Abstract
In this forum piece, we review progress in exploiting diet and nutrition for enhancing tissue regeneration with a particular emphasis on how dietary composition and diet-induced physiology influence adult stem cell biology.
Introduction
The idea of promoting health by nutrition modification has a long history. Over 400 years ago, Luigi Cornaro described “How to Live One Hundred Years” with dietary restriction. In the past century, studies in model organisms have demonstrated that a reduction in total calorie intake (i.e., caloric restriction [CR]) promotes longevity in species ranging from yeast to mammals. More recently, restriction of specific nutritional components (i.e., dietary restriction [DR]) such as the amino acid methionine mimic lifespan extension by CR (Trepanowski et al., 2011). To date, a variety of caloric and dietary restriction regimens have been shown to improve human health. Here, we will discuss our perspective of how viewing diet through the lens of regenerative health may offer new translational insights to the traditional approach of calorie counting.
Looking Within the Stem Cells
It is impossible to discuss pro-regenerative diets without acknowledging recent advances in understanding stem cell metabolism. New methods that include high-resolution metabolomics, proteomics, and transcriptomics have allowed biologists to study cell metabolism in a cell-type- and cell-state-specific manner. In the past decade, for example, studies have identified unique metabolic signatures of stem cells (Chandel et al., 2016; Shapira and Christofk, 2020). Besides drawing correlations, the metabolic cell-fate determinants can now be functionally screened in vitro, owing to the technical advances in 3D organoid cultures and genome editing and validated in vivo using the inducible cell-type-specific transgenic mouse models in combination with relevant dietary models (Figure 1).
Figure 1. Deciphering the Metabolic Regulation of Adult Stem Cell Fate.
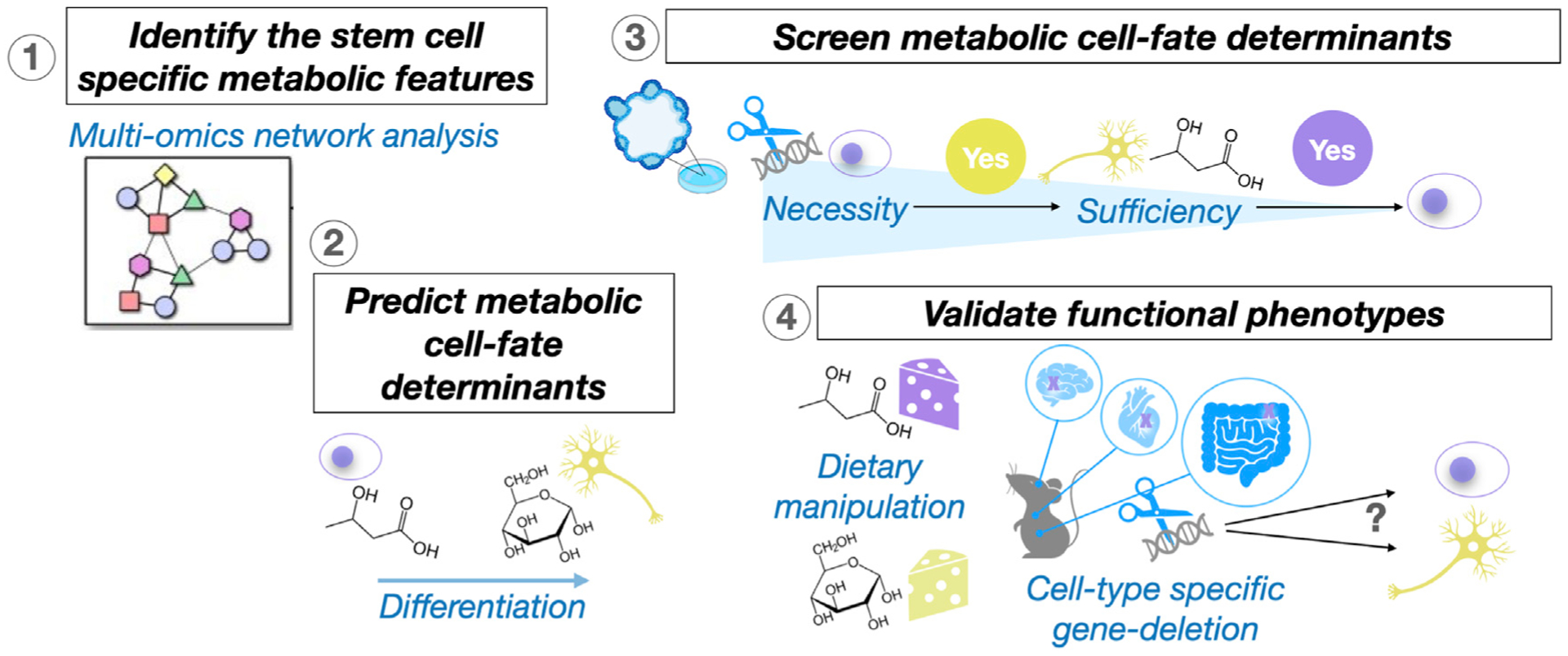
Omics analyses uncover the association between the metabolic features and cell identities at single-cell resolution. Organoid cultures and genome-editing technology enable large-scale screening for the metabolic cell-fate determinants. Precision dietary and genetically engineered mouse models help determine in vivo functional phenotypes.
Years of function-driven characterization in multiple adult stem cell systems have yielded strong causal evidence implicating involvement of tissue- or cell-type-dependent metabolic programs in influencing signaling pathways for stem cell self-renewal and fate specification (Chandel et al., 2016; Shapira and Christofk, 2020). Once thought to be a mere consequence of the cell state, the emerging view of stem cell metabolism is that it can also play an instructive role in fate decisions. Diets that alter nutrient availability and energy metabolism at the cellular level, therefore, can be used as an effective approach to modulate stem cell activity for tissue regenerative purposes (Shapira and Christofk, 2020; Spehar et al., 2020).
The Pro-regenerative Diets
A hallmark of aging is the decline of stem cell function; thus, dietary models that promote life- or health-span have been intuitive approaches for restoring aged tissue health (Spehar et al., 2020). Three prototypical regenerative diets include (1) CR that reduces caloric intake for an extended period of time (i.e., months to years), (2) fasting and fasting-mimicking interventions that deprive food or macro-nutrients (i.e., carbohydrates, proteins, and fats) for a short period of time (24–48 h) and that can include cycles of fasting and refeeding, and (3) the ketogenic diets that emulate the metabolic changes noted in CR and fasting without reduced calorie intake (Figure 2A).
Figure 2. Developing Pro-regenerative Dietary Approaches.
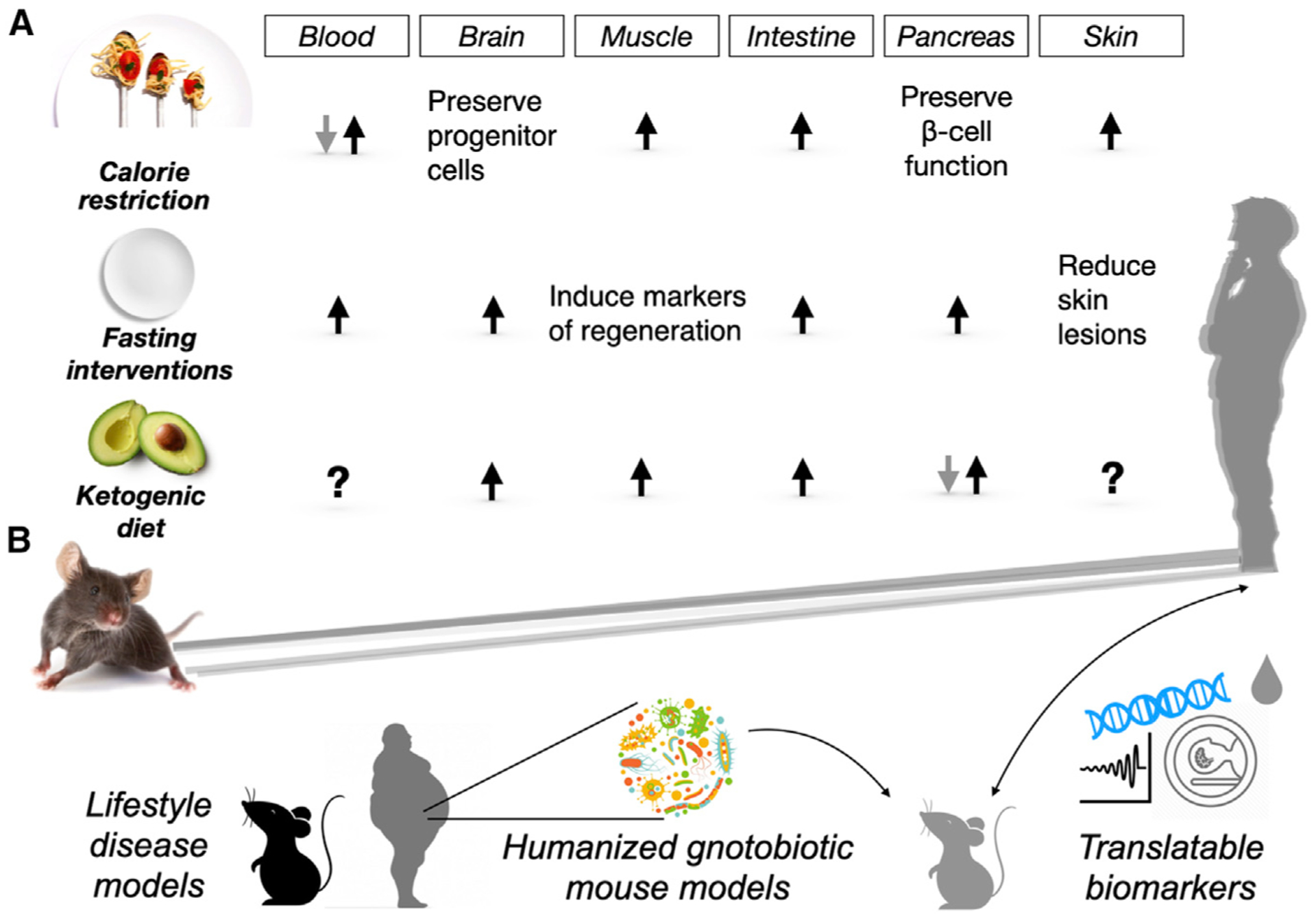
(A) Pro-regenerative effects of longevity- and health-promoting interventions. Calorie restriction, fasting interventions, and ketogenic diets exert beneficial effects on adult stem and progenitor cells in many tissues and organs, highlighting their potential for tissue regeneration. Black arrows indicate activation, while gray arrows denote inhibition of regenerative activity. Question marks highlight unanswered areas. (B) Approaches to overcome challenges in translating discoveries from mouse models to humans. Lifestyle disease models address prevalence of metabolic disorders such as obesity and diabetes in human populations. Humanized microbiome gnotobiotic mouse models help delineate the effects of processed food on the gut microbiome. Translatable biomarkers increase the relevance and solidify the promises of pre-clinical studies.
Caloric Restriction
In mice, periods of CR (10% to 40% restriction) elevate stem cell numbers and tissue repair after injury in a multitude of tissues such as the muscle (3-month CR), intestine (7-month CR), and skin (6-month CR). However, the beneficial effects are also tissue-dependent (Spehar et al., 2020). In the brain, although CR (stepwise fashion to 40% of free feeding weight by 16 weeks) preserves the number of progenitor cells committed to becoming neurons and reduces senescence in the subventricular zone, it does not protect against the age-related remodeling of the vasculature and other degenerative phenotypes. In the hematopoietic system, the onset and duration of CR also determine the outcomes. Whereas long-term (9-month) young-onset CR improves hematopoietic stem cells’ (HSCs) self-renewal and reduces myeloid-biased HSCs in aged mice, short-term (4-month) mid-age-onset CR fails to benefit aged HSCs in terms of line-age-biased differentiation (Spehar et al., 2020). In the pancreas, short-term CR prevents the loss of pancreatic β cells in a type 2 diabetes (T2D) mouse model, although it is unclear whether this occurs via β cell regeneration or protection from damage/insults (Asif et al., 2020). A caveat relevant to most diet-based studies is that variation in experimental conditions such as diet timing or composition limits the ability to generalize effects across distinct tissues.
In spite of the long history of CR research in model organisms including primates, little has been done to further extend these findings to humans. In 2002, the NIH sponsored the first clinical trial to elucidate the effects of sustained CR in humans—CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy). Results continue to be published since 2005 and suggest that CR, through cellular and metabolic adaptations, dampens age-related risks for diabetes, heart disease, and stroke. Although tissue regeneration is outside the scope of CALERIE, the CALERIE datasets and biospecimens are invaluable resources that may enable the investigation of how CR and aging alter tissue remodeling.
Fasting Interventions
In contrast to CR, fasting regimens primarily focus on the length of time between meals and not total calories, as they have no restriction on calories during feeding intervals. Fasting can be applied chronically as cycles of intermittent fasting (IF; overnight fasting, daily) or prolonged fasting (PF; 48 h or longer, every other week). Short-term fasting (24 h) enhances mouse intestinal stem cell (ISC) function during aging and intestinal epithelial repair after injury (Di Francesco et al., 2018; Shapira and Christofk, 2020). Chronic IF promotes neurogenesis and improves cognitive functions (Di Francesco et al., 2018), and PF cycles protect HSCs against chemotoxicity and restore lineage-balanced differentiation (Chandel et al., 2016). However, fasting interventions are not easy to implement or feasible in daily life and carry risk for frail patients including diabetics or the very elderly. As an alternative, low-calorie (>50% reduced) fasting-mimicking diets (FMDs) with sugars, protein, and unsaturated fats were developed to emulate a fasting physiology: reduced levels of insulin, insulin-like growth factor 1 (IGF-1), and glucose (Di Francesco et al., 2018). Mice fed periodically with an FMD have an extended healthspan, reduced inflammation, lower cancer incidence, and enhanced cognitive performance. Furthermore, FMD feeding is accompanied by improved multisystem regeneration. More recently, FMD has been proposed to exert a therapeutic, anti-diabetic effect by fostering pancreatic β cell regeneration that restores insulin secretion and glucose homeostasis in mice (Di Francesco et al., 2018).
To assess the feasibility and efficacy of FMD in humans, a randomized control trial was conducted to compare the outcomes of subjects who consumed the FMD for five consecutive days per month for three months (Di Francesco et al., 2018). Results indicate that three FMD cycles are well-tolerated and sufficient to reduce body fat and decrease levels of circulating IGF-1. A post hoc analysis further revealed that FMD decreases not only BMI but also blood glucose, triglycerides, cholesterol, and inflammation markers, indicating that such interventions may counter metabolic syndrome or age-related diseases. Nevertheless, extensive studies in patients with defined diseases or risk factors are needed to further validate the extent to which FMD prevents or treats disease.
Ketogenic Diet
The low-carbohydrate, high-fat ketogenic diet (KD) is perhaps the first therapeutic diet-based intervention, created for the treatment of epilepsy in the 1920s. Recent clinical and pre-clinical studies also report that a KD exerts neuroprotective effects on degenerative neural diseases, such as Alzheimer’s and Parkinson’s disease, and prevents muscle and nerve degeneration in injury models (Yildiran et al., 2020). Despite the success of the KD in treating neurodegeneration, the mechanism by which it does so is not clear. Adult mice fed isocaloric amounts of a KD have an extended median lifespan compared to controls, raising the possibility that reduced calories are not required for the pro-longevity effects of a KD. In the intestine, a short-term (4 weeks) KD increases stem cell numbers and enhances post-injury repair (Shapira and Christofk, 2020). How KDs impact diabetes and pancreatic β cell regeneration is a matter of debate. Rodent studies illustrate that a long-term KD (22 weeks) decreases glucose tolerance due to the loss of insulin-secreting β cells. However, human studies support KD’s effect on improving β cell function (Bolla et al., 2019). One explanation for this interspecies difference may involve how the gut microbiota responds to the disease and/or diet (Bolla et al., 2019). For example, type 1 diabetic (T1D) patients have enrichment of Bacteroides and depletion of lactate- and butyrate-producing bacteria, yet these microbiome-related changes are not seen in rodent studies. Future studies will be needed to gain a broader appreciation of how a KD contributes to regeneration and whether secondary changes in the microbiome or immune system undergird some of its effects.
Of note, most human diets that stimulate ketosis consist of 5%–10% kcal carbohydrate (CHO) and ≥20% kcal protein. On the other hand, ketosis induction in rodents necessitates a diet with <1% kcal CHO and <10% kcal protein and requires the micronutrient choline as a critical catalyst (Puchalska and Crawford, 2017). Currently, there are more than two hundred clinical trials investigating the therapeutic potential of the KD. A majority of the studies focus on remedying epilepsy, brain injury, Alzheimer’s disease, and Parkinson’s disease—conditions that would benefit from augmented neural regeneration. Also, other trials are underway to evaluate the efficacy of a KD in treating T1D by stimulating β cell function or regeneration. It is worth mentioning that another category of clinical trials is deciphering the effects that exogenous ketone body supplements have on various diseases. The comparisons between KDs and ketone body supplements will uncouple the actions of ketone body metabolites from that of KD-induced lipid metabolism, which is critical for future therapeutic trials.
Moving Beyond the Calories
Nutrients and metabolites may exert specific effects independent of total calorie intake. For example, restriction of the dietary amino acid valine impairs HSC self-renewal and helps condition the niche for bone marrow transplantation, while enrichment of the dietary amino acid methionine impairs liver regeneration following partial hepatectomy (Shapira and Christofk, 2020). Also, supplementation of micronutrients such as vitamin C has emerged as a new strategy to improve leukemia treatments by targeting nutritional dependencies of (pre)malignant HSCs (Shapira and Christofk, 2020). There are also endogenous metabolites such as the ketone body beta-hydroxybutyrate (βOHB) with both energetic and signaling properties, the latter of which include inhibition of histone deacetylases (HDACs). In ISCs, βOHB, through HDAC inhibition, reinforces the NOTCH program to maintain the stem cell function (Puchalska and Crawford, 2017; Shapira and Christofk, 2020). Interestingly, elevated ketone bodies are a shared physiologic feature of CR, fasting, and the KD, highlighting their probable role in actuating aspects of these diets’ regenerative outcomes.
Beyond the dietary calories and cellular energy, diet-induced endocrinology informs organismal dietary responses by coordinating long-distance interorgan communication. An important question is how endocrine signaling influences the nutrient-sensing and cell-fate decision of adult stem cells in different systems. The differential expression of hormone receptors may explain in part how diet couples stem cell function with physiology. In the bone marrow, for example, leptin receptor (LepR)-expressing cells are responsible for osteogenic, chondrogenic, and adipogenic regeneration involved in fracture healing (Ambrosi et al., 2019). Therefore, it is crucial to consider the interplay between local and systemic nutrient-sensing factors when studying stem cell dietary responses.
Conclusions
100 years of modern dietary research has brought promises but also faces challenges in exploiting diet and nutrition for tissue regeneration. The obstacles come from the scientific and cultural blind spots in interpreting the dietary studies in mice and translating these findings back to humans. First, the prevalence of metabolic disorders in urban populations sets a discrete baseline for human studies. Taking the US populations as an example, more than 30%–40% of adults are pre-diabetic, obese, or both. Dietary interventions that rely on healthy lipid metabolism may not be feasible for these populations. Second, humans consume greater amounts of processed food in the modern era. Food processing steps such as heating, freezing, seasoning, coloring, and sterilizing with antibiotics modify the nutritional value of food as well as impact the gut microbiome. Finally, food is a pivotal part of culture and is enmeshed in ethnic, religious, and social histories. Thus, unlike taking a small-molecule pharmaceutic, adopting a dietary intervention is often considered a lifestyle choice rather than a medical decision. These challenges make dietary interventions less acceptable, especially when not accompanied with biomarkers to comprehensively monitor therapeutic efficacy. Encouragingly, scientists are now overcoming the challenges mentioned above with the lifestyle disease models, humanized gnotobiotic mice, and more translatable biomarkers (Figure 2B). In the next 100 years, nutritional strategies may evolve into a holistic medical approach that exploits diet-imposed nutrient-sensing and metabolic pathways in stem cells to execute coordinated multisystem regeneration in disease and aging.
REFERENCES
- Ambrosi TH, Longaker MT, and Chan CKF (2019). A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol 7, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif S, Morrow NM, Mulvihill EE, and Kim KH (2020). Understanding Dietary Intervention-Mediated Epigenetic Modifications in Metabolic Diseases. Front. Genet 11, 590369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla AM, Caretto A, Laurenzi A, Scavini M, and Piemonti L (2019). Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 11, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, Jasper H, Ho TT, and Passegué E (2016). Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat. Cell Biol 18, 823–832. [DOI] [PubMed] [Google Scholar]
- Di Francesco A, Di Germanio C, Bernier M, and de Cabo R (2018). A time to fast. Science 362, 770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalska P, and Crawford PA (2017). Multidimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 25, 262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira SN, and Christofk HR (2020). Metabolic Regulation of Tissue Stem Cells. Trends Cell Biol. 30, 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehar K, Pan A, and Beerman I (2020). Restoring aged stem cell functionality: Current progress and future directions. Stem Cells 38, 1060–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Canale RE, Marshall KE, Kabir MM, and Bloomer RJ (2011). Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: a summary of available findings. Nutr. J 10, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiran H, Macit MS, and Özata Uyar G (2020). New approach to peripheral nerve injury: nutritional therapy. Nutr. Neurosci 23, 744–755. [DOI] [PubMed] [Google Scholar]


