ABSTRACT
Here, we present the genome sequence of Pseudomonas sp. strain MM211, which was isolated from garden soil. The complete circular genome consists of a 5,281,862-bp chromosome, with a GC content of 61.5%.
ANNOUNCEMENT
The Gram-negative rod-shaped bacterial genus Pseudomonas lives in diverse habitats (1–3) and is well characterized (4). Currently, 258 validated species are published (5), including human, animal, and plant pathogens (6). In addition, some species interact with plants and can promote plant growth and influence resistance against plant diseases (7, 8). Some Pseudomonas species are able to grow in association with other organisms in highly polluted environments and degrade various substances (9). Because of these many different properties, the organisms of this genus have great potential to be some of the most influential bacteria in research and development (10).
We isolated Pseudomonas sp. strain MM211 from a soil sample obtained in Langenfeld, North Rhine-Westphalia, Germany (51°06′31.1″N, 6°56′40.2″E), from dark humus at a depth of 10 cm. The sample was diluted with 0.9 NaCl, filtered (431015; Macherey-Nagel, Düren, Germany), plated (1.5% agar, 1% peptone from soy, 0.3% NaCl, 0.1% sucrose, 0.1% cellulose, 0.1% xylan, 0.1% chitin, and 0.05% Tris-HCl), and incubated at 28°C until colonies were observed. DNA was isolated from a single colony with a NucleoSpin microbial DNA minikit (Macherey-Nagel) with RNA digestion. DNA was barcoded with the native barcoding kit (Oxford Nanopore Technologies, Oxford, UK) and sequenced on a GridION system with a R9.4.1 flow cell (Oxford Nanopore Technologies). Sequences were called using the super accuracy base-calling model in MinKNOW (v1.4.3; Oxford Nanopore Technologies). Adapters were trimmed using Porechop (v0.2.4) (11). The genome was assembled with Canu (v2.1.1) (12) set to a genome size of 8 Mb and was polished with Racon (v1.4.20) (13) in combination with BWA (v0.7.17) (14) and Medaka (v1.4.3; Oxford Nanopore Technologies). Completeness was examined with Benchmarking Universal Single-Copy Orthologs (BUSCO) (v5.1.2) (15) set to genome, with the lineage set to pseudomonadales_odb10. The final single-contig assembly was circularized and oriented with berokka (v0.2.3) (https://github.com/tseemann/berokka) and uploaded to NCBI. Default settings were used for all tools unless stated otherwise. All relevant assembly statistics, including BUSCO results, are listed in Table 1.
TABLE 1.
Sequencing and assembly statistics for Pseudomonas sp. strain MM211
| Parametera | Finding |
|---|---|
| Raw read sequencing | |
| No. of reads | 168,644 |
| N50 (bp) | 13,834 |
| Total length (bp) | 1,579,810,087 |
| Assembly | |
| Coverage (×) | 286 |
| GC content (%) | 61.5 |
| Length (bp) | 5,281,862 |
| Annotation | |
| Total no. of genes | 4,853 |
| No. of coding genes | 4,645 |
| BUSCO results (%) | |
| Complete | 98.8 |
| Single copy | 98.3 |
| Duplicated | 0.5 |
| Fragmented | 0.4 |
| Missing | 0.8 |
Coverage was based on mapping of the trimmed reads to the assembly with SAMtools (v1.12) (25). Annotation was based on NCBI PGAP (v5.3) annotation of GCA_020386635.1 on 15 November 2021 (26). BUSCO values represent complete, single copy, duplicated, fragmented, and missing single-copy orthologue genes.
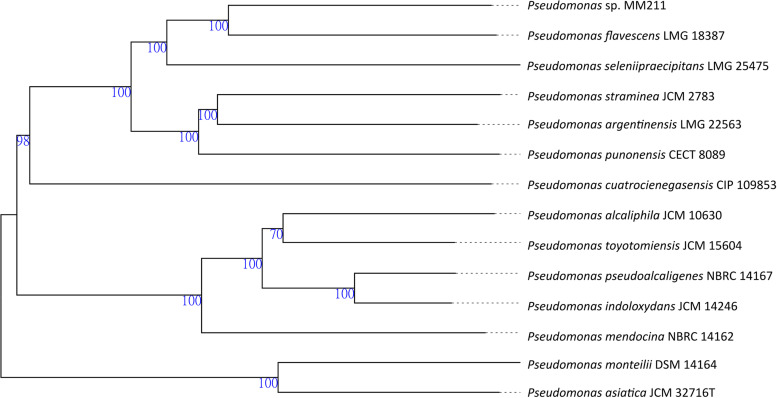
The genome sequence of Pseudomonas sp. strain MM211 presented here has Pseudomonas flavescens LMG 18387 (GenBank accession number GCA_900100535.1) (16) and Pseudomonas seleniipraecipitans LMG 25475 (GenBank accession number GCA_900102335.1) (17) as its closest relatives (Fig. 1). The digital DNA-DNA hybridization (dDDH) shows values of 41.8% with P. flavescens LMG 18387 and 36.4% with P. seleniipraecipitans LMG 25475, both well below the 70% cutoff value for dDDH (18). A carotenoid biosynthetic gene cluster was identified using the antiSMASH server (19, 20). A KEGG analysis showed that Pseudomonas sp. strain MM211 is likely able to grow a flagellum (21). Furthermore, MM211 may be auxotrophic for biotin. P. flavescens, the most closely related species, is also capable of producing a flagellum and pigments (16).
FIG 1.
Genome BLAST Distance Phylogeny (GBDP) tree. The phylogenetic tree was created with the Type (Strain) Genome Server (TYGS) (22). The tree was inferred with FastME (v2.1.6.1) (23) from GBDP distances calculated from genome sequences. The branch lengths are scaled in terms of GBDP distance formula d5. The numbers at the branches are GBDP pseudo-bootstrap support values of >60% from 100 replications, with an average branch support of 100.0%. The tree was rooted at the midpoint (24).
Data availability.
The MM211 assembly, RefSeq annotation, and reads are available at NCBI GenBank under accession numbers GCA_020386635.1, CP081942.1, and SRR15526917, respectively.
ACKNOWLEDGMENTS
We thank Kristin Rojek for assistance with isolation of bacteria, Andrea Bräutigam for proofreading, and Jörn Kalinowski for providing the flow cell and sequencing platform.
Sequences were generated, assembled, and analyzed as part of a molecular biology of microorganisms (MM) course at Bielefeld University in 2021.
This work was supported by the BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI) (031A532B, 031A533A, 031A533B, 031A534A, 031A535A, 031A537A, 031A537B, 031A537C, 031A537D, 031A538A). Furthermore we acknowledge support for the publication costs by the Open Access Publication Fund of Bielefeld University and the Deutsche Forschungsgemeinschaft (DFG).
Contributor Information
Bart Verwaaijen, Email: bverwaai@cebitec.uni-bielefeld.de.
Catherine Putonti, Loyola University Chicago.
REFERENCES
- 1.Lopes LD, Davis EW, II, Pereira E, Silva MC, Weisberg AJ, Bresciani L, Chang JH, Loper JE, Andreote FD. 2018. Tropical soils are a reservoir for fluorescent Pseudomonas spp. Environ Microbiol 20:62–74. doi: 10.1111/1462-2920.13957. [DOI] [PubMed] [Google Scholar]
- 2.Amoozegar MA, Shahinpei A, Sepahy AA, Makhdoumi-Kakhki A, Seyedmahdi SS, Schumann P, Ventosa A. 2014. Pseudomonas salegens sp. nov., a halophilic member of the genus Pseudomonas isolated from a wetland. Int J Syst Evol Microbiol 64:3565–3570. doi: 10.1099/ijs.0.062935-0. [DOI] [PubMed] [Google Scholar]
- 3.Vyas P, Rahi P, Gulati A. 2009. Stress tolerance and genetic variability of phosphate-solubilizing fluorescent Pseudomonas from the cold deserts of the trans-Himalayas. Microb Ecol 58:425–434. doi: 10.1007/s00248-009-9511-2. [DOI] [PubMed] [Google Scholar]
- 4.Lalucat J, Mulet M, Gomila M, García-Valdés E. 2020. Genomics in bacterial taxonomy: impact on the genus Pseudomonas. Genes 11:139. doi: 10.3390/genes11020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LPSN. 2021. Genus Pseudomonas. https://lpsn.dsmz.de/genus/pseudomonas. Accessed 22 July 2021.
- 6.Hesse C, Schulz F, Bull CT, Shaffer BT, Yan Q, Shapiro N, Hassan KA, Varghese N, Elbourne LDH, Paulsen IT, Kyrpides N, Woyke T, Loper JE. 2018. Genome-based evolutionary history of Pseudomonas spp. Environ Microbiol 20:2142–2159. doi: 10.1111/1462-2920.14130. [DOI] [PubMed] [Google Scholar]
- 7.Scales BS, Dickson RP, LiPuma JJ, Huffnagle GB. 2014. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin Microbiol Rev 27:927–948. doi: 10.1128/CMR.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke KA, Becker MG, Girard IJ, Millar JL, Dilantha Fernando WG, Belmonte MF, de Kievit TR. 2017. The biocontrol agent Pseudomonas chlororaphis PA23 primes Brassica napus defenses through distinct gene networks. BMC Genomics 18:467. doi: 10.1186/s12864-017-3848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts C, Edwards S, Vague M, León-Zayas R, Scheffer H, Chan G, Swartz NA, Mellies JL. 2020. Environmental consortium containing Pseudomonas and Bacillus species synergistically degrades polyethylene terephthalate plastic. mSphere 5:6. doi: 10.1128/mSphere.01151-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiers AJ, Buckling A, Rainey PB. 2000. The causes of Pseudomonas diversity. Microbiology (Reading, England) 146:2345–2350. doi: 10.1099/00221287-146-10-2345. [DOI] [PubMed] [Google Scholar]
- 11.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom 3:e000132. doi: 10.1099/mgen.0.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaser R, Sović I, Nagarajan N, Šikić M. 2017. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res 27:737–746. doi: 10.1101/gr.214270.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 1303.3997v2. https://arxiv.org/abs/1303.3997.
- 15.Manni M, Berkeley MR, Seppey M, Simao FA, Zdobnov EM. 2021. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol 38:4647–4654. doi: 10.1093/molbev/msab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildebrand DC, Palleroni NJ, Hendson M, Toth J, Johnson JL. 1994. Pseudomonas flavescens sp. nov., isolated from walnut blight cankers. Int J Syst Bacteriol 44:410–415. doi: 10.1099/00207713-44-3-410. [DOI] [PubMed] [Google Scholar]
- 17.Hunter WJ. 2014. Pseudomonas seleniipraecipitans proteins potentially involved in selenite reduction. Curr Microbiol 69:69–74. doi: 10.1007/s00284-014-0555-2. [DOI] [PubMed] [Google Scholar]
- 18.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. 2013. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T. 2021. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49:W29–W35. doi: 10.1093/nar/gkab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedkova N, Tao L, Rouvière PE, Cheng Q. 2005. Diversity of carotenoid synthesis gene clusters from environmental Enterobacteriaceae strains. Appl Environ Microbiol 71:8141–8146. doi: 10.1128/AEM.71.12.8141-8146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. 2007. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res 35:W182–W185. doi: 10.1093/nar/gkm321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier-Kolthoff JP, Göker M. 2019. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun 10:2182. doi: 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefort V, Desper R, Gascuel O. 2015. FastME 2.0: a comprehensive, accurate, and fast distance-based phylogeny inference program. Mol Biol Evol 32:2798–2800. doi: 10.1093/molbev/msv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farris JS. 1972. Estimating phylogenetic trees from distance matrices. Am Nat 106:645–668. doi: 10.1086/282802. [DOI] [Google Scholar]
- 25.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, O’Neill KR, Haft DH, DiCuccio M, Chetvernin V, Badretdin A, Coulouris G, Chitsaz F, Derbyshire MK, Durkin AS, Gonzales NR, Gwadz M, Lanczycki CJ, Song JS, Thanki N, Wang J, Yamashita RA, Yang M, Zheng C, Marchler-Bauer A, Thibaud-Nissen F. 2021. RefSeq: expanding the Prokaryotic Genome Annotation Pipeline reach with protein family model curation. Nucleic Acids Res 49:D1020–D1028. doi: 10.1093/nar/gkaa1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The MM211 assembly, RefSeq annotation, and reads are available at NCBI GenBank under accession numbers GCA_020386635.1, CP081942.1, and SRR15526917, respectively.