Abstract
This study aimed to estimate the effect of cobalt nanoparticles (Co NPs) with different concentrations against multidrug-resistant (MDR) pathogenic bacteria. Three isolates of Staphylococcus aureus (gram-positive), Proteus spp. (gram-negative), and Escherichia coli (gram-negative) bacteria were extracted from various clinical examples utilizing routine methods on bacteriological culture media. The antibacterial sensitivity of commercial antibiotics such as Ciprofloxacin, Cefotaxime, Gentamycin, and Amoxicillin was broken down on a Muller Hinton agar plate and evaluated using the disk diffusion method. The study results demonstrated the antibacterial effect of the Co NPs against the bacterial isolates with three different concentrations utilized in the study. The results indicated that the Co NPs showed the highest antibacterial activity when utilizing 100 μg/ml against Escherichia coli followed by Proteus spp and Staphylococcus aureus with zones of inhibition measured as 22.2±0.1 mm, 20.3±0.15 mm, and 15.8±0.1 mm; respectively. Co NPs at a 100 μg/mL concentration showed higher inhibition zones than several common antibiotics except for Ciprofloxacin, which demonstrated better antibacterial activity against the bacterial isolates employed in this study. Scanning Electron Microscope (SEM)and X-Ray diffraction (XRD)studies confirmed that Cobalt nanoparticles (Co NPs) were synthesized from cobalt sulphate solution with a size ranging from 40 nm to 60 nm. The nanoparticles showed a crystalline structure with a round shape and smooth surface. The antibacterial resistance of Co NPs against three common bacteria such as Staphylococcus aureus, Proteus spp, and Escherichia coli was assessed in this study. The optimum concentration of the Co NPs was identified as 100 μg/ml, which could provide a similar or higher antibacterial effect.
Keywords: cobalt nanoparticle, nanomedicine, pathogenic bacteria, antimicrobial activity, antibiotics
Keywords: AI – Activity index, Co NPs – Cobalt nanoparticles, MH – Muller-Hinton agar medium, MDR – Multidrug-resistant, SEM – Scanning Electron Microscope, XRD – X-Ray diffraction
Introduction
Diminishing antimicrobial resistance is quickly becoming a worldwide concern with a fast increase in multidrug-resistant (MDR) bacteria [1]. Escherichia coli, Staphylococcus aureus, and Klebsiella pneumonia are the most well-known MDR bacteria related to nosocomial infections [2–3]. In recent years, the utilization of nanotechnology and the blend and production of nanoparticles (NPs) have brought new expectations for the fight against MDR bacteria [4]. In addition, nanoscale materials have appeared as the new antimicrobial agents. Some classes of antimicrobial NPs and nanosized carriers for antibiotics delivery have demonstrated their efficiency in treating infectious diseases in vitro, including the antimicrobial-resistant ones [5]. The rapid development of nanotechnology has provided several materials for biomedical applications, including those used as anti-microbes [4]. Modern drug delivery techniques operate on the highly beneficial principle of site-specific or targeted therapy, and the use of nanoparticles in various medical applications has allowed for drug therapy and various applications related to visualization, sensing, and gene delivery [6]. NPs have attracted extraordinary interest in their improvement as potential antibacterial drugs [7–8]. Over the most recent years, many studies have investigated the structure and chemical behavior of some metals and metal oxides to discover new drugs with antibacterial capabilities. Among them, Ag, Au, Co, TiO2, ZnO, CuO, Fe2O3 etc, have proven their ability to act as an antibiotic [4–9]. Cobalt complex showed promises as a good drug of choice to manage bacterial, fungal, or amoebal diseases as outlined in recent publications [10–13]. Over the past years, several studies have been carried out on the antibacterial activity of Co NPs [14–18]. Igwe et al. concluded that Co NPs with hexagonal shapes and sizes ranging from 20–49 nm could be employed for treating infections by inhibiting the growth of Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, and Streptococcus pyogene [14]. Kharade et al. [15] demonstrated excellent antibacterial activity of green synthesized Co NPs with an average size of 20.88 nm using Hibiscus cannabinus leaf extract against Bacillus substilis and Escherichia coli. Co NP was synthesized using Raphanussativus var. longipinnatus leaf extract showed effective antibacterial activity against gram-negative bacteria such as Pseudomonas putida and Klebsiella pneumonia [16]. In this case, the NPs was characterized as a spherical shape of slightly bigger size of 80 nm. Raza et al. [17] presented the results of the antibacterial performance of three bacteria, E. coli, P. aeruginosa, and B. subtilis, at different concentrations of the Co NPs 1 mg/ml, 50 mg/ml, and 100 mg/ml, respectively. It was concluded that the best performance was obtained at the highest concentration. Shahzadi et al. [18] tested the performance of CoNPs with an average particle size of 27.42 nm on antimicrobial activity. Co NPs showed lower activity against gram-positive bacteria B. subtilis (inhibition zone diameter: 42.18 mm) compared to the gram-negative E. coli 51.83 mm. However, compared with a reference antibacterial drug Rifampicin, both bacteria showed lower inhibition zone diameters. In a more recent study, Gupta et al. [19] reported that Co NPs demonstrated better antibacterial efficacy than their bulk form. Furthermore, the Co NPs were effective even at lower concentrations (0.125 μg/ml) against S. aureus and E. coli and showed better efficacy than standard antibiotics. Satpathy G. and Manikandan E. [20] reported that cobalt nanoparticles have a sensitive antiseptic effect for the gram-negative Escherichia coli strains and present the results of the inhibition zone (mm) of different concentrations of Co NPs of about 18, 20, 25, 27 in diameter for 5 μg/ml 15 μg/ml, 25 μg/ml, and 35 μg/ml of Co NPs concentrations, respectively. Anwar et al. [21] reported that Co NPs prepared by different techniques (utilizing hydrothermal and ultrasonication) were used as novel nanotherapeutics against Acanthamoeba castellanii. At present, the limited information available in the literature about the effectiveness of the cobalt nanoparticle (Co NPs) as an antibacterial agent on varieties of bacteria demands further investigation. Although several reports are available on killing bacteria with Co or cobalt complex NPs, to the best of the author’s knowledge, no studies have determined the antibacterial effect of Co NPs against all three selected bacteria in this study. Furthermore, studies related to comparative assessment on the antibacterial effects between the Co NPs and commonly available antibiotics are still lacking. Therefore, the present study was conducted to determine the physical properties and to estimate the antimicrobial activity of the Co NPs against three multi-drug resistant bacteria.
Material and Methods
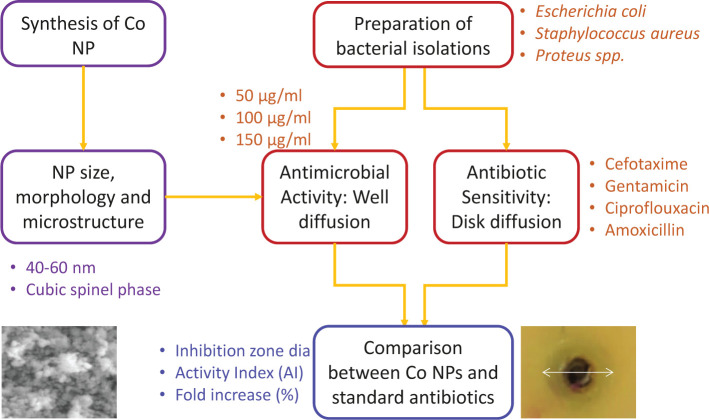
The study design to synthesize Co NPs and assess their antibacterial effect is presented in Figure 1.
Figure 1.
Methodology adopted for evaluating the antibacterial effect of Co NPs.
Preparation and characterization of Cobalt Nanoparticles (Co NPs)
Hydrazine monohydrate and cobalt sulfate heptahydrate (CoSO4.7H2O) were utilized as the precursors for synthesizing Co NPs [22]. A 0.2 M solution of sodium citrate dihydrate was added to 10 ml of 0.1 M aqueous cobalt sulfate solution. The solution was kept up at a foreordained temperature and permitted to respond for 60 min to 120 min. Afterward, the solution was centrifuged at 4000 rpm for 1hr; the suspensions were taken out and washed a few times with distilled water and dried utilizing a vacuum dryer at a temperature of 80°C. The nanoparticles on a glass slide were placed in a scanning electron microscope (SEM) to observe the particle morphology (Inspect S50, FEI company, Netherlands) at an accelerating voltage of 12.5 kV. The phase structure and orientation of the Co NPs were determined by X-ray diffraction (XRD) technique using a Shimadzu-XRD 6000 (Shimadzu Company, Japan) diffractometer with a Bragg Brentano geometry and employing a CuKα source (40 kV, 30 mA) at an incident angle of 2°. The average crystallite dimensions were estimated by the Scherrer formula [23].
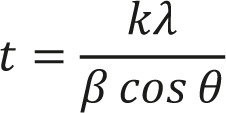 |
Where k is the shape factor (usually 0.9), λ is the X-ray wavelength, θ is the Bragg diffraction angle, β is the full width at half maximum (FWHM) in radians.
Bacterial Isolation and Identification
During the study, three isolates of Escherichia coli, Staphylococcus aureus, and Proteus spp. were isolated from different clinical samples, and bacteria were identified using blood agar, MacConkeyagar, and Nutrient agar (Difco, USA). The plates were incubated for 24h at 37°C. The classical method was utilized to identify bacteria by comparing with systematic Bacteriology Bergey’s Manual.
Antimicrobial Activity Measurements of Co NPs
An agar well diffusion method was used to assess the antibacterial activities of Co NPs against the isolates of Escherichia coli, Staphylococcus aureus, and Proteus spp. [19–24]. All bacteria were suspended in sterile water and diluted to 1×108CFU/ml. The suspension was spread over Mueller Hinton’s agar by sterile cotton swab, and after 15 min, wells (8mm) were cut into the agar using a sterilized cork borer. The lower ends of the wells were closed with molten agar to prevent any leakage of the tested nanomaterial. An equal volume of 100 μl Co NPs was taken from different concentrations of the suspensions 50, 100, 150 μg/ml using a micropipette and separately poured onto the wells. The Petri plates were incubated at 37°C for 24h, and the inhibition zones were measured for each concentration and microbes. The inhibition zone is defined as the clear zone created around the wells by the antibacterial action of the Co NPs. Negative controls were set using sterile water, and the positive control using antibiotics. As expected, no inhibition zone was observed in the case of the negative control. The antibacterial activity was assessed by determining the diameters of inhibition zones of the tested bacteria according to National Committee for Clinical Laboratories Standard rules [25]. The greater the inhibition zone, the greater the antibacterial activity. All measurements were executed three times to obtain an average result.
Antibiotic Sensitivity Testing
The disk diffusion method on Muller-Hinton agar medium (MH) (Oxoid, UK) was performed to assess the sensitivity of Staphylococcus aureus, Escherichia coli, and Proteus spp. isolates against standard antibiotic disks of 8 mm, including Cefotaxime, Gentamicin, Ciprofloxacin, and Amoxicillin (Himedia, India). The isolates were suspended in sterile water and diluted according to MacFarland microbial suspension, which approximately contained 1×108 CFU/ml. The cultures were incubated at 37°C for 18 hr, according to Kirby-Baur. The zones of inhibition were determined by the National Committee for Clinical Laboratories Standard rules [25]. All measurements were executed three times to obtain an average result. Activity Index (AI) and Fold Increase were calculated based on the inhibition zone diameters using Equation 2 and Equation 3 to compare the performance of Co nanoparticles compared to standard antibiotics [19–24].
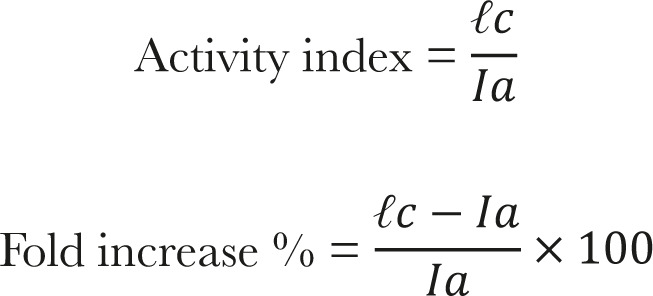 |
Where Ic is the inhibition zone diameter of Co NPs and Ia is the inhibition zone diameter of antibiotics.
Statistical analysis
A statistical design of experiments was used to study the antibacterial effect of Co NPs at different concentrations, the inhibition zone diameters of cobalt nanoparticles compared with the antibiotics, and the activity index (AI) of cobalt nanoparticles compared with the antibiotics.
Results
Structural characteristics of Co NPs
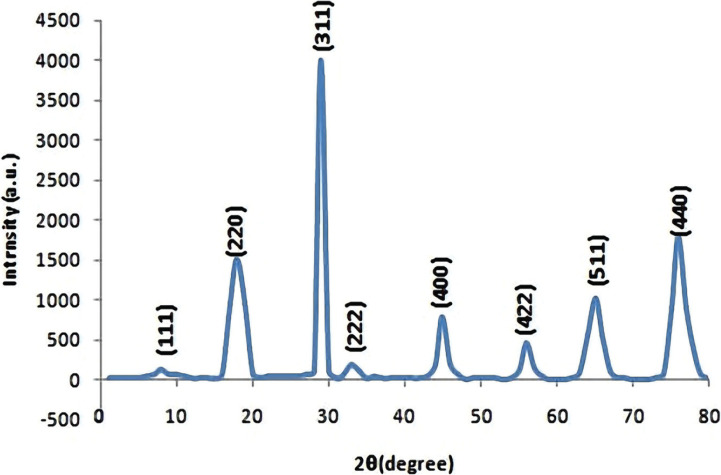
The Co NPs crystalline structure was established by XRD, as revealed in Figure 2. The XRD patterns showed a diffraction line assigned to the pure cubic spinel phase. Well-developed peaks corresponded to 111, 220, 311, 222, 400, 422, 511, and 440 crystal planes. All measured XRD peaks match well with the standard patterns of Co. The sharp peaks confirmed the crystallinity of the Co NPs. It was also concluded that the Co NPs were pure as no peaks related to other materials were observed [26]. The particle size was estimated in the range of 50 nm to 55 nm using the Scherrer formula.
Figure 2.
XRD pattern of synthesized CoNPs.
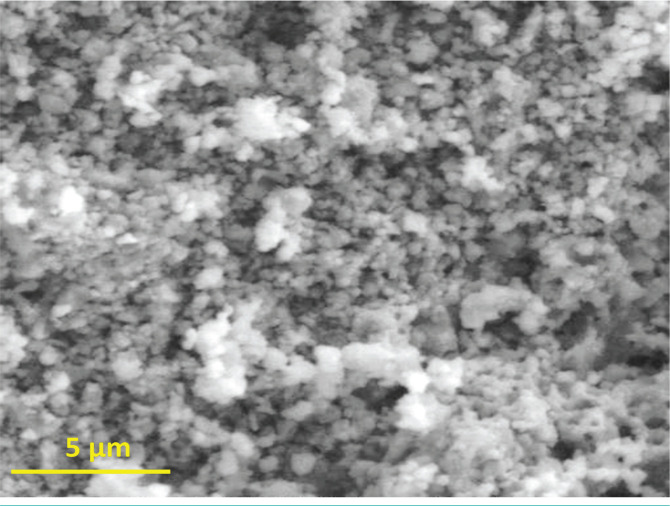
The morphological image of the CoNPs shown in Figure 3, was taken by a scanning electron microscope (SEM). The SEM image confirmed the formation of nanosized crystallites with spherical shapes. The average grain size was found to be in the range of 40 nm to 60 nm when measured using the linear intercept method. Furthermore, smaller particles were amalgamated to form clusters of larger size. Other researchers made similar observations about the Co NPs [26–27]. In general, the particle shape and size were uniform with a smooth surface, which could be related to contact with the bacteria to demonstrate increased antibacterial activities [27–28].
Figure 3.
SEM Image of Co nanoparticles.
Assessment of Antibacterial effect: Well diffusion
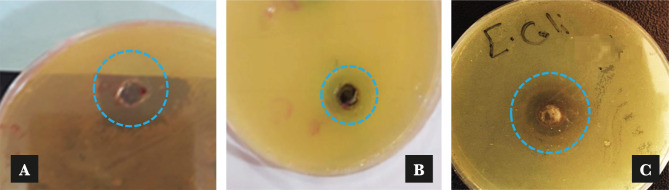
Table 1 presents the means and standard deviations of inhibition zone diameters at three different Co NPs concentrations. The results of the well diffusion method exhibited the highest antibacterial activity against Escherichia coli followed by Proteus spp. and Staphylococcus aureus indicated by the zones of inhibition reaching 22.2±0.1 mm, 20.3±0.15 mm, and 15.8±0.1 mm, respectively when a Co NPs concentration of 100 μg/ml was used. The antibacterial effect of Co NPs at different concentrations was defined by a clear zone around the wells. While the lowest inhibition zone was recorded against Proteus spp. with a diameter of 10.26±0.15 mm at 50 μg/ml of Co NPs. However, the inhibition zones were 11.5±0.1 mm and 13.23±0.15 mm against each Staphylococcus aureus and Escherichia coli, when the Co NPs concentration was 50 μg/ml. While 150 μg/ml of Co NPs recorded lower effects than the 100 μg/ml concentration having a lowest mean inhibition zone of 12.26±0.15 mm against the Proteus spp. Figure 4 presents example images of inhibition zones for different bacteria. These results agreed with Satpathy G., Manikandan E. [20], and Moradpoor et al. [29], who reported that the Co NPs showed antibacterial activity when exposed to pathogenic activity bacteria using the wells diffusion technique.
Figure 4.
Inhibition zones of bacterial isolates with 100 μg/ml concentration of Co NPs: (a) Staphylococcus aureus, (b) Proteus spp. and (c) Escherichia coli (not to be scaled).
Table 1.
Antibacterial effects of the cobalt nanoparticles using well diffusion method.
| Bacterial type | Mean±SD of inhibition zone diameters (mm) at different Co NP concentrations | ||
|---|---|---|---|
| 50 μg/ml | 100 μg/ml | 150 μg/ml | |
| Proteus spp. | 10.26±0.15 | 20.3±0.15 | 12.26±0.15 |
| Staphylococcus aureus | 11.5±0.1 | 15.8±0.1 | 13.2±0.1 |
| Escherichia coli | 13.23±0.15 | 22.2±0.1 | 15.16±0.5 |
Assessing the effect of antibiotic: Disk diffusion
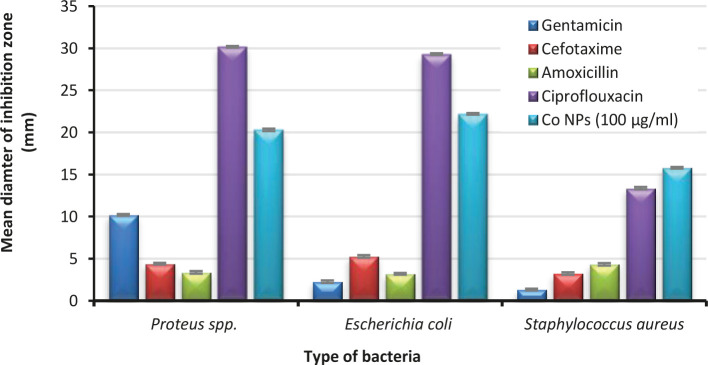
Figure 5 presents the diameters of the inhibition zone when anti-microbials sensitivity was measured against the Staphylococcus aureus, Escherichia coli, and Proteus spp. isolates controlled by a disk diffusion method. All the isolates demonstrated a high resistant rate (inhibition zone≤5 mm) to the most antibiotics utilized in this study, including Gentamicin, Ceftriaxone, and Amoxicillin except for Ciprofloxacin, which recorded the zones of inhibition extended to 30.17±0.1mm, 29.30±0.1 mm, and 13.37±0.15 mm against Proteus spp., Escherichia coli, and Staphylococcus aureus respectively.
Figure 5.
Mean of inhibition zone diameters of 100 μg/ml of cobalt nanoparticle compared with the antibiotics.
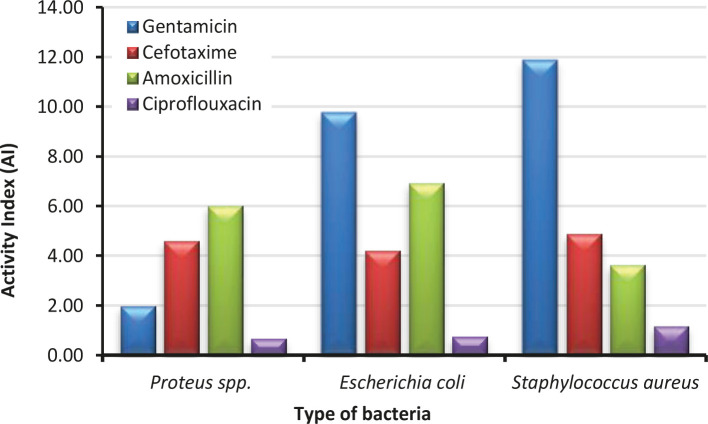
Figure 6 presents the Activity Index (AI) of Co NPs. AI is a relative measure of the effectiveness of one antimicrobial drug compared to another antibiotic drug. In general, if the AI value is greater than unity, the tested material (Co NPs) would be better than an antibiotic drug against a particular strain of bacteria [19]. The AIs were calculated for all four standard antibiotics, Gentamicin, Cefotaxime, Amoxicillin, and Ciprofloxacin, against the three selected bacteria in this study. In the case of Proteus spp. AI values of Co NPs compared to Gentamicin 1.99, Cefotaxime 4.61, and Amoxicillin 6.02 were higher than 1, indicating its better antibacterial effect. However, an AI value 0.67 lower than 1 was found for Co NP compared to Ciprofloxacin. This result led to believe that Co NPs might not be better than all antibiotics. In the case of Escherichia coli, the AI values of Co NPs compared to Gentamicin 9.78, Cefotaxime 4.21, and Amoxicillin 6.94 were higher than 1 except Ciprofloxacin 0.76. In the case of Staphylococcus aureus, the AI values of Co NPs compared to Gentamicin 11.88, Cefotaxime 4.89, Amoxicillin 3.65, and Ciprofloxacin 1.18 were higher than 1. In summary, Co NPs at a concentration of 100 μg/ml showed better bacteria-killing ability against all three strains than all four antibiotics except Ciprofloxacin against Proteus spp. and Escherichia coli.
Figure 6.
Activity Index (AI) of 100 μg/ml of cobalt nanoparticle compared with the antibiotics.
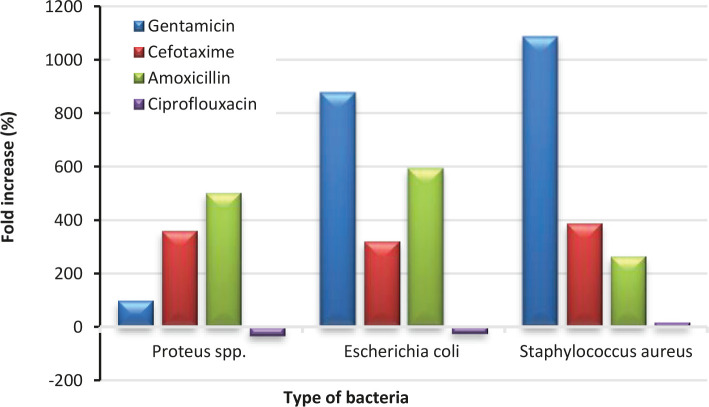
Fold increase (%) of Co nanoparticles also represented another relative measure of its antibacterial actions compared to the standard antibiotics used, as shown in Figure 7. A positive fold increase value indicated a better efficacy of the Co NP than the tested antibiotics and vice versa. Again, similar conclusions could be drawn from the results as determined by the AI results. Negative fold increases of Co NPs with respect to Ciprofloxacin against Proteus spp -32.71% and Escherichia coli -24.23% revealed slightly poorer antibacterial action than this particular antibiotic. However, significantly higher positive fold increases compared to the other three antibiotics demonstrate the superior antibacterial performance of the Co NPs.
Figure 7.
Fold increase (%) of 100 μg/ml of cobalt nanoparticle compared with the antibiotics.
Discussions
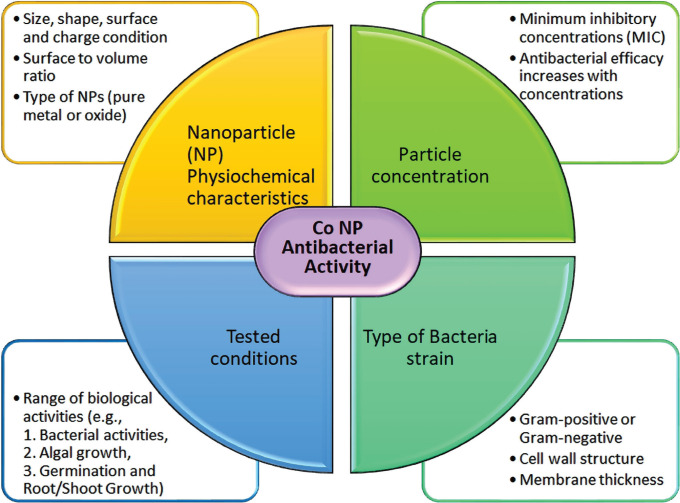
Variations were noticed in the inhibition zone diameters between this study and other studies reported in the literature. In general, for a relatively high concentration of Co NPs the inhibition zone diameters in this study were lower than the other reported values. For example, Satpathy and Manikanda evaluated the prophylactic activity of Cobalt nanoparticles towards isolated Escherichia coli with the infusion prepared at four concentrations from 5–35 μg/ml [20]. The optimum concentration was identified as 35 μg/ml, which generated an inhibition zone diameter of 27 mm. However, in this study, for the same bacteria, the inhibition zone diameter was 13 mm even at a slightly higher concentration of 50 μg/ml. This could be due to the difference in size and characteristics of the Co NPs used and their interaction with the bacteria cells. The Co NPs played a role as a potential antiseptic to control the infections by other bacteria such as B. Subtilis and Pseudomonas Sp. [10–27]. It was also suggested that Co NPs were biosafe when cell cytotoxicity tests produced minimal damage to human cells at a nanoparticle concentration of 100 μg/ml [13]. Other than Co or cobalt oxide, nanocomposite made of graphene-cobalt oxide also showed potential for antibacterial activity [30]. Literature studies reported that the inhibition zone diameter continuously increased with the increase in NP concentration [19–27]. However, for all three bacteria in this study, the inhibition zone diameters decreased at 150 μg/ml when compared to the concentration of 100 μg/ml. The exact reason for this decrease was not entirely clear. Therefore, further studies are required to explore the antibacterial effect beyond a Co NPs concentration of 150 μg/ml. A similar observation was also made by Gupta et al. [19] that the diameter of inhibition zones increased up to Co NPs concentration of 128.0 μg/ml against S. aureus and E. coli. and the inhibition zone started to shrink beyond this concentration. The authors reasoned that the nanoparticles might start to agglomerate beyond an optimum concentration and gradually lose their ability to penetrate the bacteria cell. It was reported that the size of the nanoparticles could play an important role in the antibacterial activity as the surface-to-volume ratio increases with a reduction in the size of the nanoparticles [9]. Kong et al. [26] evaluated the effects of two different Co NPs sizes on the antibacterial characteristics. Group A nanoparticle had a size ranging between 10 nm to 30 nm with some particles larger than 50 nm, whereas Group B mainly had a range between 80 nm to 150 nm with many over 200 nm. In particular, it was confirmed that there was a clear indication that smaller CoNPs showed a higher 1.2-to-1.5-fold inhibitory effect compared to the larger nanoparticle group with the tested conditions. It was hypothesized that the physical characteristics of the particles were more impactful compared to the antibacterial action caused by the ions released by the CoNPs. In general, the effect of Co NPs depends on the NP’s physical & chemical characteristics, particle concentration, the type of bacteria strains, and the tested conditions, Figure 8 [26–28].
Figure 8.
Factor affecting Co NPs effectiveness in antibacterial action.
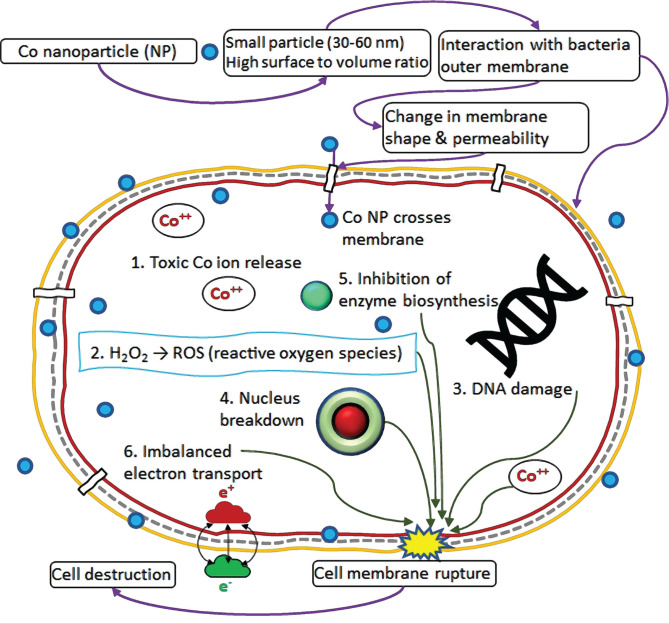
It was clear from Table 1 that the inhibition zones for the gram-negative bacteria (Proteus spp. and Escherichia coli) were bigger than that of the gram-positive bacteria (Staphylococcus aureus). Other researchers noted similar observations for Co NPs [26–31]. This behavior could generally happen due to the difference in the bacterial cell wall structure and/or size, shape, surface, and charging states of the metallic nanoparticles used. This could be perceived because the cell walls of the gram-negative bacteria (Proteus spp. and Escherichia coli) were comprised of thin layers of peptidoglycan 8 nm under the outer membrane lipopolysaccharides (1–3 μm thick). On the other hand, the gram-positive bacteria (Staphylococcus aureus) possess a thicker peptidoglycan 80 nm layer with a porous structure. This difference in cell structure makes the gram-negative bacteria more susceptible to cell destruction [28–32]. Further details about the gram-positive and gram-negative bacteria were provided by Hoseinzadeh et al. [7]. Other physicochemical characteristics of the nanoparticles used and their interaction with the cell wall can play an important role in showing strong antibacterial action against gram-negative bacteria. The positively charged Co ions could be attracted by the cell of the gram-negative bacteria and led to inhibition of different biological processes. Furthermore, a greater tendency of the Co NPs to agglomerate might reduce its antibacterial action against the gram-positive Staphylococcus aureus [33]. However, for the cases of Co oxide and other oxide NPs, evidence of the opposite trend was also noticed [24–27]. The higher effectiveness of the Co oxide NPs against gram-positive bacteria could be related to an enhanced cell wall permeability resulting from the interactions with the nanoparticles [12]. At present, there is still limited understanding on the exact mechanism of the antibacterial action of the metallic nanoparticles. However, based on different explanations provided in the literature [3, 11, 13, 32–35], the antibacterial actions of the Co NPs could be summarized as in Figure 9. First, the small Co NPs with a high surface-to-volume ratio interacted with the bacteria’s outer membrane and caused a change in its shape and permeability. This higher permeability allowed the NPs to enter the cell. Toxic Co ions were then released by the nanoparticles and induced the synthesis of highly reactive oxygen species and cellular oxidative stress [36]. This could cause damage to the DNA [37], nucleus breakdown, and imbalance in electron transport across the cell wall. Furthermore, the interaction of Co NPs with thiol groups (-SH) of enzymes in bacteria causing inactivation and death of pathogens could be a potential mechanism for the antibacterial action [27]. The existence of metal ions on the bacterial cell surface is facilitated by the thiol groups (-SH) of proteins. The produced proteins with the carried nutrients penetrated the cell membrane, wherein the inactivation of proteins was initiated by the NPs, thus causing the bacterial demise [11]. Finally, Co NPs showed remarkable inhibition effects against the growth of E. coli and S. aureus; they can be deployed for treating infectious diseases that occurred by these pathogenic organisms.
Figure 9.
Possible cell death mechanism of bacteria in contact with Co nanoparticles
One of the objectives of the present study was to estimate the antibiotic resistance of the clinical isolates [38]. Bacteria developed different methods to be resistant against anti-microbials. For example, resistance to β lactam antibiotics (Ceftriaxone and Amoxicillin) was essentially caused by the production of β-lactamases, which was noticed among all the isolates in the present study. The β- lactamases enzyme plays a major role in the resistance to β-lactam antibiotics. Also, Staphylococcus aureus becomes resistant to the β-lactam drug by decreasing the permeation through the outer membrane, which reduces the affinity for penicillin-binding protein [38–39]. Furthermore, some aminoglycosides and Ciprofloxacin might show in vitro action against Escherichia coli and Proteus spp. isolates, leaving a long way off the β-lactam antibiotics. It was interesting to note that although Ciprofloxacin was the best among the antibiotics used, again, similar to Co NPs, it showed better action against the gram-negative bacteria than the gram-positive one. Poor resistance of gentamicin against Escherichia coli 2.27±0.15 mm and Staphylococcus aureus 1.33±0.1 mm might be due to the production of various active enzymes, including aminoglycoside modifying enzymes (AME) which could damage the power of antibiotics [40]. Several fluoroquinolone antibiotics, such as ciprofloxacin, displayed a high action of fluoroquinolones to prevent bacterial growth by inhibiting DNA replication. More lately, researchers have recognized that plasmid-mediated horizontally transferable genes (Qnr) are capable of protecting DNA gyrase from quinolones [41]. Varaprasad et al. evaluated the antibacterial performance of green synthesized Co NPs (48 nm) using the disc diffusion with a concentration of 50 μg/ml against human pathogenic gram-negative bacteria (S. dysenteriae and E. faecalis) and compared to a standard antibiotic [42]. The inhibition zone diameters for these two bacteria (14 mm and 13 mm, respectively) were slightly lower than Ciprofloxacin (15 mm), indicating that Co NPs demonstrated effective antibacterial activity on the pathogenic bacteria. Gupta et al. studied the antibacterial activity of Co NPs from very low (0.125 μg/ml) to high (128.0 μg/ml) concentrations against S. aureus and E. coli [19]. The results clearly indicated better antibacterial action of Co NPs compared to the bulk Co and gentamicin at all concentrations but slightly poorer than Oxytetracycline. This result agreed well with the present study. Hafeez et al. also found that Co oxide nanoparticles showed better antibacterial action against two gram-positive bacteria, Bacillus subtilis and Bacillus licheniformis, compared to bacitracin antibiotics [27]. Al-Tamimi [34] showed high growth inhibition against Staphylococcus aureus and Escherichia coli with Co3O4 NPs than amoxicillin at 100 μg/ml concentration. However, not many antibiotics were tested in the above studies, unlike four antibiotics in this study.
It was clear that Co NPS were more effective against the two gram-negative bacteria compared to the one gram-positive bacteria used in this study. Further studies could explore its effectiveness on other gram-positive bacteria to identify a clear trend. This finding also indicated that Co NPs alone are not equally effective against all types of bacteria. Therefore, the applications of multiple nanoparticles [43] or a combination of standard antibiotics and Co NPs [44–45] can be further studied. The efficiency of Co NPs synthesized by a green route can be compared as well [10]. With all these development opportunities, Co NPs can play a vital role against antibacterial infections for therapeutical applications and create a revolution in the healthcare sector with applications including medical devices such as catheters, wound care, dental hygiene, and antibacterial soaps.
Furthermore, it is envisaged that the applications of Co NPs can go far beyond the healthcare sector, such as the garments industry, food and drink industry, care homes, water purification, air purifications, manufacturing industry, chemicals, and pharmaceuticals industry etc with Co NPs based products in the forms of a solution, paste, thin coating, or solid nanocomposite material. In real-life scenarios, there could be more than one MDR bacteria present in the same location. This study demonstrated that the Co NPs were equally effective against some common MDR gram-positive and gram-negative bacteria. Therefore, in the future, Co NPs give hope of developing new therapeutic medicine to replace the less effective traditional standard antibiotic.
Conclusion
In summary, SEM and XRD studies confirmed that Cobalt nanoparticles (Co NPs) were synthesized from cobalt sulphate solution with a size ranging from 40 nm to 60 nm. The nanoparticles showed a crystalline structure with a round shape and smooth surface. The antibacterial resistance of Co NPs against three common bacteria such as Staphylococcus aureus, Proteus spp., and Escherichia coli was assessed in this study. The results demonstrated that the Co NPs possessed strong antibacterial activity during the in vitro examination and may help kill multidrug-resistant (MDR) bacteria and be suitable for treating disease. The Co NPs were effective for both gram-positive and gram-negative bacteria. The optimum concentration of the Co NPs was identified as 100 μg/ml, which could provide a similar or higher antibacterial effect than several standard antibiotics available in the market. Moreover, this study suggested that the CoNPs can be used in medical instrumentation, antibiotics disinfection, and detergents. The prepared CoNPs showed amazing antibacterial activity against Staphylococcus aureus (gram-positive), Proteus spp. (gram-negative) and Escherichia coli (gram-negative) bacteria. The results obtained confirm that green synthesized CoNPs using Hibiscus cannabinus leaf extract will bring a promising application in the field of medicine.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest.
Authorship
AAA contributed to methodology, data characterization of the microbiology part, writing the original draft, results, discussion. WMA contributed to methodology, data characterization of the physical part, writing the original draft, results, and discussion. WKA and NHA contributed to writing the introduction. JH contributed to writing the original draft, results, and discussion.
References
- 1.Roca I, Akova M, Baquero F, Carlet J, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015 Apr 16;6:22–9. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, Harbarth S, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet Infectious Diseases. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Hajipour MJ, Fromm KM, Ashkarran AA, de Abeasturi DJ, et al. Antibacterial properties of nanoparticles. Trends in Biotechnology. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Baptista PV, McCusker MP, Carvalho A, Ferreira DA, et al. Nano-Strategies to Fight Multidrug Resistant Bacteria-“A Battle of the Titans”. Front Microbiol. 2018 Jul 2;9:1441. doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011 Dec 10;156(2):128–45. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Shrivastava S, Bera T, Roy A, Singh G, et al. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22):225103. doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- 7.Hoseinzadeh E, Makhdoumi P, Taha P, Hossini H, et al. A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms. Curr Drug Metab. 2017;18(2):120–128. doi: 10.2174/1389200217666161201111146. [DOI] [PubMed] [Google Scholar]
- 8.Vimbela GV, Ngo SM, Fraze C, Yang L, Stout DA. Antibacterial properties and toxicity from metallic nanomaterials [published correction appears in Int J Nanomedicine. 2018 Oct 16;13:6497]. Int J Nanomedicine. 2017;12:3941–3965. doi: 10.2147/IJN.S134526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azam A, Ahmed AS, Oves M, Khan MS, et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012;7:6003–9. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waris A, Din M, Ali A, Afridi S, et al. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021;16(1):14–30. doi: 10.1515/biol-2021-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chemistry. 2020;22(9):2643–2661. doi: 10.1039/D0GC00885K.. [DOI] [Google Scholar]
- 12.David SA, Veeraputhiran V, Vedhi C. Biosynthesis of cobalt oxide nanoparticles—A Short Review. J. Nanosci. Tech. 2019;5:734–737. doi: 10.30799/jnst.S01.19050308. [DOI] [Google Scholar]
- 13.Anwar A, Numan A, Siddiqui R, et al. Cobalt nanoparticles as novel nanotherapeutics against Acanthamoebacastellanii. Parasites Vectors. 2019;12:280. doi: 10.1186/s13071-019-3528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igwe OU, Ekebo ES. Biofabrication of cobalt Nanoparticles using leaf extract of Chromolaenaodorata and their potential antibacterial application. Research Journal of Chemical. 2018;8(1):11–17. https://doi.org/10.1515 . [Google Scholar]
- 15.Suvarta KD, Gurunath NH, Gavade Shuhangi MJ, Sachinkumar PR, Kishor GV. Biogenic synthesis of cobalt nanoparticles using Hibiscus cannabinus leaf extract and their antibacterial activity. Res J Chem Environ. 2020;24(5):9–13. [Google Scholar]
- 16.Koyyati R, Kudle KR, Padigya PR. Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanussativus var. longipinnatus leaf extract. Int J PharmTech Res. 2016;9(3):466–72. doi: 10.1515/biol-2021-0003. [DOI] [Google Scholar]
- 17.Raza MA, Kanwal Z, Riaz S, Naseem S. Synthesis, characterization and antibacterial properties of nanosized cobalt particles. In Proceedings of the 2016 World Congress on Advances in Civil, Environmental, and Materials Research (ACEM16), Jeju Island, Korea. 2016. p. 28.
- 18.Shahzadi T, Zaib M, Riaz T, Shehzadi S, et al. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian J Sci Eng. 2019;44(7):6435–44. doi: 10.1007/s13369-019-03937-0. [DOI] [Google Scholar]
- 19.Gupta V, Kant V, Sharma AK, Sharma M. Comparative assessment of antibacterial efficacy for cobalt nanoparticles, bulk cobalt and standard antibiotics: A concentration dependent study. Nanosystems: Physics, Chemistry, Mathematics. 2020;11(1):78–85. doi: 10.17586/2220-8054-2020-11-1-78-85. [DOI] [Google Scholar]
- 20.Satpathy G, Manikandan E. Cobalt Nanoparticle As The Antibacterial Tool: In Vitro. International Journal of Engineering and Advanced Technology (IJEAT) 2019;8:3684–3687. doi: 10.35940/ijeat.F9374.088619. [DOI] [Google Scholar]
- 21.Anwar A, Numan A, Siddiqui R, Khalid M, Khan NA. Cobalt nanoparticles as novel nanotherapeutics against Acanthamoeba castellanii. Parasites & Vectors. 2019;12(1):280. doi: 10.1186/s13071-019-3528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salman SA, Usami T, Kuroda K, Okido M. Synthesis and Characterization of Cobalt Nanoparticles Using Hydrazine and Citric Acid. Journal of Nanotechnology. 2014;2014:e525193. doi: 10.1155/2014/525193. [DOI] [Google Scholar]
- 23.Cullity BD. 3rd edition. Addison-Wesley, Boston, Mass, USA: Elements of X-Ray Diffraction; p. 2001. [Google Scholar]
- 24.Omran BA, Nassar HN, Younis SA, El-Salamony RA, et al. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillusbrasiliensis ATCC 16404-optimization, characterization and antimicrobial activity. Journal of applied microbiology. 2020;128(2):438–457. doi: 10.1111/jam.14498. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. Approved standard M07-A8. Wayne, Pa: Clinical and Laboratory Standards Institute; 2010. Performance Standards for Antimicrobial Susceptibility Testing; 20th Informational Supplement. [Google Scholar]
- 26.Kong IC, Ko KS, Koh DC, Chon CM. Comparative Effects of Particle Sizes of Cobalt Nanoparticles to Nine Biological Activities. Int. J. Mol. Sci. 2020;21:6767. doi: 10.3390/ijms21186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafeez M, Shaheen R, Akram B, Haq S, et al. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Materials Research Express. 2020;7(2):025019. doi: 10.1088/2053-1591/ab70dd. [DOI] [Google Scholar]
- 28.Bankier C, Matharu RK, Cheong YK, Ren GG, et al. Synergistic antibacterial effects of metallic nanoparticle combinations. Scientific reports. 2019;9(1):1–8. doi: 10.1038/s41598-019-52473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moradpoor H, Safaei M, Rezaei F, Golshah A, et al. Optimisation of cobalt oxide nanoparticles synthesis as bactericidal agents. Open access Macedonian journal of medical sciences. 2019;7(17):2757. doi: 10.3889/oamjms.2019.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsharaeh E, Mussa Y, Ahmed F, Aldawsari Y, et al. Novel route for the preparation of cobalt oxide nanoparticles/reduced graphene oxide nanocomposites and their antibacterial activities. Ceram Int. 2016;42(2):3407–3410. doi: 10.1016/j.ceramint.2015.10.135. [DOI] [Google Scholar]
- 31.Zaib M, Shahzadi T, Muzammal I, Farooq U. Catharanthusroseus extract mediated synthesis of cobalt nanoparticles: evaluation of antioxidant, antibacterial, hemolytic and catalytic activities. Inorganic and Nano-Metal Chemistry. 2020;50(11):1171–1180. doi: 10.1080/24701556.2020.1737819. [DOI] [Google Scholar]
- 32.Slavin YN, Asnis J, Häfeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. Journal of nanobiotechnology. 2017;15(1):1–20. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Tamimi AM, Hummadi EH, Hammadi M. Antibacterial activity of ZnO and Co3O4 nanoparticles synthesized by co-precipitation method, Biochemical and Cellular Archives. 2020;19(2):3489–3494. doi: 10.35124/bca.2019.19.2.3489. [DOI] [Google Scholar]
- 35.Ajarem JS, Maodaa SN, Allam AA, Taher MM, Khalaf M. Benign Synthesis of Cobalt Oxide Nanoparticles Containing Red Algae Extract: Antioxidant, Antimicrobial, Anticancer, and Anticoagulant Activity. Journal of Cluster Science. 2021. pp. 1–12. [DOI]
- 36.Abdal Dayem A, Hossain MK, Lee SB, Kim K, et al. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int J Mol Sci. 2017 Jan 10;18(1):120. doi: 10.3390/ijms18010120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol. 2005 Nov;71(11):7589–93. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson E, Kaur P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Frontiers in Microbiology. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995 Oct;8(4):557–84. doi: 10.1128/CMR.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005 Oct;18(4):657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 2006 Oct;6(10):629–40. doi: 10.1016/S1473-3099(06)70599-0. [DOI] [PubMed] [Google Scholar]
- 42.Barros JC, Pinheiro SR, Bozza M, Gueiros-Filho FJ, et al. Evidences of gentamicin resistance amplification in Klebsiellapneumoniae isolated from faeces of hospitalized newborns. Mem Inst Oswaldo Cruz. 1999 Nov-Dec;94(6):795–802. doi: 10.1590/S0074-02761999000600016. [DOI] [PubMed] [Google Scholar]
- 43.Varaprasad T, Govindh B, Rao BV. Green synthesized cobalt nanoparticles using Asparagus racemosus root extract & evaluation of antibacterial activity. International Journal of ChemTech Research. 2017;10:339–345. doi: 10.18488/journal.64. [DOI] [Google Scholar]
- 44.Kanwal Z, Raza MA, Riaz S, Manzoor S, et al. Synthesis and characterization of silver nanoparticle-decorated cobalt nanocomposites (Co@AgNPs) and their density-dependent antibacterial activity. Royal Society Open Science. 6(5):182135. doi: 10.1098/rsos.182135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mu H, Tang J, Liu Q, Sun C, Wang T, Duan J. Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria. Sci Rep. 2016;6(1):18877. doi: 10.1038/srep18877. [DOI] [PMC free article] [PubMed] [Google Scholar]