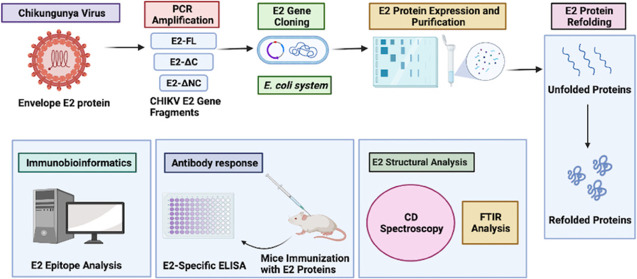
Abstract
Chikungunya virus (CHIKV) is a mosquito-transmitted alphavirus, which causes severe illness in humans and is responsible for epidemic outbreaks in Africa, Asia, North and South America, and Europe. Despite its increased global prevalence, no licensed vaccines are available to date for treating or preventing CHIKV infection. The envelope E2 protein is one of the promising subunit vaccine candidates against CHIKV. In this study, we describe successful cloning, expression, and purification of CHIKV E2 full-length (E2-FL) and truncated (E2-ΔC and E2-ΔNC) proteins in the Escherichia coli expression system. The recombinant E2 proteins were purified from inclusion bodies using Ni-NTA chromatography. Further, we describe a detailed refolding procedure for obtaining the CHIKV E2-FL protein in native conformation, which was confirmed using circular dichroism and Fourier transform infrared spectroscopy. BALB/c mice immunized with the three different E2 proteins exhibited increased E2-specific antibody titers compared to sham-immunized controls, suggesting induction of strong humoral immune response. On analyzing the E2-specific antibody response generated in immunized mice, the CHIKV E2-FL protein was observed to be the most immunogenic among the three different CHIKV E2 antigens used in the study. Our B-cell and T-cell epitope mapping results indicate that the presence of specific immunogenic peptides located in the N-terminal and C-terminal regions of the CHIKV E2-FL protein may contribute to its increased immunogenicity, compared to truncated CHIKV E2 proteins. In summary, our study provides a detailed protocol for expressing, purifying, and refolding of the CHIKV E2-FL protein and provides an understanding of its immunogenic epitopes, which can be exploited for the development of novel multiepitope-based anti-CHIKV vaccine strategies.
1. Introduction
Chikungunya is a viral disease caused by chikungunya virus (CHIKV). CHIKV is a single-stranded RNA virus belonging to the Togaviridae family and genus Alphavirus.1 It is transmitted to humans by infected Aedes aegypti and/or Aedes albopictus mosquitoes.2,3 Apart from mosquito transmission, some of the recent epidemics have arisen from maternal-fetal transmission as well.4 Chikungunya virus (CHIKV) causes severe illness and fever in humans, which is associated with other symptoms like rashes, myalgia, headache, and debilitating polyarthralgia.5 Although chikungunya fever is generally considered as self-limiting and nonfatal, casualties and complications have been seen in patients with comorbidities.6 While the acute stage of chikungunya infection typically lasts for only 1–2 weeks, severe joint pain, critical morning stiffness, swelling, chronic fatigue, and consistent inflammatory arthritis may persist for weeks, months, or years in affected individuals.7 The laboratory diagnosis of CHIKV infection is based on viral RNA detection through RT-PCR and the presence of IgM and IgG antibodies through serological tests.8 Several studies have also reported the recurrence of the disease in patients infected with CHIKV after the initial infection or relapse of chikungunya infection.9,10 The case fatality rate has been estimated to be 1 in 1000, with most deaths occurring in newborn children, the elderly, and adults with underlying medical conditions.11−13 CHIKV was originally isolated in 1953 from the Makonde plateau in Tanzania, followed by numerous outbreaks in Africa and Asia.14−16 Globally, the viral transmission is responsible for epidemic outbreaks in Africa, Europe, Southeast Asia, India, North America, and South America.17 In spite of its geographical expansion and outbreak, no specific treatment and effective licensed vaccines are currently available to prevent CHIKV infection. Hence, there is a need to understand and develop anti-CHIKV immunity through either vaccination or passive immunization strategies.
CHIKV is a spherical alphavirus, consisting of an enveloped particle comprising a nucleocapsid core containing positive-sense single-stranded RNA as a genetic material. The CHIKV genome consists two long open reading frames (ORFs). The genome of CHIKV (approximately 12 kb) encodes for five structural (envelope proteins E1, E2, E3, capsid, and 6K) and four nonstructural (nsP1–4) proteins. The E1 and E2 glycoproteins are mainly responsible for membrane fusion and virus entry into host cells, where E2 interacts with the cellular receptor and functions in attachment to cells and E1 participates in virus fusion to the cell membrane.18 The E2 protein contains three distinct domains: A (16–134 aa), B (173–231 aa), and C (269–341 aa), which are involved in receptor binding and are considered immunogenic.19,20 Literature reports show increasing evidence for the importance of antibody-mediated protection against CHIKV, and E2 glycoprotein has been implicated as the main target for the anti-CHIKV antibody response during the entire course of disease (from the convalescent phase to the recovery phase).21,22 Neutralizing antibodies raised against the E2 protein are believed to be crucial for protection in animal models.23,24 Since humoral immunity (conferred by B cells and antibodies) is an essential component of protective host response against CHIKV,25 passive immunotherapy using E2-specific monoclonal antibodies as well as polyclonal human immunoglobulin (Ig) has been explored with considerable success in CHIKV infection.26 Among some of the diverse vaccine strategies being explored for CHIKV, including live-attenuated or whole-inactivated virus, recombinant virus-vectored vaccines, inactivated viruslike particles (VLPs), nucleic acid vaccines, and subunit vaccine formulations focusing on the CHIKV envelope E2 protein have shown various advantages like safety, efficacy, scalable production, and cost-effectiveness during development.27,28
In the present study, we describe the cloning, expression, and purification of the CHIKV E2-FL protein along with its two truncated versions using an Escherichia coli-based prokaryotic expression system. The CHIKV E2-FL and truncated proteins were solubilized from inclusion bodies and purified using Ni-NTA chromatography. The E2 proteins were successfully refolded using a multistep dialysis procedure, and a secondary structure of refolded CHIKV E2 proteins was confirmed by circular dichroism (CD) and Fourier transform infrared (FTIR) spectroscopies. On testing the immunogenic nature of E2 proteins, the E2-FL protein was seen to exhibit significantly higher antibody titers in immunized mice when compared with sham-immunized controls. Our results from B-cell and T-cell epitope analyses using CHIKV E2-FL and truncated proteins demonstrate that specific epitopes located exclusively in the N-terminal and C-terminal regions of the CHIKV E2-FL protein may confer increased immunogenicity and provides insights into the development of a multiepitope and/or multivalent anti-CHIKV vaccine. In summary, the simplicity and cost-effectiveness of the recombinant CHIKV E2-FL protein purification methodology described herein are not only important for resource-limiting conditions but also can be exploited for developing fast and effective CHIKV E2-protein-based diagnostic or serological assays and can be utilized further in designing novel vaccine strategies.
2. Results
2.1. Sequence Alignment of Full-Length and Truncated E2 Proteins
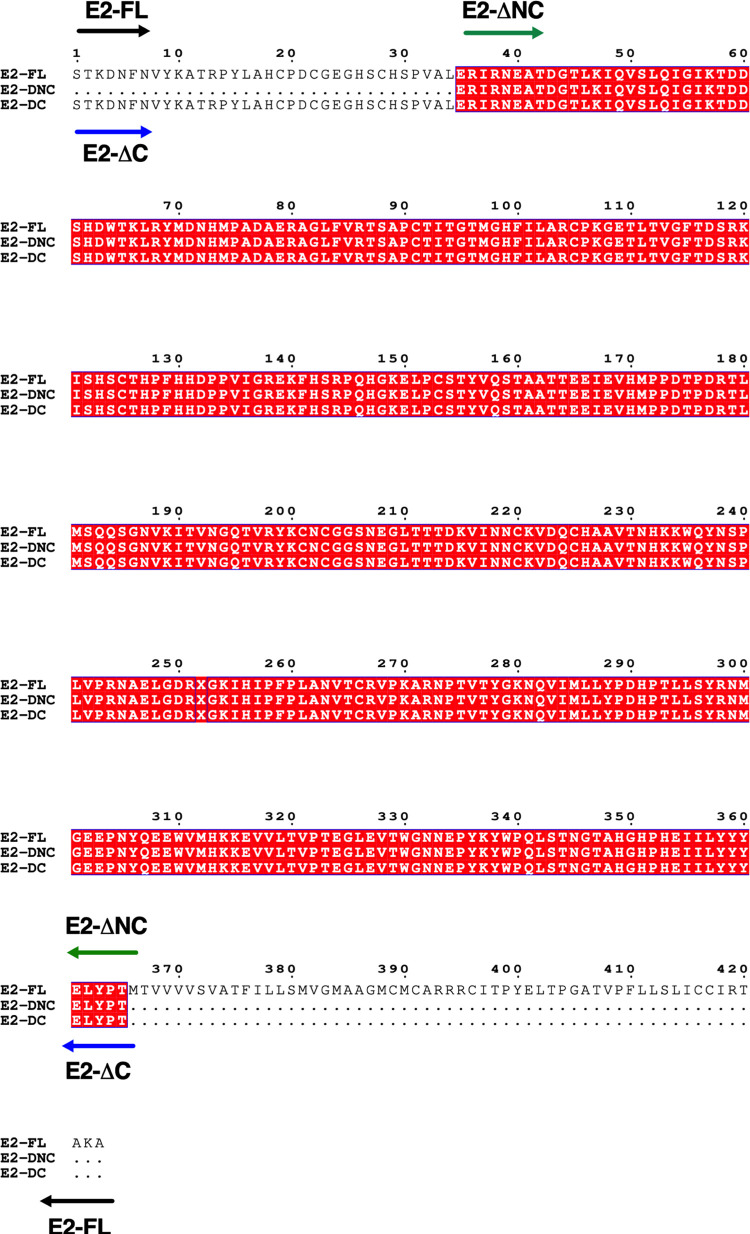
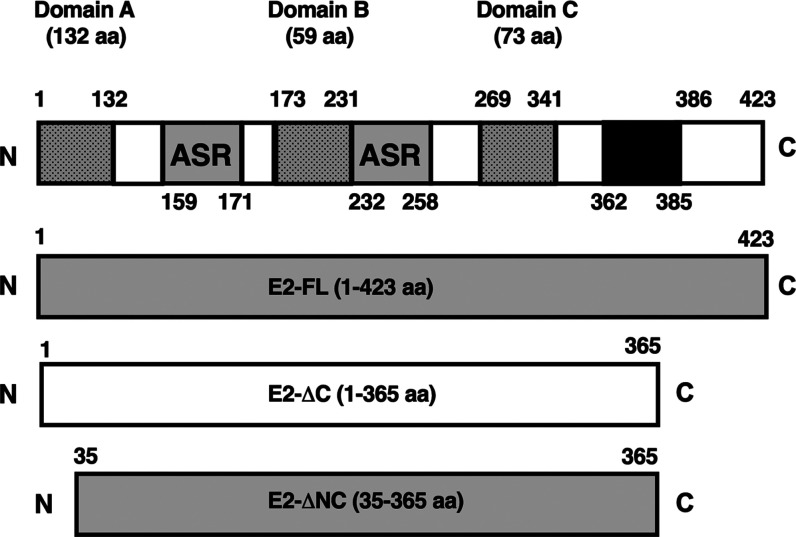
Sequence analysis of CHIKV E2-FL and E2 truncated proteins was performed using Clustal software. The amino acid sequence alignment of the CHIKV E2-FL and truncated protein fragments used in this study is shown in Figure 1. The regions for CHIKV E2-FL (1–423 aa), E2-ΔC (1–365 aa), and E2-ΔNC (35–365 aa) are indicated using black, blue, and green arrows, respectively. The CHIKV E2 protein (1–423 aa) contains a total of 17 cysteine residues, which may cause protein aggregation.29 Therefore, besides the CHIKV E2-FL protein, we also synthesized two truncated E2 protein fragments, namely, (a) the E2-ΔC (1–365 aa) fragment missing five cysteine residues from the C-terminal and (b) the E2-ΔNC (35–365 aa) fragment, missing five cysteine residues from the C-terminal and an additional three cysteine residues from the N-terminal end. While the E2-ΔC (1–365 aa) fragment had a truncation of 58 aa from the C-terminal portion, the E2-ΔNC (35–365 aa) fragment had a truncation of 58 aa from the C-terminal portion as well as 34 aa from the N-terminal end (330 aa). Figure 2 shows a schematic representation of the full-length and truncated CHIKV E2 fragments used in the study. CHIKV E2-FL contains three structural domains, Domain A (132 aa, spanning 1–132 aa), Domain B (59 aa, spanning 173–231 aa), and Domain C (73 aa, spanning 269–341 aa), two acid-sensitive regions (ASRs; from 159–171 aa and from 232–258 aa), and a 23 aa-long C-terminal transmembrane region (spanning 362–385 aa).20,21
Figure 1.
Amino acid sequence alignment of the CHIKV E2-FL and truncated E2 protein fragments used in the study. The 5′ start and 3′ stop regions for E2-FL (1–423 aa), E2-ΔC (1–365 aa), and E2-ΔNC (35–365 aa) proteins are indicated using black, blue, and green arrows, respectively.
Figure 2.
Schematic representation of full-length and truncated CHIKV E2 proteins used in the study. The three different E2 domains (dotted gray boxes) are indicated on top. The C-terminal transmembrane region (362–385 aa) is depicted in black. ASR, acid-sensitive regions (gray boxes). The C-terminal deleted (E2-ΔC) protein fragment (1–365 aa) and both C-terminal and N-terminal deleted (E2-ΔNC) protein fragment (35–365 aa) are outlined below the full-length E2 protein (1–423 aa).
2.2. PCR Amplification and Cloning of Full-Length and Truncated E2 Proteins
Total RNA was extracted from the chikungunya viral lysate (Ind-06-Guj), and virus cDNA was synthesized using the oligo-dT primer as mentioned in Experimental section. The gene-specific primers designed for amplifying E2-FL, E2-ΔC, and E2-ΔNC fragments are outlined in Table 1. The full-length CHIKV E2 gene (referred to as E2-FL) corresponding to 1269 bp was PCR-amplified using CHIKV cDNA and gene-specific primers as mentioned in the Experimental section (Figure 3A). PCR reactions for amplifying the two different truncated E2 fragments were carried out using the full-length E2 gene as a template. Single specific bands corresponding to 1095 bp and 993 bp were obtained for truncated CHIKV E2-ΔC and E2-ΔNC gene fragments, respectively (Figure 3B). The PCR amplicons were subjected to double digestion with restriction enzymes (BamH1 and HindIII) and cloned into the digested pQE-30 Xa vector having corresponding overhangs for enabling directional cloning. The ligated products were transformed in the E. coli XL1-Blue strain. The colonies obtained after transformation were subjected to plasmid isolation, and positive clones were identified by restriction analysis and confirmed by sequencing. The CHIKV E2-FL, E2-ΔC, and E2-ΔNC positive clones showed fallout of the correct size insert genes. Sanger sequencing results showed no mutation or reading-frame shift in any of the CHIKV E2 fragments used in this study.
Table 1. Primer Sequences Used for PCR Amplification and Generation of Recombinant Full-Length and Truncated CHIKV E2 Fragments.
| E2-regions | primers used | primer sequencec | base pairs | position | amino acidsd |
|---|---|---|---|---|---|
| CHIKV E2-FL | E2-FL FP | CCCCGGATCCAGCACCAAGGACAACTTCAATG | 1269 bp | 1–423 aa | 423 aa |
| E2-FL RP | CCCCCAAGCTTCGCTTTAGCTGTTCTGATGCAG | ||||
| CHIKV E2-ΔCa | E2-FL FP | CCCCGGATCCAGCACCAAGGACAACTTCAATG | 1095 bp | 1–365 aa | 365 aa |
| E2-TC RP | CCCCCAAGCTTAGTAGGGTACAGCTCATAATAATAC | ||||
| CHIKV E2-ΔNCb | E2-TNC FP | CCCCGGATCCGAACGCATCAGAAATGAAGCGAC | 993 bp | 35–365 aa | 331 aa |
| E2-TC RP | CCCCCAAGCTTAGTAGGGTACAGCTCATAATAATAC |
The CHIKV E2-ΔC fragment was obtained using listed forward and reverse primers and the CHIKV E2-FL cloned plasmid as the template.
The CHIKV E2-ΔNC fragment was obtained using listed forward and reverse primers and the CHIKV E2-FL cloned plasmid as the template.
The underlined nucleotides in forward and reverse primer sequences represent BamHI and HindIII restriction enzyme sites, respectively.
Besides the corresponding amino acids for CHIKV E2 fragments (shown in bold), all constructs included additional 31 aa from the vector (28 aa from the N-terminal and 3 aa from the C-terminal).
Figure 3.
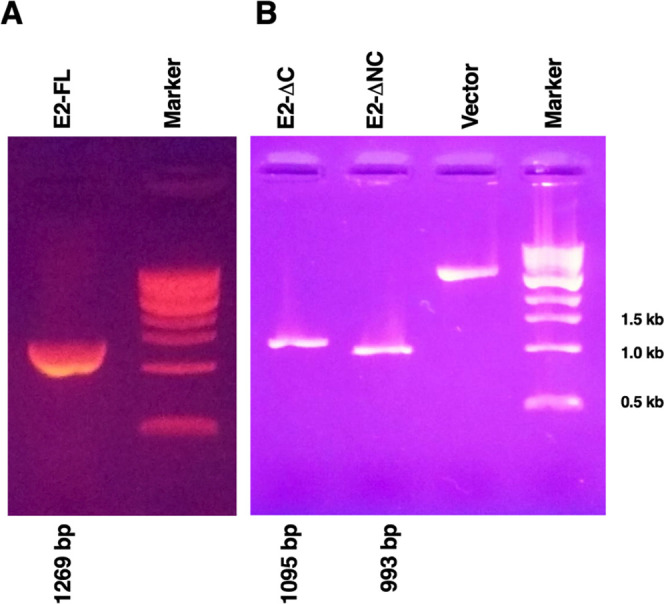
PCR amplification of CHIKV E2-FL and truncated fragments used in the study. (A) PCR amplification of the CHIKV E2 full-length gene (1269 bp). (B) PCR amplification of truncated E2 fragments, E2-ΔC (1095 bp), and E2-ΔNC (993 bp). Vector: linearized pQE-30 Xa vector. Marker: 1 Kb DNA ladder; last three marker bands are indicated on the right.
2.3. Expression of Full-Length and Truncated E2 Proteins
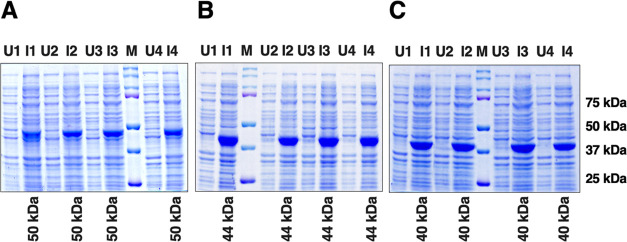
The E. coli SG13009 (Qiagen)-competent cells were transformed with recombinant CHIKV E2-FL, E2-ΔC, and E2-ΔNC plasmids in the pQE-30 Xa vector backbone carrying an N-terminal 6-His tag. The CHIKV E2-FL and truncated proteins were expressed in E. coli cells under IPTG induction. Next, overexpression of recombinant CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins under IPTG induction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The molecular weights of each of the three proteins, viz. E2-FL, E2-ΔC, and E2-ΔNC, were calculated using online tools and were found to be approximately 50, 44, and 40 kDa, respectively. SDS-PAGE gels stained with Coomassie brilliant blue staining show the presence of distinct bands migrating alongside the molecular weight marker bands, corresponding to their calculated molecular weights (Figure 4). Specific bands were obtained for histidine-tagged CHIKV E2 fusion proteins corresponding to E2-FL (50 kDa, Figure 4A), E2-ΔC (44 kDa, Figure 4B), and E2-ΔNC (40 kDa, Figure 4C). To investigate whether the full-length and truncated E2 proteins were expressed in the soluble or insoluble form inside E. coli, bacterial cultures obtained after IPTG induction were centrifuged. Bacterial pellets were subsequently analyzed on SDS-PAGE gels. The expressed CHIKV E2-FL and truncated proteins were found in the insoluble fraction (inclusion bodies), which was processed for further purification steps.
Figure 4.
Protein induction of CHIKV E2 full-length and truncated fragments used in the study. (A) CHIKV E2 full-length protein (423 aa; ∼50 kDa). (B) CHIKV E2 C-terminal truncated fragment E2-ΔC (365 aa; ∼44 kDa). (C) CHIKV E2 N-terminal and C-terminal truncated fragment E2-ΔNC (331 aa; ∼40 kDa). Lanes U: uninduced samples; Lanes I: induced samples; M: protein marker; marker bands are indicated on the right.
2.4. Purification of Full-Length and Truncated E2 Proteins
The T5 promoter/lac operator transcription–translation system-based pQE-30 Xa vector with an N-terminal 6-His tag offers an affinity-based purification strategy of recombinant fusion proteins expressed in E. coli. Nickel nitrilotriacetic acid (Ni-NTA) chromatography was chosen to purify the His-tagged CHIKV E2-FL and truncated proteins. Since the recombinant proteins were seen to accumulate in inclusion bodies, purification was performed under denaturing conditions in the presence of urea. The E. coli SG13009 cells harboring CHIKV E2-FL, E2-ΔC, and E2-ΔNC constructs were harvested from a 500 mL culture volume, and their cell weights were measured. The pellets were stored in Lysis buffer (300 mM NaCl, 25 mM Tris pH 7.3, 8 M urea, 5 mM β-ME, 0.1% Triton X-100, 10% glycerol) along with 1 mM PMSF and proteinase inhibitor cocktail at −80 °C overnight. The cells were then lysed using a Lysis buffer supplemented with lysozyme and mechanical sonication followed by syringe passing. The inclusion bodies were extracted by centrifugation, and the supernatants obtained were incubated with Ni-NTA slurry (Qiagen) and loaded into columns. After obtaining flowthrough, the columns were washed with a lysis buffer supplemented with 20 mM, 50 mM, and 100 mM imidazole. Finally, the His-tagged fusion proteins were eluted using a lysis buffer supplemented with 250 mM imidazole. During column washing steps in Ni-NTA chromatography, wash buffers were supplemented with 0.1% Triton X-114 for removing endotoxin contamination in purified CHIKV E2 proteins.
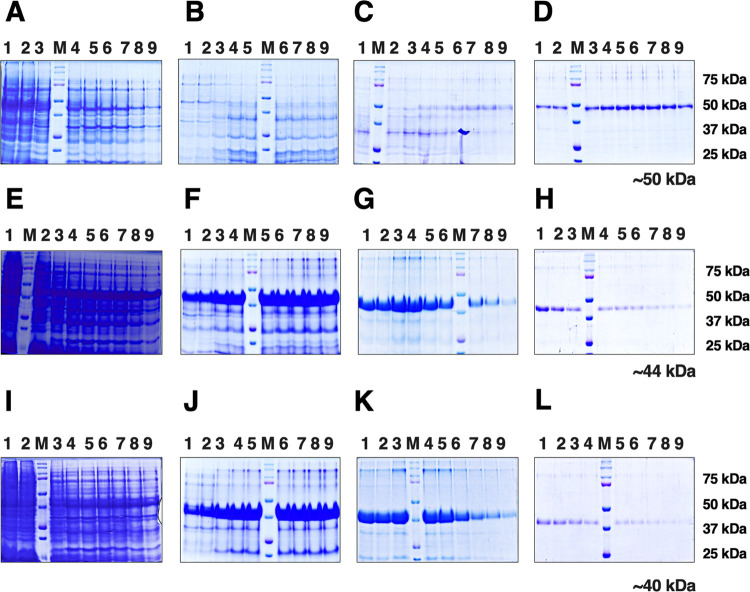
The purities of CHIKV E2-FL and E2 truncated protein fractions were estimated by SDS-PAGE electrophoresis, wherein the purified E2-FL and truncated proteins migrated through denaturing SDS-PAGE gels according to their expected molecular weight and were visualized using Coomassie brilliant blue staining. Figure 5 shows the SDS-PAGE analysis of washed and eluted fractions obtained under denaturing conditions (8.0 M urea, pH 7.3) using different imidazole concentrations for histidine-tagged CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins used in this study. For purifying the CHIKV E2-FL protein, the column was initially washed with 20 mM imidazole wash buffer and the fractions obtained are shown in lanes 1–9 (Figure 5A). Bacterial impurities in CHIKV E2-FL protein preparation were further removed by washing the column with 50 mM imidazole wash buffer, as indicated in lanes 1–9 (Figure 5B). To further purify the CHIKV E2-FL protein, columns were washed with 100 mM imidazole wash buffer, as shown in lanes 1–9 (Figure 5C). Subsequently, the imidazole concentration was increased to 250 mM in the elution buffer for eluting the CHIKV E2-FL protein (Figure 5D). The purified CHIKV E2-FL protein was seen to correspond to ∼50 kDa size as per the protein marker. For purifying the CHIKV E2-ΔC protein, the column was washed with 20 mM imidazole wash buffer, and the fractions obtained are shown in lanes 1–9 (Figure 5E). On using wash buffer supplemented with 50 mM imidazole, fractions containing impurities were observed in lanes 1–9 (Figure 5F). The bacterial impurities were further removed by washing the column with 100 mM wash buffer, and the fractions obtained are shown in lanes 1–9 (Figure 5G). Subsequently, the column was then passed with a 250 mM imidazole elution buffer for eluting the CHIKV E2-ΔC protein fractions shown in lanes 1–9 (Figure 5H). The purified CHIKV E2-ΔC protein corresponded to ∼44 kDa size as per the protein marker. For purifying the CHIKV E2-ΔNC protein, the Ni-NTA column was washed with wash buffer containing 20 mM imidazole, and the fractions obtained are depicted in lanes 1–9 (Figure 5I). Next, the column was washed with wash buffer containing 50 mM imidazole, and the fractions obtained are shown in lanes 1–9 (Figure 5J). Bacterial impurities were removed by passing the column with wash buffer containing 100 mM imidazole elution buffer, as shown in lanes 1–9 (Figure 5K). Finally, the CHIKV E2-ΔNC protein was eluted by passing the column with elution buffer containing 250 mM imidazole, and fractions obtained are shown in lanes 1–9 (Figure 5L). The purified CHIKV E2-ΔNC protein corresponded to ∼40 kDa size as per the protein marker.
Figure 5.
SDS-PAGE analysis of purified full-length and truncated His-tagged CHIKV E2 proteins used in the study. Fractions of purified proteins obtained under denaturing conditions (8.0 M urea, pH 7.3) using different imidazole concentrations in washed and elution buffers: Gels A, E, and I: 20 mM imidazole; gels B, F, and J: 50 mM imidazole; gels C, G, and K: 100 mM imidazole; and gels D, H, and L: 250 mM imidazole. (A–D) CHIKV E2 full-length protein (423 aa; ∼50 kDa). (E–H) CHIKV E2 C-terminal truncated fragment E2-ΔC (365 aa; ∼44 kDa). (I–L) CHIKV E2 N-terminal and C-terminal truncated fragment E2-ΔNC (331 aa; ∼40 kDa). Lanes 1–9: washed or eluted samples obtained at a given buffer concentration; M, protein marker; marker bands are indicated on the right.
2.5. Western Blotting of Full-Length and Truncated E2 Proteins
After purification, a single specific band corresponding to 50 kDa, 44 kDa, and 40 kDa was observed for CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins, devoid of any bacterial impurities. To confirm that the 50 kDa, 44 kDa, and 40 kDa bands observed on SDS-PAGE gels were of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins, a western blot hybridization was performed using the anti-His antibody (Figure 6). The band sizes at 50 kDa, 44 kDa, and 40 kDa, which correspond to His-tagged CHIKV E2-FL, E2-ΔC, and E2- ΔNC fusion proteins were detected, suggestive of the successful cloning, expression, and purification of CHIKV E2-FL and truncated proteins. CHIKV E2 protein estimation was performed using the BCA assay as per the commercially available kit. The physicochemical properties of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins were predicted by the ProtParam analysis tool on the Expasy website (Table 2).
Figure 6.
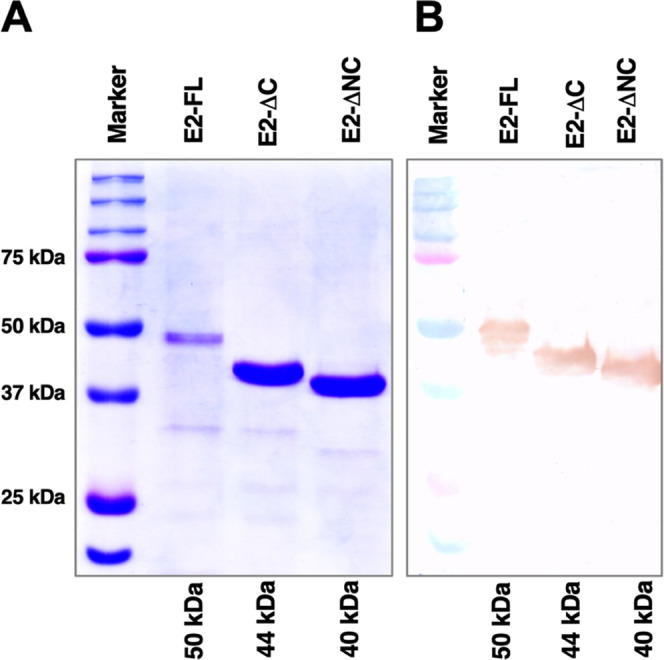
Western blotting of full-length and truncated CHIKV E2 proteins used in the study. (A) SDS-PAGE using Coomassie brilliant blue staining. (B) Western blot using the DAB substrate. Histidine-tagged full-length (E2-FL, ∼50 kDa), C-terminal truncated E2 (E2-ΔC, ∼44 kDa), and both N-terminal and C-terminal truncated E2 (E2-ΔNC, ∼40 kDa) proteins are shown. Protein marker band sizes are indicated on the left.
Table 2. Analysis of Physicochemical Properties of Full-Length and Truncated CHIKV E2 Protein Fragments Using the ProtParam Server.
| criteria | E2-FL | E2-ΔC | E2-ΔNC |
|---|---|---|---|
| number of amino acids | 454 | 396 | 362 |
| molecular weight (Da) | 50 523.73 | 44 359.06 | 40 657.94 |
| theoretical pI | 8.48 | 7.97 | 8.05 |
| number of negative amino acids (Asp+Glu) | 40 | 39 | 36 |
| number of positive amino acids (Arg+Lys) | 46 | 41 | 38 |
| instability index | 36.66 (stable protein) | 35.45 (stable protein) | 34.51 (stable protein) |
| grand average of hydropathicity (GRAVY) | –0.462 (hydrophilic protein) | –0.710 (hydrophilic protein) | –0.722 (hydrophilic protein) |
2.6. Refolding of Full-Length and Truncated E2 Proteins
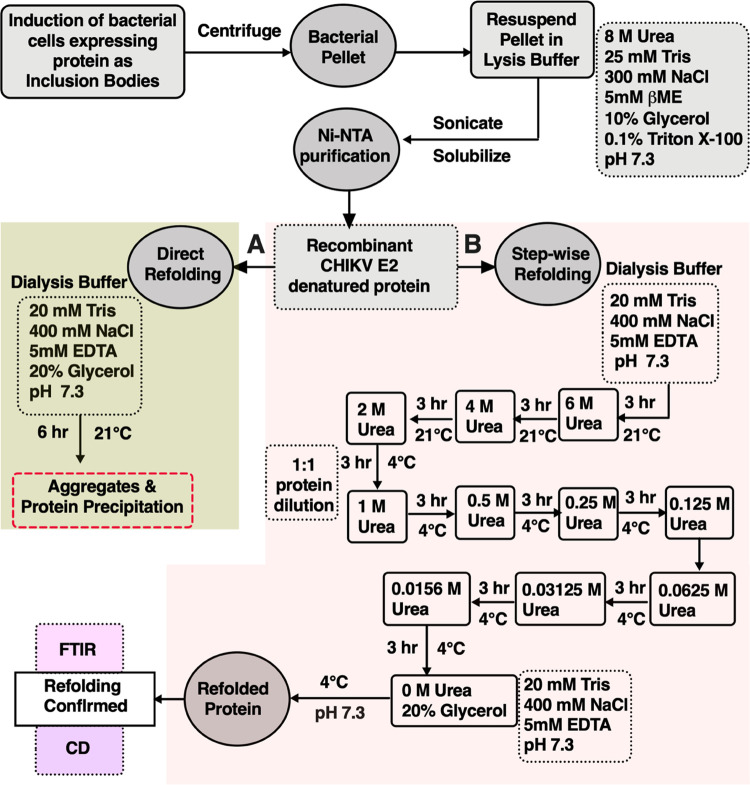
After protein purification with Ni-NTA chromatography, refolding of the denatured protein was attempted using the direct refolding (single-step) or stepwise refolding (multistep) dialysis procedure. In the direct refolding method, purified and truncated E2 protein fractions were dialyzed against the refolding buffer (containing 20 mM Tris pH 7.3, 400 mM NaCl, 5 mM EDTA, and 20% glycerol) at pH 7.3 for 6 h at 21 °C. After 6 h, a visible protein precipitate was observed. For the improvement of the refolding efficiency of CHIKV E2-FL and E2 truncated proteins, we modified and further optimized the refolding procedure. Instead of decreasing the urea concentration in a single step, we optimized the refolding of CHIKV E2-FL and E2 truncated proteins by decreasing the urea concentration in a total of 10 steps. Figure 7 shows a schematic representation of the detailed refolding procedure of CHIKV E2-FL and truncated proteins used in this study.
Figure 7.
Schematic representation of the refolding procedure of CHIKV E2 full-length and truncated proteins after solubilizing inclusion bodies. (A) Direct refolding method involving single-step dialysis for the refolding of the CHIKV E2 denatured protein, which led to protein precipitation. (B) Stepwise refolding method involving the multistep dialysis procedure optimized for the refolding of full-length and truncated CHIKV E2 denatured protein samples.
In the stepwise refolding method, the purified CHIKV E2-FL and E2 truncated protein fractions were dialyzed against the refolding buffer (containing 20 mM Tris pH 7.3, 400 mM NaCl, 5 mM EDTA) at pH 7.3 for 3 h. The protein fractions were dialyzed with 6 M urea at 21 °C for 3 h. Subsequently, the E2 protein fractions were subjected to refolding steps in 4 and 2 M urea at 21 °C for a time duration of 3 h each. The protein samples in 2 M urea were diluted to a final concentration of 1 M urea by addition of an equal volume of urea-free dialysis buffer, followed by dialysis against a buffer containing 1 M urea for 3 h. After 3 h, the protein fractions were dialyzed with gradually decreasing concentrations of urea (0.5, 0.25, and 0.125 M) at 4 °C and a time duration of 3 h each. The urea concentration was further decreased gradually to 0.0625, 0.03125, and 0.0156 M concentrations, and protein fractions were dialyzed at 4 °C and a time duration of 3 h each. Finally, the CHIKV E2 protein fractions were subjected to dialysis in a urea-free (0 M urea) dialysis buffer (20 mM Tris pH 7.3, 400 mM NaCl, 5 mM EDTA, and 20% glycerol) at pH 7.3 and 4 °C for 3 h with continuous stirring to obtain the refolded CHIKV E2-FL and truncated protein fragments.
We observed that the direct refolding method for the refolding of the CHIKV E2 unfolded protein in urea led to formation of protein precipitates. On the other hand, by optimizing a stepwise refolding method for the refolding of CHIKV E2-FL and E2 truncated protein samples, no visible protein precipitates were observed. Both unfolded and refolded protein fractions were used for subsequent structural analysis.
2.7. Structural Analysis of Full-Length and Truncated E2 Proteins
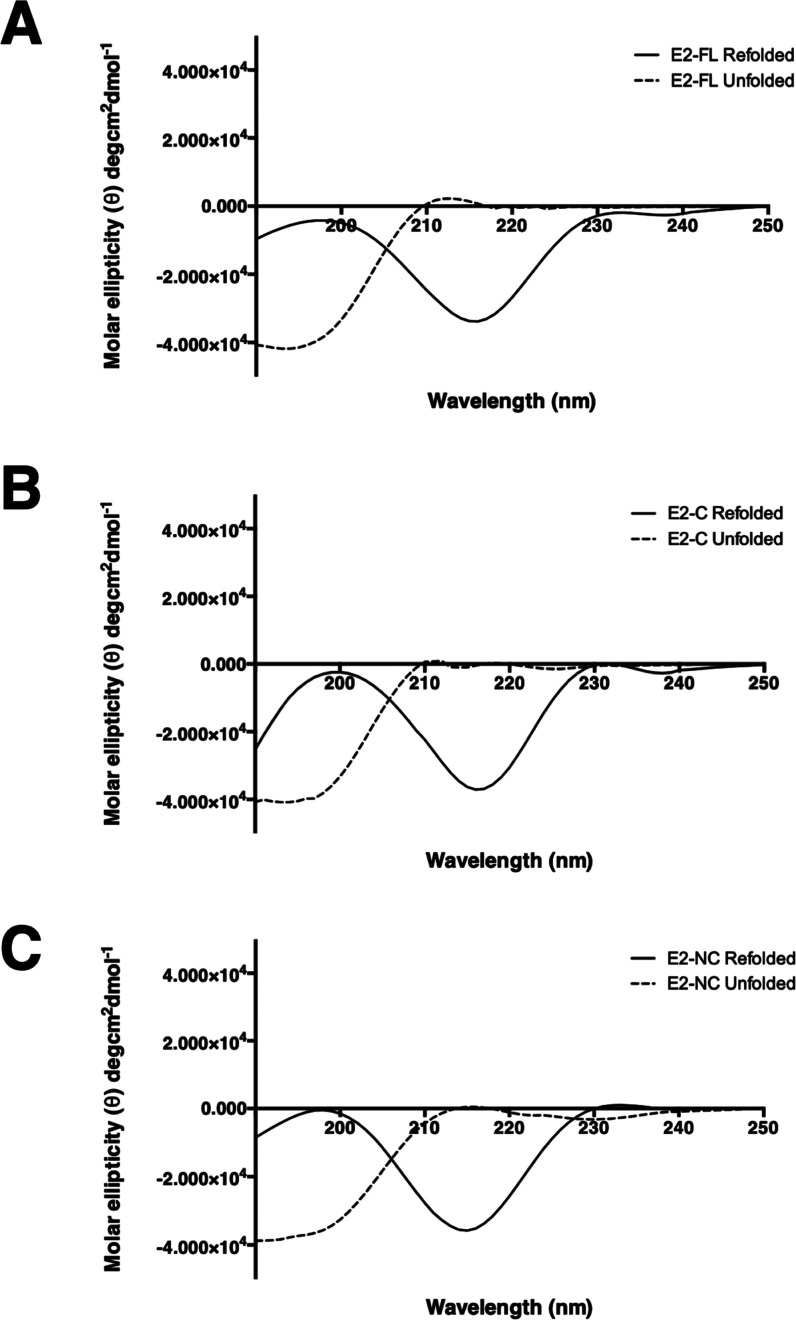
We used CD spectroscopy to analyze the secondary structure of denatured and refolded CHIKV E2-FL and truncated proteins used in the study. The CD spectral data were analyzed and deconvoluted using the CD spectra algorithm available online, as mentioned in the Methods section (Figure 8). Molar ellipticity is depicted on the y-axis with wavelength on the x-axis. The CD spectra were compared for denatured and refolded forms of CHIKV E2-FL (Figure 8A), E2-ΔC (Figure 8B), and E2-ΔNC (Figure 8C) proteins. On comparing CD spectra for each of the refolded and denatured CHIKV E2-FL and truncated protein samples, a change in CD spectra was observed in the folded sample when compared with the denatured samples. As shown in Figure 8A–C, the dip seen around 214 nm in the CD spectra for the refolded protein samples suggests the presence of secondary structure elements, especially β-strands,30 whereas the denatured samples depicted a predominantly random coil structure.
Figure 8.
Far UV-CD spectra analysis of denatured and refolded CHIKV E2 full-length and truncated proteins. CD spectra obtained for (A) E2-FL, (B) E2-ΔC (B), and (C) E2-ΔNC proteins are shown. Molar ellipticity is depicted on the y-axis with wavelength on the x-axis. The denatured and refolded samples are shown in dotted lines and black lines, respectively.
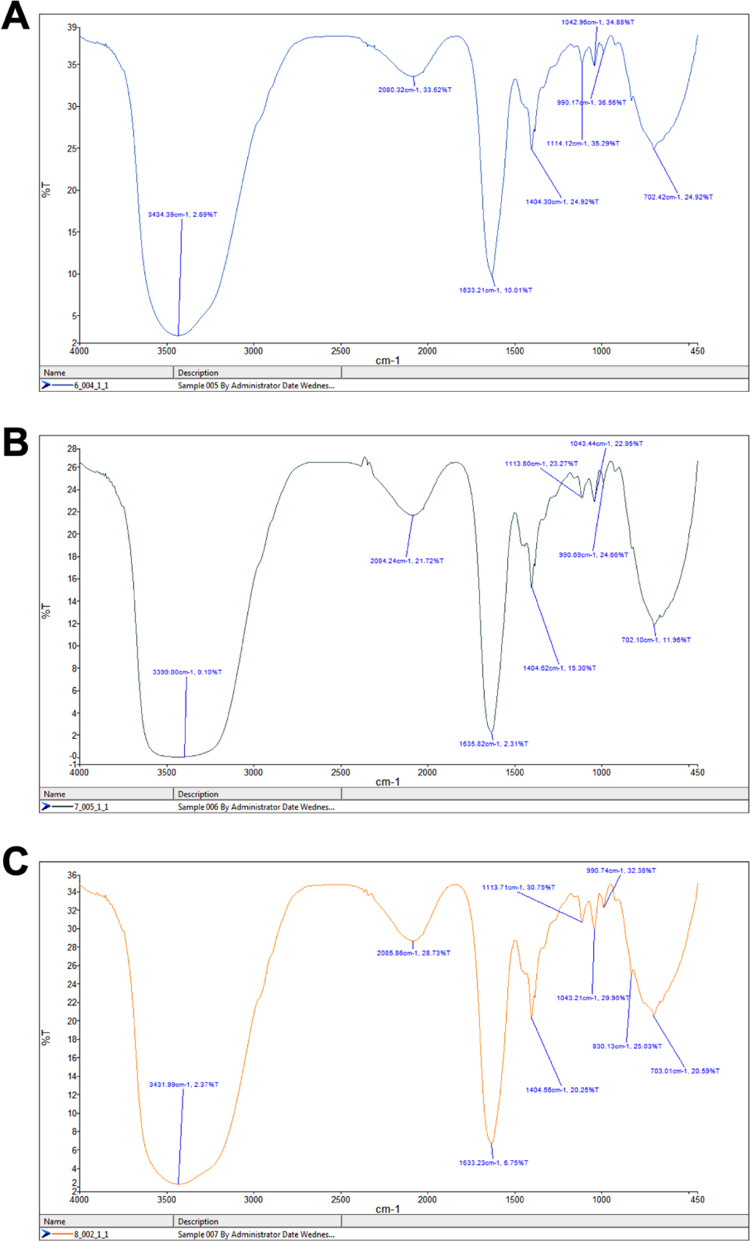
To further affirm the correctly folded state of the in vitro refolded E2 proteins, the respective samples of CHIKV E2-FL (1–423 aa), E2-ΔC (1–365 aa), and E2-ΔNC (35–365 aa) were subjected to FTIR spectroscopy. The IR spectral data of proteins is usually interpreted in terms of vibrations of secondary structure repeat units, which give rise to nine absorption bands, namely, amides A, B, and I–VII. Among these, the amide-I band (1700–1600 cm–1), which is generated by the C=O stretch vibrations of the peptide linkages, is the most useful in predicting the secondary structure of proteins. The amide bands in the regions of 1642–1624 and 1696–1691 cm–1 correspond to β-sheets, whereas the amide bands in the regions of 1656–1651 and 1685–1667 cm–1 correspond to α-helix and β-turn secondary structures, respectively.31 In the case of each refolded E2 protein sample, a sharp and distinct amide band was observed at 1633/1635 cm–1 corresponding to β-sheet structures (Figure 9).
Figure 9.
FTIR spectra of refolded CHIKV E2 full-length and truncated protein samples used in the study. (A) E2 full-length (1–423 aa), (B) E2-ΔC (1–365 aa), and (C) E2-ΔNC (35–365 aa) protein samples. In the FTIR spectra, the sharp and prominent peaks obtained at around 1633/1635 cm–1 indicate the presence of β-sheet-rich structures in the refolded protein samples.
The FTIR results obtained for unfolded E2-FL, E2-ΔC, and E2-ΔNC proteins are shown in Figure S1. The peaks obtained at 1633/1635 cm–1 for unfolded E2 protein samples were not as sharp compared to the peaks obtained for refolded protein samples. The spectral data are depicted for refolded CHIKV E2-FL (Figure 9A), E2-ΔC (Figure 9B), and E2-ΔNC (Figure 9C) proteins. This spectral data also correlate with the crystal structures of the E2 protein reported earlier, wherein it has been shown that all three domains of the E2 protein are composed of β-sandwich motifs.19 Therefore, the CD and FTIR data collectively suggest that the refolded CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins have attained their native conformations.
2.8. Antibody Immune Responses against Full-Length and Truncated E2 Proteins
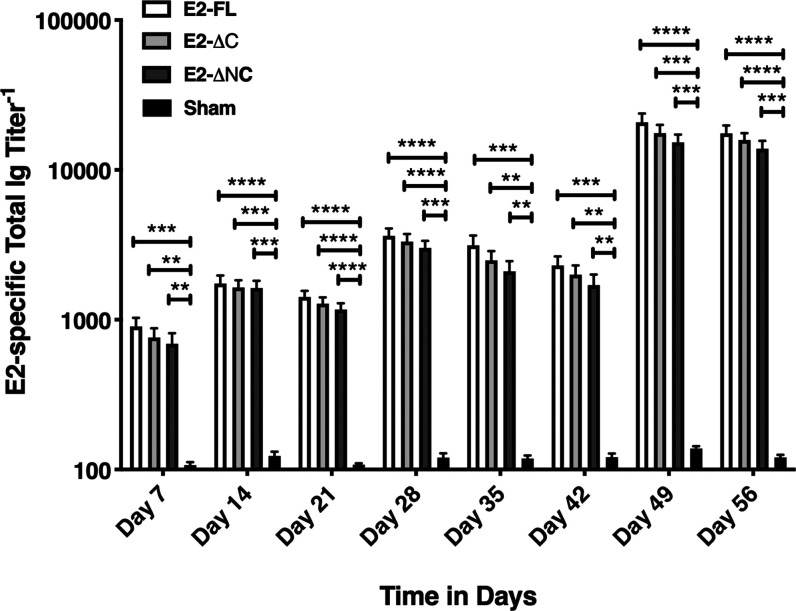
To evaluate the immunogenic efficiencies of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins, different groups of wild-type BALB/c mice were immunized with CHIKV E2-FL and E2 truncated proteins along with alum as the adjuvant. Before mice immunization, endotoxin levels in purified CHIKV E2 proteins were quantified using a commercial kit and the endotoxin levels in all immunogens were found to be between 0.5 and 0.1 EU/mL. Serum obtained from CHIKV E2-immunized and sham-immunized mice was tested by ELISA to detect the presence of antibodies specific to the CHIKV E2-FL protein. The CHIKV E2-specific total Ig titers in different groups of mice immunized on day 0, day 14, and day 42 with CHIKV E2-FL, E2-ΔC, and E2-ΔNC antigens and sham controls are shown (Figure 10). Total Ig antibody levels in CHIKV E2-immunized mice were found to be increased compared to those in sham-immunized controls, suggesting the induction of strong humoral immune response. Compared with sham-immunized controls, mice immunized with the CHIKV E2-FL protein exhibited significantly increased Ig titers as opposed to mice immunized with E2-ΔC and E2-ΔNC proteins on days 7, 14, 21, 28, 35, 42, 49, and 56 post immunization. At each timepoint, no significant difference (p > 0.05) was noted when antibody titers for the three different CHIKV E2 proteins were compared. CHIKV E2-specific Ig titers increased significantly after each booster dose of the E2 antigen, with peak antibody titers observed on day 49, one week after the second booster dose. Of note, we found that E2-specific antibody titers obtained after CHIKV E2-FL immunization were consistently higher than antibody titers obtained after immunization with E2-ΔC and E2-ΔNC proteins, at all times examined. Sham-immunized mice had very low or negligible antibody titers, which indicate specific antibody response generated only against CHIKV E2 proteins in immunized mice. Our results demonstrate that although immunization with CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins could efficiently produce high antibody titers in mice, the CHIKV E2-FL protein was seen to be the most immunogenic among the three different E2 antigens used in the study, making it a potent immunogenic antigen against CHIKV infection.
Figure 10.
CHIKV E2-specific total Ig titers in different groups of BALB/c mice immunized on day 0, day 14, and day 42 with E2-FL (open bars), E2-ΔC (gray bars), and E2-ΔNC (dark gray) antigens and sham controls (black bars). Antibody titers are shown on the y-axis, and time in days is indicated on the x-axis. All serum samples were tested in duplicate. Bars represent mean ± scanning electron microscopy (SEM) (n = 5). P values are determined using ordinary one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. Differences between groups are indicated by bars and symbols: *, P<0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
2.9. Epitope Analysis of Full-Length and Truncated E2 Proteins
2.9.1. B-Cell Epitope Analysis
Tertiary (three-dimensional, 3D) structure modeling was performed using SWISS-MODEL (https://swissmodel.expasy.org) and I-TASSER (I-TASSER server for protein structure and function prediction (zhanggroup.org)) for all three CHIKV E2 protein fragments based on available structure templates. The predicted structures of the three CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins were visualized in PyMOL graphics (Figure 11).
Figure 11.
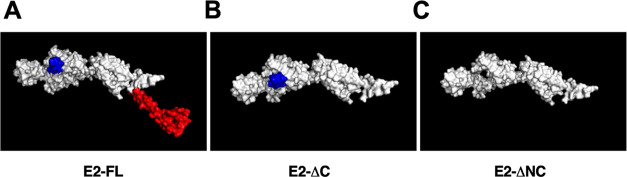
Tertiary structures of (A) CHIKV E2-FL, (B) E2-ΔC, and (C) E2-ΔNC proteins. The N-terminal B-cell epitopes (1–19 and 16–32 aa) present only in E2-FL and E2-ΔC proteins are shown in blue. The extra C-terminal region (366–423 aa), present only in the E2-FL protein (containing four additional B-cell epitopes), is shown in red.
To further identify the unique epitopes and investigate the immunogenicity difference in all three CHIKV E2 protein fragments used in this study, we performed B-cell epitope analysis using two different approaches. In the first approach, we analyzed the presence or absence of B-cell epitopes identified in previously published studies, which included experimental validation of these epitopes in the CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins. A number of previously published studies have experimentally mapped and identified CHIKV E2 protein B-cell epitopes in human, mouse, and nonhuman primates using synthetic overlapping peptides.24,28,32,33 On comparing the sequences of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins for the presence or absence of 10 B-cell epitopes identified by Kam et al.,32,34 we found the presence of all 10 epitopes in the CHIKV E2-FL protein (Table 3). However, E2-ΔC and E2-ΔNC protein fragments showed the presence of only 9 epitopes and 8 epitopes, respectively. Specifically, the N-terminal epitope (1–19 aa) was found absent in the E2-ΔNC protein fragment, while the C-terminal epitopes (378–411) were found absent in both the E2-ΔC and E2-ΔNC protein fragments (shown in blue and red, Figure 11). On comparing the sequences of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins for the presence or absence of seven B-cell epitopes identified by Chua et al.,24 we found the presence of all seven epitopes in the CHIKV E2-FL and E2-ΔC protein fragments (Table 3). However, the E2-ΔNC protein fragment showed the presence of only six epitopes, wherein the N-terminal epitope (16–30 aa) was found absent (shown in blue, Figure 11). On comparing the sequences of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins for the presence or absence of 17 B-cell epitopes identified by Verma et al.,29 we found all 17 epitopes to be present in the CHIKV E2-FL and E2-ΔC protein fragments (Table 3). However, the E2-ΔNC protein fragment showed the presence of only 16 epitopes, wherein the N-terminal epitope (18–32 aa) was found absent (shown in blue, Figure 11).
Table 3. B-Cell Epitopes (as per References Cited) Present in CHIKV E2 Full-Length and Truncated Proteins Used in the Study¶.
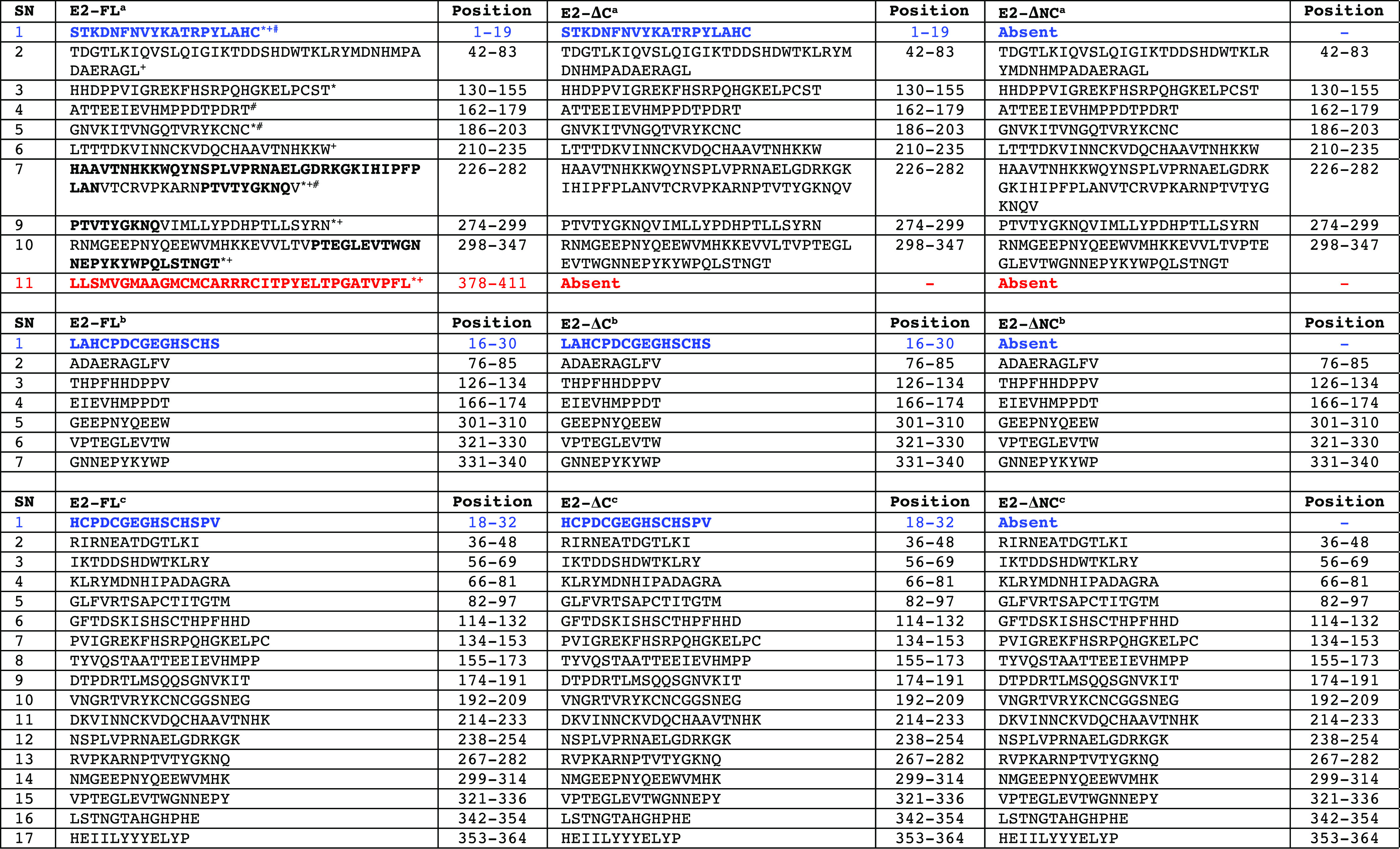
E2 epitopes identified as per ref (32).
E2 epitopes identified as per ref (24).
E2 epitopes identified as per ref (70).
E2 epitopes identified in humans as per ref (22).
E2 epitopes identified in mouse as per ref (25).
E2 epitopes identified in macaque as per ref (33).
E2 epitope residues conserved in humans and mouse are indicated in bold. The N-terminal epitope (1–19 aa and 16–32 aa) is listed in blue, present in CHIKV E2-FL and E2-ΔC proteins. The C-terminal epitope (378–411 aa) present only in the CHIKV E2-FL protein is shown in red.
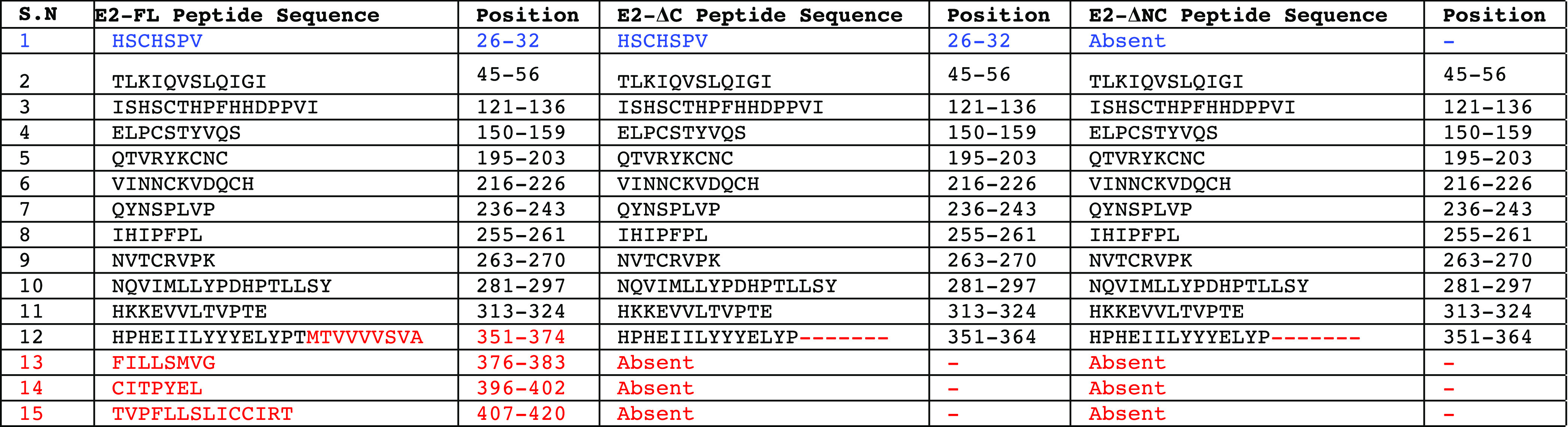
In the second approach, we performed in silico B-cell epitope analysis for CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins used in the study using two distinct methods, including ABCpred (https://webs.iiitd.edu.in/raghava/abcpred/ABC_submission.html) and BCEpred (https://webs.iiitd.edu.in/raghava/bcepred/bcepred_submission.html) servers using their default threshold values. Using the ABCpred server, we identified varying numbers of potential B-cell epitopes in CHIKV E2-FL (n = 41), CHIKV E2-ΔC (n = 36), and CHIKV E2-ΔNC (n = 33) using a window size of 16-mers at 0.51 threshold value. The ABCpred online server predicted four potential B-cell epitopes in the extra C-terminal region of the CHIKV E2-FL protein, which were absent in both the E2-ΔC and E2-ΔNC proteins (shown in red, Figure 11) (Table 4). From the BCEpred server, the potential B-cell epitopes were predicted as follows: CHIKV E2-FL (n = 15), CHIKV E2-ΔC (n = 12), and CHIKV E2-ΔNC (n = 11). According to the BCEpred server (based on accessibility), three extra potential B-cell epitopes were identified in the extra C-terminal region of the CHIKV E2-FL protein at 1.9 threshold value (shown in red, Figure 11) (Table 5). These three epitopes overlapped with the four unique epitopes found in the extra C-terminal region of CHIKV E2-FL as per ABCpred server results.
Table 4. List of Predicted B-Cell Epitopes in CHIKV E2 Protein Fragments Using the ABCpred Server (Epitope Threshold = 0.51).
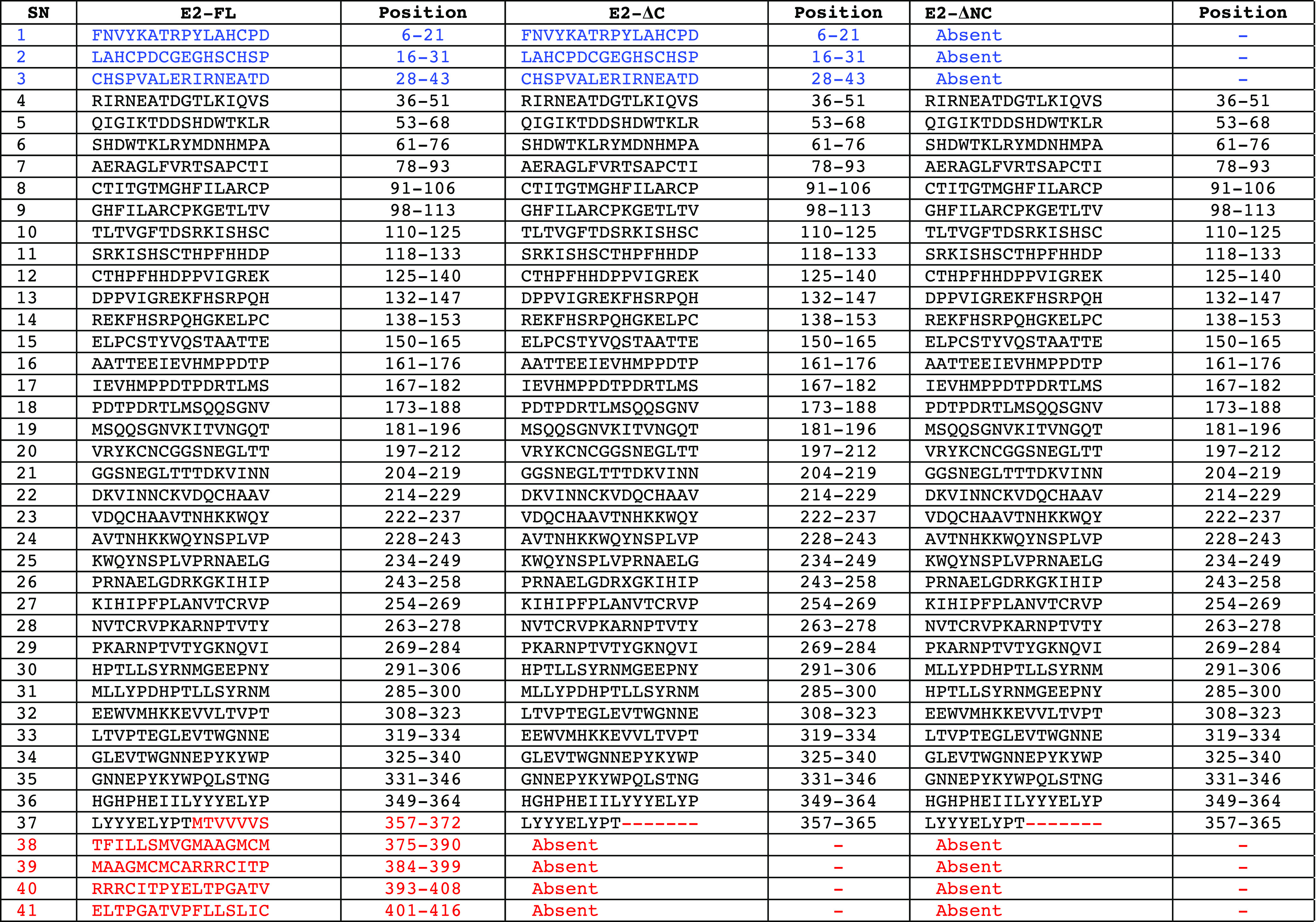
Table 5. List of Predicted B-Cell Epitopes in CHIKV E2 Protein Fragments Using the BCEpred Server (Epitope Threshold = 1.9).
2.9.2. T-Cell Epitope Analysis
The use of the CHIKV E2-FL protein is expected to improve the efficacy and therapeutic potential of the subunit vaccine against chikungunya infection, as compared to vaccine formulations using truncated fragments of the CHIKV E2 protein, which lack the C-terminal epitopes. To test whether the CHIKV E2-FL protein fares better than the truncated fragments in terms of immunogenic epitopes, we investigated if the extra C-terminal region of the CHIKV E2-FL protein contains immunogenic peptides or better epitopes, which are not present in the truncated CHIKV E2-ΔC and E2-ΔNC fragments. For identifying these T-cell epitopes, we performed in silico analysis of the extra C-terminal region of the CHIKV E2-FL protein using T-cell epitope prediction servers available online, which calculate the binding affinity and antigenicity by generating small peptide sequences from the submitted sequence, on the basis of predicted scores above a certain threshold value. Since MHC binding is a prerequisite for T-cell-based immune responses against antigenic peptides, we used this as a parameter for identifying the potential antigenic peptides in the extra region of the CHIKV E2-FL protein.
For identifying human MHC class I binding T-cell epitopes present in the extra region of the CHIKV E2-FL protein, we used the Immune Epitope Database and the Analysis Resource (IEDB) T-cell epitope prediction server, wherein the CHIKV E2-FL protein C-terminal sequence spanning 366–423 aa (58 aa) was submitted as the query sequence. The IEDB-recommended NetMHCpan EL 4.1 (http://tools.iedb.org/mhci/, version 2020.09) method was used to predict the MHC class I binding affinity of T-cell epitopes (of 8–14 mer length) for 77 human MHC class I alleles present in the IEDB server. The server predicts the binding affinity of each peptide with multiple MHC alleles, which resulted in the total number of peptides being more than the total overlapping peptides possible in the extra region of the protein. We found a total of 2673 CD8 T-cell potential peptides binding to 27 human MHC I alleles. As per published reports,35,36 T-cell epitopes with an MHC class I binding score>0.75 are considered good binders. We identified two potential T-cell epitopes (ATVPFLLSL: 0.87 and YELTPGATV: 0.85) in the CHIKV E2-FL extra C-terminal region binding exclusively to HLA-A*02:06 and HLA-B*40:01 MHC class I alleles, respectively, whose score value>0.75 (Table 6). These two potential epitopes were absent in both the truncated (E2-ΔC and E2-ΔNC) fragments.
Table 6. List of MHC Class I and MHC Class II T-Cell Epitopes in the Extra C–Terminal Region of the CHIKV E2-FL Protein Predicted Using the IEDB Online Server Along with Their Alleles.
| SN | peptide | position (aa) | identity of human MHC I alleles |
|---|---|---|---|
| 1 | ATVPFLLSL | 406–414 | HLA-A*02:06 |
| 2 | YELTPGATV | 400–408 | HLA-B*40:01 |
| SN | peptide | position (aa) | identity of human MHC II alleles |
|---|---|---|---|
| 1 | AAGMCMCARRRCITP | 385–399 | HLA-DRB5*01:01, HLA-DRB1*11:01 |
| 2 | AGMCMCARRRCITPY | 386–400 | HLA-DRB1*11:01 |
| 3 | ATFILLSMVGMAAGM | 374–388 | HLA-DRB1*01:01, HLA-DRB1*04:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*11:01, HLA-DRB1*04:05, HLA-DRB4*01:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*15:01, HLA-DRB1*09:01, HLA-DRB5*01:01, HLA-DRB1*03:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*07:01 |
| 4 | ATVPFLLSLICCIRT | 406–420 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*04:05, HLA-DPA1*01:03/DPB1*02:01, HLA-DRB1*15:01 |
| 5 | CITPYELTPGATVPF | 396–410 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*09:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*11:01 |
| 6 | ELTPGATVPFLLSLI | 401–415 | HLA-DPA1*03:01/DPB1*04:02, HLA-DPA1*02:01/DPB1*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DPA1*01:03/DPB1*02:01 |
| 7 | FILLSMVGMAAGMCM | 376–390 | HLA-DRB1*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*04:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*04:05, HLA-DRB1*11:01, HLA-DRB1*09:01, HLA-DRB4*01:01, HLA-DRB1*15:01, HLA-DRB1*07:01 |
| 8 | GATVPFLLSLICCIR | 405–419 | HLA-DRB1*01:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*07:01, HLA-DPA1*01:03/DPB1*02:01 |
| 9 | GMAAGMCMCARRRCI | 383–397 | HLA-DRB5*01:01, HLA-DRB1*11:01 |
| 10 | ILLSMVGMAAGMCMC | 377–391 | HLA-DRB1*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*04:01 |
| 11 | ITPYELTPGATVPFL | 397–411 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*09:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*11:01 |
| 12 | LLSMVGMAAGMCMCA | 378–392 | HLA-DRB1*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*04:01 |
| 13 | LSMVGMAAGMCMCAR | 379–393 | HLA-DRB1*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DQA1*01:02/DQB1*06:02 |
| 14 | LTPGATVPFLLSLIC | 402–416 | HLA-DPA1*03:01/DPB1*04:02, HLA-DPA1*02:01/DPB1*01:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DRB1*07:01 |
| 15 | MAAGMCMCARRRCIT | 384–398 | HLA-DRB5*01:01, HLA-DRB1*11:01 |
| 16 | MTVVVVSVATFILLS | 366–380 | HLA-DPA1*02:01/DPB1*01:01, HLA-DRB1*07:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DRB1*01:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*13:02, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB5*01:01, HLA-DRB1*15:01, HLA-DRB1*09:01, HLA-DRB1*08:02, HLA-DQA1*05:01/DQB1*03:01, HLA-DPA1*02:01/DPB1*05:01, HLA-DRB1*03:01, HLA-DRB1*04:05, HLA-DRB4*01:01 |
| 17 | MVGMAAGMCMCARRR | 381–395 | HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*01:01, HLA-DRB5*01:01, HLA-DQA1*05:01/DQB1*03:01 |
| 18 | PFLLSLICCIRTAKA | 409–423 | HLA-DRB1*01:01, HLA-DRB1*11:01, HLA-DRB1*04:01, HLA-DRB1*07:01, HLA-DRB4*01:01 |
| 19 | PGATVPFLLSLICCI | 404–418 | HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DPA1*02:01/DPB1*01:01 |
| 20 | PYELTPGATVPFLLS | 399–413 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*09:01 |
| 21 | RCITPYELTPGATVP | 395–409 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*09:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*11:01 |
| 22 | RRCITPYELTPGATV | 394–408 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*09:01, HLA-DRB1*11:01 |
| 23 | SMVGMAAGMCMCARR | 380–394 | HLA-DRB1*01:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DQA1*05:01/DQB1*03:01 |
| 24 | SVATFILLSMVGMAA | 372–386 | HLA-DRB1*01:01, HLA-DRB1*04:01, HLA-DRB1*11:01, HLA-DRB1*15:01, HLA-DRB1*04:05, HLA-DRB4*01:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DQA1*01:02/DQB1*06:02, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*09:01, HLA-DRB5*01:01, HLA-DRB1*03:01, HLA-DPA1*02:01/DPB1*01:01, HLA-DRB1*07:01, HLA-DRB1*12:01 |
| 25 | TFILLSMVGMAAGMC | 375–389 | HLA-DRB1*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*04:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*04:05, HLA-DRB1*11:01, HLA-DRB1*03:01, HLA-DRB1*09:01, HLA-DRB1*15:01, HLA-DRB4*01:01 |
| 26 | TPGATVPFLLSLICC | 403–417 | HLA-DPA1*03:01/DPB1*04:02, HLA-DPA1*02:01/DPB1*01:01, HLA-DPA1*01:03/DPB1*02:01 |
| 27 | TPYELTPGATVPFLL | 398–412 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*09:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*11:01 |
| 28 | TVPFLLSLICCIRTA | 407–421 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*04:05, HLA-DPA1*03:01/DPB1*04:02, HLA-DPA1*01:03/DPB1*02:01 |
| 29 | TVVVVSVATFILLSM | 367–381 | HLA-DPA1*02:01/DPB1*01:01, HLA-DRB1*07:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*01:01, HLA-DRB1*13:02, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*15:01, HLA-DRB5*01:01, HLA-DRB1*09:01, HLA-DPA1*02:01/DPB1*05:01, HLA-DRB1*04:05, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*08:02, HLA-DRB1*03:01, HLA-DRB1*04:01, HLA-DRB4*01:01 |
| 30 | VATFILLSMVGMAAG | 373–387 | HLA-DRB1*01:01, HLA-DRB1*04:01, HLA-DRB1*11:01, HLA-DRB1*04:05, HLA-DRB1*15:01, HLA-DRB4*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*09:01, HLA-DRB5*01:01, HLA-DRB1*03:01, HLA-DRB1*12:01, HLA-DRB1*07:01, HLA-DPA1*02:01/DPB1*01:01 |
| 31 | VGMAAGMCMCARRRC | 382–396 | HLA-DQA1*01:02/DQB1*06:02, HLA-DRB5*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*01:01 |
| 32 | VPFLLSLICCIRTAK | 408–422 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DRB1*11:01, HLA-DRB1*04:05, HLA-DPA1*03:01/DPB1*04:02 |
| 33 | VSVATFILLSMVGMA | 371–385 | HLA-DRB1*01:01, HLA-DPA1*02:01/DPB1*01:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DPA1*01:03/DPB1*02:01, HLA-DRB1*11:01, HLA-DRB4*01:01, HLA-DRB1*15:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*04:01, HLA-DRB1*04:05, HLA-DRB1*03:01, HLA-DRB5*01:01, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*12:01, HLA-DRB1*09:01 |
| 34 | VVSVATFILLSMVGM | 370–384 | HLA-DRB1*01:01, HLA-DPA1*02:01/DPB1*01:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DPA1*01:03/DPB1*02:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*07:01, HLA-DRB1*11:01, HLA-DRB4*01:01, HLA-DRB1*15:01, HLA-DRB1*04:01, HLA-DRB1*04:05, HLA-DRB5*01:01 |
| 35 | VVVSVATFILLSMVG | 369–383 | HLA-DPA1*02:01/DPB1*01:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*07:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*01:01, HLA-DRB4*01:01, HLA-DRB1*15:01, HLA-DRB1*13:02, HLA-DPA1*02:01/DPB1*05:01, HLA-DRB1*11:01, HLA-DRB1*04:05 |
| 36 | VVVVSVATFILLSMV | 368–382 | HLA-DPA1*02:01/DPB1*01:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DRB1*07:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*01:01, HLA-DQA1*01:02/DQB1*06:02, HLA-DRB1*15:01, HLA-DRB4*01:01, HLA-DRB1*13:02, HLA-DPA1*02:01/DPB1*05:01, HLA-DRB1*08:02, HLA-DRB1*04:05, HLA-DRB1*04:01 |
| 37 | YELTPGATVPFLLSL | 400–414 | HLA-DRB1*01:01, HLA-DRB1*07:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DQA1*05:01/DQB1*03:01, HLA-DRB1*09:01, HLA-DPA1*01:03/DPB1*02:01, HLA-DPA1*02:01/DPB1*01:01 |
Further, we analyzed the MHC class II binding T-cell epitopes present in the extra region of the CHIKV E2-FL protein using the default IEDB-recommended 2.22 method (http://tools.iedb.org/mhcii/). The human MHC class II binding T-cell epitopes (of default 15-mer length) were analyzed with respect to 1190 human MHC II alleles present in the IEDB server. The server predicts the binding affinity of each peptide with multiple MHC alleles, resulting in the total number of peptides being more than the total overlapping peptides possible in the extra region of the protein. We obtained a total of 1188 CD4 T-cell peptides in the selected CHIKV E2 C-terminal sequence, binding to 28 human MHC II alleles. As per the literature, the standard peptide affinity measurements were followed, viz., IC50 values <50 nM considered as higher affinity, IC50 values <500 nM considered as intermediate affinity, and IC50 < 5000 nM indicated a lower affinity of the epitopes.35,37 By assigning a cutoff value of IC50 < 500 nM, we identified a total of 253 peptides with good binding affinities, out of which 37 were unique T-cell peptides (listed in Table 6 along with IC50 values), which showed good binding to 19 human MHC class II alleles.
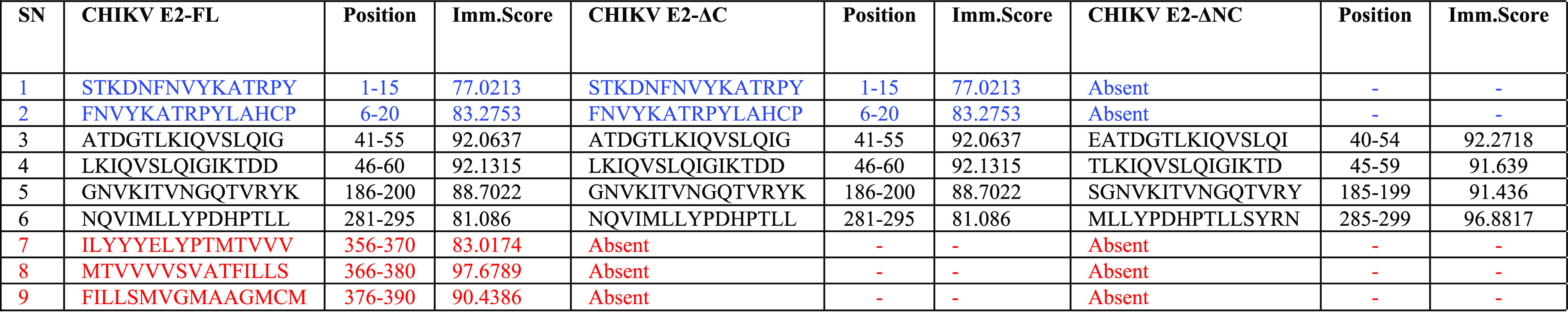
Although peptide binding to MHC class I and MHC class II molecules is necessary for T-cell responses, it does not guarantee its immunogenic nature. To investigate how many of the good binding peptides are immunogenic, we used the IEDB CD4 T-cell immunogenicity prediction program (http://tools.iedb.org/CD4episcore/) to filter for immunogenic peptides. We found a total of nine immunogenic peptides in the CHIKV E2-FL protein, six in CHIKV E2-ΔC, and four T-cell-specific immunogenic peptides in the CHIKV E2-ΔNC fragment. On keeping a cutoff score>90, we found two highly immunogenic peptides (MTVVVVSVATFILLS: 366–380 aa position; immunogenicity score: 97.67; and FILLSMVGMAAGMCM: 376–390 aa position; immunogenicity score: 90.43) in the extra C-terminal region of CHIKV E2-FL (Table 7). Interestingly, the highest predicted immunogenic peptide sequence (MTVVVVSVATFILLS; score 97.67) among all three CHIKV E2 fragments analyzed was located in the extra region of the full-length protein and is absent in the two truncated E2 fragments.
Table 7. List of Immunogenicity Score for Each MHC Class II Alleles to the Predicted CHIKV E2-FL, E2-ΔC, and E2-ΔNC Proteins.
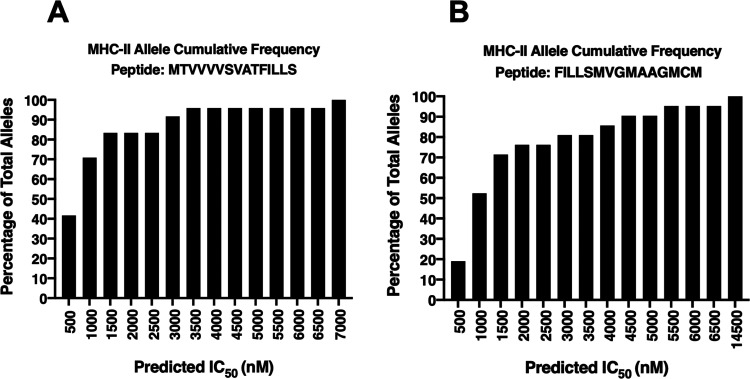
Since we wanted to check what fraction of human MHC alleles can potentially present these good binding peptides during CHIKV infection, the allele cumulative percentage frequency graphs were plotted for the selected two highly immunogenic epitopes in the C-terminal region of CHIKV E2-FL, based upon IC50 < 500 nM (Figure 12). The alleles that exhibit good binding affinities with these two peptides are listed in Table 8. Human MHC genes are highly polymorphic, which means that many different alleles exist in different individuals inside a population. T-cells recognize a complex between a specific MHC molecule and an antigenic epitope, playing an important role in immune function. Useful antigenic epitopes bind to the large spectrum of MHC alleles. Therefore, in the design and the development of vaccines, selecting epitopes binding to multiple MHC alleles will increase population coverage. If an epitope binds to all alleles of the MHC class, then it would be able to generate effective immune response in the entire population. However, there may not be a single antigenic peptide that binds to all MHC alleles in a population. We investigated the coverage of selected immunogenic epitopes present in the extra C-terminal region of the CHIKV E2-FL protein with multiple MHC class II alleles. We calculated the fractions of human MHC class II alleles with different binding affinities to two highly immunogenic T-cell epitopes predicted from the extra C-terminal region of the E2-FL CHIKV protein. From the allele cumulative frequency distribution graphs, we found that the predicted T-cell epitope MTVVVVSVATFILLS (score 97.67) binds to 41% of the class II MHC alleles (IC50 < 500 nM) (Figure 12A). The other predicted T-cell epitope FILLSMVGMAAGMCM (score 90.43) showed 19% binding to MHC class II alleles (Figure 12B). This implies that these two peptide epitopes, which are present only in the CHIKV E2-FL protein, are capable of mounting a diverse immune response in a large fraction of the population. It is likely that a vaccine containing such types of peptide epitopes will have a large population coverage and will provide future direction for vaccine design and development against chikungunya infection.
Figure 12.
Graphical representation of the predicted binding affinity of potential T-cell epitopes (A) MTVVVVSVATFILLS and (B) FILLSMVGMAAGMCM from the extra C-terminal region of the CHIKV E2 protein to MHC class II alleles. The percentage of total MHC class II alleles is depicted on the y-axis, with the predicted affinities (IC50) to MHC class II depicted on the x-axis.
Table 8. List of MHC Class II Alleles and Predicted IC50 (nM) to Both Peptides: MTVVVVSVATFILLS (366–380 aa) and FILLSMVGMAAGMCM (376–390 aa).
| peptide | MTVVVVSVATFILLS (366–380 aa) | predicted IC50 < 500 nM |
|---|---|---|
| S.N | hMHCII Allele | Predicted IC50 (nM) |
| 1 | HLA-DPA1*02:01/DPB1*01:01 | 16 |
| 2 | HLA-DRB1*07:01 | 19 |
| 3 | HLA-DPA1*01:03/DPB1*02:01 | 26 |
| 4 | HLA-DRB1*01:01 | 44 |
| 5 | HLA-DPA1*03:01/DPB1*04:02 | 57 |
| 6 | HLA-DRB1*13:02 | 92 |
| 7 | HLA-DQA1*01:02/DQB1*06:02 | 164 |
| 8 | HLA-DRB5*01:01 | 230 |
| 9 | HLA-DRB1*15:01 | 234 |
| 10 | HLA-DRB1*09:01 | 245 |
| 11 | HLA-DRB1*08:02 | 254 |
| 12 | HLA-DQA1*05:01/DQB1*03:01 | 322 |
| 13 | HLA-DPA1*02:01/DPB1*05:01 | 351 |
| 14 | HLA-DRB1*03:01 | 369 |
| 15 | HLA-DRB1*04:05 | 401 |
| 16 | HLA-DRB4*01:01 | 455 |
| peptide | FILLSMVGMAAGMCM (376–390 aa) | predicted IC50 < 500 nM |
|---|---|---|
| S.N | hMHCII allele | predicted IC50 (nM) |
| 1 | HLA-DRB1*01:01 | 9 |
| 2 | HLA-DQA1*05:01/DQB1*03:01 | 83 |
| 3 | HLA-DRB1*04:01 | 96 |
| 4 | HLA-DQA1*01:02/DQB1*06:02 | 232 |
| 5 | HLA-DRB1*04:05 | 250 |
| 6 | HLA-DRB1*11:01 | 277 |
| 7 | HLA-DRB1*09:01 | 310 |
| 8 | HLA-DRB4*01:01 | 329 |
| 9 | HLA-DRB1*15:01 | 355 |
| 10 | HLA-DRB1*07:01 | 431 |
We also performed the analysis for the identification of T-cell epitopes for the extra region of the CHIKV E2-FL protein using the IEDB server for mouse MHC class I and class II alleles. We did not find any antigenic peptide in the extra region of the E2-FL protein whose binding score was >0.75 when the analysis was performed for mouse MHC class I alleles. Similarly, we did not find any antigenic peptide in the extra region of the CHIKV E2-FL protein whose binding affinity (IC50) was less than 500 nM for mouse MHC class II alleles.
3. Discussion
Ongoing research efforts are underway to develop new methodologies for efficient diagnosis, monitoring, and controlling of chikungunya infections. The development of anti-CHIKV subunit vaccines and more efficient serological tests depends on the isolation and purification of surface immunogenic viral antigens, among which the envelope E2 protein is a prime vaccine candidate, successfully evaluated in separate studies.38,39 The CHIKV E2 protein is a highly immunogenic viral protein, involved in host–cell interaction and capable of inducing strong antibody response against chikungunya infection.32 Multiple studies have reported cloning, expression, and purification of CHIKV E2 proteins using the prokaryotic expression system.19,29,38,40−42 However, almost all of the previously reported studies have described expression and purification of the truncated E2 protein. Additionally, few studies have demonstrated expression and purification of the CHIKV E2 protein using the baculovirus-based insect cell expression system and HEK-293T mammalian cells, which allow post-translational modifications like glycosylation.43−45 However, on comparing the performance of the two CHIKV E2 antigens, a recent study found that both the prokaryotically and eukaryotically expressed E2 proteins demonstrated similar performance for indirect ELISA using anti-CHIKV E2 IgG antibodies.45 Since recombinant proteins expressed in the prokaryotic system are much simpler, faster, cost-effective, and usually obtained in higher quantities when compared to proteins expressed in the eukaryotic system, we attempted cloning, expression, and purification of the full-length CHIKV E2 protein in E. coli in our study.
In the present study, we describe optimized protocols for cloning, expression, and purification of CHIKV E2-FL and two truncated proteins using bacterial expression vectors and E. coli strains. The recombinant E2-FL and truncated proteins were expressed as the His-tagged fusion protein in E. coli. For the production of CHIKV E2-FL and truncated proteins, the E. coli bacterial cultures were induced during the log phase with 1 mM IPTG at 0.2 OD for 18 h, which resulted in a high protein yield. After centrifugation, the uninduced and induced bacterial pellets were subjected to SDS-PAGE analysis and visualized by Coomassie brilliant blue staining. Clear bands of corresponding molecular weights of 50, 44, and 40 kDa were observed for CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins, respectively (Figure 4). Solubilization of inclusion bodies in E. coli is considered a convenient way to recover target recombinant proteins.40,46 Inclusion bodies are protein aggregates that are formed in E. coli during the expression of the recombinant protein and can be highly contaminated with bacterial cell wall components. Therefore, for extracting purified proteins, removal of contaminants is essential, which can be performed using guanidine hydrochloride or urea.47 Cloning the CHIKV E2 proteins in the pQE-30 Xa vector allowed addition of the N-terminal histidine tag. Since Ni-NTA chromatography is a widely employed strategy for purification of histidine-tagged recombinant proteins, the His-tagged E2 full-length and truncated fusion proteins were loaded onto the Ni-NTA affinity chromatography column after bacterial lysis. Protein purification was performed under denaturing conditions using urea. The column was washed with a wash buffer containing different concentrations of imidazole (20, 50, and 100 mM). Finally, the column was eluted with 250 mM imidazole elution buffers and the obtained protein fractions were analyzed using SDS-PAGE. We observed clear specific bands for CHIKV E2-FL and truncated proteins with corresponding molecular weights of approximately 50, 44, and 40 kDa for CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins, respectively (Figure 5). A western blot hybridization was performed using the anti-His antibody, which further confirmed the successful purification of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins (Figure 6).
Since the advantages of high-level expression of recombinant proteins as inclusion bodies are offset by heavy losses in yield encountered along the process of refolding in the form of precipitation of misfolded protein, we performed in vitro refolding of denatured CHIKV E2-FL and truncated proteins according to a previously published study.48 For the improvement of the refolding efficiency of CHIKV E2-FL and E2 truncated proteins, we modified and further optimized the refolding procedure. We observed that the direct refolding method for the refolding of CHIKV E2 denatured proteins led to the formation of protein aggregates and precipitates. On the other hand, by employing a stepwise refolding method, wherein the concentration of urea was decreased gradually in 10 steps (6, 4, 2, 1, 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.0156, and 0 M), no aggregates or protein precipitates were found (Figure 7). Our results indicated a refolding yield of ∼98% obtained for the three CHIKV E2 proteins used in the study.
We performed structural analysis of the refolded CHIKV E2 proteins using CD and FTIR spectroscopies. Evaluating the CHIKV E2 proteins experimentally by CD measurements indicated that purified CHIKV E2 proteins were present in a refolded conformation. In addition, comparative analysis of the CD spectra obtained for each of the refolded and denatured CHIKV E2 protein samples showed a change in the CD spectra: from a random coil structure in denatured E2 protein samples to a predominantly β-sheet secondary structure in refolded CHIKV E2 protein samples (Figure 8). The CHIKV E2-FL and truncated protein were subjected to FTIR spectroscopy to further affirm the correctly folded state of the in vitro refolded CHIKV E2 proteins. The FTIR analysis was performed according to a previously published study.31 We found that in the case of each refolded CHIKV E2-FL and E2 truncated protein sample, a sharp and distinct amide band was observed at 1633/1635 cm–1 wavenumbers, which corresponds to β-sheet structures (Figure 9). The results of our CD and FTIR spectroscopy experiments indicate that the purified refolded CHIKV E2-FL and truncated proteins were indeed present in their near-native conformation.19
It has been reported by many studies that CHIKV envelope proteins could generate protective immune response by producing neutralizing antibodies, making it a promising target for diagnostic and vaccine development against CHIKV.39,49,50 An E2-based subunit vaccine was demonstrated to be protective in mice studies,38 and subunit vaccine formulations based on the recombinant envelope E2 protein elicited balanced Th1/Th2 response and virus-neutralizing antibodies in mice.39 In the present study, the immunogenic efficiency of CHIKV E2 proteins was evaluated by immunizing BALB/c mice with E2-FL and truncated proteins along with an alum adjuvant. We performed an ELISA assay for the analysis of antibody titers produced against the CHIKV E2 antigen in immunized mice (Figure 10). We have reported that recombinant E2 protein immunization induced a high titer of the E2-specific antibody titers in mice. We found that E2-specific antibody titers for the CHIKV E2-FL antigen were consistently higher, while antibody titers obtained after the immunization of E2-ΔC and E2-ΔNC antigens were comparatively less.
Epitope analysis was performed to understand the differences in immunogenicity observed for CHIKV E2-FL, E2-ΔC, and E2-ΔNC antigens used in the study. Various studies have shown that the majority of CHIKV IgG reactive antigenic sequences constitute mainly of continuous linear22,25,32 or few discontinuous conformational51,52 epitopes mapped on the CHIKV E2 protein. Different studies have identified several epitopes spanning the entire E2 protein.34,53 On comparing the presence or absence of identified B-cell epitopes in the three CHIKV E2 protein fragments, we found that the CHIKV E2-FL protein contained the N-terminal epitopes (1–19 and 16–32 aa) along with a few unique epitopes present in the extra C-terminal region (spanning 365–423 aa), compared to the truncated E2 protein fragments. Our findings suggest that the absence of these epitopes may possibly result in reduced immunogenicity in the truncated E2 protein fragments. As opposed to the 11 different regions in the CHIKV E2 protein identified to contain linear epitopes recognized by the anti-CHIKV antibodies during CHIKV infection,22 we found the presence of all 11 regions in the CHIKV E2-FL protein but the absence of the N-terminal (in the E2-ΔNC protein fragment) and C-terminal (in both E2-ΔC and E2-ΔNC protein fragments) regions in truncated E2 proteins. Of note, the N-terminal epitope (STKDNFNVYKATRPYLAH; spanning 1–18 aa), referred to as E2EP3 in earlier studies, is prominently exposed on the viral envelope and has been implicated in the early IgG response to CHIKV in multiple studies.32,54,55 However, a separate study demonstrated that the use of the small linear epitope L (spanning 1–12 aa) was not sufficient to induce a protective immune response in mice when used in isolation.56
On mapping anti-CHIKV E2 mouse monoclonal antibodies using a total of 84, 15-mer synthetic peptides (each with a 10-mer overlap) covering the E2 protein (1–362 aa), Chua et al. found that mouse B-cell epitopes were distributed at different functional domains of the E2 glycoprotein, namely, at domain A (16–30 and 76–85 aa), junctions of β-ribbons with domains A and B (126–135 aa and 166–175 aa), and domain C (301–310, 321–330, and 331–340 aa).24 In this study, we further identified a few unique B-cell epitopes using two distinct methods ABCpred and BCEpred. Our ABCpred results predicted four potential B-cell epitopes in the extra C-terminal region of the CHIKV E2-FL protein, which were absent in both the truncated CHIKV E2-ΔC and E2-ΔNC proteins (shown in red, Figure 11). Our results using BCEpred predicted three epitopes, which overlapped with the already predicted four unique B-cell epitopes predicted through ABCpred. The importance of epitopes located in the C-terminal region of the CHIKV E2-FL protein has also been reported by Lum et al., who found that the anti-CHIKV antibodies generated in CHIKV-infected mice targeted epitopes located mainly at the C-terminus of the virus E2 glycoprotein (spanning 130–411 aa), along with the N-terminal epitope (1–19 aa).25 Using a bacteriophage Qβ viruslike particle (VLP) platform, Basu et al. reported that epitopes spanning 1–18 and 226–259 aa elicited high-titer antibodies and the C-terminal epitope (spanning 378–411 aa) was the least reactive, possibly due to the truncated E2 protein fragment used in this study.57 In an in silico B-cell epitope prediction study, the epitope residues at 386 and 388 aa positions were predicted to evoke significant immune response.58 Of note, an immuno-informatics study by Hasan et al. reported the peptide region spanning 9 aa from 397 to 405 aa as the most potential B-cell epitope, along with epitopes identified in the N-terminal region (1–29 aa).59 Rodriguez et al. also predicted B-cell epitopes located in the N-terminal (spanning 14–22 and 27–35 aa) and C-terminal (spanning 367–389 aa) regions, suggesting that the presence of these amino acids in the CHIKV E2-FL protein may be contributing in its increased immunogenicity.60
Since T-cell epitope content is one of the factors that contributes to protein antigenicity, we performed in silico T-cell epitope prediction for the extra C-terminal region of the CHIKV E2-FL protein for understanding immunogenicity differences observed in E2-FL with respect to E2-ΔC and E2-ΔNC proteins. The binding strength of T-cell epitopes to the major histocompatibility complex (MHC or HLA) molecules is a key determinant in T-cell epitope immunogenicity. Using the IEDB T-cell epitope prediction server, we identified two potential T-cell epitopes (ATVPFLLSL: 0.87 and YELTPGATV: 0.85) in the CHIKV E2-FL extra C-terminal region binding exclusively to MHC class I alleles (score value > 0.75) spanning 406–414 and 400–408 aa, respectively (Table 6). These two potential T-cell epitopes were absent in the truncated forms of E2 fragments. A study by Sreekumar et al. also identified the FLLSLICCI epitope (410–418 aa) present in the C-terminal region of the CHIKV E2-FL protein among the six T-cell epitopes identified using the EpiJen online server and implicated the importance of C416G mutation present in the cytoplasmic domain of the E2 protein.61 Including CTL epitopes in multiepitope peptide-based CHIKV vaccine is extremely beneficial as they are necessary for the clearance of intracellular pathogens by arbitrating cell-mediated immunity. It has been demonstrated that epitope-based vaccines containing epitopes recognizing multiple HLA molecules can provide the broadest possible coverage of the human population. In a separate study, the CHIKV E2 peptide KTDDSHD (spanning 57–63 aa) was predicted to be the most probable T-cell epitope and the peptide FVRTSAPCT (84–92 aa) was predicted to be the common T- and B-cell epitope having a high antigenicity.62 While both of these peptides were located in Domain A wherein the major secondary structure was β-strands, it was reported that Domain C of the E2 protein, which consisted of a long α-helix (362–423 aa), comprised the highly conserved “TPY” domain (398–400 aa) and the transmembrane domain (365–385 aa).62 In addition to the above-mentioned reports, MHC class I peptides “TAECKDKNL” and “VTWGNNEPY” were predicted to be extremely antigenic in studies by Qamar et al. and Narula et al., respectively.63,64 Using an in silico immunoinformatic approach, Khan et al. identified key mutations in the CHIKV E2 region of circulating Pakistani isolates along with the CHIKV-S27 strain and predicted a total of 18 potential CTL epitopes using bioinformatics tools.65 Among the 18 potential CTL epitopes predicted, four epitopes were located in the extra C-terminal region of the CHIKV E2-FL protein sequence as per our study.
On analyzing the MHC class II binding of helper T-cell (HTL) epitopes present in the extra region of the CHIKV E2-FL protein, we identified a total of 37 unique T-cell epitopes, which showed binding to MHC class II alleles (Table 6). On performing immunogenicity predictions using the IEDB server for evaluating the immunogenicity of these HTL epitopes, we found two highly immunogenic peptides (MTVVVVSVATFILLS: 366–380 aa position; immunogenicity score: 97.67 and FILLSMVGMAAGMCM: 376–390 aa position; immunogenicity score: 90.43) in the extra C-terminal region of CHIKV E2-FL (Table 7). The peptide sequence (MTVVVVSVATFILLS; score 97.67) having the highest predicted immunogenicity was exclusively located in the extra C-terminal region of the CHIKV E2-FL protein and was absent in the two truncated E2 protein fragments. From the allele cumulative frequency distribution graphs, we found that the predicted T-cell epitope MTVVVVSVATFILLS (score 97.67) binds to 41% of MHC class II alleles, while FILLSMVGMAAGMCM (score 90.43) showed 19% binding to MHC class II alleles (Figures 12A,B). The MHC class II alleles that exhibit good binding affinities (IC50 < 500 nM) with these two peptides are listed in Table 8. Our results indicate that 16 MHC class II alleles can interact with MTVVVVSVATFILLS and 10 MHC class II alleles can interact with FILLSMVGMAAGMCM epitopes, present in the E2-FL protein. Since the epitopes that interact with numerous alleles are considered more efficient for imparting broad population coverage, including these two immunogenic epitopes located in the extra C-terminal region of the CHIKV E2-FL protein in a multiepitope-based anti-CHIKV vaccine may prove beneficial. Using an immune-informatics approach, a previous study identified the IMLLYPDHPTLLSYR epitope spanning 284–298 aa in the CHIKV E2 glycoprotein as a potent HTL epitope, which could interact with five MHC class II alleles (IC50 < 250).66 Interestingly, a proteome-wide screening study by Teo et al. identified a dominant CD4+ T-cell epitope in the N-terminal E2EP3 region (spanning 1–19 aa) of the E2 protein, by performing ELISPOT assays on splenocytes obtained from CHIKV-infected mice.67 It is likely that a vaccine containing such a type of peptide epitope will have a large population coverage and will provide future direction for vaccine design and development against chikungunya infection. Furthermore, a multiepitope-based peptide can be used as an alternate antigen to CHIKV E2-FL for immunodiagnosis of CHIKV infections with high sensitivity and specificity.68 In summary, we identified the potential B- and T-cell epitopes present in the extra C-terminal region of the CHIKV E2-FL protein. Since the extra C-terminal region of the CHIKV E2-FL protein contains extra immunogenic epitopes that are absent in the truncated fragments and can bind with a more diverse human MHC allele repertoire, it is expected to significantly enhance the effective immune response and therefore may be advantageous to be included when designing multiepitope vaccines or immunotherapies for CHIKV.
4. Conclusions
Several reports have shown that the CHIKV E2 protein can serve as an effective subunit vaccine candidate against chikungunya infection. This study provides a detailed methodology optimized for cloning, expression, and purification of CHIKV E2-FL and E2 truncated proteins using a prokaryotic expression system. Using an E. coli-based expression system is a cost-effective option and makes the process amenable to inexpensive scale-up. The CHIKV E2 proteins were purified using Ni-NTA chromatography and were refolded in vitro for obtaining proteins in native conformation (devoid of any aggregates or protein precipitates). Structural analysis performed using CD and FTIR spectroscopies confirmed the presence of CHIKV E2 proteins in their near-native state. The biological function of the refolded and purified recombinant CHIKV E2-FL protein was confirmed by its ability to generate high titers of E2-specific antibodies in BALB/c mice. The recombinant CHIKV E2 proteins expressed in E. coli retained their immunogenicity and could induce high antibody titers in BALB/c mice, as evaluated using E2-specific ELISA. By performing B-cell and T-cell epitope analyses using various in silico approaches, our study identified highly immunogenic unique peptides present in the CHIKV E2-FL protein. Specifically, both the N-terminal region (1–32 aa) and the C-terminal region (366–423 aa) present in the CHIKV E2-FL protein demonstrated the presence of unique highly immunogenic peptides. We anticipate that our predicted B-cell and T-cell epitopes could aid the design of a prospective anti-CHIKV multiepitope vaccine. In conclusion, the results outlined in this study could be valuable for the development of novel and effective multiepitope subunit vaccine strategies for prevention of CHIKV infections and also help in producing purified antigens in large amounts for use in anti-CHIKV diagnostic and/or serological tests. Furthermore, this approach of expressing and purifying the CHIKV E2 protein in E. coli with a high yield may also offer a promising method for the production of other viral recombinant proteins.
5. Experimental Section
5.1. Materials
Chikungunya virus strain isolate (Ind-06-Guj, Gen Bank Accession No. JF274082) was kindly provided by Dr. Sudhanshu Vrati (RCB, Faridabad, India). The plasmid vector pQE-30 Xa along with the bacterial host E. coli strains XL1-Blue and SG13009 were kindly provided by Dr. Devinder Sehgal (NII, New Delhi, India). Media for bacterial growth was purchased from Himedia Laboratories, India. All restriction enzymes, PCR components, and DNA Ligase were procured from NEB (New England Biolabs). Oligoes were synthesized from Eurofins, India. Ni-NTA resin was purchased from Qiagen, Germany. Dialysis tubing was obtained from Spectrum Labs. Antibodies for Western blotting were purchased from Thermofisher Scientific. All chemicals were procured from Sigma.
5.2. PCR Amplification and Cloning of E2 Gene Constructs
The nucleotide sequence of CHIKV E2 was downloaded from the NCBI database (Accession No. JF274082) and used as a template to design gene-specific primers. Total RNA was isolated from the chikungunya viral lysate (Ind-06-Guj) using the High Pure Viral RNA kit as per the manufacturer’s instructions (Roche Life Science). Total RNA was used for cDNA synthesis following the commercial protocol and the Oligo-dT primer (Verso cDNA Synthesis kit, Thermo Fisher). The CHIKV E2-FL nucleotide sequence (1269 bp; corresponding to 1–423 amino acid residues) was PCR-amplified using high-fidelity Phusion DNA Polymerase (NEB, England) and gene-specific forward (5′-CCCCGGATCCAGCACCAAGGACAACTTCAATG) and reverse (5′-CCCCCAAGCTTCGCTTTAGCTGTTCTGATGCAG) primers, respectively (Table 1). The underlined nucleotides in forward and reverse primer sequences signify BamHI and HindIII restriction enzyme sites, respectively. The 25 μl PCR reaction mix comprised 1× DNA polymerase buffer, 200 μM dNTP mix, 0.2 μM of each primer, 5 μL of cDNA, and 1 U of Phusion DNA polymerase. PCR was performed using a programmable thermal cycler (GeneAmp PCR system 2720; Applied Biosystems) starting with an initial denaturation step at 95 °C for 2 min. This was followed by 25 cycles consisting of denaturation at 95 °C for 1 min, annealing at 60 °C for 1 min, and an extension at 72 °C for 1 min. A final extension step was conducted at 72 °C for 10 min. The PCR amplicon (1269 bp) was subsequently digested with BamH1 and HindIII restriction enzymes. The digested product was ligated into BamH1-HindIII digested E. coli expression vector pQE-30 Xa using T4 DNA ligase. The ligated product was transformed in the E. coli XL1-Blue strain. Positive clones were identified by restriction analysis and confirmed by sequencing.
The truncated CHIKV E2-ΔC fragment (1095 bp; corresponding to 1–365 amino acids) was obtained through PCR amplification using the full-length E2 plasmid DNA as the template along with gene-specific forward (5′-CCCCGGATCCGAACGCATCAGAAATGAAGCGAC) and reverse (5′-CCCCCAAGCTTAGTAGGGTACAGCTCATAATAATAC) primers. The truncated CHIKV E2-ΔNC fragment (993 bp; corresponding to 35–365 amino acids) was obtained through PCR amplification using CHIKV E2-FL plasmid DNA as the template along with gene-specific forward (5′-CCCCGGATCCGAACGCATCAGAAATGAAGCGAC) and reverse (5′-CCCCCAAGCTTAGTAGGGTACAGCTCATAATAATAC) primers, respectively. The PCR components and cycling conditions used were the same as mentioned above. The amplicons obtained were digested with BamH1 and HindIII enzymes and cloned into the digested pQE-30 Xa vector. Positive clones were identified by restriction analysis and confirmed by sequencing.
5.3. Expression of Recombinant E2 Proteins
For expression purposes, the CHIKV E2-FL and truncated E2 constructs were transformed in the E. coli expression strain SG13009 (Qiagen). Bacterial cultures were grown in a Luria-Bertani medium containing ampicillin (100 μg/mL) and kanamycin (25 μg/mL) at 37 °C overnight with shaking at 220 rpm. The secondary cultures were induced at 0.2 OD600 by adding 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 18 h at 18 °C. Bacteria were harvested by centrifugation at 8000 rpm for 30 min at 4 °C. Uninduced and induced bacterial pellets were analyzed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized using Coomassie brilliant blue R-250 staining. For determining protein solubility, bacterial cell pellets obtained after induction were lysed using a Lysis buffer (containing 25 mM pH 7.3, 300 mM NaCl, and 5 mM β-mercaptoethanol) supplemented with 1× Protease inhibitor cocktail (Roche). The lysate was sonicated briefly and centrifuged at 12 000 rpm for 30 min at 4 °C. The soluble (supernatant) and insoluble (pellet) protein fractions obtained were analyzed on 10% SDS-PAGE gel. The expressed protein found in the insoluble fraction was processed for subsequent purification steps.
5.4. Purification of Recombinant E2 Proteins
The recombinant CHIKV E2-FL and truncated E2 proteins were purified using Ni-NTA affinity chromatography under denaturing conditions with some modifications from the manufacturer’s protocol (Qiagen, Germany). Briefly, the cell pellet obtained after induction was lysed in a Lysis buffer (containing 8 M urea, 25 mM Tris pH 7.3, 300 mM NaCl, and 5 mM β-mercaptoethanol) supplemented with 1× Protease inhibitor cocktail (Roche). Cell lysates were prepared by 20 sonication cycles of a 10 s duration pulse with a 10 s time interval between pulses. The lysate was centrifuged at 12 000 rpm for 30 min at 4 °C, and the supernatant containing the fusion protein was loaded onto a column containing 1 mL of equilibrated Ni-NTA resin and allowed to bind at room temperature for 15 minutes. The column was washed with wash buffers (lysis buffer with 8 M urea supplemented with increasing concentrations of imidazole (20, 50, and 100 mM)). Finally, the column was washed with an elution buffer (lysis buffer with 8 M urea, supplemented with 250 mM imidazole). The washed and eluted fractions were analyzed on 10% SDS-PAGE for purity estimation. During column washing steps in Ni-NTA chromatography, wash buffers had 0.1% Triton X-114 added as the default as per the manufacturer’s instructions, for removing endotoxin contamination. The endotoxin levels in all immunogens were found to be between 0.5 and 0.1 EU/mL, using the Pierce LAL Chromogenic Endotoxin Quantitation kit (Thermo scientific).
5.5. Protein Refolding
The purified recombinant CHIKV E2-FL and E2 truncated protein fractions were refolded using a single step or the stepwise dialysis method. In the single-step dialysis procedure carried out for 6 h at 21 °C with continuous stirring, a visible protein precipitate was observed. Subsequently, refolding was optimized using a stepwise dialysis procedure. Briefly, eluted fractions were pooled and dialyzed against refolding buffer (containing 20 mM Tris pH 7.3, 5 mM DTT, 400 mM NaCl, and 5 mM EDTA), which had decreasing amounts of urea in subsequent buffer changes. The concentration of urea was decreased gradually (6, 4, 2, 1, 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.015, and 0 M), keeping the concentration of the rest of the constituents constant. Each dialysis step was carried out at room temperature (21 °C) at pH 7.3 for 3 h with gentle stirring. All dialysis procedures were performed at 4 °C once the urea concentration was decreased to 2 M. While performing urea-free dialysis in the last step, the dialysis buffer was supplemented with 20% glycerol. The refolded protein samples were removed from the dialysis bags and centrifuged to remove any residual aggregates. The dialyzed and undialyzed protein samples were stored at −20 °C and analyzed on 10% SDS-PAGE gel. The concentration of the refolded protein was estimated spectrophotometrically by the BCA protein microassay (Genetix) using bovine serum albumin (BSA) as the standard. The in vitro refolding yield was calculated as per the following formula
5.6. Western Blotting
Purified N-terminal histidine-tagged full-length and truncated E2 fusion proteins were separated on a 10% SDS-PAGE gel along with protein marker, positive control (histidine-tagged fusion protein), and negative control (bovine serum albumin, BSA) proteins and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). The membrane was blocked using 3% (w/v) BSA in Tris-buffered saline (TBS) for 1 h at room temperature. Subsequently, the membrane was washed four times with 1× TBS containing 0.05% Tween-20 (TBST). Next, the membrane was incubated with the anti-His monoclonal antibody (Santa Cruz Biotechnology) at 1:1000 dilution for 1 h at room temperature. After washing four times with TBST, the membrane was incubated for 1 h at room temperature with HRP-conjugated goat antimouse IgG (Santa Cruz Biotechnology) at 1:5000 dilution. After washing four times with TBST and once with TBS, the membrane was incubated with a 3,3′-diaminobenzidine (DAB) substrate solution (0.5 mg/mL) at room temperature. Color development was stopped by washing with water, following which the blots were air-dried and stored.
5.7. Circular Dichroism
To evaluate the secondary structure content of purified recombinant CHIKV E2 proteins, both the unfolded and refolded CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins were subjected to CD analysis using a Chirascan Circular Dichroism Spectrometer (Applied Photophysics Ltd., Surrey KT22 7PB, United Kingdom). CD spectra were collected using a 1 mm quartz cell under constant nitrogen purge between 190 and 250 nm in 1 nm wavelength steps and an average time of 2.0 s at 18 °C. Protein samples at concentrations of 0.5 mg/mL were analyzed in a 20 mM Tris, pH 7.3 buffer containing 400 mM NaCl, 5 mM EDTA, and 20% glycerol. For each sample, five scans were collected and averaged. The final average spectrum of all of the samples was estimated by subtracting the baseline spectrum of the corresponding buffer. The data was analyzed by the CAPITO web tool (https://capito.uni-jena.de). Thereafter, the secondary structure-specific graphs were obtained by plotting the molar ellipticity against wavelength. Recorded CD data in millidegrees were converted to molar ellipticity as per the following formula: molar ellipticity (θ) = (m° × M)/(10 × L × C), where θ (theta) is the molar ellipticity in deg cm2/dmol, m° is the recorded ellipticity in millidegrees, M is the mean residual weight (g/mol), L is the path length in cm, and C is the concentration in g/L.69 Smoothening of the raw CD data was performed using the Savitzky–Golay filter on CAPITO software online.
5.8. FTIR Spectroscopy
The secondary structure elements of the denatured and refolded E2 protein samples were further analyzed by Fourier transform infrared (FTIR) spectroscopy. A PerkinElmer Spectrum Two FTIR spectrometer was used to measure the spectrum in the spectral range of 400–4000 cm–1 at a resolution of 8 cm–1 and an average of 16 scans, using potassium bromide (KBr) pellets. FTIR spectroscopy was performed at the Department of Chemistry, IIT Roorkee.
5.9. Mice Immunization
Five- to six-week-old inbred male BALB/c mice were obtained from CDRI (Lucknow, India) and were housed in the Institute Animal Facility at IIT Roorkee (Uttarakhand, India). All animals were maintained at a 12:12 h light/dark cycle at 25 °C and were provided with a pellet diet and water ad libitum. Approval from the Institutional Animal Ethics Committee was obtained for all experimental procedures involving mice for this study. Different groups of BALB/c mice (n = 5) were immunized subcutaneously with 10 μg of purified recombinant CHIKV E2-FL and E2 truncated proteins along with 50 μg of alum (Pierce) as the adjuvant in PBS. After 3 weeks (day 21) and 6 weeks (day 42), mice were boosted with the same dose of antigen and adjuvant. Sham-immunized mice received an equal amount of adjuvant in saline on day 0, day 21, and day 42. Mice (n = 5) were bled retro-orbitally every week, at indicated timepoints (days 7, 14, 19, 28, 35, 42, 49, 56, 63). The isolated blood samples were allowed to clot for 2–3 h at room temperature and later centrifuged to separate serum. The serum samples were removed by aspiration and stored at −20 °C for further analysis.
5.10. E2-Specific ELISA
For determining E2-specific antibody titers, 96-well ELISA plates (Nunc MaxiSorp, Thermo) were coated with 5 μg/mL recombinant full-length E2 protein in a carbonate-bicarbonate buffer overnight at 4 °C. All blocking steps were performed using 5% milk protein in PBS for 1 h at 37 °C, and plates were washed with 0.05% Tween-20 in PBS (PBST) before use. The plates were then incubated with serially diluted serum samples obtained from mice immunized with CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins along with commercially available monoclonal antibody (18H01) purchased from The Native Antigen (Oxford, United Kingdom) as a positive control. Serum samples were incubated for 1 h at 37 °C, following which the plates were washed, and CHIKV E2-specific antibody levels were detected with the HRP-conjugated goat antimouse IgG secondary antibody (Southern Biotechnology) (1:5000 dilution) for 1 h at 37 °C. Plates were subsequently developed with 0.4 mg/mL o-phenylenediamine dihydrochloride (SRL, India) dissolved in citrate-phosphate buffer (pH 5.0). The reaction was stopped with 2 N H2SO4, and optical density at 490 nm was measured using a microplate reader (Tecan, USA). Titers were defined as the highest dilution of serum that gave an optical density at least twice the mean background reading (wells with all reagents except sera).
5.11. Epitope Analysis
5.11.1. B-Cell Epitope Analysis
The B-cell epitopes of CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins are delineated as per previously published reports, which included experimental validation of these epitopes.24,70 Since B-cell epitopes are characterized by four parameters, namely, surface accessibility, antigenicity, flexibility, and hydrophilicity, tertiary 3D structure modeling was performed using both SWISS-MODEL (https://swissmodel.expasy.org) and I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/download/) for CHIKV E2-FL, E2-ΔC, and E2-ΔNC antigens based on available structure templates. The modeled 3D structures of the three CHIKV E2 proteins were superimposed using Pymol 3D structure visualization software (http://www.pymol.org/) for comparison of their respective epitope structures and surface accessibility. The physical and chemical properties {including amino acid composition, molecular weight, theoretical isoelectric point, instability coefficient, and grand average of hydropathicity (GRAVY)} of the three different CHIKV E2 proteins and their respective epitopes were analyzed using ProtParam (http://web.expasy.org/protparam/). Additionally, two freely available online epitope prediction servers, namely, ABCpred (https://webs.iiitd.edu.in/raghava/abcpred/ABC_submission.html) and Bcepred (http://www.imtech.res.in/raghava/bcepred/), were used to predict the B-cell epitopes using their default threshold values for CHIKV E2-FL, E2-ΔC, and E2-ΔNC antigens used in this study.
5.11.2. T-Cell Epitope Prediction
The MHC I binding affinity of T-cell epitopes was predicted using the default IEDB-recommended 2020.09 NetMHCpan EL 4.1 method (http://tools.iedb.org/mhci/), and the MHC II binding affinity of T-cell epitopes was identified using the default IEDB-recommended 2.22 method (http://tools.iedb.org/mhcii/). Further, the immunogenicity values of the in silico-predicted MHC class II binding peptides of the E2-FL and truncated CHIKV E2 proteins used in the study were calculated using the IEDB CD4 T-cell immunogenicity (http://tools.iedb.org/CD4episcore/) tool against seven reference sets of human HLA alleles.
Acknowledgments
The authors thank Ravikant Ranjan for technical assistance and suggestions on the manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05975.
FTIR spectra results for unfolded CHIKV E2-FL, E2-ΔC, and E2-ΔNC proteins (Figure S1) (PDF)
(PDF)
Author Contributions
M.S.: methodology, investigation, and original draft preparation. P.C.: writing, reference collection, and proofreading. S.T.: methodology. N.K.: methodology. S.P.M.: methodology. S.R.: conceptualization, methodology, supervision, writing, and reviewing. All authors contributed to the manuscript. All authors have read and agreed to the final version of the manuscript.
This work was supported by the Ramalingaswami grant (Dept. of Biotechnology, Govt. of India) and the Early Career Research Award (Science and Engineering Research Board, Govt. of India) to S.R. The authors acknowledge the DBT-JRF Fellowship grant to MS, ICMR-JRF Fellowship grant to P.C, and research and infrastructural facilities at IIT-Roorkee.
The authors declare no competing financial interest.
Supplementary Material
References
- Morrison T. E. Reemergence of Chikungunya Virus. J. Virol. 2014, 88, 11644–11647. 10.1128/jvi.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwameis M.; Buchtele N.; Wadowski P. P.; Schoergenhofer C.; Jilma B. Chikungunya vaccines in development. Hum. Vaccin. Immunother. 2016, 12, 716–731. 10.1080/21645515.2015.1101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers A. M.; Logue C. H. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007, 2363–2377. 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- Chen C. I.; Clark D. C.; Pesavento P.; Lerche N. W.; Luciw P. A.; Reisen W. K.; Brault A. C. Comparative pathogenesis of epidemic and enzootic Chikungunya viruses in a pregnant Rhesus macaque model. Am. J. Trop. Med. Hyg. 2010, 83, 1249–1258. 10.4269/ajtmh.2010.10-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiboutot M. M.; Kannan S.; Kawalekar O. U.; Shedlock D. J.; Khan A. S.; Sarangan G.; Srikanth P.; Weiner D. B.; Muthumani K. Chikungunya: A potentially emerging epidemic?. PLoS Neglected Trop. Dis. 2010, 4, e623 10.1371/journal.pntd.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A.; Gwan S.; Vasileva D.; Yaghoubi S. International Journal of Infectious Diseases Prevalence of chronic comorbidities in chikungunya: A systematic review and meta-analysis. Int. J. Infect. Dis. 2018, 67, 107–113. 10.1016/j.ijid.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgherini G.; Poubeau P.; Jossaume A.; Gouix A.; Cotte L.; Michault A.; Arvin-Berod C.; Paganin F. Persistent arthralgia associated with chikungunya virus: A study of 88 adult patients on Reunion Island. Clin. Infect. Dis. 2008, 47, 469–475. 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- Phommanivong V.; Kanda S.; Shimono T.; Lamaningao P.; Darcy A. W.; Mishima N.; Phaytanavanh B.; Nishiyama T. Co-circulation of the dengue with chikungunya virus during the 2013 outbreak in the southern part of Lao PDR. Trop. Med. Health 2016, 44, 1–10. 10.1186/s41182-016-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F.; Javelle E.; Oliver M.; Leparc-Goffart I.; Marimoutou C. Chikungunya virus infection. Curr. Infect. Dis. Rep. 2011, 13, 218–228. 10.1007/s11908-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilte C.; Staikovsky F.; Couderc T.; Madec Y.; Carpentier F.; Kassab S.; Albert M. L.; Lecuit M.; Michault A.. Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Neglected Trop. Dis. 2013, 7 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérardin P.; Barau G.; Michault A.; Bintner M.; Randrianaivo H.; Choker G.; Lenglet Y.; Touret Y.; Bouveret A.; Grivard P.; et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Réunion. PLoS Med. 2008, 5, e60 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault P.; Solet J. L.; Sissoko D.; Balleydier E.; Larrieu S.; Filleul L.; Lassalle C.; Thiria J.; Rachou E.; De Valk H.; et al. A major epidemic of chikungunya virus infection on Réunion Island, France, 2005-2006. Am. J. Trop. Med. Hyg. 2007, 77, 727–731. 10.4269/ajtmh.2007.77.727. [DOI] [PubMed] [Google Scholar]
- Schwartz O.; Albert M. L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- Robinson M. C. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53 I. Clinical Features. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 28–32. 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- Petersen L. R.; Powers A. M. Chikungunya: Epidemiology. F1000Research 2016, 5, 1–8. 10.12688/f1000research.7171.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiberville S. D.; Moyen N.; Dupuis-Maguiraga L.; Nougairede A.; Gould E. A.; Roques P.; de Lamballerie X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013, 99, 345–370. 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller H.; Van Bortel W.; Sudre B. Chikungunya: Its history in Africa and Asia and its spread to new regions in 2013-2014. J. Infect. Dis. 2016, 214, S436–S440. 10.1093/infdis/jiw391. [DOI] [PubMed] [Google Scholar]
- Khan A. H.; Morita K.; Parquet C.; Hasebe F.; Mathenge E. G. M.; Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J. Gen. Virol. 2002, 3075–3084. 10.1099/0022-1317-83-12-3075. [DOI] [PubMed] [Google Scholar]
- Voss J. E.; Vaney M. C.; Duquerroy S.; Vonrhein C.; Girard-Blanc C.; Crublet E.; Thompson A.; Bricogne G.; Rey F. A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010, 468, 709–712. 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]
- Weger-Lucarelli J.; Aliota M. T.; Kamlangdee A.; Osorio J. E. Identifying the Role of E2 Domains on Alphavirus Neutralization and Protective Immune Responses. PLoS Neglected Trop. Dis. 2015, 9, e0004163 10.1371/journal.pntd.0004163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahata W.; Nabel G. J. A Specific Domain of the Chikungunya Virus E2 Protein Regulates Particle Formation in Human Cells: Implications for Alphavirus Vaccine Design. J. Virol. 2012, 86, 8879–8883. 10.1128/jvi.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Y.-W.; Lee W. W. L.; Simarmata D.; Harjanto S.; Teng T.-S.; Tolou H.; Chow A.; Lin R. T. P.; Leo Y.-S.; Renia L.; et al. Longitudinal Analysis of the Human Antibody Response to Chikungunya Virus Infection: Implications for Serodiagnosis and Vaccine Development. J. Virol. 2012, 86, 13005–13015. 10.1128/jvi.01780-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh L. Y. H.; Hobson-Peters J.; Prow N. A.; Gardner J.; Bielefeldt-Ohmann H.; Pyke A. T.; Suhrbier A.; Hall R. A. Neutralizing monoclonal antibodies to the E2 protein of chikungunya virus protects against disease in a mouse model. Clin. Immunol. 2013, 149, 487–497. 10.1016/j.clim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Chua C. L.; Chan Y. F.; Sam I. C. Characterisation of mouse monoclonal antibodies targeting linear epitopes on Chikungunya virus E2 glycoprotein. J. Virol. Methods 2014, 195, 126–133. 10.1016/j.jviromet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Lum F.-M.; Teo T.-H.; Lee W. W. L.; Kam Y.-W.; Rénia L.; Ng L. F. P. An Essential Role of Antibodies in the Control of Chikungunya Virus Infection. J. Immunol. 2013, 190, 6295–6302. 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A. M. Monoclonal antibodies as prophylactic and therapeutic agents against chikungunya virus. J. Infect. Dis. 2016, 214, S506–S509. 10.1093/infdis/jiw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M.; Chauhan A.; Goyal V.; Jaiswal N.; Singh S.; Singh M. Recent development in the strategies projected for chikungunya vaccine in humans. Drug Des., Dev. Ther. 2018, 12, 4195–4206. 10.2147/DDDT.S181574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S.; Song S.; Zhang L. Recent Progress in Vaccine Development Against Chikungunya Virus. Front. Microbiol. 2019, 10, 2881 10.3389/fmicb.2019.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A.; Chandele A.; Nayak K.; Kaja M. K.; Arulandu A.; Lodha R.; Ray P. High yield expression and purification of Chikungunya virus E2 recombinant protein and its evaluation for serodiagnosis. J. Virol. Methods 2016, 235, 73–79. 10.1016/j.jviromet.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Corrêa D.; Ramos C. The use of circular dichroism spectroscopy to study protein folding, form and function. Afr. J. Biochem. Res. 2009, 3, 164–173. 10.5897/AJBR.9000245. [DOI] [Google Scholar]
- Kong J.; Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Kam Y.; Lum F.; Teo T.; Lee W. W. L.; Simarmata D.; Harjanto S.; Chua C.; Chan Y.; Wee J.; Chow A.; et al. Early neutralizing IgG response to Chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol. Med. 2012, 330–343. 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Y. W.; Lee W. W. L.; Simarmata D.; Le Grand R.; Tolou H.; Merits A.; Roques P.; Ng L. F. P. Unique epitopes recognized by antibodies induced in chikungunya virus-infected non-human primates: Implications for the study of immunopathology and vaccine development. PLoS One 2014, 9, e95647 10.1371/journal.pone.0095647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum F. M.; Ng L. F. P. Cellular and molecular mechanisms of chikungunya pathogenesis. Antiviral Res. 2015, 120, 165–174. 10.1016/j.antiviral.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Hoque H.; Islam R.; Ghosh S.; Rahaman M. M.; Jewel N. A.; Miah M. A. Implementation of in silico methods to predict common epitopes for vaccine development against Chikungunya and Mayaro viruses. Heliyon 2021, 7, e06396 10.1016/j.heliyon.2021.e06396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakhar R.; Gakhar S. K. An immunoinformatics study to predict epitopes in the envelope protein of SARS-CoV-2. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 7079356 10.1155/2020/7079356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Sidney J.; Dow C.; Mothé B.; Sette A.; Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 2008, 4, e1000048 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Sudeep A. B.; Arankalle V. A. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine 2012, 30, 6142–6149. 10.1016/j.vaccine.2012.07.072. [DOI] [PubMed] [Google Scholar]
- Khan M.; Dhanwani R.; Rao P. V. L.; Parida M. Subunit vaccine formulations based on recombinant envelope proteins of Chikungunya virus elicit balanced Th1/Th2 response and virus-neutralizing antibodies in mice. Virus Res. 2012, 167, 236–246. 10.1016/j.virusres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Tripathi N. K.; Priya R.; Shrivastava A. Production of recombinant Chikungunya virus envelope 2 protein in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 2461–2471. 10.1007/s00253-013-5426-4. [DOI] [PubMed] [Google Scholar]
- Dudha N.; Rana J.; Gabrani R.; Gupta A.; Chaudhary V. K.; Gupta S. Small scale expression, solubilization, and characterization of Chikungunya virus structural proteins. Asian J. Pharm. Clin. Res. 2014, 7, 268–271. [Google Scholar]
- Tripathi N. K.; Priya R.; Shrivastava A. Immunogenicity of Escherichia coli expressed envelope 2 protein of chikungunya virus. Bioengineered 2014, 5, 198–203. 10.4161/bioe.28336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S. W.; Geertsema C.; Martina B. E.; Andrade P.; Heldens J. G.; Van Oers M. M.; Goldbach R. W.; Vlak J. M.; Pijlman G. P. Functional processing and secretion of Chikungunya virus E1 and E2 glycoproteins in insect cells. Virol. J. 2011, 8, 353 10.1186/1743-422X-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua C. L.; Sam I. C.; Chan Y. F. Expression and purification of E2 glycoprotein from insect cells (Sf9) for use in serology. Methods Mol. Biol. 2016, 1426, 51–61. 10.1007/978-1-4939-3618-2_5. [DOI] [PubMed] [Google Scholar]
- Bagno F. F.; Godói L. C.; Figueiredo M. M.; Rodrigues Sérgio S. A.; de Fátima Silva Moraes T.; de Castro Salazar N.; Kim Y. C.; Reyes-Sandoval A.; Guimarães da Fonseca F. Chikungunya E2 protein produced in E. coli and HEK293-T cells—comparison of their performances in ELISA. Viruses 2020, 12, 939 10.3390/v12090939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. M.; Panda A. K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. 10.1263/jbb.99.303. [DOI] [PubMed] [Google Scholar]
- Palmer I.; Wingfield P. T. Preparation and extraction of insoluble (Inclusion-body) proteins from Escherichia coli. Curr. Protoc. Protein Sci. 2012, 1, 70 10.1002/0471140864.ps0603s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapryal S.; Krishnan L.; Batra J. K.; Kaur K. J.; Salunke D. M. Cloning, expression and efficient refolding of carbohydrate-peptide mimicry recognizing single chain antibody 2D10. Protein Expr. Purif. 2010, 72, 162–168. 10.1016/j.pep.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Kumar J. S.; Khan M.; Gupta G.; Bhoopati M.; Lakshmana Rao P. V.; Parida M. Production, characterization, and application of monoclonal antibodies specific to recombinant (E2) structural protein in antigen-capture ELISA for clinical diagnosis of chikungunya virus. Viral Immunol. 2012, 25, 153–160. 10.1089/vim.2011.0068. [DOI] [PubMed] [Google Scholar]
- Metz S. W.; Martina B. E.; van den Doel P.; Geertsema C.; Osterhaus A. D.; Vlak J. M.; Pijlman G. P. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine 2013, 31, 6092–6096. 10.1016/j.vaccine.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Bréhin A.-C.; Rubrecht L.; Navarro-Sanchez M. E.; Maréchal V.; Frenkiel M. P.; Lapalud P.; Laune D.; Sall A. A.; Desprès P. Production and characterization of mouse monoclonal antibodies reactive to Chikungunya envelope E2 glycoprotein. Virology 2008, 371, 185–195. 10.1016/j.virol.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Fong R. H.; Banik S. S. R.; Mattia K.; Barnes T.; Tucker D.; Liss N.; Lu K.; Selvarajah S.; Srinivasan S.; Mabila M.; et al. Exposure of Epitope Residues on the Outer Face of the Chikungunya Virus Envelope Trimer Determines Antibody Neutralizing Efficacy. J. Virol. 2014, 88, 14364–14379. 10.1128/jvi.01943-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey S. H.; Kashyap R. S.; Purohit H. J.; Taori G. M.; Daginawala H. F. An approach towards peptide-based antibody detection for diagnosis of Chikungunya infection. Biomarkers 2010, 15, 546–552. 10.3109/1354750X.2010.494200. [DOI] [PubMed] [Google Scholar]
- Chua C. L.; Sam I. C.; Merits A.; Chan Y. F. Antigenic Variation of East/Central/South African and Asian Chikungunya Virus Genotypes in Neutralization by Immune Sera. PLoS Neglected Trop. Dis. 2016, 10, e0004960 10.1371/journal.pntd.0004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua C. L.; Sam I. C.; Chiam C. W.; Chan Y. F. The neutralizing role of IgM during early Chikungunya virus infection. PLoS One 2017, 12, e0171989 10.1371/journal.pone.0171989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C.; Büchner S. M.; Schnierle B. S. A Small Antigenic Determinant of the Chikungunya Virus E2 Protein Is Sufficient to Induce Neutralizing Antibodies which Are Partially Protective in Mice. PLoS Neglected Trop. Dis. 2015, 9, e0003684 10.1371/journal.pntd.0003684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R.; Zhai L.; Rosso B.; Tumban E. Bacteriophage Qβ virus-like particles displaying Chikungunya virus B-cell epitopes elicit high-titer E2 protein antibodies but fail to neutralize a Thailand strain of Chikungunya virus. Vaccine 2020, 38, 2542–2550. 10.1016/j.vaccine.2020.01.091. [DOI] [PubMed] [Google Scholar]
- Khan N.; Bhat R.; Jain V.; Raghavendhar B S.; Patel A. K.; Nayak K.; Chandele A.; Murali-Krishna K.; Ray P. Epidemiology and molecular characterization of chikungunya virus from human cases in North India, 2016. Microbiol. Immunol. 2021, 65, 290–301. 10.1111/1348-0421.12869. [DOI] [PubMed] [Google Scholar]
- Hasan M. A.; Khan M. A.; Datta A.; Mazumder M. H. H.; Hossain M. U. A comprehensive immunoinformatics and target site study revealed the corner-stone toward Chikungunya virus treatment. Mol. Immunol. 2015, 65, 189–204. 10.1016/j.molimm.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues R. L.; Menezes G. D. L.; Saivish M. V.; Da Costa V. G.; Pereira M.; Moreli M. L.; Silva R. A. Da Prediction of MAYV peptide antigens for immunodiagnostic tests by immunoinformatics and molecular dynamics simulations. Sci. Rep. 2019, 9, 13339 10.1038/s41598-019-50008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar E.; Issac A.; Nair S.; Hariharan R.; Janki M. B.; Arathy D. S.; Regu R.; Mathew T.; Anoop M.; Niyas K. P.; et al. Genetic characterization of 2006-2008 isolates of Chikungunya virus from Kerala, South India, by whole genome sequence analysis. Virus Genes 2010, 40, 14–27. 10.1007/s11262-009-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A.; Das B.; Das M.; Patra A.; Biswal S.; Kar S. K.; Hazra R. K. Genetic characterization of E2 region of Chikungunya virus circulating in Odisha, Eastern India from 2010 to 2011. Infect. Genet. Evol. 2013, 18, 113–124. 10.1016/j.meegid.2013.04.037. [DOI] [PubMed] [Google Scholar]
- Tahir Ul Qamar M.; Bari A.; Adeel M. M.; Maryam A.; Ashfaq U. A.; Du X.; Muneer I.; Ahmad H. I.; Wang J. Peptide vaccine against chikungunya virus: Immuno-informatics combined with molecular docking approach. J. Transl. Med. 2018, 16, 298 10.1186/s12967-018-1672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula A.; Pandey R. K.; Khatoon N.; Mishra A.; Prajapati V. K. Excavating chikungunya genome to design B and T cell multi-epitope subunit vaccine using comprehensive immunoinformatics approach to control chikungunya infection. Infect., Genet. Evol. 2018, 61, 4–15. 10.1016/j.meegid.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Khan B. A.; Saifullah; Lail A.; Khan S. Sub-genomic analysis of chikungunya virus e2 mutations in pakistani isolates potentially modulating b-cell & t-cell immune response. Pakistan J. Med. Sci. 2020, 37, 1–6. 10.12669/pjms.37.1.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bappy S. S.; Sultana S.; Adhikari J.; Mahmud S.; Khan M. A.; Kibria K. M. K.; Rahman M. M.; Shibly A. Z. Extensive immunoinformatics study for the prediction of novel peptide-based epitope vaccine with docking confirmation against envelope protein of Chikungunya virus: a computational biology approach. J. Biomol. Struct. Dyn. 2021, 39, 1139–1154. 10.1080/07391102.2020.1726815. [DOI] [PubMed] [Google Scholar]
- Teo T. H.; Chan Y. H.; Lee W. W. L.; Lum F. M.; Amrun S. N.; Her Z.; Rajarethinam R.; Merits A.; Rötzschke O.; Rénia L.; et al. Fingolimod treatment abrogates chikungunya virus-induced arthralgia. Sci. Transl. Med. 2017, 9, 1–12. 10.1126/scitranslmed.aal1333. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S.; Kumar P.; Mohan T.; Verma P.; Parida M. M.; Hoti S. L.; Rao D. N. Evaluation of multiple antigenic peptides based on the Chikungunya E2 protein for improved serological diagnosis of infection. Viral Immunol. 2015, 28, 107–112. 10.1089/vim.2014.0031. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Singh V. K.; Vasam M.; Patil P. S.; Dhaked R. K.; Ansari A. S.; Lohiya N. K.; Parashar D.; Tapryal S. An in vitro refolding method to produce oligomers of anti-CHIKV, E2-IgM Fc fusion subunit vaccine candidates expressed in E. coli. J. Immunol. Methods 2020, 487, 112869 10.1016/j.jim.2020.112869. [DOI] [PubMed] [Google Scholar]
- Verma P.; Bhatnagar S.; Kumar P.; Chattree V.; Parida M. M.; Hoti S. L.; Ali S.; Rao D. N. Analysis of antibody response (IgM, IgG, IgG3) to Chikungunya virus using a panel of peptides derived from envelope protein for serodiagnosis. Clin. Chem. Lab. Med. 2014, 52, 297–307. 10.1515/cclm-2013-0363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.