Abstract
Encephalitozoon cuniculi infects a wide range of mammalian hosts. Three genotypes based on the number of GTTT repeats in the internal transcribed spacer (ITS) of the rRNA have been described, of which genotypes I and III have been identified in humans. In this study, the genetic diversity of E. cuniculi was examined at the polar tube protein (PTP) and spore wall protein I (SWP-1) loci. Nucleotide sequence analysis of the PTP gene divided 11 E. cuniculi isolates into three genotypes in congruence with the result of analysis of the ITS of the rRNA gene. The three PTP genotypes differed from one another by the copy number of the 78-bp central repeat as well as point mutations. These E. cuniculi isolates also differed from one another in the number of 15- and 36-bp repeats in the SWP-1 gene. In addition, some E. cuniculi isolates had heterogeneous copies of the SWP-1 gene with various numbers of repeats. Intragenotypic variation was also observed at the SWP-1 locus. Based on the length polymorphism and sequence diversities of the PTP and SWP-1 genes, two simple PCR tests were developed to differentiate E. cuniculi in clinical samples.
Encephalitozoon cuniculi is a common microsporidian parasite that infects various mammals, such as rabbits, rats, mice, horses, foxes, cats, dogs, muskrats, leopards, baboons, and humans (7, 13). Recent characterization of the internal transcribed spacer (ITS) of the rRNA gene has identified three genotypes of E. cuniculi based on the number of GTTT repeats present: a genotype or strain I from rabbits containing three repeats, a genotype or strain II from mice containing two repeats, and a genotype or strain III from dogs containing four repeats (9). Thus far, both genotype or strain I and genotype or strain III genotypes of E. cuniculi have been found in humans, indicating that human E. cuniculi infection can be of zoonotic origin (6, 8, 18, 20, 21). The number of human E. cuniculi isolates characterized so far, however, is very small (13).
Currently, genotyping of E. cuniculi involves mostly DNA sequencing of ITS, which is not practical in most diagnostic laboratories because of the technical demands and high cost. Thus, there is a need for the development of simpler genotyping tools that are more cost-effective and can be performed in most diagnostic laboratories. This will allow the genotyping of E. cuniculi in large numbers of laboratories and better characterization of the epidemiology of human E. cuniculi infection. Recently, genes coding for the polar tube protein (PTP) and spore wall protein I (SWP-1) of E. cuniculi were reported (2, 5). Because the gene has long central repeats of 78 bp in PTP and 15 and 36 bp in SWP-1 and the number of repeats in repetitive proteins tends to vary in other parasites such as Plasmodium spp., we examined the sequence diversity of PTP and SWP-1 genes among various isolates of E. cuniculi. This study has led to the development of two simple PCR-based molecular diagnostic tests.
MATERIALS AND METHODS
Parasite isolates.
The E. cuniculi isolates used in this study included seven isolates from humans and one isolate from a rabbit (Table 1). Three previously characterized reference strains, strain I from a rabbit, strain II from a mouse, and strain III from a dog (9) were also used as controls. Most isolates were maintained in E6 and HLF cell cultures after inoculation with biopsy samples, with the exception of isolates CDC:V428A, CDC:V449A, and 3275, for which infected tissues were directly used in molecular analysis.
TABLE 1.
E. cuniculi isolates used in this study and genotyping results from analyses of repetitive genes
| ID | Host | Tissue | Location | ITS genotype | PTP genotype | SWP-1 genotype |
|---|---|---|---|---|---|---|
| CDC:V282 | Human | Kidney | Colorado | III | III | IIIa |
| USP A-1a | Human | Sputum | Spain | III | III | IIIa |
| USP A-2a | Human | Kidney | Spain | III | III | IIIa |
| CDC:V449A | Human | Kidney | Michigan | III | III | IIIb |
| 3275 | Human | Kidney | Italy | III | III | NTb |
| CDC:V385 | Human | Kidney | California | I | I | Ia |
| CDC:V446 | Human | Kidney | Italy | I | I | Ib |
| CDC:V428A | Rabbit | Lens tissue | Ohio | I | I | Ia |
| Ca-d | Dog | Kidney | Texas | III | III | IIIb |
| Strain I (Ja-r) | Rabbit | Kidney | Texas | I | I | Ia |
| Strain II (Va-m) | Mouse | Kidney | New York | II | II | II |
| Strain III (Ja-d) | Dog | Kidney | Texas | III | III | IIIb |
These two isolates were from different samples from the same patient at the same time.
NT, not tested.
DNA extraction.
DNA was extracted from biopsy specimens or cultured parasites using a previously described method (10). Briefly, tissues or cultured organisms were washed twice in phosphate-buffered saline before they were placed in 300 μl of lysis buffer containing 10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 20 mM dithiothreitol, 2 mg of proteinase K per ml, and 250 U of lyticase (Sigma Co., St. Louis, Mo.). After 15 min of incubation, mechanical disruption was performed with 0.5-mm-diameter glass beads (Biospec Products, Bartlesville, Okla.) and a mini-bead beater (Biospec Products) at 5,000 rpm for 3 min. The suspension was then incubated at 37°C for 18 h. After addition of 150 μl of 2% sodium dodecyl sulfate and 20 μl of proteinase K (20 mg/ml), the incubation was continued at 50°C for 72 h. DNA was extracted with phenol-chloroform and precipitated with ethanol. Nucleic acid from each sample was resuspended in 50 μl of distilled water and stored at −20°C before being used in PCR.
Sequence analysis of the ITS.
The biopsy specimens or cultured parasites were genotyped by sequence analysis of the ITS of the rRNA gene (24). Briefly, the 3′ end of the small subunit rRNA, ITS, and the 5′ end of the large subunit rRNA were amplified from extracted DNA by PCR using primers ss530f [5′-GTGCCAGC(C/A)GCTGGCAC-3′] and ls212r1 [5′-GTT(G/A)GTTTCTTTTCCTC-3′]. The PCR product was sequenced in both directions on an ABI377 autosequencer (Applied Biosystems, Foster City, Calif.). E. cuniculi genotype was determined by the number of the GTTT repeats present in the ITS region: two repeats for genotype or strain II, three repeats for genotype or strain I, and four repeats for genotype or strain III (9).
Sequence analysis of PTP.
A 1,076-bp fragment of the PTP was amplified from DNA of all E. cuniculi isolates by PCR, using primers 5′-ATGAAAGGTATTTCTAAGAT-3′ (nucleotides 345 to 364), and 5′-GCCTCCATGGCATACTGC-3′ (nucleotides 1403 to 1420), based on a PTP sequence (AJ005666) previously published by Delbac et al. (5). PCR products were sequenced in both directions, and the sequences obtained were aligned with each other and the published sequence using the Wisconsin Package Version 9.0 (Genetics Computer Group, Madison, Wis.). Restriction enzyme mapping of PTP sequences was also conducted using the same software.
Genotyping by direct PCR analysis of PTP.
Based on the results of PTP gene sequencing, a simple length polymorphism-based PCR genotyping technique was developed. A 363-bp fragment of the PTP was amplified from E. cuniculi DNA by PCR, using primers 5′-GCAGTTCCAGGCTACTAC-3′ (nucleotides 840 to 857 of AJ005666), and 5′-AGGAACTCCGGATGTTCC-3′ (nucleotides 1185 to 1202 of AJ005666) (5). The PCR products were differentiated by electrophoresis in agarose gel, using 100-bp ladders (Life Technologies, Grand Island, N.Y.) as molecular markers. To differentiate E. cuniculi genotype I and genotype II, 20 μl of the 363-bp PTP PCR products were digested with 20 U of Sau96I (New England BioLabs, Beverly, Mass.), under conditions recommended by the supplier. Genotypes were differentiated by the banding patterns in agarose gel electrophoresis.
Sequence analysis of SWP-1.
A fragment of the SWP-1 of the expected size of 399 bp was amplified from DNA of all E. cuniculi isolates by PCR, using primers 5′-ACTGACAAGTACCACATC-3′ (nucleotides 995 to 1002) and 5′-TTGGACTCACACATTAGG-3′ (nucleotides 1376 to 1393), based on a SWP-1 sequence (AJ133745) previously published by Bohne et al. (2). PCR products were sequenced in both directions, and the sequences obtained were aligned with each other and the published sequence as described above. A neighbor-joining tree of the sequences was constructed based on genetic distance calculated using the Kimura 2-parameter model and the program TreeconW (23).
Nucleotide sequence accession number.
The nucleotide sequences of E. cuniculi were deposited in the GenBank database under accession no. AF310677 through AF310679 (for PTP) and AF340007 through AF340012 (for SWP-1).
RESULTS
Sequence analysis of ITS.
All E. cuniculi isolates used in this study were sequenced for the ITS gene. Nucleotide sequences obtained from the three reference isolates were identical to those previously reported, with genotype I having three GTTT repeats, genotype II having two GTTT repeats, and genotype III having four GTTT repeats. As expected, a wild isolate (CDC:V428A) from a rabbit had the strain I sequence. This genotype was also found in two of the human samples (CDC:V385 and CDC:V446). Five other isolates from four humans, however, were identified to have genotype III (Table 1). One nucleotide difference was also seen in the 3′ end of the small subunit rRNA gene: genotype I had A (5′-CGGGACAGTG-3′) whereas genotypes II and III had T (5′-CGGGACTGTG-3′) at nucleotide position 716 of L07255.
Sequence analysis of PTP.
Nucleotide sequences of a 1,076-bp fragment of the PTP gene were determined from all isolates used. Three types of PTP sequences were obtained: (i) the genotype I sequence was identical to the previously published sequence (AJ005666) and was obtained from the reference isolate strain I, the rabbit isolate (CDC:V428A), and two human isolates (CDC:V385 and CDC:V446); (ii) the genotype II sequence had four base pair differences from genotype I (Fig. 1) and was obtained from the reference strain II; and (iii) genotype III had a deletion of one of the 78-bp repeats and two base pair differences from genotype I (Fig. 1) and was obtained from the reference isolate strain III and five human isolates (CDC:V282, USP A-1, USP A-2, CDC:V449A, and 3275). There was a complete concordance in genotyping results of samples between sequence analyses of ITS and PTP (Table 1).
FIG. 1.
Sequence diversity among three genotypes (I, II, and III) of E. cuniculi in the PTP gene. Dots denote sequence identity to genotype I and dashes denote nucleotide deletions. The repeat region is underlined.
Genotyping of E. cuniculi by direct PTP PCR.
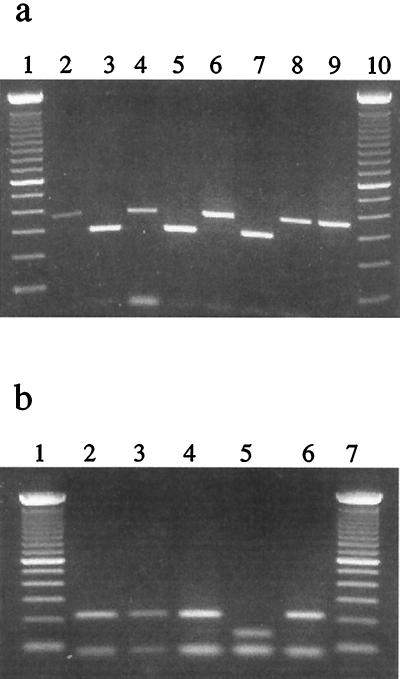
Because of the length polymorphism of the two human pathogenic E. cuniculi genotypes (genotypes I and III), a set of primers (5′-GCAGTTCCAGGCTACTAC-3′ and 5′-AGGAACTCCGGATGTTCC-3′) were developed for the detection and differentiation of human E. cuniculi by direct PCR. This primer set generated a 363-bp product for genotypes I and II and a 285-bp product for genotype III. These two products were easily differentiated by agarose gel electrophoresis (Fig. 2a). No amplification was produced with purified DNA from Encephalitozoon hellem, Encephalitozoon intestinalis, and Enterocytozoon bieneusi (data not shown).
FIG. 2.
Genotyping E. cuniculi isolates by PCR analysis of the PTP gene. (a) Differentiation of genotype III from genotypes I and II by eletrophoresis of PCR products: lanes 1 and 10, 100-bp ladders; lanes 2 (strain I), 4 (CDC:V385), 6 (CDC:V446), and 9 (CDC:V428A), genotype I; lane 8 (strain II), genotype II; and lanes 3 (CDC:V282), 5 (USP A-1), and 7 (3275), genotype III. (b) Differentiation of genotype I from genotype II by restriction digestion of PCR products with Sau96I: lanes 2 (strain I), 3 (CDC:V385), 4 (CDC:V446), and 6 (CDC:V428A), genotype I; and lane 5 (strain II), genotype II.
Differentiation of genotypes I and II by RFLP analysis of PTP.
Although E. cuniculi genotypes I and II both generated PCR products of the same size, computer analysis of the PTP sequences obtained indicated that the 363-bp products of these two genotypes could be differentiated by the use of restriction enzymes AlwI, MboI, BstYI, DpnI, Fnu4HI, TseI, BsaJI, BbvI, AvaII, PpuMI, or Sau96I. Sau96I was chosen a the restriction enzyme for restriction fragment length polymorphism (RFLP) analysis, which would cleave genotype I products at two sites, leading to three fragments of predicted sizes of 209 bp, 76 bp, and 78 bp, and genotype II at three sites, leading to four fragments of predicted sizes of 131, 78, 76, and 78 bp. Digestion of the 363-bp PTP DNA fragment with Sau96I produced banding patterns similar to those predicted by computer analysis: two visible bands for both genotypes I and II, with a size difference in the upper band for each genotype (Fig. 2b).
Sequence analysis of SWP-1.
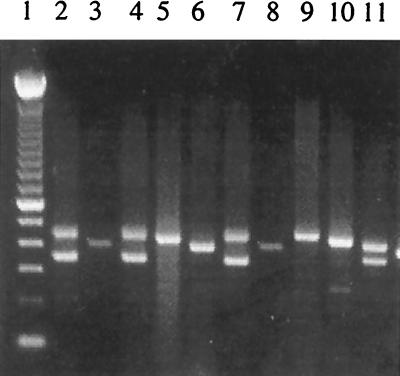
The repeat region of the SWP-1 gene was amplified using primers based on sequences flanking the repeats in the published sequence (AJ133745) (2). Only three isolates (CDC:V385, CDC:V428A, and the reference strain I) produced a PCR fragment of the expected 399-bp size (Fig. 3). Other isolates yielded either a single band of different sizes or double bands (Fig. 3). Consistent results were obtained even after changing the annealing temperature and magnesium concentration in the PCR.
FIG. 3.
Genotyping E. cuniculi isolates by PCR analysis of SWP-1. Lane 1, 100-bp ladder; lanes 2 (CDC:V282), 4 (USP A-1), and 7 (USP A-2), genotype IIIa; lanes 3 (CDC:V428A), 6 (strain I), and 8 (CDC:V385), genotype Ia; lanes 5 (CDC:V449A) and 10 (strain III), genotype IIIb; lane 9 (CDC:V446), genotype Ib; lane 11 (strain II), genotype II.
Sequence analyses of 399-bp PCR products revealed a nucleotide sequence identical to the published sequence AJ133745. As expected, the repeat region contained five 36-bp repeats and five 15-bp repeats (Table 2). DNA sequencing of other PCR products, including those of the double bands, produced nucleotide sequences similar but not identical to AJ133745. All together, six types of SWP-1 sequences were obtained, and they differed from one another in the numbers of 36- and 15-bp repeats in the repeat region, which was responsible for the size differences in the electrophoresis of PCR products (Table 2).
TABLE 2.
Differences among E. cuniculi genotypes in the repetitive region of the SWP-1 gene
| Genotype | PCR product length (bp) | Repeat length (bp) | No. of repeats | Repeat ordera |
|---|---|---|---|---|
| Ia | 399 | 255 | 10 | 36, 15, 36, 15, 36, 15, 36, 15, 36, 15 |
| Ib | 450 | 306 | 12 | 36, 15, 36, 15, 36, 15, 36, 15, 36, 15, 36, 15 |
| II | 363 | 219 | 9 | 15, 36, 15, 36, 15, 36, 15, 36, 15 |
| 414 | 270 | 11 | 15, 36, 15, 36, 15, 36, 15, 36, 15, 36, 15 | |
| IIIa | 348 | 204 | 8 | 15, 36, 15, 36, 36, 15, 36, 15 |
| 429 | 285 | 12 | 15, 36, 15, 36, 15, 15, 15, 36, 36, 15, 36, 15 | |
| IIIb | 429 | 285 | 12 | 15, 36, 15, 36, 15, 15, 15, 36, 36, 15, 36, 15 |
36, 36-bp repeat; 15, 15-bp repeat.
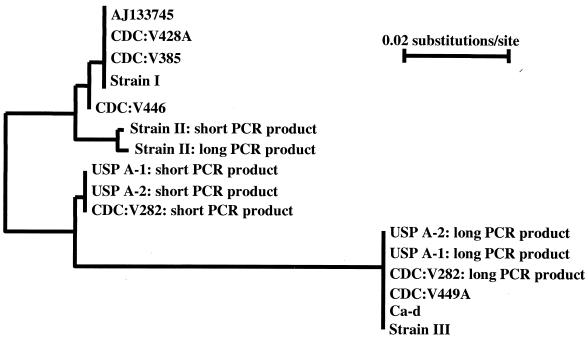
The 10 E. cuniculi isolates sequenced were divided into five genotypes based on SWP-1 banding patterns and sequences: (i) genotype Ia had one PCR band and 10 repeats; (ii) genotype Ib had one PCR band and 12 repeats; (iii) genotype II had two PCR bands, one with 9 repeats and one with 11 repeats; (iv) genotype IIIa had two PCR bands, one with 8 repeats and one with 12 repeats; (v) genotype IIIb had one PCR band with a sequence identical to the longer PCR product with 12 repeats in genotype IIIa (Tables 1 and 2). A neighbor-joining tree constructed with all SWP-1 sequences suggested that the long and short PCR products were related to each other within genotype II or IIIa and genotype Ia was related to genotype Ib whereas genotype IIIa was related to genotype IIIb (Fig. 4).
FIG. 4.
Genetic relatedness of spore wall protein gene sequences based on a neighbor-joining analysis.
DISCUSSION
Genetic characterization of pathogenic microorganisms has led to the development of molecular diagnostic tools, which have helped the understanding of the transmission of pathogens and epidemiologic investigations. While considerable progress has been made with several pathogenic enteric parasites, very few molecular epidemiologic studies have been conducted for the pathogens that cause human microsporidiosis. Since the discovery of three genotypes of E. cuniculi in 1995 (9), several studies of genetic variation in microsporidian parasites, including E. cuniculi, E. hellem, E. intestinalis, and E. bieneusi have been published (1, 3, 4, 11–15, 17–19). With a few exceptions using pulsed-field gel electrophoresis and karyotyping (2, 22), most of the genotyping studies have employed the ITS as the diagnostic target. In addition to those of E. cuniculi, different genotypes have been found for E. bieneusi and E. hellem (11, 14, 15, 17–19). Because most of the genotyping techniques involve DNA sequencing, the use of these techniques is largely restricted to a few research laboratories and only a few samples have been characterized. Thus, alternative, simpler techniques and additional genetic loci are needed for better characterization of the pathogen and its epidemiology.
Results of this study suggest that the PTP and SWP-1 genes can be good targets for genotype analysis. Sequence comparison of the PTP gene divided E. cuniculi into three genotypes. The segregation of genotypes was congruent with that at the ITS locus. The advantage with the PTP gene is the length polymorphism, which allows the development of simple PCR-based genotyping tools. Genotype III has a deletion of one copy of the 78-bp central repeats, enabling it to be differentiated from genotypes I and II by electrophoresis of PCR products. Because genotype II has not yet been found in humans, this size difference of PCR products allows the differentiation of two human-pathogenic E. cuniculi genotypes in clinical samples by agarose gel electrophoresis of PCR products. In addition, genotypes I and II can be differentiated from each other by RFLP analysis, which provides a mechanism for confirmation and circumvents the need for DNA sequencing. Data on E. cuniculi genetic polymorphism at the PTP locus and the PCR-RFLP tool developed in this study are very similar to those reported by another research group in an independent study (16), which was published recently while this paper was under review.
Similar results were observed for the SWP-1 gene. Length polymorphism originating from variations of repeat numbers also existed among the three E. cuniculi genotypes. This length variation was further seen within some genotypes and between different copies of the SWP-1 gene, which divided the genotypes I and III into several subgenotypes. The resolution of typing is likely to increase if the analyses involve more samples. The previous observation of the single-copy nature of the SWP-1 gene in E. cuniculi (2) is probably incorrect, because in this study we found that at least some isolates have two heterogeneous copies of the SWP-1 gene.
The linkage disequilibrium among the ITS, PTP, and SWP-1 E. cuniculi genotypes suggests a need for extensive genetic characterization and determination of the population structure of microsporidian parasites. Although a clonal reproduction of E. cuniculi can be argued based on findings of identical genotypes in different geographic regions and the linkage disequilibrium at the ITS, PTP, and SWP-1 loci, genetic characterizations of more loci are needed before such a conclusion can be made. Understanding the extent and nature of genetic variations in microsporidian parasites, however, is a prerequisite in characterizing the epidemiology and transmission of these pathogens. In particular, knowing whether genotypes of microsporidian parasites are stable (because of clonal propagation) or unstable (because of frequent genetic recombination) will be useful to the development of diagnostic tools.
ACKNOWLEDGMENTS
We thank C. del Aguila, J. Yee, E. Pozzio, A. Tosoni, M. Scaglia, and L. Felchle for providing various culture and tissue samples and Irshad Sulaiman for molecular analysis of one isolate.
REFERENCES
- 1.Biderre C, Mathis A, Deplazes P, Weber R, Metenier G, Vivares C P. Molecular karyotype diversity in the microsporidian Encephalitozoon cuniculi. Parasitology. 1999;118:439–445. doi: 10.1017/s0031182099004023. [DOI] [PubMed] [Google Scholar]
- 2.Bohne W, Ferguson D J, Kohler K, Gross U. Developmental expression of a tandemly repeated, glycine- and serine-rich spore wall protein in the microsporidian pathogen Encephalitozoon cuniculi. Infect Immun. 2000;68:2268–2275. doi: 10.1128/iai.68.4.2268-2275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitenmoser A C, Mathis A, Burgi E, Weber R, Deplazes P. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology. 1999;118:447–453. doi: 10.1017/s0031182099004229. [DOI] [PubMed] [Google Scholar]
- 4.del Aguila C, Izquierdo F, Navajas R, Pieniazek N J, Miro G, Alonso A I, Da Silva A J, Fenoy S. Enterocytozoon bieneusi in animals: rabbits and dogs as new hosts. J Eukaryot Microbiol. 1999;46:8S–9S. [PubMed] [Google Scholar]
- 5.Delbac F, Peyret P, Metenier G, David D, Danchin A, Vivares C P. On proteins of the microsporidian invasive apparatus: complete sequence of a polar tube protein of Encephalitozoon cuniculi. Mol Microbiol. 1998;29:825–834. doi: 10.1046/j.1365-2958.1998.00975.x. [DOI] [PubMed] [Google Scholar]
- 6.Deplazes P, Mathis A, Muller C, Weber R. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in faecal samples of pigs. J Eukaryot Microbiol. 1996;43:93S. doi: 10.1111/j.1550-7408.1996.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 7.Didier E S, Didier P J, Snowden K F, Shadduck J A. Microsporidiosis in mammals. Microbes Infect. 2000;2:709–720. doi: 10.1016/s1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 8.Didier E S, Visvesvara G S, Baker M D, Rogers L B, Bertucci D C, De Groote M A, Vossbrinck C R. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J Clin Microbiol. 1996;34:2835–2837. doi: 10.1128/jcm.34.11.2835-2837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D C, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–421. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 10.Fedorko D P, Nelson N A, Cartwright C P. Identification of Microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol. 1995;33:1739–1741. doi: 10.1128/jcm.33.7.1739-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liguory O, David F, Sarfati C, Derouin F, Molina J M. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. J Clin Microbiol. 1998;36:1882–1885. doi: 10.1128/jcm.36.7.1882-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liguory O, Fournier S, Sarfati C, Derouin F, Molina J M. Genetic homology among thirteen Encephalitozoon intestinalis isolates obtained from human immunodeficiency virus-infected patients with intestinal microsporidiosis. J Clin Microbiol. 2000;38:2389–2391. doi: 10.1128/jcm.38.6.2389-2391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathis A. Microsporidia: emerging advances in understanding the basic biology of these unique organisms. Int J Parasitol. 2000;30:795–804. doi: 10.1016/s0020-7519(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 14.Mathis A, Breitenmoser A C, Deplazes P. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite. 1999;6:189–193. doi: 10.1051/parasite/1999062189. [DOI] [PubMed] [Google Scholar]
- 15.Mathis A, Tanner I, Weber R, Deplazes P. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int J Parasitol. 1999;29:767–770. doi: 10.1016/s0020-7519(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 16.Peuvel I, Delbac F, Metenier G, Peyret P, Vivares C P. Polymorphism of the gene encoding a major Polar Tube Protein PTP1 in two microsporidia of the genus Encephalitozoon. Parasitology. 2000;121:581–587. doi: 10.1017/s0031182000006910. [DOI] [PubMed] [Google Scholar]
- 17.Rinder H, Katzwinkel-Wladarsch S, Loscher T. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi. Parasitol Res. 1997;83:670–672. doi: 10.1007/s004360050317. [DOI] [PubMed] [Google Scholar]
- 18.Rinder H, Katzwinkel-Wladarsch S, Thomschke A, Loscher T. Strain differentiation in microsporidia. Tokai J Exp Clin Med. 1998;23:433–437. [PubMed] [Google Scholar]
- 19.Rinder H, Thomschke A, Dengjel B, Gothe R, Loscher T, Zahler M. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J Parasitol. 2000;86:185–188. doi: 10.1645/0022-3395(2000)086[0185:CGRBEB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Rossi P, La Rosa G, Ludovisi A, Tamburrini A, Gomez Morales M A, Pozio E. Identification of a human isolate of Encephalitozoon cuniculi type I from Italy. Int J Parasitol. 1998;28:1361–1366. doi: 10.1016/s0020-7519(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 21.Snowden K, Logan K, Didier E S. Encephalitozoon cuniculi strain III is a cause of encephalitozoonosis in both humans and dogs. J Infect Dis. 1999;180:2086–2088. doi: 10.1086/315154. [DOI] [PubMed] [Google Scholar]
- 22.Sobottka I, Albrecht H, Visvesvara G S, Pieniazek N J, Deplazes P, Schwartz D A, Laufs R, Elsner H A. Inter- and intra-species karyotype variations among microsporidia of the genus Encephalitozoon as determined by pulsed-field gel electrophoresis. Scand J Infect Dis. 1999;31:555–558. doi: 10.1080/00365549950164427. [DOI] [PubMed] [Google Scholar]
- 23.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 24.Weiss L M, Vossbrinck C R. Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia. In: Wittner M, editor. Microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 129–195. [Google Scholar]