Abstract
Sporulation during Clostridioides difficile infection (CDI) contributes to recurrent disease. Cell division and sporulation both require peptidoglycan biosynthesis. We show C. difficile growth and sporulation is attenuated by antisenses to murA and murC or the MurA inhibitor fosfomycin. Thus, targeting the early steps of peptidogly can biosynthesis might reduce the onset of recurrent CDI.
Keywords: Peptidoglycan, Mur ligases, antibiotic drug-targets heat-resistant spore cortex
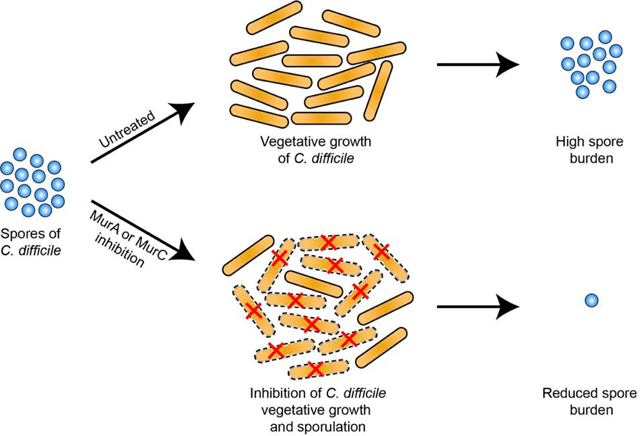
Graphical Abstract
Antibiotic options to treat Clostridioides difficile infections (CDI) are fidaxomicin, vancomycin and metronidazole [1]. However, 20% or more of patients treated for CDI go on to experience recurrence of diarrhea following treatment [2, 3]. Following treatment, the failure of the normal gut microbiota to repopulate the large bowel is thought to allow C. difficile to recolonize the bowel and cause recurrent CDI (rCDI). In this regard, during infection, the formation and survival of spores that are intrinsically resistant to antibiotics is a major contributor to rCDI and transmission of the disease [4]. It is estimated that half or more of the cases of rCDI is due to endogenous spores, while reinfection with environmental spores account for other cases [5].
Given the link between endogenous spores and rCDI, there is a need for therapeutics that block sporogenesis. Current efforts to find anti-sporulation drug targets focus on proteins that primarily affect sporogenesis, but are not required for vegetative growth [6, 7]. These ongoing efforts might lead to sporulation inhibitors that can serve as adjuvants to standard of care antibiotics. However, there is a continuing need for new anti-C. difficile antibiotics as resistance has emerged to all currently used first-line antibiotics [8, 9]. We propose that enzymes involved in cytoplasmic steps of peptidoglycan biosynthesis could be suitable antibacterial and antisporulation drug targets, because they play essential roles in vegetative cells and spores [9–12]. Peptidoglycan is critical for maintaining cell shape and rigidity to prevent osmotic lysis, while in sporogenesis, as seen in Bacillus spp., it enables mother cell engulfment of the forespore and eventual formation of the peptidoglycan spore cortex that confers the heat resistance of spores [10].
Peptidoglycan biosynthesis is a multi-step process involving: (1) formation of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) disaccharide pentapeptide peptidoglycan precursors; (2) transport across the cell envelope and (3) assembly of the precursors into the growing peptidoglycan layer. Among the enzymes required for synthesis of the disaccharide pentapeptide are the Mur enzymes. Furthermore, in B. subtilis, depletion of the Mur enzyme UDP-N-acetylenolpyruvoylglucosamine reductase (MurB) decreases sporulation and causes cells to be more sensitive to cell wall synthesis inhibitors [10]. We therefore investigated Mur enzymes, UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA) and UDP-N-acetylmuramate-L-alanine ligase (MurC), which catalyze the first and third steps of peptidoglycan precursor biosynthesis [13]. Many Gram-positive bacteria harbor two copies of murA [14], but C. difficile expresses a single murA that is predicted to be located in an operon (Figure S1) with sporulation genes SpoIIC (Stage II sporulation protein D) and SpoIIID (Stage III sporulation protein D). The genetic arrangement of sporulation and cell wall biosynthesis genes in operons is also evident in B. subtilis [10] and in C. perfringens ATCC 13124 (accession no. NC_008261). Because C. difficile murC is monocistronic we selected it for antisense analysis, to confirm that anti-sporulation outcomes from murA depletion was not due to polar effects on downstream sporulation genes.
Strains and plasmids used in this study are indicated in Table 1; primers and antisense sequences are in Table S1. Antisense RNAs (asRNAs) to murA, murC, spo0A were expressed under the anhydrotetracycline (ATc) inducible pTet promoter in plasmid pMSPT, which carries a paired terminus to stabilize the antisense RNA [15]. The asRNAs were 100 bp in length; for murA and spo0A targeted the upstream region of the gene, including the ribosome binding site, whereas that to murC targeted the middle region of the gene (Table S1). The antisense fragments to murA, murC and spo0A were cloned into XhoI and SphI sites of pMSPT. Constructs were conjugated into C. difficile CD630 as described [15]. Antisenses were induced with ATc. Susceptibility to fosfomycin and vancomycin were determined by microdilution in 96-well plate, using two-fold antibiotic dilutions and inocula of 105 CFU/ml of C. difficile. Antibiotic minimum inhibitory concentrations (MICs) were defined as the lowest concentration of compound inhibiting visible growth. Automated growth kinetics were performed in 96-well using logarithmic cultures in a Versa max microplate reader, which recorded OD600nm every 30 min for 24 h, with shaking before each read, in a Coy anaerobic chamber.
Table 1.
Strains and plasmids used in this study.
| Strain | Plasmid | Description | Source |
|---|---|---|---|
| C. difficile CD630 | pMSPT | pRPF185 derivative in which the gusA gene was replaced with paired termini sequence | [15] |
| C. difficile CD630 | pMSPT-murAi | pMSPT containing antisense murA RNA | This work |
| C. difficile CD630 | pMSPT-murCi | pMSPT containing antisense murC RNA | This work |
| C. difficile CD630 | pMSPT-spo0Ai | pMSPT containing antisense spo0A RNA | [15] |
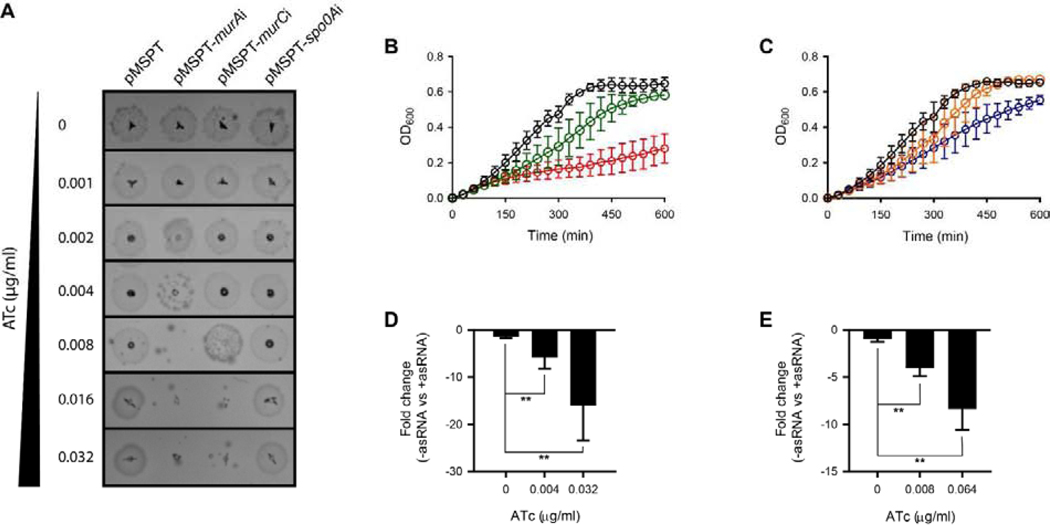
Induction of asRNA to murA mRNA inhibited growth of C. difficile CD630 on agar in a dose-dependent manner at ATc concentrations of ≥0.004 μ g/mL and above (Figure 1A); similarly, expression of asRNA to murC mRNA inhibited grown on agar with ATc at ≥0.008μ g/mL (Figure 1A). In broth, strong dose-dependent growth inhibition was also seen for asRNAs to murA and murC (Figure 1B–C). The test concentrations of ATc did not inhibit growth of the control strain carrying the empty vector pMSPT (Figure 1A and S2). To analyze the effect of antisense fragments on RNA levels, RT-qPCR was performed on four biological replicates as described previously [15] and values analyzed by t-test in GraphPad prism 8. ATc concentrations were chosen to represent ½× and 4× the inducer concentration that results in growth inhibition, to assess dose response. As shown in Figure 1D, induction of asRNA to murA at 0.004 μ g/ml of ATc reduced cDNA formation by 5.18 ± 2.6 (p = 0.0087) (Figure 1D), while a 8-fold increase in ATc to 0.032 μ g/ml caused a 13.5 ± 8.1- fold reduction in cDNA (p = 0.0077). Transcript levels were unaffected for upstream (CD630_01220, encoding a hypothetical protein) and downstream (spoIIC, stage II sporulation protein D and CD630_01250, encoding a hypothetical protein) genes in the operon (Figure S3) and murC (Figure S3). This is consistent with inhibition of translation by asRNAs designed to resemble naturally occurring antisenses, as previously described [15]. Against murC, cDNA formation was reduced by 3.51 ± 1.7 fold (p = 0.0020) with ATc at growth inhibitory concentration of 0.008 μ g/mL (Figure 1E); further reduction in cDNA formation (6.28 ± 3.6- fold; p = 0.0025) occurred at ATc 0.064 μ g/mL. Expression of the antisense to murC did not affect murA transcript levels (Figure S4). Consistent with the effect of cell wall synthesis inhibition [16], antisenses to murA and murC caused cell elongation (Figure S5). As a further control, we tested spo0A, an early stage sporulation gene, which does not mediate vegetative cell growth; induction of asRNA to spo0A did not affect growth on agar (Figure 1A). Taken together these observations confirms the essentiality of MurA and MurC for vegetative growth of C. difficile.
Figure 1. Impact of antisenses to murA and murC on C. difficile growth and gene expression.
(A) C. difficile strain carrying empty plasmid or plasmids encoding antisense RNA were analyzed on BHI agar. Three micro liters of overnight cultures were spotted on BHI agar plates containing required concentrations of anhydrotetracycline (ATc); representative results from >3 independent experiments are shown. (B & C) Growth kinetics (n=4 biological replicates) for the strains were analyzed by microdilution in 96-well plate. Growth was analyzed for C. difficile CD630 strain harboring asRNA to murA (B) and asRNA to murC (C). The growth was analyzed at different concentrations of ATc (μ g/ml) i.e. at 0 (black), 0.004 (green), 0.008 (orange), 0.032 (red) and 0.064 (blue). (D & E) mRNA levels for murA (D) and murC (E) genes were analyzed by RT-qPCR on 4 biological replicates. The fold-change was calculated for difference in mRNA levels between strains containing empty vector and those with antisense constructs. Data in D and E were analyzed by t-test in GraphPad prism 8 and significance at p<0.01 is shown by asterisks.
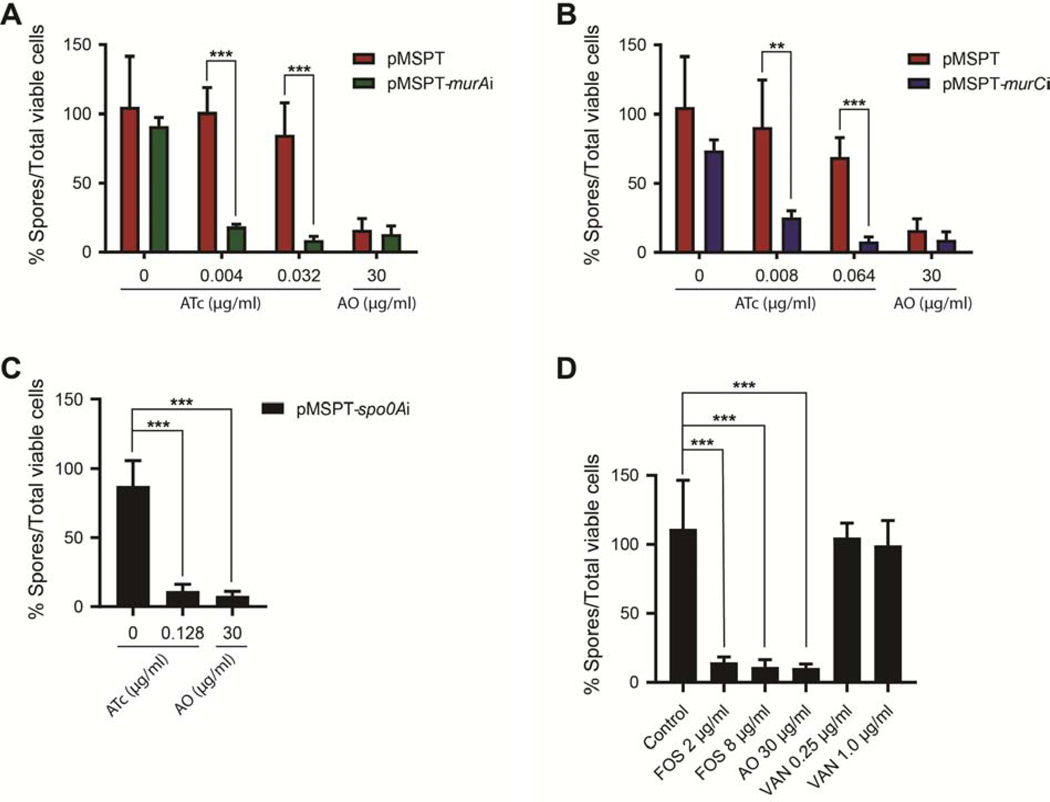
To study whether murA and murC were also essential for C. difficile spore formation, we analyzed sporulation in presence and absence of asRNA to respective genes. Sporulation was analyzed as described previously [15], using four biological replicates to determine the number of spores produced by cells at day 5. There was a significant reduction in sporulation by cells expressing asRNA to murA upon induction with 0.004 and 0.032 μ g/mL of ATc; these concentrations represent ½× and 4× the inducer concentration that results in growth inhibition. Spore formation was reduced as the total population contained 8.8–18.6% of spores (Figure 2A), in contrast to the empty vector control in which spore formation was not inhibited by the respective ATc concentrations. Induction of asRNA to murC (0.008 and 0.064 μ g/mL of ATc or ½× and 4× the inducer concentration causing growth inhibition) also reduced spore as there were 8.1–25.1% spores in the total population (Figure 2B). The percent of spores in the total viable population for asRNA to murA and murC were comparable to cells expressing asRNA to spo0A, which encode a key transcriptional regulator of the initial stages of sporulation (Figure 2C).
Figure 2. Inhibition of murA and murC gene activity affects C. difficile sporulation.
Spore production was analyzed at day 5, following induction by anhydrotetracycline (ATc) of antisenses to murA (A), murC (B) and spo0A (C). (D) Comparison of inhibition of sporulation by fosfomycin (FOS [MIC = 8 μ g/mL]) and vancomycin (VAN [MIC = 0.25 μg/mL]). Acridine orange (AO) served as a positive control. Data from four biological replicates were analyzed by t-test in GraphPad prism 8 and significance at p<0.01 and p<0.001 are shown by asterisks.
To confirm findings from the above genetic studies, we tested the antibiotic fosfomycin, which inhibits MurA by covalently binding to a conserved cysteine in its active site. Fosfomycin at ¼× and 1× its MIC (8 μg/mL) reduced spore formation, as there were 11.3–14.7% of spores in the population (Figure 2D). Inhibition of sporulation by fosfomycin was comparable to the positive control acridine orange (AO). In contrast, vancomycin, which inhibits the final stage of cell wall assembly at the cell envelope, did not inhibit sporulation in cells exposed to ¼× and 1×its MIC (0.25 and 1.0 μg/mL). Sub-inhibitory fosfomycin (¼× MIC) did not affect toxin production (Figure S6). These findings further support the essentiality of MurA for sporulation and vegetative growth of C. difficile.
Although traditional antibiotics metronidazole and vancomycin inhibit C. difficile growth, the growth inhibited cells are still capable of undergoing sporulation, which contrasts with the newer drug fidaxomicin that inhibits sporulation and toxin production by inhibiting gene transcription [17, 18]. Because alternative antibiotics will be needed to cover emerging antibiotic-resistant C. difficile, we reason that discovery efforts should focus on antibacterial drug targets that affect C. difficile sporulation and/or toxin production. Recently, we reported that sporulation is decreased upon inhibition of the enoyl ACP-reductase FabK enzyme, which catalyzes the final step of bacterial fatty acid biosynthesis [15]. We now show that MurA and MurC enzymes could also be potential drug targets for the discovery of anti-sporulation antibacterial agents. The enzymatic mechanisms of Mur ligases are well known, allowing for high throughput assays to identify enzyme inhibitors [19]. A potential challenge will be identifying compounds that selectively inhibit C. difficile, avoiding inhibition of gut microbiota that also carry these enzymes. However, narrow-spectrum inhibitors of C. difficile Mur ligases may be developed with an increase in current understanding of substrate transporters in C. difficile that are absent in key gut flora [20, 21]. This may allow engineering of antibiotics that use the designated transporters to reach their cytoplasmic targets. Additionally, Mur ligase inhibitors may be designed with physicochemical properties favoring their uptake into Gram-positive bacteria [22]. We anticipate that such inhibitors would be valuable in reducing the risk of rCDI.
Supplementary Material
Highlights.
Early stage cell wall synthesis is essential for vegetative cells and sporogenesis.
MurA and MurC catalyzes the first and third steps in cell wall synthesis.
Growth and sporogenesis were affected by genetic knockdown of murA and murC genes.
Sporogenesis was inhibited by the antibiotic fosfomycin, targeting MurA.
Recurrent C. difficile infection (rCDI) is linked survival of endogenous spores.
Early stage cell wall synthesis enzymes are potential drug targets to prevent rCDI.
Acknowledgments
This study was in part supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health grant R21AI126755. MS acknowledges support from the University of Texas at Arlington.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at
Declaration of interest
None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis 66 (2018) 987–94. [DOI] [PubMed] [Google Scholar]
- [2].Petrella LA, Sambol SP, Cheknis A, Nagaro K, Kean Y, Sears PS, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin. Infect. Dis 55 (2012) 351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med 372 (2015) 825–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, et al. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun 80 (2012) 2704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chilton CH, Pickering DS, Freeman J.. Microbiologic factors affecting Clostridium difficile recurrence. Clin. Microbiol. Infect 24 (2018) 476–82. [DOI] [PubMed] [Google Scholar]
- [6].Edwards AN, McBride SM.. Initiation of sporulation in Clostridium difficile: a twist on the classic model. FEMS Microbiol. Lett 358 (2014) 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ciftci Y, Girinathan BP, Dhungel BA, Hasan MK, Govind R.. Clostridioides difficile SinR’ regulates toxin, sporulation and motility through protein-protein interaction with SinR. Anaerobe (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Adler A, Miller-Roll T, Bradenstein R, Block C, Mendelson B, Parizade M, et al. Anational survey of the molecular epidemiology of Clostridium difficile in Israel: the dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn. Microbiol. Infect. Dis 83 (2015) 21–4. [DOI] [PubMed] [Google Scholar]
- [9].Schwanbeck J, Riedel T, Laukien F, Schober I, Oehmig I, Zimmermann O, et al. Characterization of a clinical Clostridioides difficile isolate with markedly reduced fidaxomicin susceptibility and a V1143D mutation in rpoB. J. Antimicrob. Chemother 74 (2019) 6–10. [DOI] [PubMed] [Google Scholar]
- [10].Real G, Henriques AO.. Localization of the Bacillus subtilis murB gene within the dcw cluster is important for growth and sporulation. J. Bacteriol 188 (2006) 1721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tocheva EI, Lopez-Garrido J, Hughes HV, Fredlund J, Kuru E, Vannieuwenhze MS, et al. Peptidoglycan transformations during Bacillus subtilis sporulation. Mol. Microbiol 88 (2013) 673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vasudevan P, Weaver A, Reichert ED, Linnstaedt SD, Popham DL.. Spore cortex formation in Bacillus subtilis is regulated by accumulation of peptidoglycan precursors under the control of sigma K. Mol. Microbiol 65 (2007) 1582–94. [DOI] [PubMed] [Google Scholar]
- [13].Smith CA.. Structure, function and dynamics in the mur family of bacterial cell wall ligases. J. Mol. Biol 362 (2006) 640–55. [DOI] [PubMed] [Google Scholar]
- [14].Du W, Brown JR, Sylvester DR, Huang J, Chalker AF, So CY, et al. Two active forms of UDP-N-acetylglucosamine enolpyruvyl transferase in gram-positive bacteria. J. Bacteriol 182 (2000) 4146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marreddy RKR, Wu X, Sapkota M, Prior AM, Jones JA, Sun D, et al. The fatty acid synthesis protein enoyl-ACP reductase II (FabK) is a target for narrow-spectrum antibacterials for Clostridium difficile infection. ACS Infect. Dis 5 (2019) 208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nonejuie P, Burkart M, Pogliano K, Pogliano J.. Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules. Proc. Natl. Acad. Sci. U S A 110 (2013) 16169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen L, Sonenshein AL.. Fidaxomicin inhibits spore production in Clostridium difficile. Clin. Infect. Dis 55 Suppl 2 (2012) S162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu X, Alam MZ, Feng L, Tsutsumi LS, Sun D, Hurdle JG.. Prospects for flavonoid and related phytochemicals as nature-inspired treatments for Clostridium difficile infection. J. Appl. Microbiol 116 (2014) 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kouidmi I, Levesque RC, Paradis-Bleau C.. The biology of Mur ligases as an antibacterial target. Mol. Microbiol 94 (2014) 242–53. [DOI] [PubMed] [Google Scholar]
- [20].Gutierrez-Preciado A, Torres AG, Merino E, Bonomi HR, Goldbaum FA, Garcia-Angulo VA.. Extensive identification of bacterial riboflavin transporters and their distribution across bacterial species. PLoS One 10 (2015) e0126124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steglich M, Hofmann JD, Helmecke J, Sikorski J, Sproer C, Riedel T, et al. Convergent loss of ABC transporter genes from Clostridioides difficile genomes is associated with impaired tyrosine uptake and p-Cresol production. Front. Microbiol 9 (2018) 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Silver LL.. Challenges of antibacterial discovery. Clin. Microbiol. Rev 24 (2011) 71–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.