were very low (12.25 nmol/L) (Table 1) (Fig. 1). Concurrently, there were high levels of intact parathyroid hormone (PTH) (108 pg/mL) with normal renal function (creatinine 61.61 µmol/L, estimated glomerular function rate (eGFR)> 60 mL/min), which suggested hyperparathyroidism secondary to vitamin D deficiency (Table 1) (Fig. 1). Cholecalciferol supplementation was started with an initial oral dose of 70,000 UI/day. In March 2015, normal calcium-phosphorus metabolism and renal function were documented, while 25OH-vitamin D serum concentrations were already above the upper limit of the normal range (>375 nmol/L) after only 2 months of therapy, with a cumulative dose of 4,200,000 UI of cholecalciferol. Although serum vitamin D levels were above the normal range, the dosage of cholecalciferol supplementation was progressively increased as follows: 100,000 UI/day from March 2015 to July 2015, then 140,000 UI/day to April 2016, and finally up to 160,000 UI/day. At subsequent examinations performed during 20 months of treatment, the biochemical pattern remained quite stable, except for a progressive reduction of PTH, until September 2016, when she entered our inpatient Clinic (Table 1).
Table 1.
Time course of biochemical parameters of our patient during high-dose cholecalciferol supplementation (grey area) and after its discontinuation.
| - | Normal Range | Jan-15 | Mar-15 | Jul-15 | Nov-15 | Apr-16 | Sep-16 | Oct-16 | Nov-16 | Jan-17 | Apr-17 | Jan-18 | May-18 | Nov-18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Months from Baseline | - | 0 | 2 | 6 | 9 | 14 | 19 | 20 | 21 | 23 | 26 | 35 | 39 | 45 |
| Cumulative dose of cholecalciferol (x103 UI) | 0 | 4,200 | 16,200 | 33,000 | 54,000 | 78,000 | - | - | - | - | - | - | - | |
| Average of daily dose of cholecalciferol (UI/day) | 0 | 70,000 | 90,000 | 110,000 | 120,000 | 130,000 | - | - | - | - | - | - | - | |
| 25-OH Vitamin D (nmol/L) | 75 – 250 | 12.3 | >375 | >375 | >375 | >375 | 920 | 688 | 1028 | 610 | 788 | 273 | 239 | 223 |
| Serum Calcium (mmol/L) | 2.13 – 2.63 | 2.38 | 2.33 | 2.28 | 2.23 | 2.40 | 3.23 | 2.68 | 2.75 | 2.55 | 2.43 | 2.43 | 2.28 | 2.45 |
| Serum Phosphorus (mmol/L) | 0.81 – 1.65 | 1.06 | 1.03 | 1.13 | 1.19 | 1.29 | 0.90 | 0.97 | 1.00 | 0.94 | 1.06 | 1.06 | 0.87 | 1.00 |
| Serum Ca/P ratio | <2.6 | 2.23 | 2.25 | 2.02 | 1.86 | 1.86 | 3.57 | 2.76 | 2.75 | 2.73 | 2.28 | 2.28 | 2.61 | 2.45 |
| Serum PTH (pg/mL) | 15 – 88 | 108 | 47.1 | 40 | 25 | 19 | 2.3 | 6.7 | 7.7 | 36.8 | 46.7 | 30.7 | 86.2 | - |
| Creatinine (µmol/L) | 44.0 – 105.6 | 61.6 | 70.4 | 70.4 | 70.4 | 79.2 | 220.1 | 180.4 | 117.9 | 95.9 | 79.2 | 78.3 | 81.9 | 82.7 |
| eGFR (ml/min/1.73m2) | >60 | >60 | >60 | >60 | >60 | >60 | 20 | 23 | 30 | 57 | >60 | >60 | >60 | >60 |
Abbreviations: Ca/P: serum calcium to phosphorous ratio; eGFR: estimated Glomerular filtration rate (calculated using the CKD-EPI formula normalized for body surface).
Fig. (1).
Temporal evolution of serum vitamin D (A), creatinine (B), PTH (C), serum calcium (D), and Calcium to Phosphorus ratio (E) during the ultra-high dose supplementation of cholecalciferol (grey area) ended at the moment of clinical intoxication, and during the subsequent follow-up. Grey area from January 2015 to September 2016 defines the period of supplementation with high doses of vitamin D. In box A: levels of vitamin D from March 2015 to September 2016 are above 375 nmol/L, but not assessed through dilution, and they are represented by the red dashed line. In boxes B, D and E: horizontal dashed lines represent the upper limit of normal ranges of creatinine, calcium, and Ca/P ratio. In box C: horizontal dashed lines represent the upper and the lower limits of normal range of PTH. Abbreviations: Ca, calcium; P, phosphorus; PTH, parathyroid hormone. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
At admission, the patient had normal vital signs, diffuse abdominal pain, and dehydration of mucous membranes and skin. Heart rhythm was normal. Hypercalcemia (3.23 mmol/L) and acute renal injury (serum creatinine 220.05 µmol/L, eGFR 20 mL/min) were identified. The suspicion of vitamin D intoxication was confirmed by the very high levels of 25OH-vitamin D (920 nmol/L) assessed by a chemiluminescence assay (Diasorin, Inc., Stillwater, MN). Accordingly, PTH was below the normal range (6.7 pg/mL, normal range 15-88 pg/mL). Routine chest and abdomen X-rays were negative. Moreover, bone mineral density (BMD) was evaluated upon intoxication by Dual-Energy-X-ray-Absorptiometry (DXA) (DXA, Hologic-QDR-2000 densitometer, Inc., Waltham, MA). According to the World Health Organization (WHO) criteria [9], T-scores at the lumbar spine (L1 to L4) and total hip were defined as normal (i.e. T-score > -1.0), and BMD did not significantly differ over time compared to a previous DXA performed in 2014, before the start of vitamin D integration.
Vitamin D integration was immediately stopped and high-flow hydration (physiological solution 170 cc/hour), intravenous diuretics (furosemide 40 mg/day), and steroids (hydrocortisone 200-300 mg/day) were administered. Serum calcium returned to normal in a week and renal function gradually improved.
In April 2017, i.e. six months after the discontinuation of cholecalciferol, serum calcium remained within the normal range (2.43 mmol/L), renal function returned to normal (creatinine 79.22 µmol/L, eGFR >60 mL/min) and PTH levels increased (46.7 pg/mL), while 25OH-vitamin D levels were still high (787.50 nmol/L). It took about 18 additional months to observe 25OH-vitamin D concentrations below the intoxication threshold of 250 nmol/L in May 2018 (Table 1, Fig. 1).
3. REVIEW OF THE LITERATURE
3.1. Methods
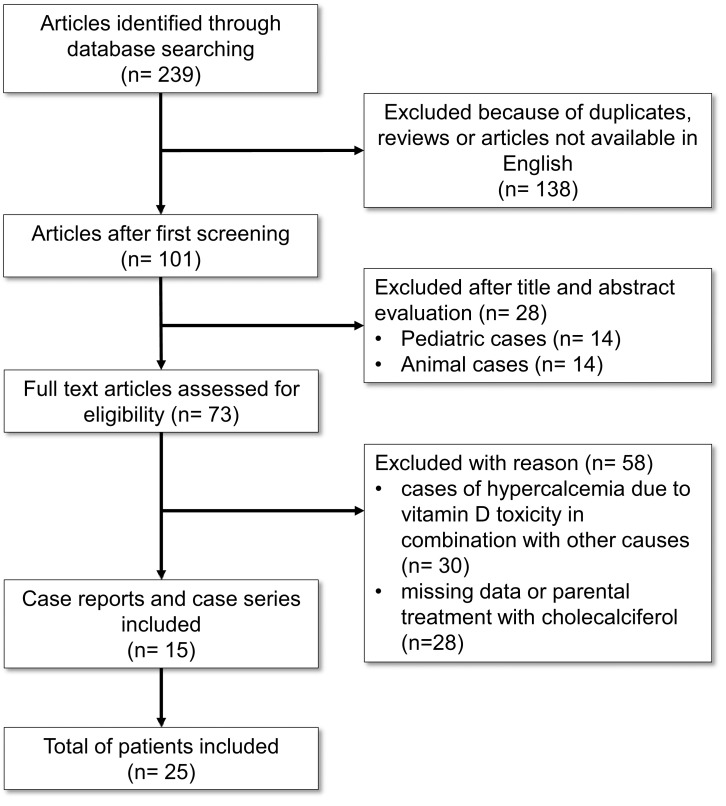
Pubmed, Medline, Google Scholar and Science Direct were searched for cases of vitamin D toxicity published between 1949 and 2020. To this end, the following MeSH terms were used in the search: ‘vitamin D toxicity cases,’ ‘vitamin D intoxication cases,’ ‘vitamin D overdose cases,’ ‘cholecalciferol intoxication cases.’ On the basis of such criteria, a total of 239 peer-reviewed articles were identified, 101 of which were written in English (Fig. 2). After examining the title, abstract, and laboratory findings, 15 publications for a total of 25 clinical cases of vitamin D intoxication in adult patients reporting the biochemical examinations upon admission, the amount and the duration of vitamin D per os intake were included (Table 2, Fig. 2). Publications that were rejected included animal studies (n = 14), cases of hypercalcemia owing to vitamin D toxicity combined with other causes (n = 14), pediatric cases (n = 30), and case reports with missing data or other than oral treatment (i.e. parenteral) with cholecalciferol (n = 28) (Fig. 2).
Fig. (2).
Flow-chart of the literature review.
4. DISCUSSION
This case describes the biochemical effects of prolonged exposure to an ultra-high dose of inactivated vitamin D therapy. After an average cholecalciferol supplementation of 130,000 IU per day for almost 2 years, our patient developed hypercalcemia and acute kidney injury.
The use of vitamin D analogues has been extraordinarily increased, and it is supported by many clinical and nutritional guidelines. There is heterogeneity among these guidelines about the recommended dose of vitamin D intake, ranging from 800 to 2,000 UI per day, and the serum 25OH-vitamin D target levels to be pursued [10]. Notwithstanding the widespread use of vitamin D supplements, evidence about ultra-high dose-related complications is very limited, and the upper limits of safe intake have not been precisely defined. Few studies have assessed the effects of daily vitamin D doses at or above the tolerable upper intake level (up to 10,000 UI per day), or greater for 12 months, reporting a low incidence of adverse events (i.e. mostly hypercalcemia and hypercalciuria) [11]. The knowledge about toxicity from hypervitaminosis D in terms of dosage and duration is limited. Because of the high heterogeneity in reported amounts of vitamin D intoxication cases, it is not possible to calculate a reliable mean value that will invariably induce toxic effects. The highest daily dose hitherto reported in the literature is about 2,000,000 UI/day that has led to intoxication in a couple of months [12] (Table 2). Moreover, the highest cumulative dose (657,000,000 UI) leading to toxicity was obtained after 36 months of treatment with cholecalciferol 600,000 UI/day [13] (Table 2). In our case, a cumulative dose of about 78,000,000 UI of cholecalciferol was calculated based on the patient’s medical history. In cases exposed to a daily dose of Vitamin D in the order of magnitude such as ours, the intoxication occurred in a time-lapse ranging between 2 months [14, 15] and 2 years [16], the latter quite close to the duration of this case; all other cases developed intoxication more rapidly than our patient. For instance, in one case report, signs of intoxication occurred in only 2 months of treatment with a daily dose much lower than ours but with hypercalcemia and impaired renal function even at the beginning of cholecalciferol supplementation [17] (Table 2). The lack of baseline biochemical data of other reported cases does not allow to explore the reason for this heterogeneity in cholecalciferol dosage and duration, leading to toxicity.
Table 2.
Dosage information and biochemical tests of adult patients with vitamin D intoxication.
| Reference | Sex, Age (Years) | Country of Residence | Cholecalciferol Supplementation | Biochemical Parameters at Intoxication | Time to Normalize calcium (Weeks) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Dose (x103 UI) | Daily Dose (UI/day) | Duration (Months) | 25OH- Vitamin D (nmol/L) | Serum Ca (mmol/L) | Serum P (mmol/L) | Serum Creatinine (µmol/L) | Serum PTH (pg/mL) | ||||
| Araki, 2011 (a) [12] | M, 58 | USA | 111,840 | 1,864,000 | 2 | 2800 | 3.75 | ↔ | 158.4 | ↓ | 4 |
| Araki, 2011 (b) [12] | M, 40 | USA | 29,100 | 970,000 | 1 | 1613 | 3.30 | ↔ | 176.0 | ↓ | 4 |
| Auguste, 2019 [18] | M, 54 | Canada | 9,125 | 10,000 | 30 | 240 | 3.05 | 0.94 | 378.5 | 4.72 | 24 |
| Bell, 2013 [13] | F, 67 | Australia | 657,000 | 600,000 | 36 | 913 | 3.28 | - | 325.7 | 20.7 | 20 |
| Chowdry, 2017 (a) [26] | F, 84 | India | 3,600 | 60,000 | 2 | 1155 | 3.80 | 0.90 | 167.2 | 18.0 | - |
| Chowdry, 2017 (b) [26] | F, 71 | India | 5,760 | 64,000 | 3 | 510 | 3.50 | 1.32 | 308.1 | 9 | - |
| Chowdry, 2017 (c) [26] | M, 49 | India | 4,500 | 60,000 | 2.5 | 885 | 3.13 | 1.00 | 246.5 | 13 | - |
| Chowdry, 2017 (d) [26] | F, 56 | India | 6,300 | 60,000 | 3.5 | 1110 | 3.55 | 1.13 | 290.5 | 15.5 | - |
| Chowdry, 2017 (e) [26] | M, 51 | India | 4,500 | 60,000 | 2.5 | 770 | 3.13 | 1.10 | 484.1 | 14.3 | - |
| Chowdry, 2017 (f) [26] | F, 61 | India | 5,640 | 56,400 | 3.4 | 770 | 3.78 | 1.19 | 281.7 | 10 | - |
| Feige, 2018 [27] | M, 53 | Austria | 9,000 | 50,000 | 6 | >400 | 3.30 | 1.48 | 598.5 | 7 | 11 |
| Granado-Lorencio, 2012 [28] | F, 51 | Ecuador | 18,000 | 600,000 | 1 | 2742 | 3.23 | 1.64 | 255.3 | 12 | 4 |
| Guerra, 2016 [17] | M, 70 | Brazil | 90 | 2,000 | 1.5 | 375 | 3.35 | 1.87 | 501.7 | 15.3 | 28 |
| Kaptein, 2010 [14] | F, 75 | Netherlands | 9,000 | 150,000 | 2 | 1375 | 4.00 | 0.87 | 272.9 | 9.4 | 3 |
| Kaur, 2015 (a) [29] | F, 83 | India | 2,880 | 32,000 | 3 | >400 | 2.78 | 1.29 | 96.8 | - | - |
| Kaur, 2015 (b) [29] | M, 61 | India | 3,600 | 60,000 | 2 | 973 | 3.43 | 1.16 | 179.6 | 17.9 | - |
| Kaur, 2015 (c) [29] | M, 46 | India | 2,760 | 46,000 | 2 | 913 | 2.80 | 1.03 | 96.8 | 14.8 | - |
| Kaur, 2015 (d) [29] | M, 51 | India | 3,600 | 60,000 | 2 | 723 | 2.90 | 1.29 | 79.2 | 23 | - |
| Kim, 2017 (a) [8] | M, 75 | USA | 600* | 50,000* | 12 | 608 | 3.85 | - | - | 3.0 | 3 |
| Kim, 2017 (b) [8] | F, 60 | USA | 400 | 40,000 | 10 | 1198 | 2.48 | - | - | 17 | - |
| Klontz, 2007 [15] | F, 58 | USA | 10,416 | 186,900 | 2 | 1173 | 3.75 | 0.80 | 264.1 | 12.0 | 2 |
| Koutkia, 2001 [16] | M, 42 | USA | 113,880 | 156,000 | 24 | 1218 | 3.75 | - | 220.1 | ↔ | 8 |
| Leu, 2008 [30] | F, 60 | Dominican Republic | 42,000 | 600,000 | 2.3 | >375 | 3.80 | - | - | ↓ | 1.4 |
| Marins, 2014 [31] | F, 53 | Brazil | - | 4,000,000 | ‘Some’ | >250 | 3.25 | - | 237.7 | 17.5 | 8 |
| Vieth, 2002 [32] | M, 63 | Canada | 11,900 | 1,700,000 | 7 | 1550 | 3.83 | - | 440.1 | <1 | - |
Abbreviations: USA: United States of America; Ca: calcium; P: phosphorous; PTH: parathyroid hormone. *Ergocalciferol (vitamin D2) and 500 mg calcium-citrate daily.
Furthermore, in our patient, serum creatinine was documented to be normal at the beginning of high-dose vitamin integration. Conversely, data about baseline renal function are missing in other published cases. Therefore, it is reasonable to assume that a normal renal function at baseline is a key factor in delaying the presence of toxicity owing to high-dose vitamin D supplementation. In the literature, data about long-term renal outcomes are lacking, and magnitude as well as time course of improvement in serum creatinine has not been clearly established. In this case, the hypervitaminosis D-induced renal injury did not require dialysis once the supplements were discontinued and serum calcium levels were managed. In our case, renal failure underwent full resolution in about 6 months, following the discontinuation of cholecalciferol (Table 1, Fig. 1). This is at odds with residual chronic kidney impairment reported in several cases [13, 18].
The minimum amount of vitamin D analogue required to cause intoxication cannot be inferred by our report. However, the daily dose and the calculation of cumulative dose at the moment of intoxication in our patient, together with other available cases, collectively allow us to speculate that a very high dose (at least 5-fold greater than the maximum recommended dose in the setting of normal renal function) of vitamin integration is dangerous in individuals with baseline normal renal function. We highlight that the doses assumed by this patient for at least 18 months are substantially higher than doses which are used both in clinical practice and in clinical trials.
With the increasing use of vitamin D products, there are reports of vitamin D toxicity in the literature related to prescribing errors, the use of high-dose supplements and manufacturing errors [19]. Among the published reports of vitamin D intoxication, very few provide information about biochemical parameters before the intoxication occurs. Our case is valuable because periodic examinations from baseline to the moment of intoxication were available. This gave us the opportunity to observe the time course of vitamin D-dependent parameters, especially serum calcium and phosphorous, in relation to vitamin D concentrations itself (Table 1, Fig. 1). From a speculative point of view, we were thrilled by the search for a potential predictive factor that can forewarn clinicians about the possible risk of toxicity. Whereas vitamin D levels were not of help as above the normal range for all the duration of high-dose supplementation, serum PTH had a decreasing trend and values near the lower limit of the normal range may potentially alert to the risk of intoxication. Considering the trend of serum calcium, this parameter progressively decreased in the first phase of cholecalciferol supplementation in accordance with the decreasing serum PTH trend (Fig. 1). Then, after about 14 weeks of treatment, serum calcium began to rise in contrast with the progressive decrease of PTH, in a non-physiological manner (Fig. 1). The uncoupling of calcium from PTH levels may be a clue to impending vitamin D intoxication. Similarly, an increase in calcium to phosphorous ratio, particularly when above 3.5 [20], suggests an impaired metabolism of calcium-phosphorous, which is another reason for concern.
Our patient’s baseline laboratory profile indicates secondary hyperparathyroidism, characterized by increased serum PTH alongside a severe deficiency of vitamin D. As expected, with the start of cholecalciferol integration, PTH and vitamin D concentrations showed an opposite trend: PTH levels were normalized in only 2 months and continued to decrease almost up to a complete suppression in concomitance with the highest levels of vitamin D. Therefore, in our case, a cholecalciferol dose of 70,000 UI/day for 60 days (total dose 4,200,000 UI) was able to over-correct hypovitaminosis D and its related secondary hyperparathyroidism restoring a normal parathyroid function, but without impairing the calcium-phosphorous metabolism. Once again, applying this finding to real-life practice, clinical guidelines recommend doses of vitamin D supplementation that does not cause toxicity and clinicians may safely prescribe them without the necessity to re-assess serum 25-OH Vitamin D [10].
In line with these observations, we believe that, beyond serum calcium, serum phosphorous should be assessed to evaluate calcium homeostasis rather than serum vitamin D. Indeed, despite clinical guidance to restrict testing for vitamin D deficiency, there have been reports showing increasing worldwide rates of routine testing over the past decade [21-23]. This is accompanied with a heavy economic burden, especially after the diffusion of last-generation laboratory assays that have caused an increment in the test cost [23]. Repeated measurements of vitamin D levels do not add any value to clinical management of patients with ongoing supplementation, with a detrimental cost-effectiveness ratio. Our experience with this case is in agreement with the recommendation to not screen the general population routinely for vitamin D and not to repeatedly assess vitamin D in patients with ongoing supplementation [24, 25].
CONCLUSION
This case confirms that vitamin D intoxication is indeed possible albeit, with a high cumulative dose (78,000,000 UI), obtained with a mean daily dose (130,000 UI per day) 65-fold greater than the maximum recommended dose. The doses used in clinical practice are far below those used by this patient. Therefore, intoxication is very unlikely even without knowing the starting vitamin D levels, suggesting that repeated measurements of serum vitamin D are not necessary for patients under standard integrative therapy. However, both patients and clinicians should be more aware of the potential dangers of vitamin D overdose. When ultra-high dose vitamin D supplementation is used, regular monitoring of serum calcium, phosphorous, PTH, and creatinine is prompted.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- BMD
Bone Mineral Density
- DXA
Dual-Energy-X-ray-Absorptiometry
- eGFR
Estimated Glomerular Function Rate
- PTH
Parathyroid Hormone
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient prior to the publication of the study.
STANDARD OF REPORTING
CARE guidelines and methodology were followed to conduct the study.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Autier P., Boniol M., Pizot C., Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 2.Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascherio A., Munger K.L., Simon K.C. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9(6):599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 4.Pierrot-Deseilligny C., Souberbielle J.C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017;14:35–45. doi: 10.1016/j.msard.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Zheng C., He L., Liu L., Zhu J., Jin T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2018;23:56–61. doi: 10.1016/j.msard.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson D.W., Peiris A.N. The lack of vitamin D toxicity with megadose of daily ergocalciferol (D2) therapy: a case report and literature review. South. Med. J. 2009;102(7):765–768. doi: 10.1097/SMJ.0b013e3181a8d1e4. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty S., Sarkar A.K., Bhattacharya C., Krishnan P., Chakraborty S. A nontoxic case of vitamin D toxicity. Lab. Med. 2015;46(2):146–149. doi: 10.1309/LM5URN1QIR7QBLXK. [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Stephens L.D., Fitzgerald R.L. How much is too much? Two contrasting cases of excessive vitamin D supplementation. Clin. Chim. Acta. 2017;473:35–38. doi: 10.1016/j.cca.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 10.Cesareo R., Attanasio R., Caputo M., Castello R., Chiodini I., Falchetti A., Guglielmi R., Papini E., Santonati A., Scillitani A., Toscano V., Triggiani V., Vescini F., Zini M. AME and Italian AACE Chapter. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients. 2018;10(5):E546. doi: 10.3390/nu10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burt L.A., Billington E.O., Rose M.S., Raymond D.A., Hanley D.A., Boyd S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA. 2019;322(8):736–745. doi: 10.1001/jama.2019.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araki T., Holick M.F., Alfonso B.D., Charlap E., Romero C.M., Rizk D., Newman L.G. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J. Clin. Endocrinol. Metab. 2011;96(12):3603–3608. doi: 10.1210/jc.2011-1443. [DOI] [PubMed] [Google Scholar]
- 13.Bell D.A., Crooke M.J., Hay N., Glendenning P. Prolonged vitamin D intoxication: presentation, pathogenesis and progress. Intern. Med. J. 2013;43(10):1148–1150. doi: 10.1111/imj.12269. [DOI] [PubMed] [Google Scholar]
- 14.Kaptein S., Risselada A.J., Boerma E.C., Egbers P.H., Nieboer P. Life-threatening complications of vitamin D intoxication due to over-the-counter supplements. Clin. Toxicol. (Phila.) 2010;48(5):460–462. doi: 10.3109/15563650.2010.486382. [DOI] [PubMed] [Google Scholar]
- 15.Klontz K.C., Acheson D.W. Dietary supplement-induced vitamin D intoxication. N. Engl. J. Med. 2007;357(3):308–309. doi: 10.1056/NEJMc063341. [DOI] [PubMed] [Google Scholar]
- 16.Koutkia P., Chen T.C., Holick M.F. Vitamin D intoxication associated with an over-the-counter supplement. N. Engl. J. Med. 2001;345(1):66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 17.Guerra V., Vieira Neto O.M., Laurindo A.F., Paula F.J., Moysés Neto M. Hypercalcemia and renal function impairment associated with vitamin D toxicity: case report. J. Bras. Nefrol. 2016;38(4):466–469. doi: 10.5935/0101-2800.20160074. [DOI] [PubMed] [Google Scholar]
- 18.Auguste B.L., Avila-Casado C., Bargman J.M. Use of vitamin D drops leading to kidney failure in a 54-year-old man. CMAJ. 2019;191(14):E390–E394. doi: 10.1503/cmaj.180465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor P.N., Davies J.S. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br. J. Clin. Pharmacol. 2018;84(6):1121–1127. doi: 10.1111/bcp.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madeo B., De Vincentis S., Kara E., Vescini F., Trenti T., Guaraldi G., Rochira V. Reliability of calcium-phosphorus (Ca/P) ratio as a new, accurate and inexpensive tool in the diagnosis of some Ca-P disorders. J. Endocrinol. Invest. 2019;42(9):1041–1049. doi: 10.1007/s40618-019-01025-6. [DOI] [PubMed] [Google Scholar]
- 21.Crowe F.L., Jolly K., MacArthur C., Manaseki-Holland S., Gittoes N., Hewison M., Scragg R., Nirantharakumar K. Trends in the incidence of testing for vitamin D deficiency in primary care in the UK: a retrospective analysis of The Health Improvement Network (THIN), 2005-2015. BMJ Open. 2019;9(6):e028355. doi: 10.1136/bmjopen-2018-028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodford H.J., Barrett S., Pattman S., Vitamin D. Vitamin D: too much testing and treating? Clin. Med. (Lond.) 2018;18(3):196–200. doi: 10.7861/clinmedicine.18-3-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sattar N., Welsh P., Panarelli M., Forouhi N.G. Increasing requests for vitamin D measurement: costly, confusing, and without credibility. Lancet. 2012;379(9811):95–96. doi: 10.1016/S0140-6736(11)61816-3. [DOI] [PubMed] [Google Scholar]
- 24.Rosen C.J., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., Kovacs C.S., Manson J.E., Mayne S.T., Ross A.C., Shapses S.A., Taylor C.L. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Chowdry A.M., Azad H., Najar M.S., Mir I. Acute kidney injury due to overcorrection of hypovitaminosis D: A tertiary center experience in the Kashmir Valley of India. Saudi J. Kidney Dis. Transpl. 2017;28(6):1321–1329. doi: 10.4103/1319-2442.220873. [DOI] [PubMed] [Google Scholar]
- 27.Feige J., Salmhofer H., Hecker C., Kunz A.B., Franzen M., Moré E., Sellner J. Life-threatening vitamin D intoxication due to intake of ultra-high doses in multiple sclerosis: A note of caution. Mult. Scler. 2019;25(9):1326–1328. doi: 10.1177/1352458518807059. [DOI] [PubMed] [Google Scholar]
- 28.Granado-Lorencio F., Rubio E., Blanco-Navarro I., Pérez-Sacristán B., Rodríguez-Pena R., García López F.J. Hypercalcemia, hypervitaminosis A and 3-epi-25-OH-D3 levels after consumption of an “over the counter” vitamin D remedy. a case report. Food Chem. Toxicol. 2012;50(6):2106–2108. doi: 10.1016/j.fct.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Kaur P., Mishra S.K., Mithal A. Vitamin D toxicity resulting from overzealous correction of vitamin D deficiency. Clin. Endocrinol. (Oxf.) 2015;83(3):327–331. doi: 10.1111/cen.12836. [DOI] [PubMed] [Google Scholar]
- 30.Leu J.P., Weiner A., Barzel U.S. Vitamin D toxicity: caveat emptor. Endocr. Pract. 2008;14(9):1188–1190. doi: 10.4158/EP.14.9.1188. [DOI] [PubMed] [Google Scholar]
- 31.Marins T.A., Galvão Tde.F., Korkes F., Malerbi D.A., Ganc A.J., Korn D., Wagner J., Guerra J.C., Borges Filho W.M., Ferracini F.T., Korkes H. Vitamin D intoxication: case report. Einstein (Sao Paulo) 2014;12(2):242–244. doi: 10.1590/S1679-45082014RC2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieth R., Pinto T.R., Reen B.S., Wong M.M. Vitamin D poisoning by table sugar. Lancet. 2002;359(9307):672. doi: 10.1016/S0140-6736(02)07814-5. [DOI] [PubMed] [Google Scholar]