Abstract
Background:
Complex medication regimens, often present in heart failure with preserved ejection fraction, may increase the risk of adverse drug effects and harm. We sought to characterize this complexity by determining the prevalence of polypharmacy, potentially inappropriate medications, and therapeutic competition (where a medication for one condition worsens another condition) in one of the few dedicated heart failure with preserved ejection fraction programs in the United States.
Methods and Results:
We conducted chart review on 231 patients with heart failure with preserved ejection fraction seen in the University of Michigan’s Heart Failure with Preserved Ejection Fraction Clinic between July 2016 and September 2019. We recorded: 1) standing medications to determine the presence of polypharmacy, defined as ≥ 10 medications; 2) potentially inappropriate medications based on the 2016 American Heart Association Scientific Statement on drugs that pose a major risk of causing or exacerbating heart failure, the 2019 Beers Criteria update, or a previously-described list of medications associated with geriatric syndromes; and 3) competing conditions and subsequent medications that could create therapeutic competition. The prevalence of polypharmacy was 74%, and the prevalence of potentially inappropriate medications was 100%. Competing conditions were present in 81% of patients, of whom 49% took a medication that created therapeutic competition.
Conclusion:
In addition to confirming that polypharmacy was highly prevalent, we found that potentially inappropriate medications and therapeutic competition were also frequently present. This supports the urgent need to develop patient-centered approaches to mitigate the negative effects of complex medication regimens endemic to adults with heart failure with preserved ejection fraction.
Keywords: medications, polypharmacy, heart failure, geriatrics
Graphical abstract: Overview of medication complexity in heart failure with preserved ejection fraction
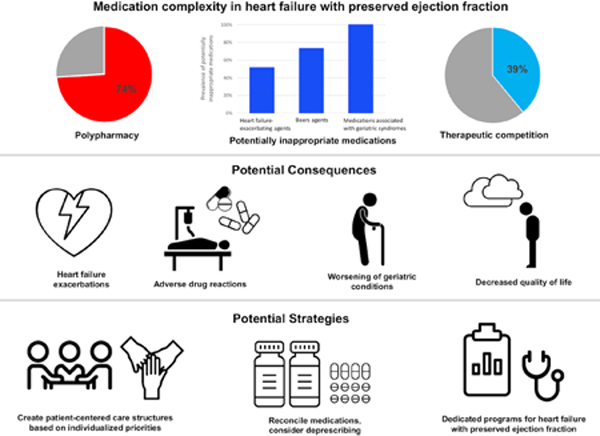
Several factors contribute to medication complexity, including polypharmacy, use of potentially inappropriate medications, and the presence of therapeutic competition. With increased medication complexity, patients with heart failure with preserved ejection fraction are at higher risk for adverse outcomes. Patient-centered approaches are needed to mitigate the negative effects of medication complexity in heart failure with preserved ejection fraction.
INTRODUCTION
Successful management of heart failure with preserved ejection fraction is challenging, but not just due to the current lack of interventions that consistently improve cardiovascular outcomes.1, 2 Patients with heart failure with preserved ejection fraction are disproportionately older adults, and geriatric conditions such as frailty, cognitive impairment, falls, reduced appetite, urinary incontinence, and/or depression are often present and impair quality of life.3–5 Multimorbidity (the presence of multiple comorbid conditions) is the rule rather than the exception in heart failure with preserved ejection fraction and is believed to closely intertwine with its pathophysiology.4, 6–8
Despite the link between heart failure with preserved ejection fraction and multimorbidity, there are few data about the accompanying complexity of medication regimens in this population.9, 10 Polypharmacy defined as taking a high burden of medications, potentially inappropriate medications defined as medications which pose risks that may outweigh benefits in older adults and therapeutic competition defined as the scenario where a medication for one condition may worsen a coexisting condition all contribute to this complexity.11–18 While these factors are well-described in the geriatrics literature and adversely affect outcomes in older adults, little has been discussed in the setting of heart failure with preserved ejection fraction, a condition that has been described as a “geriatric syndrome.” 4, 19 We accordingly sought to address this gap by examining medication patterns in adults seen in one of the few dedicated heart failure with preserved ejection fraction programs in the United States.
METHODS
We examined 231 patients seen in the University of Michigan’s Frankel Cardiovascular Center Heart Failure with Preserved Ejection Fraction Clinic between July 2016 and September 2019. This study was approved by the institutional review board of the University of Michigan, Ann Arbor, MI (HUM00052866).
Polypharmacy
Polypharmacy is broadly described as the condition of taking many medications and has been variably defined in the literature.11–14 Prior observations indicate that a large proportion of patients with heart failure take at least 10 medications and that the risk for adverse drug events is incrementally increased beyond 9 medications.20–23 Accordingly, we defined polypharmacy as ≥ 10 medications because we felt that this would identify individuals with the greatest degree of medication complexity and also those at the highest risk for adverse events.24 To determine the number of medications taken, we reviewed the first note from the Heart Failure with Preserved Ejection Fraction Clinic within the electronic medical record and counted each unique standing medication. We included all listed prescription and over-the-counter scheduled medications because both contribute to medication burden and complexity.
Potentially Inappropriate Medications
We defined potentially inappropriate medications based on their presence on one of the following 3 lists: 1) 2016 American Heart Association Scientific Statement on drugs that pose a major risk of causing or exacerbating heart failure, 2) 2019 Beers Criteria update, which outlines medications that the American Geriatric Society recommends avoiding in most older adults, in most circumstances, and 3) medications associated with geriatric syndromes, defined as one of 513 medications that are associated with or may contribute to at least one of 6 geriatric syndromes (cognitive impairment, delirium, falls, reduced appetite or weight loss, urinary incontinence, and/or depression).15, 17, 25 We opted to include medications associated with geriatric syndromes because most adults with heart failure with preserved ejection fraction—a condition that disproportionately affects older adults—either already have these conditions or are at risk for developing them. 4, 7, 19, 26, 27 To identify and count medications that would be considered potentially inappropriate medications, we cross-referenced each class of potentially inappropriate medications with the medications listed in the first note from the Heart Failure with Preserved Ejection Fraction Clinic within the electronic medical record.
Therapeutic competition
Therapeutic competition is a type of disease-drug interaction in which a guideline-recommended medication for one condition may adversely affect a coexisting condition or compete with a medication for the coexisting condition. Lorgunpai et al reviewed clinical practice guidelines for 14 common chronic conditions and identified 25 pairs of chronic conditions for which a recommended oral or inhaled prescription medication might lead to therapeutic competition.18 These conditions included atrial fibrillation or atrial flutter, benign prostatic hyperplasia, coronary artery disease, chronic obstructive pulmonary disease, dementia, depression, diabetes, gastroesophageal reflux disease or peptic ulcer disease, heart failure, hyperlipidemia, hypertension, hypothyroidism, osteoarthritis, and osteoporosis. For each pair of competing health conditions, the authors identified medications which may result in therapeutic competition. For example, the use of nonsteroidal anti-inflammatory drugs in patients with heart failure and osteoarthritis, or the use of thiazolidinediones in patients with heart failure and diabetes. To identify therapeutic competition, we reviewed the problems on the electronic medical record structured problem list as well as problems included in the first note from the Heart Failure with Preserved Ejection Fraction Clinic since free-text notes have been shown to be more sensitive then electronic medical record problem lists when assessing common comorbidities.28–32 We also reviewed medications listed in the first clinic note, as described above.
Statistical methods
We calculated medians and interquartile ranges (IQRs) for continuous variables and percentages with 95% confidence intervals for categorical variables to summarize participant characteristics and medication patterns.
RESULTS
Among 231 patients with heart failure with preserved ejection fraction, the median age was 70 years (IQR [59–76]), 64% were women, and 90% were white. The most common comorbidities were hypertension (83%), obesity (72%), obstructive sleep apnea (68%), dyslipidemia (68%), and diabetes (48%) (Table 1). The distribution of the medication count is shown in Figure 1. Polypharmacy was present in 74% of patients, with an overall median of 12 medications (IQR [9–15]).
Table 1.
Patient characteristics
| All (N=231) | With polypharmacy (n=170) | Without polypharmacy (n=61) | |
|---|---|---|---|
| Sociodemographic factors | |||
| Age in years, median (IQR a) | 70 (59–76) | 70 (58–76) | 69 (59–77) |
| Age ≥ 65 years, n (%) | 146 (63) | 108 (64) | 38 (51) |
| Female, n (%) | 147 (64) | 112 (66) | 35 (57) |
| White, n (%) | 209 (90) | 152 (89) | 57 (93) |
| Medicare as primary insurance payer, n (%) | 181 (78) | 139 (82) | 42 (69) |
| Count of comorbid conditions, median (IQR) | 7 (5–8) | 7 (6–8) | 5 (3–6) |
| Prevalence of comorbid conditions, n (%) | |||
| Hypertension | 191 (83) | 146 (86) | 45 (74) |
| Obesity | 167 (72) | 127 (75) | 40 (66) |
| Obstructive sleep apnea | 156 (68) | 121 (71) | 35 (57) |
| Dyslipidemia | 156 (68) | 131 (71) | 25 (41) |
| Diabetes | 112 (48) | 96 (56) | 16 (26) |
| Atrial fibrillation or atrial flutter | 105 (45) | 76 (45) | 29 (48) |
| Gastroesophageal reflux disease or peptic ulcer disease | 102 (44) | 87 (51) | 15 (25) |
| Chronic kidney disease | 98 (42) | 75 (44) | 23 (38) |
| Coronary artery disease | 86 (37) | 74 (44) | 12 (20) |
| Osteoarthritis | 86 (37) | 68 (40) | 18 (30) |
| Hypothyroidism | 69 (30) | 55 (32) | 14 (23) |
| Depression | 62 (27) | 53 (31) | 9 (15) |
| Chronic obstructive pulmonary disease | 62 (27) | 56 (33) | 6 (10) |
| Osteoporosis | 27 (12) | 22 (13) | 5 (8) |
| Benign prostatic hyperplasia | 12 (5) | 10 (6) | 2 (3) |
| Cirrhosis | 7 (3) | 7 (4) | 0 (0) |
| Total number of standing medications, median (IQR) | 12 (9–15) | 13 (11–16) | 7 (6–8) |
Abbreviations:
a Interquartile range
Figure 1. Distribution of medication count among patients with heart failure with preserved ejection fraction.
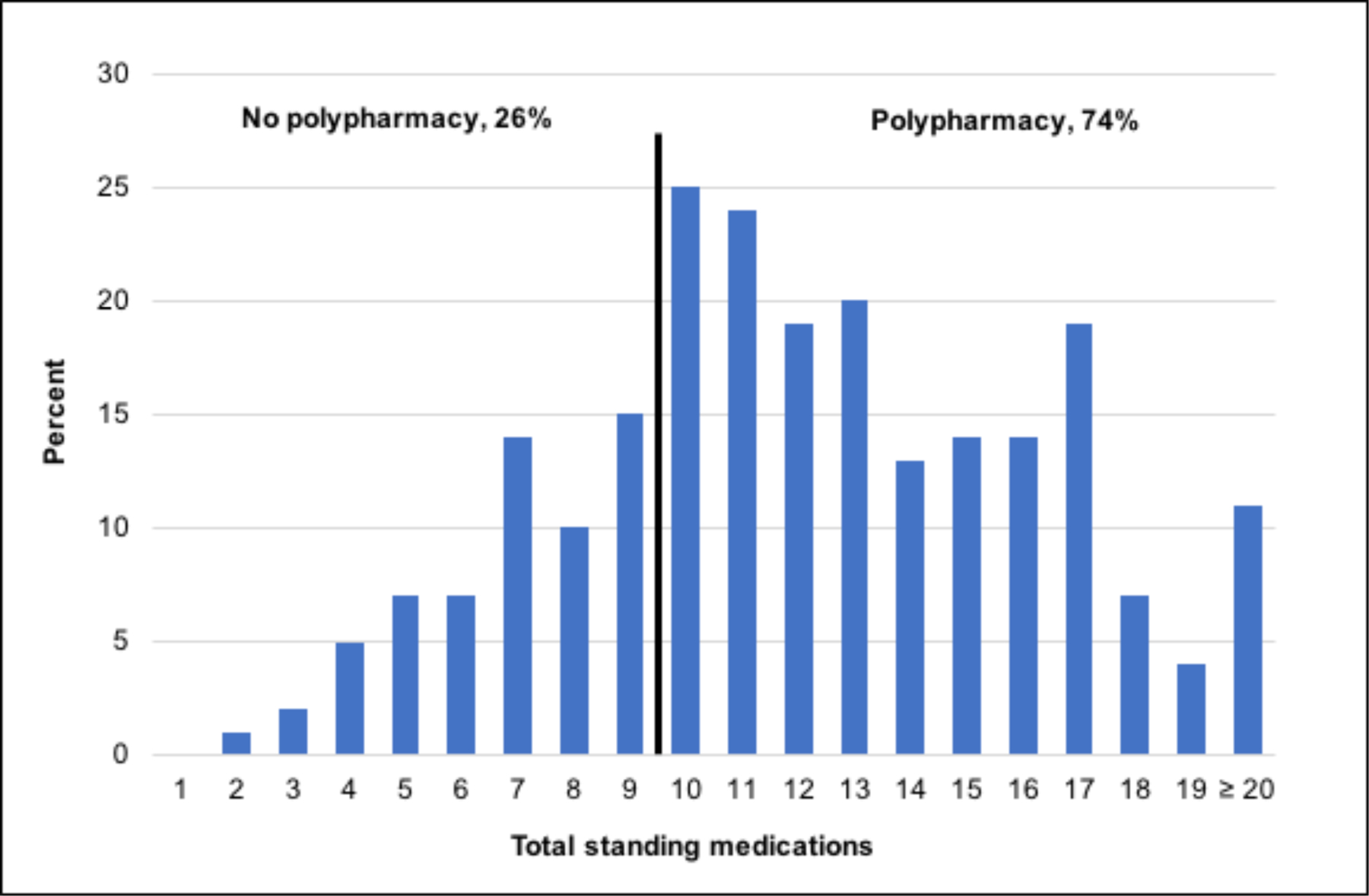
As shown, 74% of adults with heart failure with preserved ejection fraction had polypharmacy (took at least 10 medications).
As shown in Table 2, 52% of patients took at least one American Heart Association heart failure-exacerbating agent (median 1, IQR [0–1]), 73% took a least one Beers agent (median 1, IQR [0–2]), and 100% took at least one medication associated with geriatric syndromes (median 6, IQR [4–8]). The most commonly-used American Heart Association heart failure-exacerbating agents were metformin (19%) and non-dihydropyridine calcium channel blockers (11%); the most commonly-used Beers agents were proton pump inhibitors (50%); and the most commonly-used medications associated with geriatric syndromes were loop diuretics (81%) and beta-blockers (66%) (Table 2).
Table 2.
Prevalence of potentially inappropriate medications by category, stratified by presence of polypharmacy
| All patients (N=231) | With polypharmacy (n=170) | Without polypharmacy (n=61) | |
|---|---|---|---|
| Total number of any potentially inappropriate medication, median (IQRa) | 7 (5–9) | 8 (6–10) | 4 (3–5) |
| American Heart Association heart failure-exacerbating agents, n (%) | |||
| Any | 119 (52) | 97 (57) | 22 (36) |
| Metformin | 43 (19) | 37 (22) | 6 (10) |
| Non-dihydropyridine calcium channel blocker | 26 (11) | 22 (13) | 4 (7) |
| Citalopram or escitalopram | 18 (8) | 15 (9) | 3 (5) |
| Sulfonylurea | 16 (7) | 15 (9) | 0 (0) |
| Nonsteroidal anti-inflammatory drug | 16 (7) | 13 (8) | 3 (5) |
| Hydroxychloroquine | 13 (6) | 11 (6) | 2 (3) |
| Albuterol | 9 (4) | 8 (5) | 1 (2) |
| Beers agents, n (%) | |||
| Any | 169 (73) | 139 (82) | 30 (49) |
| Proton pump inhibitor | 115 (50) | 101 (59) | 14 (23) |
| Non-dihydropyridine calcium channel blocker | 26 (11) | 22 (13) | 4 (7) |
| Sulfonylurea | 16 (7) | 15 (9) | 0 (0) |
| Nonsteroidal anti-inflammatory drug | 16 (7) | 13 (8) | 3 (5) |
| Amiodarone | 13 (6) | 10 (6) | 3 (5) |
| Sliding scale insulin | 11 (5) | 11 (6) | 0 (0) |
| Paroxetine | 9 (4) | 9 (5) | 0 (0) |
| Medications associated with geriatric syndromes, n (%) | |||
| Any | 231 (100) | 170 (100) | 61 (100) |
| Loop diuretic | 186 (81) | 147 (86) | 39 (64) |
| Beta blocker | 153 (66) | 117 (69) | 36 (59) |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 112 (48) | 88 (52) | 24 (39) |
| Calcium channel blocker | 62 (27) | 51 (30) | 11 (18) |
| Selective serotonin reuptake inhibitor | 60 (26) | 49 (29) | 11 (18) |
| Insulin | 54 (23) | 52 (31) | 2 (3) |
| Thiazide diuretic | 53 (23) | 42 (25) | 11 (18) |
| Metformin | 43 (19) | 37 (22) | 6 (10) |
| Warfarin | 43 (19) | 34 (20) | 9 (15) |
| Alpha-adrenergic blocker | 26 (11) | 21 (12) | 5 (8) |
| Serotonin-norepinephrine reuptake inhibitor | 24 (10) | 22 (13) | 2 (3) |
| Histamine-2 receptor antagonist | 23 (10) | 19 (11) | 4 (7) |
Abbreviations:
a interquartile range
We found that 81% of heart failure with preserved ejection fraction patients had at least one pair of competing health conditions in which a medication recommended for one condition could worsen the other, with a median of 3 pairs (IQR 2–6) (Table 3). The most common pairs of competing conditions were heart failure and diabetes (48%), hypertension and diabetes (46%), heart failure and osteoarthritis (37%), and hypertension and osteoarthritis (31%) (Table 3). Among those with competing conditions, 49% took a medication which could negatively affect a coexisting condition, creating potential therapeutic competition in 39% of patients overall. The most common medications creating potential therapeutic competition with heart failure were beta-agonists for patients with comorbid chronic obstructive pulmonary disease, and beta-blockers with alpha-blocking activity for patients with comorbid diabetes (Table 3).
Table 3.
Prevalence of therapeutic competition scenarios
| Competing Condition pairs, n (%) | Medication causing potential therapeutic competition, n (%) | |||
|---|---|---|---|---|
| Heart failure | Diabetes mellitus | 112 (48) | Beta blocker with alpha-blocking activity | 20 (9) |
| Thiazolidinedione | 3 (1) | |||
| Hypertension | Diabetes mellitus | 106 (46) | Beta blocker with alpha-blocking activity | 19 (8) |
| Heart failure | Osteoarthritis | 86 (37) | Nonsteroidal anti-inflammatory drug | 6 (3) |
| Hypertension | Osteoarthritis | 72 (31) | Nonsteroidal anti-inflammatory drug | 4 (2) |
| Heart failure | Chronic obstructive pulmonary disease | 62 (27) | Nonselective beta blocker or
Beta blocker with alpha-blocking activity |
14 (6) |
| Hypertension | Chronic obstructive pulmonary disease | 55 (24) | Beta agonist | 31 (13) |
| Nonselective beta blocker or Beta blocker with alpha-blocking activity |
13 (6) | |||
| Corticosteroid | 3 (1) | |||
| Diabetes mellitus | Coronary artery disease | 49 (21) | Sulfonylurea | 8 (3) |
| Beta blocker with alpha-blocking activity | 8 (3) | |||
| Thiazolidinedione | 2 (1) | |||
| Hypertension | Depression | 49 (21) | Serotonin-norepinephrine reuptake inhibitor | 12 (5) |
| Diabetes mellitus | Atrial fibrillation or atrial flutter | 48 (21) | Beta blocker with alpha-blocking activity | 10 (4) |
| Coronary artery disease | Gastroesophageal reflux disease or peptic ulcer disease | 41 (18) | Clopidogrel | 8 (3) |
| 40 (17) | Warfarin | 12 (5) | ||
| Atrial fibrillation or atrial flutter | Gastroesophageal reflux disease or peptic ulcer disease | Clopidogrel | 1 (<1) | |
| Diabetes mellitus | Chronic obstructive pulmonary disease | 34 (15) | Corticosteroid | 1 (<1) |
| Chronic obstructive pulmonary disease | Gastroesophageal reflux disease or peptic ulcer disease | 31 (13) | Corticosteroid | 4 (2) |
| Atrial fibrillation or atrial flutter | Chronic obstructive pulmonary disease | 28 (12) | Beta agonist | 12 (5) |
| Nonselective beta blocker, or Beta blocker with alpha-blocking activity |
9 (4) | |||
| Coronary artery disease | Chronic obstructive pulmonary disease | 28 (12) | Beta agonist | 13 (6) |
| Nonselective beta blocker, or Beta blocker with alpha-blocking activity |
6 (3) | |||
| Diabetes mellitus | Depression | 28 (12) | Tricyclic antidepressant | 2 (1) |
| Atrial fibrillation or atrial flutter | Depression | 22 (10) | Tricyclic antidepressant | 0 (0) |
| Gastroesophageal reflux disease or peptic ulcer disease | Osteoporosis | 20 (9) | Proton pump inhibitor | 14 (6) |
| Bisphosphonate | 1 (<1) | |||
| Coronary artery disease | Depression | 18 (8) | Tricyclic antidepressant | 1 (<1) |
| Diabetes mellitus | Osteoporosis | 18 (6) | Thiazolidinedione | 0 (0) |
| Atrial fibrillation or atrial flutter | Osteoporosis | 11 (5) | Bisphosphonate | 0 (0) |
| Coronary artery disease | Osteoporosis | 8 (3) | Nonsteroidal anti-inflammatory drug | 1 (<1) |
| Chronic obstructive pulmonary disease | Osteoporosis | 7 (3) | Corticosteroid | 1 (<1) |
| Diabetes mellitus | Benign prostatic hyperplasia | 6 (3) | Alpha-adrenergic blocker | 4 (2) |
DISCUSSION
In our study of patients with heart failure with preserved ejection fraction, we characterized several aspects of medication regimen complexity that warrant increased attention to optimize outcomes in this population. Consideration of medication regimen complexity has previously been identified as an important aspect of caring for older adults with heart failure.3, 12 Prior work has primarily focused on polypharmacy, which we confirm here is highly prevalent.12, 33, 34 However, polypharmacy is only one aspect that contributes to the complexity of managing medications in patients with heart failure with preserved ejection fraction. Our findings show a high prevalence of potentially inappropriate medications and therapeutic competition, extending insights regarding medication complexity and further underscoring the need for formal processes to optimize prescribing patterns in this vulnerable population.
Potentially inappropriate medications are particularly important in older adults because they confer an increased risk for adverse drug events and hospitalizations.3, 17, 35 Our study described the prevalence of potentially inappropriate medications in heart failure with preserved ejection fraction using three different definitions. Prior work examining the prevalence of medications listed on the 2016 American Heart Association Scientific Statement, which identified medications that can cause or exacerbate heart failure, showed that 48% of ambulatory adults with heart failure (from the National Health and Nutrition Examination Survey [NHANES]) and 41% of hospitalized older adults (from the Reasons for Geographic and Racial Differences in Stroke study [REGARDS]) were taking potentially heart failure-exacerbating agents.15, 36, 37 Identifying differences across heart failure subtypes was not possible in the study of ambulatory patients from NHANES, and the study from REGARDS showed that the prevalence of heart failure-exacerbating medications among patients with heart failure with preserved ejection fraction was slightly higher than among those with heart failure with reduced ejection fraction. Our study now extends these findings by showing that the prevalence of potentially heart failure-exacerbating agents among ambulatory heart failure with preserved ejection fraction patients exceeded 50%, supporting the importance of medication reconciliation as a potential strategy to prevent heart failure exacerbations.
Since heart failure with preserved ejection fraction predominantly affects older adults, we additionally examined the prevalence of potentially inappropriate medications according to the Beers criteria, which lists medications with an increased risk for adverse effects among older adults.17 We found that about three quarters of adults with heart failure with preserved ejection fraction take at least one medication listed on the Beers criteria. Our findings, coupled with the observation that a high proportion of hospitalizations and deaths in heart failure with preserved ejection fraction are from non-cardiovascular causes, suggest that clinicians need to look beyond medications that can exacerbate heart failure and consider the potential negative effects of the entire medication list to improve outcomes in this population.38
Heart failure with preserved ejection fraction has been described as a geriatric syndrome that primarily affects older adults, a subpopulation at risk for developing geriatric conditions like frailty, cognitive dysfunction, and incontinence.4, 7, 26, 27, 39, 40 Accordingly, efforts to prevent new onset and/or worsening of these conditions may be an important aspect of managing heart failure with preserved ejection fraction. Unfortunately, we found that all heart failure with preserved ejection fraction patients take at least one medication that can contribute to or cause one or more geriatric conditions.25 The most common medications associated with geriatric syndromes were loop diuretics and beta-blockers. Diuretics are a nuisance among older adults because they can exacerbate urinary incontinence, a common condition among older adults with heart failure.27 However, diuretics are a pillar of heart failure therapy to treat and subsequently prevent congestion, a major cause of hospitalization. Accordingly, diuretics may not be an appropriate agent to discontinue in most adults with heart failure with preserved ejection fraction.
On the other hand, beta-blockers, which are frequently used in heart failure with preserved ejection fraction and can contribute specifically to geriatric syndromes including falls and depression in older adults, may be a reasonable agent to consider discontinuing when present.41, 42 To date, there are few data to support the benefits of beta-blockers in heart failure with preserved ejection fraction.43 In fact, there is a pathophysiologic rationale for beta-blockers causing harm in heart failure with preserved ejection fraction via worsening chronotropic incompetence and limiting cardiac output. Indeed, in a recent study based on data from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) study, the use of beta-blockers was associated with adverse outcomes.44 Moreover, data among older adults with functional impairment has shown that beta-blockers are associated with worse quality of life.45 While beta-blockers may be used to treat comorbid conditions commonly observed in heart failure with preserved ejection fraction such as atrial fibrillation and coronary artery disease, a recent study from REGARDS showed that almost half of adults hospitalized with heart failure with preserved ejection fraction do not have a compelling indication for a beta-blocker.46 Taken together, our findings identify beta-blockers as a medication that merits reconsideration for routine use in heart failure with preserved ejection fraction.
With a rising rate and degree of multimorbidity (>50% of older adults with heart failure now contend with at least 5 other chronic medical conditions), pharmacologic therapeutic competition is an under-recognized but important issue in heart failure with preserved ejection fraction.47 Our data show that the prevalence of competing health conditions and the number of medications causing potential therapeutic competition were higher in patients with heart failure with preserved ejection fraction compared to those observed in a random sample of Medicare beneficiaries, supporting the imperative for clinicians to consider these issues when caring for patients with heart failure with preserved ejection fraction.18 To date, specialized heart failure with preserved ejection fraction programs, which are slowly increasing in number across the country, have sought to improve the accuracy with which heart failure with preserved ejection fraction is diagnosed, provide standardized treatment, and facilitate enrollment into clinical trials.48 Our findings support the need to develop and incorporate formal strategies to manage scenarios of competing conditions and therapeutic competition into these dedicated heart failure with preserved ejection fraction programs, with a particular focus on optimal methods for communication among the many specialists that care for adults with heart failure with preserved ejection fraction.
Our findings additionally underscore the limitations of disease-specific guidelines for conditions like heart failure with preserved ejection fraction that disproportionately affect older adults. Older adults with multiple chronic conditions (and subsequently with polypharmacy and circumstances of therapeutic competition) have been excluded from many of the randomized controlled trials that underlie the heart failure practice guidelines, raising uncertainty about their applicability to this population.49 Moreover, it is likely that the risk-to-benefit ratio for older adults with multiple chronic conditions is different compared to those who are younger with fewer comorbid conditions. This further heightens the challenge of providing optimal care to adults with heart failure with preserved ejection fraction. Decision-making with regard to medications should probably be tailored to the unique vulnerability and needs of older adults.3 Patient Priorities Care is an emerging approach to care where patients’ health priorities and preferences are set as the primary focus for medical decision-making. In a recent nonrandomized trial, participants who received Patient Priorities Care experienced lower treatment burden and unwanted health care.50 Whether it is beneficial to incorporate this approach into heart failure with preserved ejection fraction programs across the country is an important question that warrants further consideration.
Study Limitations
Our findings should be viewed in light of some important limitations. This was a single-site observational study. Medication and comorbidity data were collected from structured and free-text fields in the electronic medical record, which may not be complete or accurate in all cases. For instance, the use of non-prescribed over-the-counter agents (such as nonsteroidal anti-inflammatory drugs, diphenhydramine, or proton pump inhibitors) may be underestimated if not reported by patients. Also, we included only scheduled medications in our chart review, as it was not possible to know how often patients took as-needed medications. As a result of these limitations, it is likely that the extent of polypharmacy, potentially inappropriate medications, and therapeutic competition was even greater than reported here. Although geriatric syndromes are common in this population, we did not have data on their prevalence within this cohort. As a result, we could not estimate the specific impact that medications associated with geriatric syndromes had on this population. Given that the prevalence of medications associated with geriatric syndromes in this study was 100%, future studies should examine this in greater detail.
CONCLUSIONS
In this observational study, we characterized medication regimen complexity in patients with heart failure with preserved ejection fraction, a largely geriatric population that almost universally contends with multiple chronic conditions. In addition to confirming that polypharmacy is highly prevalent, we found that potentially inappropriate medications and therapeutic competition were also frequently present. These observations support the urgent need for patient-centered approaches to mitigate the negative effects of complex medication regimens endemic to heart failure with preserved ejection fraction.
Sources of Funding:
Data collection was supported by the Cardiovascular Health Improvement Program (CHIP), a biorepository funded by the University of Michigan.
Footnotes
Disclosures:
Dr. Goyal is supported by the National Institute on Aging grant R03AG056446 and American Heart Association grant 18IPA34170185 and is a recipient of a National Institute on Aging Loan Repayment Plan. Dr. Hummel is supported by the National Heart, Lung, and Blood Institute grant R01-HL139813 and Veterans Administration grant CARA-009–16F9050. Michael Dorsch has received honoraria from Jansen and research funding from BMS/Pfizer, Amgen, Agency for Healthcare Research and Quality, NIH/National Institute of Aging, and the American Heart Association. Dr. Willer’s spouse works at Regeneron.
REFERENCES:
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res 2019;124:1598–1617. 10.1161/circresaha.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorodeski EZ, Goyal P, Hummel SL, et al. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol 2018;71:1921–1936. 10.1016/j.jacc.2018.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc 2017;65:2431–2440. 10.1111/jgs.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal P, Gorodeski EZ, Flint KM, et al. Perspectives on Implementing a Multidomain Approach to Caring for Older Adults With Heart Failure. J Am Geriatr Soc 2019;67:2593–2599. 10.1111/jgs.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail 2019;7:1001–1011. 10.1016/j.jchf.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornsby WE, Sareini MA, Golbus JR, et al. Lower Extremity Function Is Independently Associated With Hospitalization Burden in Heart Failure With Preserved Ejection Fraction. J Card Fail 2019;25:2–9. 10.1016/j.cardfail.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016;134:73–90. 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 10.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 11.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300:2867–2878. 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal P, Bryan J, Kneifati-Hayek J, et al. Association Between Functional Impairment and Medication Burden in Adults with Heart Failure. J Am Geriatr Soc 2019;67:284–291. 10.1111/jgs.15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–995. 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 2014;62:2261–2272. 10.1111/jgs.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016;134:e32–69. 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 16.Goyal P, Anderson TS, Bernacki GM, et al. Physician Perspectives on Deprescribing Cardiovascular Medications for Older Adults. J Am Geriatr Soc 2020;68:78–86. 10.1111/jgs.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019;67:674–694. 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 18.Lorgunpai SJ, Grammas M, Lee DS, McAvay G, Charpentier P, Tinetti ME. Potential therapeutic competition in community-living older adults in the U.S.: use of medications that may adversely affect a coexisting condition. PLoS One 2014;9:e89447. 10.1371/journal.pone.0089447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol 2001;87:413–419. 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 20.Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep 2014;11:212–219. 10.1007/s11897-014-0186-8. [DOI] [PubMed] [Google Scholar]
- 21.Dunlay SM, Eveleth JM, Shah ND, McNallan SM, Roger VL. Medication adherence among community-dwelling patients with heart failure. Mayo Clin Proc 2011;86:273–281. 10.4065/mcp.2010.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gastelurrutia P, Benrimoj SI, Espejo J, Tuneu L, Mangues MA, Bayes-Genis A. Negative clinical outcomes associated with drug-related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail 2011;17:217–223. 10.1016/j.cardfail.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Marcum ZA, Amuan ME, Hanlon JT, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012;60:34–41. 10.1111/j.1532-5415.2011.03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennel PJ, Kneifati-Hayek J, Bryan J, et al. Prevalence and determinants of Hyperpolypharmacy in adults with heart failure: an observational study from the National Health and Nutrition Examination Survey (NHANES). BMC Cardiovasc Disord 2019;19:76. 10.1186/s12872-019-1058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraf AA, Petersen AW, Simmons SF, et al. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J Hosp Med 2016;11:694–700. 10.1002/jhm.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warraich HJ, Kitzman DW, Whellan DJ, et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults >/=60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ Heart Fail 2018;11:e005254. 10.1161/CIRCHEARTFAILURE.118.005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang R, Chuan F, Peters R, Kuys S. Frequency of urinary incontinence in people with chronic heart failure. Heart Lung 2013;42:26–31. 10.1016/j.hrtlng.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care 2002;8:37–43 [PubMed] [Google Scholar]
- 29.Blecker S, Katz SD, Horwitz LI, et al. Comparison of Approaches for Heart Failure Case Identification From Electronic Health Record Data. JAMA Cardiol 2016;1:1014–1020. 10.1001/jamacardio.2016.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: experience from the Veterans Health Study. J Ambul Care Manage 2005;28:102–110. 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Wilshire CL, Fuller CC, Gilbert CR, et al. Electronic Medical Record Inaccuracies: Multicenter Analysis of Challenges with Modified Lung Cancer Screening Criteria. Can Respir J 2020;2020:7142568. 10.1155/2020/7142568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daskivich TJ, Abedi G, Kaplan SH, et al. Electronic health record problem lists: accurate enough for risk adjustment? Am J Manag Care 2018;24:e24–e29 [PubMed] [Google Scholar]
- 33.Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ 2003;327:513–514. 10.1136/bmj.327.7414.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011;124:136–143. 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu WH, Wen YW, Chen LK, Hsiao FY. Effect of polypharmacy, potentially inappropriate medications and anticholinergic burden on clinical outcomes: a retrospective cohort study. CMAJ 2015;187:E130–E137. 10.1503/cmaj.141219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kneifati-Hayek J, Kennel P, Bryan J, Safford MM, Goyal P. Use of Heart Failure-Exacerbating Medications Among Adults With Heart Failure. J Card Fail 2019;25:72–73. 10.1016/j.cardfail.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal P, Kneifati-Hayek J, Archambault A, et al. Prescribing Patterns of Heart Failure-Exacerbating Medications Following a Heart Failure Hospitalization. JACC Heart Fail 2019. 10.1016/j.jchf.2019.08.007. [DOI] [PMC free article] [PubMed]
- 38.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014;64:2281–2293. 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–777. 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 40.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 41.Luijendijk HJ, van den Berg JF, Hofman A, Tiemeier H, Stricker BH. beta-blockers and the risk of incident depression in the elderly. J Clin Psychopharmacol 2011;31:45–50. 10.1097/JCP.0b013e31820482c4. [DOI] [PubMed] [Google Scholar]
- 42.Ham AC, van Dijk SC, Swart KMA, et al. Beta-blocker use and fall risk in older individuals: Original results from two studies with meta-analysis. Br J Clin Pharmacol 2017;83:2292–2302. 10.1111/bcp.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer M, LeWinter MM. Heart Rate and Heart Failure With Preserved Ejection Fraction: Time to Slow beta-Blocker Use? Circ Heart Fail 2019;12:e006213. 10.1161/CIRCHEARTFAILURE.119.006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman DN, Plante TB, Infeld M, et al. Association of beta-Blocker Use With Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients With Heart Failure With a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw Open 2019;2:e1916598. 10.1001/jamanetworkopen.2019.16598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinman MA, Zullo AR, Lee Y, et al. Association of beta-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA Intern Med 2017;177:254–262. 10.1001/jamainternmed.2016.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yum B, Archambault A, Levitan EB, et al. Indications for beta-Blocker Prescriptions in Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc 2019;67:1461–1466. 10.1111/jgs.15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting Mortality Among Patients Hospitalized for Heart Failure: Derivation and Validation of a Clinical Model. JAMA 2003;290:2581–2587. 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 48.Shah SJ, Cogswell R, Ryan JJ, Sharma K. How to Develop and Implement a Specialized Heart Failure with Preserved Ejection Fraction Clinical Program. Curr Cardiol Rep 2016;18:122. 10.1007/s11886-016-0802-1. [DOI] [PubMed] [Google Scholar]
- 49.Cherubini A, Oristrell J, Pla X, et al. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med 2011;171:550–556. 10.1001/archinternmed.2011.31. [DOI] [PubMed] [Google Scholar]
- 50.Tinetti ME, Naik AD, Dindo L, et al. Association of Patient Priorities-Aligned Decision-Making With Patient Outcomes and Ambulatory Health Care Burden Among Older Adults With Multiple Chronic Conditions: A Nonrandomized Clinical Trial. JAMA Intern Med 2019. 10.1001/jamainternmed.2019.4235. [DOI] [PMC free article] [PubMed]


