Abstract
Effective wastewater surveillance of SARS-CoV-2 RNA requires the rigorous characterization of the limit of detection resulting from the entire sampling process - the process limit of detection (PLOD). Yet to date, no studies have gone beyond quantifying the assay limit of detection (ALOD) for RT-qPCR or RT-dPCR assays. While the ALOD is the lowest number of gene copies (GC) associated with a 95% probability of detection in a single PCR reaction, the PLOD represents the sensitivity of the method after considering the efficiency of all processing steps (e.g., sample handling, concentration, nucleic acid extraction, and PCR assays) to determine the number of GC in the wastewater sample matrix with a specific probability of detection. The primary objective of this study was to estimate the PLOD resulting from the combination of primary concentration and extraction with six SARS-CoV-2 assays: five RT-qPCR assays (US CDC N1 and N2, China CDC N and ORF1ab (CCDC N and CCDC ORF1ab), and E_Sarbeco RT-qPCR, and one RT-dPCR assay (US CDC N1 RT-dPCR) using two models (exponential survival and cumulative Gaussian). An adsorption extraction (AE) concentration method (i.e., virus adsorption on membrane and the RNA extraction from the membrane) was used to concentrate gamma-irradiated SARS-CoV-2 seeded into 36 wastewater samples. Overall, the US CDC N1 RT-dPCR and RT-qPCR assays had the lowest ALODs (< 10 GC/reaction) and PLODs (<3,954 GC/50 mL; 95% probability of detection) regardless of the seeding level and model used. Nevertheless, consistent amplification and detection rates decreased when seeding levels were < 2.32 × 103 GC/50 mL even for US CDC N1 RT-qPCR and RT-dPCR assays. Consequently, when SARS-CoV-2 RNA concentrations are expected to be low, it may be necessary to improve the positive detection rates of wastewater surveillance by analyzing additional field and RT-PCR replicates. To the best of our knowledge, this is the first study to assess the SARS-CoV-2 PLOD for wastewater and provides important insights on the analytical limitations for trace detection of SARS-CoV-2 RNA in wastewater.
Keywords: SARS-CoV-2, COVID-19, Detection limit, Recovery, Concentration method, Enveloped virus, Wastewater
Graphical abstract
1. Introduction
Wastewater surveillance is being utilized in many countries to monitor coronavirus disease 2019 (COVID-19) via severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA presence and levels in community wastewater. SARS-CoV-2 RNA excreted by infected individuals is diluted by many orders of magnitude in wastewater. Therefore, to achieve trace detection and quantification of RNA, the wastewater samples require the application of optimized concentration methods (with varying primary and/or secondary concentration steps) before extraction of the RNA fragments and, finally, highly-sensitive molecular assays using reverse transcription PCR (RT-PCR) or RT quantitative PCR (RT-qPCR) or RT digital PCR (RT-dPCR) (Ahmed et al., 2020a; Medema et al., 2020a; Prado et al., 2020; Randazzo et al., 2020; Bertrand et al., 2021; Gibas et al., 2021; Navarro et al., 2021; Westhaus et al., 2021).
Given the complexities of such workflows, there are many factors that may contribute to false negative and erroneous results (wastewater negative despite the presence of COVID-19 in the relevant population), which are thoroughly discussed in several review articles (Ahmed et al., 2020b; Medema et al., 2020b; Michael-Kordatou et al., 2020; Ahmed et al., 2021a; Bivins et al., 2021a). Interpreting discordant results is further complicated by the many RT-PCR assays in use. Multiple assays have been developed targeting various nucleic acid sequences of SARS-CoV-2 specific to the nucleocapsid (N), envelope protein (E), RNA dependent RNA polymerase (RdRp), open reading frame one (ORF1), membrane protein (M), and surface protein (S) genes (Li et al., 2020). While these assays were developed for clinical testing of nasopharyngeal swab samples, they are also being used for wastewater surveillance (Bertrand et al., 2021;Bivins et al., 2021b; Gibas et al., 2021; Navarro et al., 2021; Westhaus et al., 2021).
Several studies have examined the analytical sensitivity, as measured by the assay limit of detection (ALOD), of various RT-qPCR assays primarily for clinical testing. For example, Iglói et al. (2020) compared 13 commercially-available real-time RT-PCR assays using SARS-CoV-2 cell-cultured virus stock. Analytical sensitivity of assays in the different kits varied between 3.3 to 330 RNA gene copies (GC)/reaction. Muenchhof et al. (2020) compared 11 different RT-qPCR assays used in seven diagnostic laboratories in Germany using an RNA sample extracted from one SARS-CoV-2-positive stool sample. Serially-diluted RNA sub-samples were shared with participating laboratories to determine sensitivity. Most RT-qPCR assays for SARS-CoV-2 examined in their study successfully detected ∼5 GC/reaction, reflecting a high sensitivity. A reduced sensitivity was noted for the original RdRp assay from Charité Institute of Virology (Charité), which may have impacted the confirmation of some COVID-19 cases in the early weeks of the pandemic. A study by Vogels et al. (2020) compared the performance of nine primer-probe combinations targeting several genes (i.e., E, N, ORF1, RdRp) recommended by the World Health Organization (e.g., those developed by the China CDC, US CDC, Charité (Corman et al., 2020), and Hong Kong University). This comparison was performed with standard reference materials and clinical samples (e.g., nasopharyngeal swabs, saliva, urine, and rectal swabs) seeded with the reference material. The authors demonstrated that at low viral concentrations (1 to 10 GC/µL), not all assays yielded positive results; thus, suggesting that some assays may be more prone to false-negative errors than others (Vogels et al., 2020). Most notably, the RdRp reverse primer had mismatches with the reference material that were attributed to evolution of the virus, causing low analytical sensitivity.
While the ALOD is a useful assessment of the analytical sensitivity of SARS-CoV-2 RT-qPCR assays, the ALOD values of various SARS-CoV-2 assays during wastewater surveillance have ranged between 1 to 100 GC/reaction (Gerrity et al., 2021; Randazzo et al., 2020; Chavarria-Miró et al., 2021). For wastewater surveillance, the analytical sensitivity of methods must also account for the efficiency of the various processing steps, including primary concentration, loss through nucleic acid extraction, and inhibition of reverse transcription or PCR amplification. Together the RT-qPCR/dPCR ALOD and the process recovery efficiency (loss of target through all sample processing steps) determine the process limit of detection (PLOD), which has not yet been well characterized for any method used to concentrate SARS-CoV-2 RNA from wastewater. Frequently, assumed process recoveries (e.g., 100%) and empirically determined ALODs are combined to produce idealized estimates of the PLOD (Ahmed et al., 2021b), but empirical determination of the PLOD itself is vital for a robust characterization of the surveillance method.
A recent study evaluated four different SARS-CoV-2 RT-qPCR detection assays (US CDC N1, China CDC N (CCDC N), N_Sarbeco and E_Sarbeco) for the accurate and reliable quantification of SARS-CoV-2 in wastewater (Zhang et al., 2022). The authors recommended the CCDC N assay for its high sensitivity and reproducibility when analysing plasmid control material, plasmid-seeded wastewater, and real wastewater samples. While the US CDC N1 assay demonstrated high sensitivity in their ambient wastewater samples, it showed poor reproducibility and linearity at low concentrations against their plasmid-seeded samples (Zhang et al., 2022). However, the generalizability of such findings is constrained by several limitations. The RT-qPCR cycling parameters were the same for all assays and deviations from the optimized assay-specific cycling parameters may have affected the sensitivity of one assay compared to another. Furthermore, plasmid control materials, rather than SARS-CoV-2 virions, were seeded by Zhang et al. (2022) into RNA extracted from wastewater to determine the spiked mocks limit of detection using seeded wastewater. The use of plasmid control materials (which are double-stranded DNA) for SARS-CoV-2 (single-stranded RNA virus) has well documented limitations, including heterogeneity in PCR efficiencies and non-linearity for RT-qPCR experiments (Bivins et al., 2021a).
The primary objective of this study was to evaluate the PLODs of five RT-qPCR assays and one RT-dPCR assay for the detection of SARS-CoV-2 RNA in wastewater inclusive of the processing workflow efficiency. This was achieved by seeding a dilution series of known concentrations of gamma-irradiated SARS-CoV-2 virions into wastewater followed by primary concentration, nucleic acid extraction, and RT-qPCR/RT-dPCR analysis using each assay. In addition to determining the PLOD, the quantitative data from the seeding experiments were also used to assess the variation in SARS-CoV-2 RNA copy number along the dilution gradient. In conjunction with these experiments, the effect of variable seeding levels of SARS-CoV-2 in wastewater on recovery efficiency of each assay was also examined using 36 different wastewater samples.
2. Materials and methods
2.1. SARS-CoV-2 seeding materials
Gamma-irradiated SARS-CoV-2 hCoV-19/Australia/VIC01/2020 was provided by the Australian Centre for Disease Preparedness (ACDP), CSIRO. Gamma radiation was administered with a 50 kilogray or 5 Mrad dose using an MDS Nordion Irradiator. Gamma irradiation was necessary to mitigate the risk of infection associated with handling infectious SARS-CoV-2 in a biosecurity containment level 2 (BC2) laboratory where this study was conducted. The gamma-irradiated SARS-CoV-2 stock was stored at −80°C for twelve weeks before use in this study. Immediately prior to seeding experiments, the mean concentration and standard deviation (4.60 × 106 ± 2.50 × 105 GC/µL) of the SARS-CoV-2 stock was determined directly from aliquots (n = 3) of the stock suspension using the US CDC N1 RT-dPCR assay as described in the following section.
2.2. Sources of wastewater samples
Wastewater samples used in this study were from the Queensland Health Wastewater Surveillance program (https://www.qld.gov.au/health/conditions/health-alerts/coronavirus-covid-19/current-status/wastewater). In total, 36 wastewater samples were selected that had been collected between 30/08/2021 and 01/09/2021 from 36 wastewater treatment plants (WWTPs) across Queensland, Australia. At each WWTP, untreated wastewater samples ranging from 500 mL to 1 L in volume were collected as time-based composites using an automated sampler operating in time-proportional mode (taking subsamples every 15 mins for 24 h) (Ahmed et al., 2020a). These composite wastewater samples had been previously screened by RT-qPCR for SARS-CoV-2 RNA in conjunction with the surveillance program and confirmed to be negative using US CDC N1 and N2 assay (https://www.qld.gov.au/health/conditions/health-alerts/coronavirus-covid-19/current-status/wastewater).
2.3. Wastewater seeding experiments
To determine the ability of each RT-qPCR and US CDC N1 RT-dPCR assays to detect SARS-CoV-2 RNA in wastewater, known concentrations of gamma-irradiated SARS-CoV-2 were prepared by serial diluting stock suspension using DNase and RNase free water and seeding these serial dilutions into 50-mL wastewater samples. Final SARS-CoV-2 seeding levels ranged from 2.32 × 105 to 2.32 × 102 GC/50 mL along a serial dilution in 10-fold decrements to yield four unique titers of SARS-CoV-2 RNA.
2.4. Virus concentration
Viruses were concentrated from the SARS-CoV-2 seeded wastewater samples using the adsorption extraction (AE) method. This method has been commonly used to concentrate SARS-CoV-2 RNA from wastewater (Ahmed et al., 2020a; Jafferali et al., 2021; Juel et al., 2021; Sapula et al., 2021). The AE method began with the addition of dissolved MgCl2 to the sample to achieve a final concentration of 25 mM MgCl2. After amendment with MgCl2, wastewater samples were immediately filtered through a 0.45-µm pore-size, 47-mm diameter electronegative HA membrane (HAWP04700; Merck Millipore Ltd, Sydney, Australia) via a magnetic filter funnel (Pall Corporation) and filter flask (Merck Millipore Ltd.) (Ahmed et al., 2020a). Following filtration, using aseptic technique, the membrane was immediately removed, rolled, and inserted into a 5-mL-bead-beating tube (Qiagen, Valencia, CA) for nucleic acid extraction.
2.5. Nucleic acid extraction
Immediately after virus concentration, nucleic acid was extracted directly from the HA membranes using the RNeasy PowerWater Kit (Cat. No. 14700-50-NF) (Qiagen, Valencia, CA). Prior to homogenization, 990 µL of buffer PM1 and 10 µL of β-Mercaptoethanol (Sigma-Aldrich; M6250-10 mL) were added into each bead-beating tube. The bead-beating tubes were then homogenized using a Precellys 24 tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, FR) set for 3 × 15 s at 10,000 rpm at a 10 s interval. After homogenization, the tubes were centrifuged at 4,000 g for 5 min to pellet the filter debris and beads. Sample lysate supernatant ranging from 600–800 µL in volume was then used to extract nucleic acid following the manufacturer's specified protocol. Two modifications were made: (i) the use of DNase I solution was omitted from the protocol to isolate nucleic acid (i.e., both RNA and DNA); (ii) nucleic acid was eluted with 200 µL of DNase and RNase free water instead of 100 µL. Nucleic acid purity was verified by measuring 260/280 ratio using a DeNovix Spectrophotometer & Fluorometer (Wilmington, DE, USA).
2.6. Inhibition assessment
After homogenization and before completing the rest of the nucleic acid extraction, known quantities (1.5 × 104 GC) of murine hepatitis virus (MHV) were seeded into each lysate and pellet as an inhibition process control. The same quantity of MHV suspension was also added to a distilled water extraction control (same volume of lysate) and subjected to extraction. The presence of PCR inhibition in nucleic acid samples extracted from wastewater was assessed using an MHV RT-PCR assay (Besselsen et al., 2002). The reference PCR quantification cycle (Cq) values obtained for MHV seeded into distilled water for all methods were compared with the Cq values of the MHV seeded into wastewater lysate to obtain information on potential RT-qPCR inhibition. If the Cq value resulting from the sample was > 2 Cq difference from the reference Cq value for the distilled water control, the sample was considered inhibited (Ahmed et al., 2018; Ahmed et al., 2020c). In addition to the extraction control, all samples were analyzed alongside three PCR negative controls.
2.7. RT-qPCR and RT-dPCR analysis
Previously published RT-qPCR assays were used for MHV (Besselsen et al., 2002) and SARS-CoV-2 detection/quantification (US CDC, 2020; China CDC, 2020; Corman et al., 2020) (Supplementary Table ST1). For the MHV assay, positive control material in the form of gBlocks gene fragments was purchased from Integrated DNA Technologies (Integrated DNA Technology Coralville, IA, US). Gamma-irradiated SARS-CoV-2, as previously described, was used as an RT-qPCR standard for the SARS-CoV-2 US CDC N1, US CDC N2, CCDC N, CCDC ORF1ab, and E_Sarbeco assays. Prepared standard curve dilutions ranged from 6 × 105 to 0.6 GC/reaction. Primer and probe sequences, reaction concentrations, and thermal cycling conditions are listed in Table ST1.
RT-qPCR analyses were performed in 20-µL reaction mixtures using TaqManTM Fast Virus 1-Step Master Mix (Applied Biosystem, California, USA). MHV RT-qPCR mixture contained 5 µL of Supermix, 300 nM of forward primer, 300 nM of reverse primer, 400 nM of probe, and 5 µL of template RNA. US CDC N1 and N2 RT-qPCR mixture contained 5 µL of Supermix, 500 nM of forward primer, 500 nM of reverse primer, 125 nM of probe, and 5 µL of template RNA. CCDC N RT-qPCR mixture contained 5 µL of Supermix, 400 nM of forward primer, 400 nM of reverse primer, 250 nM of probe, and 5 µL of template RNA. CCDC ORF1ab RT-qPCR mixture contained 5 µL of Supermix, 300 nM of forward primer, 300 nM of reverse primer, 300 nM of probe, and 5 µL of template RNA. E_Sarbeco RT-qPCR mixture contained 5 µL of Supermix, 400 nM of forward primer, 400 nM of reverse primer, 200 nM of probe, and 5 µL of template RNA. The RT-qPCR experiments were performed using a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories, Richmond, CA, USA) using manual settings for threshold and baseline.
For digital RT-PCR (RT-dPCR), the US CDC N1 assay was performed in 40-µL reaction mixtures using the QIAcuity One-Step Viral RT-PCR Kit (Cat No. 1123145; Qiagen) and 26K 24-well Nanoplates (Cat No. 250001; Qiagen). The QIAcuity 26K 24-well Nanoplates are microfluidic dPCR plates that allow for the processing of up to 24 samples with up to 26K partitions/well. The PCR reaction occurs in each partition and the partition volume is 0.91 nL. RT-dPCR analyses were performed in 40 µL reaction mixtures using Qiacuity One-Step Viral RT-PCR Kit (Qiagen). US CDC N1 RT-dPCR mixture contained 10 µL of Supermix, 800 nM of forward primer, 800 nM of reverse primer, 200 nM of probe, 0.4 µL of reverse transcriptase and 10 µL of template RNA. Two RT-dPCR replicates were analyzed for each sample. The 40-µL RT-dPCR reactions were prepared in a 96-well pre-plate and then transferred into the 26K 24-well Nanoplate. The Nanoplate was then loaded onto the QIAcuity dPCR 5-plex platform (Qiagen) and subjected to a workflow that included: (i) a priming and rolling step to generate and isolate the chamber partitions; (ii) an amplification step using the thermal cycling protocol; and (iii) a final imaging step in the FAM channel. Each RT-dPCR experiment was performed using duplicate RT-dPCR no-template and positive (gamma-irradiated SARS-CoV-2 RNA) controls. Data were analyzed using the QIAcuity Suite Software V1.1.3 193 (Qiagen, Germany) and quantities exported as GC/µL of reaction. The RT-dPCR assays were performed using automatic settings for threshold and baseline. MIQE and dMIQE checklists are provided in Supplementary Tables ST4 and ST5, respectively.
2.8. RT-qPCR and RT-dPCR ALODs
To determine RT-qPCR and RT-dPCR assay limit of detection (ALOD), gamma-irradiated SARS-CoV-2 were diluted (6 × 105 to 0.6 GC/reaction) and analyzed using RT-qPCR and RT-dPCR. At each dilution, 15 replicates were analyzed. The 95% ALOD was defined by fitting an exponential survival model to the proportion of PCR replicates positive at each step along the gradient (Verbyla et al., 2016).
2.9. Quality control
To minimize RT-qPCR and RT-dPCR contamination, nucleic acid extraction and RT-qPCR/dPCR set up were performed in separate laboratories. A sample negative control was included during the concentration process. An extraction negative control was also included during nucleic acid extraction to account for any contamination during extraction. All sample and extraction negative controls were negative for the analyzed targets.
2.10. Data analysis
For RT-qPCR and RT-dPCR, the ALOD is defined as the minimum GC number with a 95% probability of detection and determined as previously described (Verbyla et al., 2016). For RT-qPCR, samples were considered positive (SARS-CoV-2 detected) if amplification was observed in at least one of the three replicates within 45 cycles. Samples were considered quantifiable if amplification was observed in all three replicates with concentrations above the ALOD. For RT-dPCR, samples were considered positive if there was at least one positive partition following the merging of nano wells from two replicate wells. Samples were considered quantifiable by RT-dPCR if the concentrations were above the ALOD, and the average number of partitions was >11,000 per sample well.
For RT-qPCR and RT-dPCR, the PLOD is defined as the minimum GC number with a 95% probability of detection, incorporating the loss of SARS-CoV-2 through sample concentration and RNA extraction and determined as previously described (Stokdyk et al., 2016; Verbyla et al., 2016). The PLOD was estimated for each assay using two probability models that predict the proportion of positive amplification within 45 cycles: the exponential survival model and the beta-Poisson model (Verbyla et al., 2016). For comparison, the cumulative Gaussian model (Bivins et al., 2021b; Stokdyk et al., 2016) was also used to estimate the 50 and 95% detection probabilities. Maximum likelihood estimates of model parameters were obtained by finding parameter values that minimized the deviance based on the positive and total number of replicates analyzed at the different concentrations of seeded SARS-CoV-2. Since the exponential model is a special case of the beta-Poisson model (Haas et al., 2014), a chi-squared test was performed to determine if the fit provided by the beta-Poisson model was worth the extra degree of freedom.
For the seeded levels that yielded a 100% detection rate, the variation in the estimated SARS-CoV-2 RNA GC number for each assay was assessed via the coefficient of variation (CV). The SARS-CoV-2 recovery efficiency for all RT-qPCR and RT-dPCR was calculated based on the GC quantified as follows:
At each concentration step with a 100% detection rate, differences in recovery efficiency between assays were assessed by the Kruskal-Wallis H test with Dunn's post hoc test (Kruskal and Wallis, 1952; Dunn, 1964). Differences in recovery efficiency between concentration steps for each assay were assessed by the Mann-Whitney U test (Mann and Whitney, 1947). Statistical significance was defined as p < 0.05.
3. Results
3.1. Assay performance and relevant QA/QC
A 260/280 nm absorbance ratio of nucleic acid >1.80 for wastewater RNA from all samples was considered acceptable RNA quality (Supplementary Table ST2) (Sambrook et al., 1989). The RT-qPCR standard curves prepared from gamma-irradiated SARS-CoV-2 had a linear dynamic range of quantification from 6 × 105 to 6 GC/reaction (1.2 × 105 to 1.2 GC/µL). The slopes of the standard curves ranged between -3.31 (CCDC N) and -3.48 (E_Sarbeco) (Table 1 ). The ranges for amplification efficiencies (94.0 to 100%) and y-intercepts (36.3, US CDC N1 to 39.8, E_Sarbeco) were within the prescribed range of MIQE guidelines (Bustin et al., 2009). The correlation coefficients (r 2) ranged from 0.98 (CCDC N) to 0.99 (E_Sarbeco). The ALODs for the RT-qPCR assays were between 9.50 and 48.1 GC/reaction, being the lowest for the US CDC N1 and greatest for E_Sarbeco assays, respectively (Table 1). The RT-dPCR ALOD was 3.30 GC/reaction for the US CDC N1 assay. All method, extraction, and RT-qPCR/RT-dPCR negative controls were negative. All positive controls or standard curves amplified in each PCR run. For the US CDC N1 RT-dPCR, the number of partitions ranged from 11,522 – 25,417 with a mean of 21,148 and a SD of 3,437. PCR inhibition was not identified in any RNA samples based on the seeded GC of MHV (all within 2-Cq values of the reference Cq value) (Supplementary Table ST3).
Table 1.
RT-qPCR performance characteristics and assay limit of detection (ALOD).
| Assay | Performance characteristics | ||||
|---|---|---|---|---|---|
| Efficiency (E) (%) | Linearity (R2) | Slope | Y-intercept | ALOD for SARS-CoV-2 RNA (GC/ reaction) | |
| US CDC N1 RT-qPCR | 97.7 | 0.99 | -3.37 | 36.3 | 9.50 |
| US CDC N2 RT-qPCR | 95.9 | 0.98 | -3.42 | 39.1 | 26.7 |
| CCDC N RT-qPCR | 100 | 0.98 | -3.31 | 36.9 | 16.4 |
| CCDC ORF1ab RT-qPCR | 98.0 | 0.99 | -3.37 | 37.4 | 11.6 |
| E_Sarbeco RT-qPCR | 94.0 | 0.99 | -3.48 | 39.8 | 48.1 |
| US CDC N1 RT-dPCR | - | - | - | - | 3.30 |
CCDC: China CDC.
3.2. RT-qPCR detection and PLOD values for SARS-CoV-2 seeded wastewater samples
SARS-CoV-2 RNA was detected in all wastewater samples by all six assay replicates (RT-qPCR and RT-dPCR) when seeding was 2.32 × 105 (n = 9 samples) and 2.32 × 104 GC/50 mL (n = 9 samples) (Table 2 ). At the lower seeding titer of 2.32 × 103 GC/50 mL (n = 9 samples), among the RT-qPCR assays, CCDC N provided detection in 9/9 (100%) wastewater samples, while US CDC N1 detected 8/9 (88.9%), CCDC ORF1ab 7/9 (77.8%), E_Sarbeco 6/9 (66.7%), and US CDC N2 1 of 9 samples (11.1%), respectively. At the lowest seeding level of 2.32 × 102 GC/50 mL (n = 9 samples), US CDC N1 was the most sensitive assay, providing detection in 6/9 (66.7%) samples, followed by CCDC N with 3/9 (33.3%), then both US CDC N2 and CCDC ORF1ab with detection in 1/9 (11.1%) samples, and the E_Sarbeco assay, which failed to produce any amplification (zero detection at lowest seed). Detection rates by both the US CDC N1 RT-qPCR and US CDC N1 RT-dPCR were the same for all serially diluted samples. Three RT-qPCR and two RT-dPCR replicates were analyzed for each wastewater samples. At seeding levels 2.32 × 105 and 2.32 × 104 GC/50 mL, all RT-qPCR and RT-dPCR replicates yielded positive amplifications. However, inconsistent amplification occurred among RT-qPCR and RT-dPCR replicates at seeding levels 2.32 × 103 and 2.32 × 102 GC/50 mL. Among the five RT-qPCR assays, US CDC N1 replicates positivity was greater than other assays. Between the US CDC N1 RT-qPCR and RT-dPCR, RT-dPCR replicates positivity rates were slightly better than RT-qPCR.
Table 2.
Proportion of samples and replicates positive for SARS-CoV-2 RNA in wastewater seeded at four concentrations using five RT-qPCR assays and one RT-dPCR assay.
| Concentrations of SARS-CoV-2 seeded/50 mL of wastewater | No. of wastewater samples positive/No. of samples tested (%) | |||||
|---|---|---|---|---|---|---|
| US CDC N1 RT-qPCR | US CDC N2 RT-qPCR | CCDC N RT-qPCR | CCDC ORF1ab RT-qPCR | E_Sarbeco RT-qPCR | US CDC N1 RT-dPCR | |
| 2.32 × 105 GC | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) |
| 2.32 × 104 GC | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) | 9/9 (100) |
| 2.32 × 103 GC | 8/9 (88.9) | 1/9 (11.1) | 9/9 (100) | 7/9 (77.8) | 6/9 (66.7) | 8/9 (88.9) |
| 2.32 × 102 GC | 6/9 (66.7) | 1/9 (11.1) | 3/9 (33.3) | 1/9 (11.1) | 0/9 (0) | 6/9 (66.6) |
| No. of replicates positive/no. of replicates tested (%) |
||||||
| 2.32 × 105 GC | 27/27 (100) | 27/27 (100) | 27/27 (100) | 27/27 (100) | 27/27 (100) | 18/18 (100) |
| 2.32 × 104 GC | 27/27 (100) | 27/27 (100) | 27/27 (100) | 27/27 (100) | 27/27 (100) | 18/18 (100) |
| 2.32 × 103 GC | 21/27 (77.8) | 1/27 (3.70) | 16/27 (59.3) | 15/27 (55.6) | 10/27 (37.0) | 16/18 (88.9) |
| 2.32 × 102 GC | 7/27 (25.9) | 1/27 (3.70) | 5/27 (18.5) | 1/27 (3.70) | 0/27 (0) | 8/18 (44.4) |
CCDC: China CDC.
Overall, the US CDC N1 RT-qPCR and the US CDC N1 RT-dPCR assays outperformed other assays. CDC N2 and E_Sarbeco were the least sensitive, and this was most evident at the lower seeding dilution. For all six RT-qPCR and RT-dPCR assays, the exponential survival model effectively estimated the probabilities of SARS-CoV-2 detection considering the entire methodological process (Table 3 ) (Verbyla et al., 2016). Any improvements in fit provided by the beta-Poisson model were not worth the extra degree of freedom, as indicated by chi-squared tests (p-values were all >0.05). Among the RT-qPCR assays and considering the definition of PLOD as the 95% probability of detection, the lowest PLOD value was 3,954 GC/50 mL for US CDC N1 assay followed by 6,651 GC/50 mL for CCDC N. PLOD values of US CDC N2 and E_Sarbeco were much greater than other assays. US CDC N1 RT-dPCR exhibited the lowest limits of detection, ranging from 33.4 (5% probability of detection) to 1,952 (95% probability of detection) GC/50 mL.
Table 3.
The probability of detecting SARS-CoV-2 RNA as determined using wastewater samples seeded with SARS-CoV-2, the adsorption extraction concentration method, and assayed using five RT-qPCR assays and one RT-dPCR assay. The % probability of detection was estimated using two probability models: exponential survival and the cumulative Gaussian models. The process limit of detection (PLOD) was defined as the concentration associated with a 95% probability of detection.
| % Probability of detection | Exponential survival model estimates of the SARS-CoV-2 concentration (GC/50 mL) | |||||
|---|---|---|---|---|---|---|
| US CDC N1 RT-qPCR | US CDC N2 RT-qPCR | CCDC N RT-qPCR | CCDC ORF1ab RT-qPCR | E_Sarbeco RT-qPCR | US CDC N1 RT-dPCR | |
| Model parameter ln (k) | -7.19 | -9.09 | -7.71 | -8.03 | -8.52 | -6.48 |
| 5% probability of detection | 68 | 457 | 113 | 157 | 258 | 33.4 |
| 25% probability of detection | 380 | 2563 | 639 | 880 | 1446 | 187 |
| 50% probability of detection | 915 | 6175 | 1539 | 2121 | 3484 | 452 |
| 75% probability of detection | 1829 | 12350 | 3078 | 4242 | 6969 | 902 |
| 95% (PLOD) | 3954 | 26689 | 6651 | 9167 | 15061 | 1952 |
| Cumulative Gaussian model estimates of the SARS-CoV-2 concentration (GC/50 mL) |
||||||
| Model parameter (μ) | 1188 | 10617 | 1886 | 2167 | 3266 | 448 |
| Model parameter (σ) | 1479 | 4940 | 1845 | 1083 | 2850 | 1533 |
| 50% probability of detection | 1188 | 10617 | 1886 | 2167 | 3266 | 448 |
| 95% (PLOD) | 3621 | 18743 | 4921 | 3948 | 7954 | 2970 |
CCDC: China CDC.
We also determined the 50 and 95% probabilities of detection of SARS-CoV-2 RNA for all six RT-qPCR and RT-dPCR assays using the cumulative Gaussian model (Table 3) (Bivins et al., 2021b). Among the RT-qPCR assays, the lowest 95% PLOD value estimated by the cumulative Gaussian model was again the US CDC N1 assay (3,621 GC/50 mL) followed by CCDC ORF1ab (3,948 GC/50 mL). The PLOD values of US CDC N2 and E_Sarbeco were much greater than they were for other assays. US CDC N1 RT-dPCR exhibited the lowest limits of detection, 448 (50% probability of detection) to 2,970 (95% probability of detection) GC/50 mL.
3.3. Variation in Quantification
For all RT-qPCR and RT-dPCR assays, SARS-CoV-2 RNA was only quantifiable at seeding values ≥2.32 × 104 GC/50 mL wastewater as shown in Table 4 . The US CDC N2 RT-qPCR assay demonstrated the largest variation in quantifiable samples with CVs of 89% and 147% at 2.32 × 105 GC/50 mL and 2.32 × 104 GC/50 mL, respectively. As shown in Fig. 1 , the CV of the RT-qPCR assays for CCDC N (50%, 52%), CCDC ORF1ab (51%, 40%), and E_Sarbeco (44%, 59%) were similar to that of the US CDC N1 RT-dPCR assay (52%, 54%) at each of the higher seeding levels. Besides the US CDC N2 RT-qPCR assay previously mentioned, the greatest increase in the CV between the two higher seeding levels was observed for the US CDC N1 RT-qPCR (47% to 76%). Interestingly for the CCDC ORF1ab RT-qPCR, the CV decreased from 51% to 40% suggesting improved quantitative precision as the seeded level decreased. For all others, the precision decreased with decreasing seeding level, as is expected. No assay yielded quantitative results when wastewater was seeded with 2.32 × 103 GC/50 or 2.32 × 102 GC/50 mL.
Table 4.
Coefficient of variation results for quantification of SARS-CoV-2 RNA in wastewater using five RT-qPCR and one RT-dPCR assays. CV was only calculated for concentrations for which all 27 replicates were positive.
| Concentrations of SARS-CoV-2 seeded/50 mL of wastewater | Coefficient of variation (CV) (%) or fraction of replicates positive | |||||
|---|---|---|---|---|---|---|
| US CDC N1 RT-qPCR | US CDC N2 RT-qPCR | CCDC N RT-qPCR | CCDC ORF1ab RT-qPCR | E_Sarbeco RT-qPCR | US CDC N1 RT-dPCR | |
| 2.32 × 105 GC | 47 | 89 | 50 | 51 | 44 | 52 |
| 2.32 × 104 GC | 76 | 147 | 52 | 40 | 59 | 54 |
| 2.32 × 103 GC | 21/27 | 1/27 | 16/27 | 15/27 | 10/27 | 16/18 |
| 2.32 × 102 GC | 7/27 | 1/27 | 5/27 | 1/27 | 0/27 | 9/18 |
CCDC: China CDC.
Fig. 1.
Box and whisker plots of RT-qPCRa or RT-dPCRb-measured SARS-CoV-2 RNA GC as observed for wastewater samples seeded with (A) 2.32 × 104 GC/50 mL and (B) 2.32 × 105 GC/50 mL. Upper and lower fences display the 5th and 95th percentile. Boxes display the interquartile range and median. CCDC: China CDC.
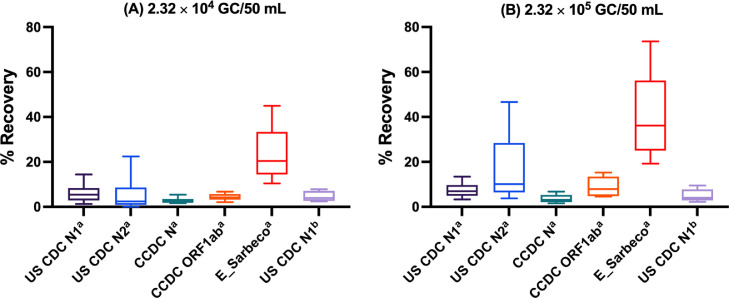
3.4. Recovery efficiency
As summarized in Table 5 , the mean recoveries for RT-qPCR and RT-dPCR assays ranged from 3.63% (CCDC N) to 41.2% (E_Sarbeco) at 2.32 × 105 GC/50 mL to 2.77% (CCDC N) to 23.8% (E_Sarbeco) at 2.32 × 104 GC/50 mL. At both these higher seeding levels, the CCDC N assay demonstrated the lowest mean recovery and the E_Sarbeco assay demonstrated the highest. As shown in Fig. 2 , the largest variation in recovery efficiency was found for the US CDC N2 RT-qPCR with a CV of 85.1% at 2.32 × 105 and 130% at 2.32 × 104 GC/50 mL. While mean recoveries did vary, statistically significant differences were only observed between the E_Sarbeco RT-qPCR assay and the US CDC N1 (p = 0.028), US CDC N2 (p = 0.024), CCDC N (p < 0.0001), and RT-dPCR N1 (p = 0.0003) assays at the higher seeding level and the E_Sarbeco RT-qPCR and the US CDC N2 (p = 0.002), CCDC N (p < 0.0001), CCDC ORF1ab (p = 0.026), and RT-dPCR N1 (p = 0.0003) at the lower seeding level. Mean recovery efficiency significantly increased with increasing seed level for the US CDC N2 RT-qPCR assay (p = 0.011), CCDC ORF1ab (p = 0.008), and E_Sarbeco (p = 0.040). The US CDC N1 RT-qPCR assay, CCDC N assay, and US CDC N1 RT-dPCR assay recovery efficiencies remained similar between the two seeding levels. Aside from the E_Sarbeco RT-qPCR assay, the mean recovery efficiencies were most often below 10% across all assays and seeding levels.
Table 5.
Recovery efficiency summary statistics for each RT-qPCR and RT-dPCR assay stratified by seeded concentration.
| Statistic | US CDC N1 RT-qPCR | US CDC N2 RT-qPCR | CCDC N RT-qPCR | CCDC ORF1ab RT-qPCR | E_Sarbeco RT-qPCR | US CDC N1 RT-dPCR |
|---|---|---|---|---|---|---|
| 2.32 × 105 GC/50 mL wastewater | ||||||
| Minimum | 3.27 | 3.74 | 1.57 | 4.55 | 19.2 | 2.22 |
| Maximum | 13.4 | 46.6 | 6.79 | 15.2 | 73.6 | 9.52 |
| Mean (%) | 7.39 | 17.1 | 3.63 | 8.79 | 41.2 | 5.15 |
| SD | 3.20 | 14.6 | 1.80 | 4.23 | 18.7 | 2.74 |
| CV (%) | 43.3 | 85.1 | 49.7 | 48.1 | 45.3 | 53.4 |
| 2.32 × 104 GC/50 mL wastewater |
||||||
| Minimum | 1.29 | 0.06 | 1.61 | 2.11 | 10.5 | 2.45 |
| Maximum | 14.4 | 22.4 | 5.42 | 6.78 | 44.9 | 7.89 |
| Mean (%) | 6.19 | 5.45 | 2.77 | 4.38 | 23.8 | 4.54 |
| SD | 3.98 | 7.10 | 1.21 | 1.46 | 11.7 | 2.19 |
| CV (%) | 64.3 | 130 | 43.8 | 33.5 | 49.3 | 48.2 |
CCDC: China CDC; SD: Standard deviation; CV: Coefficient of variation.
Fig. 2.
Box and whisker plots of RT-qPCRa or RT-dPCRb-measured SARS-CoV-2 RNA recovery efficiency as observed for wastewater samples seeded with (A) 2.32 × 104 GC/50 mL and (B) 2.32 × 105 GC/50 mL. Upper and lower fences display the 10th and 90th percentile. Boxes display the interquartile range and median. CCDC: China CDC.
4. Discussion
Many wastewater SARS-CoV-2 surveillance studies have provided ALOD values by serially-diluting standard materials and assaying the dilution series with various RT-qPCR assays (Ahmed et al., 2021b; Gerrity et al., 2021; Randazzo et al., 2020; Chavarria-Miró et al., 2021). Assuming a Poisson model for the distribution of target GC into PCR reactions, the theoretical 95% probability of detection is approximately 3 gene copies per PCR reaction (-ln(1-0.95)) (Bustin et al., 2009). But the models and methods used to determine ALODs and their estimated values vary widely between studies, even among wastewater surveillance studies for SARS-CoV-2 RNA (Bivins et al., 2021a). For example, during wastewater surveillance in Virginia using droplet dPCR (ddPCR), Gonzalez et al. (2020) determined ALODs using 60% probability of detection (N1 = 14.6 GC/reaction). While, in northern Indiana, Bivins et al. (2021b), using the same ddPCR platform, estimated the ALOD using 95% probability of detection (N1 = 3.3 GC/reaction). ALODs reported in the SARS-CoV-2 wastewater surveillance literature have ranged from 1 GC/reaction (Gerrity et al., 2021), to 50 GC/reaction (Randazzo et al., 2020), up to as high as 100 GC/reaction (Chavarria-Miró et al., 2021). However, it should be noted that the control materials and statistical methods used to estimate the ALODs are not consistent from one study to another.
In the current study, we have determined the ALOD for each RT-qPCR/dPCR assay using an exponential survival model (Verbyla et al., 2016). The exponential survival model deviates from the Poisson by incorporating a probability, r, that when the target is present describes the probability of “survival” of the target throughout the entire analytical workflow (Poisson assumes r = 1.0) to be successfully detected, so the estimated ALOD increases above the Poisson by the inverse of r ([-ln[1-0.95]/r). The observed ALODs of the RT-qPCR assays suggest that the “survival” probabilities deviate from 1 with the US CDC N1 assay demonstrating the highest survival probability (3/9.5 = 0.32) and E_Sarbeco demonstrating the lowest (3/48.1 = 0.06). Conversely, the RT-dPCR US CDC N1 assay probability of survival of 0.91 (3/3.3) was the closest to 1. These results suggest that for the RT-qPCR assays there are sources of error that reduce the detection probability even when a copy of the target has a high probability of having been added to the reaction well (according to the Poisson distribution). The sources of error could include inhibition associated with the matrix or inefficiency of the PCR reaction in amplifying the control material. In the current study, RNA extracted from gamma-irradiated SARS-CoV-2 was used as the control material, so inhibition is expected to be minimal. Since the ALODs were measured over two days with minimal freeze-thawing, the degradation of RNA during this time frame is also expected to be minimal.
While the ALOD provides information on the lowest number of GC than can be reliably detected by the RT-qPCR assays when analyzing pure culture, plasmid, or other materials, it does not incorporate the target genome loss in the sample matrix by primary, and secondary concentration and nucleic acid extraction. For clinical samples, the results of pure reverse-transcribed RNA transcript standards and seeded samples were different for CCDC-N and CCDC-ORF1ab (Vogels et al., 2020). This indicates that the comparison of RT-qPCR assays should be performed for relevant matrices, such as clinical and wastewater samples. The PLOD is the matrix-relevant limit of detection incorporating the inefficiencies of the entire workflow. Determination of PLOD is not common practice in research literature, especially for wastewater samples, but has been conducted for microbial source tracking marker genes, pathogens, and indicator viruses in surface and drinking water samples (Stokdyk et al., 2016; Symonds et al., 2016; Staley et al., 2012; Verbyla et al., 2016; Ahmed et al., 2018). In this study, we determined PLOD values of SARS-CoV-2 RNA by seeding known concentrations of SARS-CoV-2 in wastewater samples using five different RT-qPCR and one RT-dPCR assays. To the best of our knowledge, this is the first study to assess the SARS-CoV-2 PLOD for wastewater and provides insights on the analytical limitations for trace detection of SARS-CoV-2 in wastewater.
For PLOD determination, we used 36 different wastewater samples collected from 36 WWTPs representing varying wastewater characteristics. For seeding values 2.32 × 105 and 2.32 × 104 GC/50 mL, all 18 wastewater samples and PCR replicates were positive by each assay RT-qPCR and RT-dPCR assays suggesting at these seeding levels, detection of SARS-CoV-2 in wastewater may be quite straightforward using the combination of adsorption extraction and the Power Water RNeasy Kit. However, with seeding values 2.32 × 103 and 2.32 × 102 GC/50 mL, detection rates decreased with the lowest detection rates observed at the seeding level of 2.32 × 102 GC. At these two seeding levels, consistent amplification was not observed by all RT-qPCR and RT-dPCR assays. Interestingly, the US CDC N1 assay outperformed other assays regarding detection sensitivity. The superior performance of the US CDC N1 assays compared to other assays has been reported in research literature (Ahmed et al., 2022; Chavarria-Miró et al., 2021; Feng et al., 2021; Pecson et al., 2021; Pérez-Cataluña et al., 2021). Our findings also corroborate with a recent study that suggested US CDC N1 is suitable for screening SARS-CoV-2 in wastewater with low COVID-19 prevalence (Zhang et al., 2022). The performance of US CDC N1 RT-qPCR and RT-dPCR were similar, however, RT-dPCR yielded more positive replicates than RT-qPCR. The increased sensitivity of RT-dPCR for the detection of SARS-CoV-2 RNA in wastewater compared to RT-qPCR has also been reported (Ahmed et al., 2022; Ciesielski et al., 2021; Graham et al., 2020). These findings clearly suggest that sub-sampling error is occurring when the seeding level of SARS-CoV-2 RNA is <2.32 × 103 GC/50 mL wastewater (Ahmed et al., 2021b), which could introduce false negative errors in RNA detection (Taylor et al., 2019). Furthermore, factors such as stochastic amplification, measurement uncertainty, variability in virus concentration and RNA extraction methods, inefficient RT-qPCR, and stochastic levels of inhibitors could affect the PLOD and the reproducibility of results.
If the objective of the SARS-CoV-2 wastewater surveillance is early detection of COVID-19 cases in a community, then the factors mentioned above, and associated variabilities should be carefully considered by analytical laboratories and public health units. Every amplification, whether it is reproducible between replicates or not, or even with a Cq value >40 should be considered as a potential positive (given appropriate negative results in all negative controls) and reported to the relevant agencies. For a virus such as SARS-CoV-2, which is highly contagious, it may be better to be overly cautious to effectively manage outbreaks. The results of this study also highlight the importance of modelling detection limits in PCR-based methods as probabilities rather than fixed limits. This approach has been described previously to determine the ALOD (Verbyla et al., 2016; Forootan et al., 2017), but we have now applied it to determine the PLOD. Considering the loss of SARS-CoV-2 RNA throughout the sample concentration and analysis process and the observation that the survival of the target during the PCR process is frequently less than 100%, using a modelling approach can help determine a laboratory's probability of detecting the target when the concentrations are very low. For example, in this present study, we found that SARS-CoV-2 is detected using the US CDC N1 RT-qPCR assay with a probability of 50% when the concentration is 915 GC/50 mL of wastewater. Therefore, if the concentration is 915 GC/50 mL, then processing 50 mL samples in triplicate would increase the probability of detection in at least one of the field triplicates to 87.5% (1-0.53=0.875). From a public health perspective, it is important to be able to detect the virus at concentrations below the 95% PLOD; however, laboratories may not be able to simply increase the volume of sample concentrated (e.g., due to filter clogging or increasing likelihood of inhibition).
Thus, another way to improve the positive predictive value of wastewater surveillance would be to analyze field replicates, which would increase the probability of amplification in at least one replicate. Developing a probability of detection model would allow a wastewater surveillance team to weigh the costs and benefits of adding field replicates to the standard operating procedure. Characterizing the sources of variation in SARS-CoV-2 RNA measurements in wastewater and fully reporting the associated uncertainty is also an important component of effective wastewater surveillance for public health applications (McClary-Gutierrez et al., 2021a; McClary-Gutierrez et al., 2021b). The acceptable variation in PCR-based quantification systems has been formalized as the limit of quantification (ALOQ); however, allowable variation is debated with proposed CV threshold values from 25 to 35% (Klymus et al., 2020; Kralik and Ricchi, 2017; Forootan et al., 2017). For wastewater surveillance of SARS-CoV-2 RNA the intrinsic and workflow-derived variability remains largely uncharacterized due to limited method replication and the relationship between an ALOQ derived via control materials and idealized matrices and actual wastewater samples remains uncertain (McClary-Gutierrez et al., 2021a; (Medema et al., 2020b).
In the current study, we have characterized the variation through the entire sampling workflow by seeding SARS-CoV-2 RNA into wastewater from different WWTPs with replication of both filters and RT-qPCR/dPCR reactions. For all assays and measurement platforms, SARS-CoV-2 RNA was quantifiable only when seeded levels were > 2.32 × 104 GC/50 mL wastewater. The US CDC N2 RT-qPCR demonstrated the greatest variation with CVs of 89 and 147% at 2.32 × 105 GC and 2.32 × 104 GC/50 mL wastewater, respectively. The remaining RT-qPCR assays demonstrated CVs ranging from 40 to 60% across both quantifiable seeding levels, which was comparable with that demonstrated by the US CDC N1 RT-dPCR. The CVs of the US CDC N1 RT-dPCR at the two seeding levels in the current study (54%, 52%) were greater than those reported for filter replicates during wastewater surveillance in Wisconsin, USA, (24% to 29%), but it is unclear whether the results are comparable since increased variation would be expected at lower SARS-CoV-2 RNA concentrations (Feng et al., 2021). Furthermore, differences in wastewater characteristics and analysis of exogenous and endogenous SARS-CoV-2 introduce heterogeneity between studies.
During an inter-laboratory comparison of 36 sampling methods, recovery-corrected SARS-CoV-2 RNA 10th and 90th percentile measurements spanned 2.3 log10 GC across all methods that yielded quantifiable results ((Pecson et al., 2021). A smaller methods comparison in Canada found that across laboratories and methods, seeded SARS-CoV-2 and human coronavirus strain 229E concentrations were within 1 log10 GC of one another (Chik et al., 2021). A wastewater surveillance interlaboratory study conducted at three WWTPs in Utah reported 10th percentile to 90th percentile measurements spanning roughly 0.6 log10 GC (Weidhaas et al., 2021). In the current study, we observed 10th to 90th percentiles spanning 0.47 to 0.99 log10 gene copies at the 2.32 × 105 GC/50 mL seeding level and 0.42 to 2.33 log10 gene copies at the 2.32 × 105 GC/50 mL seeding level. While the variation of SARS-CoV-2 RNA measurements in the current study seems to be in reasonable agreement with those previously reported, direct comparisons are precluded by uncertainty regarding the similarity of the endogenous and seeded SARS-CoV-2 RNA concentration in the wastewater used in the studies. Nonetheless, our robust study design, which included replication of both filters and RT-qPCR/RT-dPCR reactions and wastewater samples from multiple WWTPs, indicates that after accounting for process inefficiencies the variation in measured SARS-CoV-2 RNA may exceed standards typically considered acceptable for limits of quantification.
Since we seeded known concentrations of SARS-CoV-2 in wastewater samples, we were able to calculate the full process recovery efficiency (loss of SARS-CoV-2 through concentration and extraction methods used in this study) using different assays and platforms. We calculated recovery efficiency for 2.32 × 105 and 2.32 × 104 seeding levels because all wastewater samples and associated RT-qPCR replicates were quantifiable using all RT-qPCR assays. The mean recovery efficiencies for all assays were greater for 2.32 × 105 seeding levels than 2.32 × 104, suggesting the concentrations of SARS-CoV-2 present in wastewater influence method recovery. The greater the concentration, the greater the recovery and downstream detection rates. Similar results have been reported in a recent study where greater recovery was observed for bacteriophage phi 6 surrogate with the highest seed compared to the lowest seed levels (Sangsanont et al., 2022).
This is not unexpected considering we used wastewater samples from different WWTPs with variable suspended solids (TSS = 670 to 825 mg/L), while most of the recovery assessment studies used a single or a limited number of bulk wastewater samples (Ahmed et al., 2020d). Feng et al. (2021) observed a significant difference in recovery of BCoV between the WWTPs with some WWTPs having more consistent recovery rates (0.21% to 3.0%) than others (0.89% to 28.0%).
However, relatively consistent recoveries were obtained for SARS-CoV-2 using the US CDC N1 (both RT-qPCR and RT-dPCR), CCDC N and CCDC ORF1 assays. The variations in recovery efficiencies were greater for US CDC N2 and E_Sarbeco assays suggesting these assays alone may not be sensitive enough to detect trace concentrations of SARS-CoV-2 in wastewater and should be used in combination with other assays. This is most likely due to the lower ALOD of these two assays compared to the others. However, other factors such as wastewater characteristics, RT-qPCR efficiency, and Y-intercepts and standard curve materials also introduce variability between assays. Based on the recovery data obtained in this study, we recommend recovery assessment via the most consistent assay if possible, using a dPCR platform. The recovery efficiency presented in this study should be interpreted with care because measuring the actual concentrations of SARS-CoV-2 in seeding stock is not straightforward (Kantor et al., 2021). Importantly, the findings of the current study are based on seeding wastewater with gamma-irradiated SARS-CoV-2. The behavior of an exogenous control, such as the one used in the current study, compared to an endogenous SARS-CoV-2 shed into wastewater via infected individuals remains uncharacterized.
5. Conclusions
-
•
Of the six assays evaluated, the US CDC N1 RT-dPCR, followed by its RT-qPCR assay, was most sensitive regardless of the statistical model or seeding concentrations used to determine the ALOD and PLOD associated with the AE concentration method.
-
•
The US CDC N2 and E_Sarbeco assays were the least sensitive, especially with decreasing seeding concentrations, when evaluated alone (ALOD) and as a part of the AE concentration process (PLOD).
-
•
Trends in SARS-CoV-2 RNA recovery efficiency mirrored the analytical sensitivities with recovery efficiencies being less variable for US CDC N1 RT-dPCR and RT-qPCR and most variable for US CDC N2 RT-qPCR and E_Sarbeco RT-qPCR.
-
•
The greater the SARS-CoV-2 RNA concentration in a wastewater sample, the greater the recovery and downstream detection probability. When SARS-CoV-2 RNA seeding levels were < 2.32 × 103 GC/50 mL, inconsistent amplification was observed, and detection rates decreased for all assays.
-
•
Thus, when SARS-CoV-2 RNA concentrations are expected to be low in wastewater, it may be necessary to improve the positive predictive value of wastewater surveillance by analyzing additional field and RT-PCR replicates.
-
•
Comparing the behavior of endogenous SARS-CoV-2 RNA compared to various exogeneous controls, such as the seeded SARS-CoV-2 in the current study, remains a critical research need for wastewater surveillance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank CSIRO Land and Water for strategic funding to complete this research project. We also thank University of Queensland and Urban Utilities for providing untreated wastewater samples.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2022.118132.
Appendix. Supplementary materials
References
- Ahmed W., Hamilton K.A., Lobos A., Hughes B., Staley C., Sadowsky M.J., Harwood V.J. Quantitative microbial risk assessment of microbial source tracking markers in recreational water contaminated with fresh untreated and secondary treated sewage. Environ. Int. 2018;117:243–249. doi: 10.1016/j.envint.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., Brien J.W.O., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Bertsch P.M., Bibby K., Choi P.M., Farkas K., Gyawali P., Hamilton K.A., Haramoto E., Kitajima M. Surveillance of SARS-CoV-2 RNA in wastewater: Methods optimisation and quality control are crucial for generating reliable public health information. Curr. Opin. Environ. Sci. Health. 2020;17:82–93. doi: 10.1016/j.coesh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret j., Gyawali P., Hamilton K.A., Hosegood I., Hugenholtz P., Jiang G., Kitajima M., Sichani H.T., Shi J., Shimko K.M., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Zaugg J., Mueller J.F. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Travel Med. 2020;27(5):taaa116. doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Simpson S.L., Bertsch P.M., Ehret J., Hosegood I., Metcalfe S., Smith W.J.M., Thomas K.V., Tynan J., Mueller J.F. Wastewater surveillance demonstrates high predictive value for COVID-19 infection on board repatriation flights to Australia. Environ. Int. 2021 doi: 10.1016/j.envint.2021.106938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Simpson S.L., Bertsch P.M., Bibby K., Bivins A., Blackall L.L., Bofill-Mas S., Bosch A., Brandão J., Choi P.M., Ciesielski M., Donner E., D'Souza N., Farnleitner A.H., Gerrity D., Gonzalez R., Griffith J.F., Gyawali P., Haas C.N., Hamilton K.A., Hapuarachchi C., Harwood V.J., Haque R., Jackson G., Khan S.J., Khan W., Kitajima M., Korajkic A., La Rosa G., Layton B.A., Lipp E., McLellan S., McMinn B., Medema G., Metcalfe S., Meijer W.G., Mueller J.F., Murphy H., Naughton C.C., Noble R.T., Sudhi Payyappat S., Petterson S., Pitkänen T., Rajal V.B., Reyneke B., Roman F.A., Jr., Rose J., Rusinol M., Sadowsky M., Sala-Comorera L., Steoh Y.X., Sherchan S., Sirikanchana K., Smith W., Steele J., Sabburg R., Symonds E.M., Thai P., Thomas K.V., Tynan J., Toze S., Thompson J., Whiteley A.S., Wong J.C.C., Sano D., Wuertz S., Xagoraraki I., Zhang Q., Zimmer-Faust A.G., Shanks O.C. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2021;805 doi: 10.1016/j.scitotenv.2021.149877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, W., Bivins, A., Smith, W., Metcalfe, S., Jackson, G., Phil C, Morrison M, Field D, Gyawali P, Bivins A, Kyle B, Simpson S (2022) Comparison of RT-qPCR and RT-dPCR platforms for the trace detection of SARS-CoV-2 RNA in wastewater, ACS EST Water. doi: https://doi.org/10.1021/acsestwater.1c00387. [DOI] [PMC free article] [PubMed]
- Bertrand I., Challant J., Jeulin H., Hartard C., Mathieu L., Lopez S., Obépine S.I.G., Schvoerer E., Courtois S., Gantzer C. Epidemiological surveillance of SARS-CoV-2 by genome quantification in wastewater applied to a city in the northeast of France: Comparison of ultrafiltration-and protein precipitation-based methods. Int. J. Hyg. Environ. Health. 2021;233 doi: 10.1016/j.ijheh.2021.113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselsen D.G., Wagner A.M., Loganbill J.K. Detection of rodent coronaviruses by use of fluorogenic reverse transcriptase-polymerase chain reaction analysis. Comp. Med. 2002;52(2):111–116. [PubMed] [Google Scholar]
- Bivins A., Kaya D., Bibby K., Simpson S., Bustin S., Shanks O.C., Ahmed W. Variability in RT-qPCR assay parameters indicates unreliable SARS-CoV-2 RNA quantification for wastewater surveillance. Water Res. 2021;203:117516. doi: 10.1016/j.watres.2021.117516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A, North D, Wu Z, Shaffer M, Ahmed W, Bibby K. Within-day variability of SARS-CoV-2 RNA in municipal wastewater influent during periods of varying COVID-19 prevalence and positivity. medRxiv. 2021 21253652. doi: https://doi.org/10.1101/2021.03.16.21253652. [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Martínez-Velázquez A., Vázquez-Portero M., Guix S., Paraira M., Galofré B., Sánchez G., Pintó R.M., Bosch A. Time evolution of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in wastewater during the first pandemic wave of COVID-19 in the metropolitan area of Barcelona. Spain. Appl. Environ. Microbiol. 2021;87(7):e02750. doi: 10.1128/AEM.02750-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chik A.H.S., Glier M.B., Servos M., Mangat C.S., Pang X.-L., Qiu Y., D;Aoust P.M., Burnet J-B., Delatolla R., Dorner S., Geng Q., Giesy J.P., McKay R.M., Mulvey M.R., Prystajecky N., Srikanthan N., Xie Y., Conant B., Hrudey S.E. Comparison of approaches to quantify SARS-CoV-2 in wastewater using RT-qPCR: Results and implications from a collaborative inter-laboratory study in Canada. J. Environ. Sci. 2021;107:218–229. doi: 10.1016/j.jes.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski M., Blackwood D., Clerkin T., Gonzalez R., Thompson H., Larson A., Noble R.T. Assessing sensitivity and reproducibility of RT-ddPCR and RT-qPCR for the quantification of SARS-CoV-2 in wastewater. J. Virol. Methods. 2021;297 doi: 10.1016/j.jviromet.2021.114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020. 2019-Novel Coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes.
- China CDC . China CDC; 2020. Specific Primers and Probes for Detection 2019 Novel Coronavirus. [WWW Document] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopman M.P.G. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro. Surveil. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn O.J. Multiple comparisons using rank sums. Technometrics. 1964;6(3):241–252. [Google Scholar]
- Forootan F., Sjöback R., Björkman J., Sjögreen B., Linz L., Kubista M. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR) Biomol. Detect. Quantif. 2017;12:1–6. doi: 10.1016/j.bdq.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Shuchen., Roguet A., McClary-Gutierrez Jill S., Kloczko Nathan., Meiman Jonathan G., McLellan Sandra L. Evaluation of sampling, analysis and normalization methods for SARS-CoV-2 concentrations in wastewater to assess COVID-19 burdens in Wisconsin communities. ACS ES&T Water. 2021;1(8):1955–1965. [Google Scholar]
- Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Res X. 2021;10 doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua B.V., Brazell L.R., Hinton K., Lontai J., Stark N., Young I. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., Thompson H., Keeling D., Mitchell J. Gonzalez COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.E., Loeb S.K., Wolfe M.K., Catoe D., Sinnott-Armstrong N., Kim S., Yamahara K.M., Sassoubre L.M., Mendoza Grijalva L.M., Roldan-Hernandez L., Langenfeld K. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large urban sewershed. Environ. Sci. Technol. 2020;55(1):488–498. doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Haas C.N., Rose J.B., Gerba C.P. John Wiley & Sons; 2014. Quantitative microbial risk assessment. [Google Scholar]
- Iglói Z., Leven M., Abdel-Karem Abou-Nouar Z., Weller B., Matheeussen V., Coppens J., Koopmans M., Molenkamp R. Comparison of commercial realtime reverse transcription PCR assays for the detection of SARS-CoV-2. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel M.I.A., Stark N., Nicolosi B., Lontai J., Lambirtth K., Schlueter J., Gibas C., Munir M. Performance evaluation of virus concentration methods for implementing SARS-CoV-2 wastewater-based epidemiology emphasizing quick data turnaround. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor R.S., Nelson K.L., Greenwald H.D., Kennedy L.C. Challenges in Measuring the Recovery of SARS-CoV-2 from Wastewater. Environ. Sci. Technol. 2021;55(6):3514–3519. doi: 10.1021/acs.est.0c08210. [DOI] [PubMed] [Google Scholar]
- Klymus K.E., Merkes C.M., Allison M.J., Goldberg C.S., Helbing C.C., Hunter M.E., Jackson C.A., Lance R.F., Mangan A.M., Monroe E.M., Piaggio A.J., Stokdyk J.P., Wilson C.C., Richer C.A. Reporting the limits of detection and quantification for environmental DNA assays. Environ. DNA. 2020;2(3):271–282. [Google Scholar]
- Kralik P., Ricchi M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017;8:108. doi: 10.3389/fmicb.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal W.H., Wallis W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952;47(260):583–621. [Google Scholar]
- Li D., Zhang J., Li J. Primer design for quantitative real-time PCR for the emerging coonavirus SARS-CoV-2. Theranostics. 2020;10(16):7150–7162. doi: 10.7150/thno.47649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann H.B., Whitney D.R. On a test of whether one of two random variables is stochastically larger than the other. Annals. Math. Stat. 1947;18(1):50–60. [Google Scholar]
- McClary-Gutierrez J.S., Mattioli M.C., Marcenac P., Silverman A.I., Boehm A.B., Bibby K., Balliet M., de los Reyes F.L., Gerrity D., Griffith J.F., Holden P.A., Katehis D., Kester G., LaCross N., Lipp E.K., Meiman J., Noble R.T., Brossard D., McLellan S.L. SARS-CoV-2 Wastewater Surveillance for Public Health Action. Emerg. Infect. Dis. 2021;27(9):1–8. doi: 10.3201/eid2709.210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary-Gutierrez J.S., Aanderud Z.T., Al-faliti M., Duvallet C., Gonzalez R., Guzman J., Holm R.H., Jahne M.A., Kantor R.S., Katsivelis P., Kuhn K.G., Langan L.M., Mansfeldt C., McLellan S.L., Mendoza Grijalva L.M., Murnane K.S., Naughton C.C., Packman A.I., Paraskevopoulos S., Radniecki T.S., Roman F.A., Jr., Shrestra A., Stadler L.B., Steele J.A., Swalla B.M., Vikesland P., Wartell B., Wilusz C.J., Wong J.C.C., Boehm A.B., Halden R.U., Bibby K., Vela J.D. Standardizing data reporting in the research community to enhance the utility of open data for SARS-CoV-2 wastewater surveillance. Environ. Sci.: Water Res. Technol. 2021;7:1545–1551. doi: 10.1039/d1ew00235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Medema G., Been F., Heijnen L., Petterson S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: opportunities and challenges. Curr. Opin. Environ. Sci. Health. 2020;17:49–71. doi: 10.1016/j.coesh.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8(5) doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenchhoff M., Mairhofer H., Nitschko H., Grzimek-Koschewa N., Hoffmann D., Berger A., Rabenau H., Widera M., Ackermann N., Konrad R., Zange S., Graf A., Krebs S., Blum H., Sing A., Liebl B., Wölfel R., Ciesek S., Drosten C., Protzer U., Boehm S., Keppler O.T. Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020. Euro. Surveill. 2020;25(24) doi: 10.2807/1560-7917.ES.2020.25.24.2001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A., Gómez L., Sanseverino I., Niegowska M., Roka E., Pedraccini R., Vargha M., Lettieri T. SARS-CoV-2 detection in wastewater using multiplex quantitative PCR. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.148890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecson B.M., Darby E., Haas C.N., Amha Y., Bartolo M., Danielson R., Dearborn Y., Di Giovanni G., Ferguson C., Fevig S., Gaddis E., Gray D., Lukasik G., Mull B., Olivas L., Olivieri A., Qu Y. Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: Findings from an interlaboratory methods evaluation in the U.S. Environ. Sci. Water Res. Technol. 2021;7:50. doi: 10.1039/d0ew00946f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz. 2020;2020 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsanont J., Rattanakul S., Kongprajug A., Chyerochana N., Sresung M., Sriporatana N., Wanlapakorn N., Poovorawan Y., Mongkolsuk M., Sirikanchana K. SARS-CoV-2 RNA surveillance in large to small centralized wastewater treatment plants preceding the third COVID-19 resurgence in Bangkok, Thailand. Sci. Total Environ. 2022;809 doi: 10.1016/j.scitotenv.2021.151169. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapula S.A., Whittall J.J., Pandopulos A.J., Gerber C., Venter H. An optimized and robust PEG precipitation method for detection of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;785 doi: 10.1016/j.scitotenv.2021.147270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. NY Cold Spring Harbor Laboratory press; 1989. Molecular cloning: a laboratory manual COLD Spring Harbor. [Google Scholar]
- Staley C., Gordon K.V., Schoen M.E., Harwood V.J. Performance of two quantitative PCR methods for microbial source tracking of human sewage and implications for microbial risk assessment in recreational waters. Appl. Environ. Microbiol. 2012;78(20):7317–7326. doi: 10.1128/AEM.01430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokdyk J.P., Firnstahl A.D., Spencer S.K., Burch T.R., Borchardt M.A. Determining the 95% limit of detection for waterborne pathogen analyses from primary concentration to qPCR. Water Res. 2016;96:105–113. doi: 10.1016/j.watres.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Symonds E.M., Sinigalliano C., Gidley M., Ahmed W., McQuaig-Ulrich S.M., Breitbart M. Faecal pollution along the southeastern coast of Florida and insight into the use of pepper mild mottle virus as an indicator. J Appl Microbiol. 2016;121(5):1469–1481. doi: 10.1111/jam.13252. [DOI] [PubMed] [Google Scholar]
- Taylor S.C., Nadeau K., Abbasi M., Lachance C., Nguyen M., Fenrich J. The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends. Biotechnol. 2019;37(7):P761–P774. doi: 10.1016/j.tibtech.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Verbyla M.E., Symonds E.M., Kafle R.C., Cairns M.R., Iriarte M., Mercado Guzmán A., Coronado O., Breitbart M., Ledo C., Mihelcic J.R. Managing microbial risks from indirect wastewater reuse for irrigation in urbanizing watersheds. Environ. Sci. & Technol. 2016;50(13):6803–6813. doi: 10.1021/acs.est.5b05398. [DOI] [PubMed] [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich C.C., Petrone M.E., Casanovas-Massana A., Catherine Muenker M., Moore A.J., Klein J., Lu P., Lu-Culligan A., Jiang X., Kim D.J., Kudo E., Mao T., Moriyama M., Oh J.E., Park A., Silva J., Song E., Taura M., Tokuyama M., Venkataraman A., Weizman O-E., Wong P., Yang Y., Cheemarla N.R., White E.B., Lapidus S., Earnest R., Geng B., Vijaykumar P., Odio C., Fournier J., Bermejo S., Farhadian S., Dela Cruz C.S., Iwasaki A., Ko A.I., Landry M.L., Foxman E.F., Grubaugh N.D. Analytical sensitivity and efficiency comparisons of SARSCoV-2 RT-qPCR primer-probe sets. Nat. Microbiol. 2020;5:1299–1305. doi: 10.1038/s41564-020-0761-6. Takhashi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K., Vander Laan J., LaCross N. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T. Detection of SARS-CoV-2 in raw and treated wastewater in Germany–suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li X., Shi J., Sivakumar M., Luby S., O'Brien J., Jiang G. Analytical performance comparison of four SARS-CoV-2 RT-qPCR primer-probe sets for wastewater samples. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.