Abstract
Background
The Mini Mental State Examination (MMSE) is a cognitive test that is commonly used as part of the evaluation for possible dementia.
Objectives
To determine the diagnostic accuracy of the Mini‐Mental State Examination (MMSE) at various cut points for dementia in people aged 65 years and over in community and primary care settings who had not undergone prior testing for dementia.
Search methods
We searched the specialised register of the Cochrane Dementia and Cognitive Improvement Group, MEDLINE (OvidSP), EMBASE (OvidSP), PsycINFO (OvidSP), LILACS (BIREME), ALOIS, BIOSIS previews (Thomson Reuters Web of Science), and Web of Science Core Collection, including the Science Citation Index and the Conference Proceedings Citation Index (Thomson Reuters Web of Science). We also searched specialised sources of diagnostic test accuracy studies and reviews: MEDION (Universities of Maastricht and Leuven, www.mediondatabase.nl), DARE (Database of Abstracts of Reviews of Effects, via the Cochrane Library), HTA Database (Health Technology Assessment Database, via the Cochrane Library), and ARIF (University of Birmingham, UK, www.arif.bham.ac.uk). We attempted to locate possibly relevant but unpublished data by contacting researchers in this field. We first performed the searches in November 2012 and then fully updated them in May 2014. We did not apply any language or date restrictions to the electronic searches, and we did not use any methodological filters as a method to restrict the search overall.
Selection criteria
We included studies that compared the 11‐item (maximum score 30) MMSE test (at any cut point) in people who had not undergone prior testing versus a commonly accepted clinical reference standard for all‐cause dementia and subtypes (Alzheimer disease dementia, Lewy body dementia, vascular dementia, frontotemporal dementia). Clinical diagnosis included all‐cause (unspecified) dementia, as defined by any version of the Diagnostic and Statistical Manual of Mental Disorders (DSM); International Classification of Diseases (ICD) and the Clinical Dementia Rating.
Data collection and analysis
At least three authors screened all citations.Two authors handled data extraction and quality assessment. We performed meta‐analysis using the hierarchical summary receiver‐operator curves (HSROC) method and the bivariate method.
Main results
We retrieved 24,310 citations after removal of duplicates. We reviewed the full text of 317 full‐text articles and finally included 70 records, referring to 48 studies, in our synthesis. We were able to perform meta‐analysis on 28 studies in the community setting (44 articles) and on 6 studies in primary care (8 articles), but we could not extract usable 2 x 2 data for the remaining 14 community studies, which we did not include in the meta‐analysis. All of the studies in the community were in asymptomatic people, whereas two of the six studies in primary care were conducted in people who had symptoms of possible dementia. We judged two studies to be at high risk of bias in the patient selection domain, three studies to be at high risk of bias in the index test domain and nine studies to be at high risk of bias regarding flow and timing. We assessed most studies as being applicable to the review question though we had concerns about selection of participants in six studies and target condition in one study.
The accuracy of the MMSE for diagnosing dementia was reported at 18 cut points in the community (MMSE score 10, 14‐30 inclusive) and 10 cut points in primary care (MMSE score 17‐26 inclusive). The total number of participants in studies included in the meta‐analyses ranged from 37 to 2727, median 314 (interquartile range (IQR) 160 to 647). In the community, the pooled accuracy at a cut point of 24 (15 studies) was sensitivity 0.85 (95% confidence interval (CI) 0.74 to 0.92), specificity 0.90 (95% CI 0.82 to 0.95); at a cut point of 25 (10 studies), sensitivity 0.87 (95% CI 0.78 to 0.93), specificity 0.82 (95% CI 0.65 to 0.92); and in seven studies that adjusted accuracy estimates for level of education, sensitivity 0.97 (95% CI 0.83 to 1.00), specificity 0.70 (95% CI 0.50 to 0.85). There was insufficient data to evaluate the accuracy of the MMSE for diagnosing dementia subtypes.We could not estimate summary diagnostic accuracy in primary care due to insufficient data.
Authors' conclusions
The MMSE contributes to a diagnosis of dementia in low prevalence settings, but should not be used in isolation to confirm or exclude disease. We recommend that future work evaluates the diagnostic accuracy of tests in the context of the diagnostic pathway experienced by the patient and that investigators report how undergoing the MMSE changes patient‐relevant outcomes.
Plain language summary
Mini‐Mental State Examination (MMSE) for the detection of dementia in people aged over 65
The term 'dementia' covers a group of brain problems that cause gradual deterioration of brain function, thinking skills, and ability to perform everyday tasks (e.g. washing and dressing). People with dementia may also develop problems with their mental health (mood and emotions) and behaviour that are difficult for other people to manage or deal with. The process that causes dementia in the brain is often degenerative (due to brain damage over time). Subtypes of dementia include Alzheimer's disease dementia, vascular dementia, dementia with Lewy bodies and frontotemporal dementia.
We aimed to assess the accuracy of the Mini‐Mental State Examination (MMSE), which is commonly used as part of the process when considering a diagnosis of dementia, according to the definition in the Diagnostic and Statistical Manual of Mental Disorders (DSM). The MMSE is a paper‐based test with a maximum score of 30, with lower scores indicating more severe cognitive problems. The cut point established for the MMSE defines 'normal' cognitive function and is usually set at 24, although theoretically it could fall anywhere from 1 to 30. We searched a wide range of resources and found 24,310 unique citations (hits). We reviewed the full text of 317 academic papers and finally included 70 articles, referring to 48 studies in our review. We included community studies (by which we mean people living in the community who have ) and primary care studies (by which we mean studies that had an office‐based first contact care with a non specialist clinician ‐ which would often be a GP).
Two of the studies had serious design weaknesses with regard to their methods for selecting participants, three with regard to the application of the test (MMSE), and nine with regard to the presentation of flow and timing. We were able to do a combined statistical analysis (meta‐analysis) on 28 studies in the community setting (44 articles) and 6 studies in primary care (8 articles), but we could not extract usable data for the remaining 14 community studies. Two of the six studies in primary care were conducted in people who had symptoms of possible dementia. We were able to calculate the summary diagnostic accuracy of the MMSE at three cut points in community‐based studies, but we didn't have enough data to do this in the primary care studies. A perfect test would have sensitivity (ability to identify anyone with dementia) of 1.0 (100%) and specificity (ability to identify people without dementia) of 1.0 (100%). For the MMSE, the summary accuracy at a cut point of 25 (10 studies) was sensitivity 0.87 and specificity 0.82. In seven studies that adjusted accuracy estimates for level of education, we found that the test had a sensitivity of 0.97 and specificity of 0.70. The summary accuracy at a cut point of 24 (15 studies) was sensitivity 0.85 and specificity 0.90. Based on these results, we would expect 85% of people with dementia to be correctly identified with the MMSE, while 15% would be wrongly classified as not having dementia; 90% of those tested would be correctly identified as not having dementia whilst 10% would be false positives and might be referred for further testing.
Our results support the use of the MMSE as part of the process for deciding whether or not someone has dementia, but the results of the test should be interpreted in broader context of the individual patient, such as their personality, behaviour and how they are managing at home and in daily life.
Summary of findings
Summary of findings'. 'Summary of findings table.
| What is the accuracy of the Mini Mental State Examination (MMSE) for diagnosing current dementia compared to clinical diagnosis of dementia? | ||||
|
Index test: Mini Mental State Examination (MMSE) administered to the patient. We restricted inclusion to the original 30 item MMSE but did not restrict by language. Reference test: clinical diagnosis of dementia made using any recognised classification system Studies: cross‐sectional studies but not case‐control studies Limitations: there were too few studies to perform meta‐analysis at each cut point. We could not perform bivariate meta‐analysis on studies in primary care as there were too few. Population: adults resident in the community Setting: community | ||||
| Test | Summary accuracy (95% CI) | No. of participants (studies); median (IQR) | Dementia prevalence median (IQR) | Quality, Implications and Comments |
| MMSE at cut point 24 indicating normal | Sensitivity 0.85 (0.74, 0.92) specificity 0.90 (0.82, 0.95) |
10969 (15); 435 (272 to 737) |
7.4% (5.5% to 20.1%) | Studies were generally at low risk of bias (none were at high or unclear risk in more than 1 domain). In a group of 1000 people where 7 have dementia, 105 test positive, 6 of whom have dementia, and 895 test negative, 1 of whom has dementia. A large number of people would need to be evaluated further to identify the people with dementia. 1 person with dementia is 'missed'. |
| MMSE at cut point 25 indicating normal | Sensitivity 0.87 (0.78, 0.93) specificity 0.82 (0.65, 0.92) |
5894 (10) 316 (246 to 713) |
8.4% (6.0% to 19.0%) | 1 study was high risk for patient selection and unclear risk in 2 others (Winblad 2010). 1 other study was high risk in patient flow (Eefsting 1997). Otherwise studies were at low risk of bias. In a group of 1000 people where 8 have dementia, 186 test positive, 7 of whom have dementia, and 814 test negative, 1 of whom has dementia. Impact is similar to use of the 24 cut point but more people require further evaluation. |
| MMSE at cut point adjusted for education | Sensitivity 0.97 (0.83, 1.00) specificity 0.70 (0.50, 0.85) |
8442 (7) 294 (120 to 947) |
13.8% (2.4% to 27.4%) | 2 studies were at high risk of bias for patient flow (Jacinto 2011; Lam 2008), otherwise studies were at low or unclear risk of bias. In a group of 1000 people where 14 have dementia, 309 test positive, 14 of whom have dementia, 691 test negative, none of whom have dementia. Many people need to be further evaluated to identify the people who have dementia but everybody with dementia is identified. |
CI: confidence interval; IQR: interquartile range.
Background
The protocol for this review was based on 'Neuropsychological tests for the diagnosis of Alzheimer's disease dementia and other dementias: a generic protocol for cross‐sectional and delayed‐verification studies' (Davis 2013a). This review forms part of a suite of reviews that address the accuracy of different neuropsychological tests for the cross‐sectional and delayed‐verification diagnosis of dementia in a range of populations, for example the the Montreal Cognitive Assessment for the diagnosis of Alzheimer's disease and other dementia disorders (Davis 2013b), the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of Alzheimer's disease dementia and other dementias within a general practice (primary care) setting (Quinn 2013); Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a community setting (Fage 2013) and Mini‐Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (Arevalo‐Rodriguez 2013).
This review addresses the use of the Mini‐Mental State Examination (MMSE) for the cross‐sectional (current) diagnosis of Alzheimer's dementia and other dementias when used in the community and primary care, which are populations with a relatively low prevalence of dementia (approximately 7%; Matthews 2013) compared to memory clinics (around 60%; Banerjee 2007) and secondary or inpatient care. We included studies that examine the accuracy of the MMSE in previously unevaluated people with or without symptoms (akin to screening) because we aimed to address the accuracy of the MMSE when applied to the clinically relevant question of patients and clinicians, 'Does this person have dementia now?'. A separate review evaluates the accuracy of the MMSE for delayed verification of dementia diagnosis at some future point (addressing the question, 'Is the current level of cognition sufficiently poor that this person has a pre‐dementia syndrome?').
Target condition being diagnosed
Dementia is a progressive syndrome of global cognitive impairment that affects 6.5% of the UK population aged over 65 years (Matthews 2013). There is a significant global disease burden (36 million patients worldwide) that is predicted to increase to over 115 million by 2050, particularly in developing regions (Ferri 2005; Wimo 2010). Dementia encompasses a group of neurodegenerative disorders that are characterised by a progressive loss of cognitive function and ability to perform activities of daily living that can be accompanied by neuropsychiatric symptoms and challenging behaviours of varying type and severity. Prior ability is also important: someone could have a decline in their cognition over time and meet criteria for a diagnosis of dementia while still scoring above average on a cognitive test. The underlying pathology is usually degenerative, and subtypes of dementia include Alzheimer's disease dementia, vascular dementia, dementia with Lewy bodies (pathological clusters of alpha‐synuclein protein; McKeith 2005), and frontotemporal dementia. There is considerable overlap in the clinical and pathological presentations; for example, Alzheimer's disease pathology may be present in people who have a clinical phenotype of vascular or Lewy body dementia, and vascular changes and Lewy bodies are common in the postmortem examination of brains of people with an Alzheimer's disease phenotype (CFAS 2001; Matthews 2009; Savva 2009). Some commentators have therefore advised against the use of neuropathological criteria as the gold standard for the diagnosis of dementia, including subtypes (Scheltens 2011).
The target condition in this review will be dementia or its subtypes (as defined by the reference standards described below), identified simultaneously with the administration of the index test.
Index test(s)
The Folstein Mini‐Mental State Examination (MMSE) is an 11‐item assessment of cognitive function that assesses attention and orientation, memory, registration, recall, calculation, language and ability to draw a complex polygon (Folstein 1975). The MMSE is subject to copyright restrictions (De Silva 2010), and it takes around seven minutes to administer to a person with dementia and five minutes to a person with normal cognition (Borson 2000). Scores can range from 1 to 30; the conventional cut‐off is 24, with lower scores indicating increasing cognitive impairment (Mitchell 2009), although other cut‐off points have been suggested (Crum 1993; Kukull 1994). There is a wide spectrum in the severity of disease that people with dementia have, and this will affect the diagnostic properties of a diagnostic test such as the MMSE.
Clinical pathway
Dementia develops over several years, from a presumed initial asymptomatic period where pathological changes accumulate in the absence of clinical manifestations, through subtle impairments of recent memory or changes in personality or behaviour, until the disease has become more apparent, with multiple cognitive domains involved and a noticeable decline from previous abilities in planning and performing complex tasks.
Standard diagnostic practice
Standard diagnostic assessment relates to evaluating people for whom there is concern about possible dementia, particularly to exclude alternative diagnostic hypotheses, and it includes history, clinical examination (including neurological, mental state and cognitive examination) and an interview with a relative or other informant. Before diagnosing dementia, other physical and mental disorders that might be contributing to cognitive impairment, for example hypothyroidism or depression, should be identified and if possible treated. Most recent guidelines recommend a neuroradiological examination to scan the brain (Computed Tomography (CT) or Magnetic Resonance Imaging (MRI)) to exclude structural causes for the clinical phenotype, for example a subdural haematoma (McKhann 2011; NICE 2006), but sometimes clinicians make the diagnosis on the history and presentation alone. Dementia diagnosis is defined by a deficit in more than two cognitive domains of sufficient degree to impair functional activities. These symptoms are usually progressive over a period of at least several months and should not be attributable to any other brain disorder. The International Classification of Diseases, 10th edition (ICD‐10) and the Diagnostic and Statistical Manual of Mental Disorders (DSM) general diagnostic criteria for dementia are detailed in Appendix 1 (APA 1994; WHO 1992).
Screening
Screening is the identification of unrecognised or asymptomatic disease by the administration of tests that can be applied quickly and are not intended to be diagnostic (Porta 2008). Recent UK health policy has encouraged opportunistic testing of older people attending primary care who have presented for reasons other than a memory complaint (Brunet 2012; Le Couteur 2013; Rasmussen 2013). GPs in the UK are encouraged to actively find people with dementia through routine annual questions in a Direct Enhanced Service (DOH recommendations; NICE 2013). In some cases, after further evaluation, the GP may then make a diagnosis of dementia, with or without a subtype (Ahmad 2010). Dementia screening is not recommended by the United States Preventive Services Task Force (US Preventive Services 2003), but the Patient Protection and Affordable Care Act requires an annual assessment of cognition for people who are enrolled in Medicare (Cordell 2013). The UK government has also encouraged case finding for dementia on acute admission to secondary care services (Dementia CQUIN).
Because people with dementia may not experience subjective memory problems, and a diagnosis of dementia often requires diagnostic evaluation by an experienced clinician, triage tests such as the MMSE are used in clinical practice to help rapidly identify people with a high likelihood of having normal cognition who do not require onward referral and investigation. Some investigations have blurred the distinction between the use of tests as a screening instrument in individuals without manifest disease and their use as clinical triage tools (Kamenski 2009).
Presentation to health services
In the UK people with memory problems usually present initially to their primary care practitioner, who may administer the MMSE to 'rule in' or confirm the possibility of dementia and potentially refer the patient to a specialist hospital memory clinic. Some people with dementia present much later in the disorder or follow a different pathway to diagnosis, for example, during an admission to general hospital for a physical illness. Diagnostic assessment pathways may vary in other countries, and a variety of clinicians including neurologists, psychiatrists and geriatricians, may make the diagnoses.
Role of the index test
Many countries in Europe and worldwide have been developing dementia strategies that emphasise the importance of accurate diagnosis to access appropriate health and social care services. Despite copyright restrictions, current experience is that the MMSE is still used extensively in clinical practice (Su 2014), including in the primary care setting, where clinicians may use it as either a screening test for dementia or as part of a more detailed evaluation of a person with suspected dementia. In some people, for example those who are particularly frail or unable to travel to a specialist clinic, the MMSE may be the only cognitive test used as part of the evaluation for possible dementia. A systematic evaluation of the diagnostic test accuracy of the instrument is needed to determine what confidence patients and clinicians can have in the clinical diagnosis of dementia based on the MMSE. A confirmed diagnosis of dementia is believed to offer opportunities for interventions, both social and medical, which may reduce the associated behavioural and psychiatric symptoms of dementia (Birks 2006; Clare 2003; McShane 2006), helping people with dementia, their families and potential caregivers to plan and avoid admissions to hospital or institutional care (Bourne 2007).
Prior tests
We anticipated the likelihood that no prior tests would have been performed before evaluating patients. In some settings, a two‐stage screening and assessment process takes place. Screening of people with suspected dementia usually requires a brief test of cognitive function, informant questionnaires or both, with a low score indicating a need for more in‐depth assessment (Boustani 2003). We anticipated that some studies carried out in the community and in primary care may have administered a very brief, high sensitivity instrument before applying the MMSE and investigating all of those who screened positive and a subsample of those who screened negative. In this eventuality, we planned to include the study and consider the prior test as a potential source of heterogeneity. However, no study used a test prior to the MMSE. Other tests are available to screen for dementia in primary care (Tsoi 2015), but we limited our review to the MMSE.
Rationale
Policy for dementia diagnosis is developing rapidly and has changed since the publication of the generic protocol for neuropsychological tests (Davis 2013a). There is a great need for a systematic appraisal of the diagnostic accuracy of neuropsychological tests, including the MMSE, in unselected (clinically unevaluated) populations.
Objectives
To determine the diagnostic accuracy of the Mini‐Mental State Examination (MMSE) at various cut points for dementia in people aged 65 years and over in community and primary care settings who had not undergone prior testing for dementia.
Secondary objectives
To investigate the heterogeneity of test accuracy in the included studies. We anticipated that there would be many potential sources of heterogeneity in this review, which Davis 2013a covered fully. In this review we expected that the most important sources of heterogeneity would be the characteristics of the study populations, the way investigators used the MMSE and the reference standard employed.
Methods
Criteria for considering studies for this review
Types of studies
The inclusion criteria for studies in this review were based on the generic protocol for neuropsychological tests in dementia (Davis 2013a). We reviewed the diagnostic accuracy of the MMSE when used in people aged over 65 years in non‐specialist settings, including community settings (population‐based screening) and primary care settings (where people may be screened opportunistically or present to the primary care practitioner with memory problems). We included studies that examined the diagnostic accuracy of the MMSE in people considered to have a memory problem (by patient, informant or clinician), as well as screening studies that examined the diagnostic accuracy in people regardless of a memory complaint (asymptomatic people). We analysed studies separately based on whether they were screening studies or not, as described in Investigations of heterogeneity. We included a diagnosis of dementia at any stage of disease (as long as the dementia was not previously identified by a specialist), as we considered that this pragmatic approach was most likely to be useful in informing current health policy and clinical practice. We did not examine the accuracy of MMSE for the diagnosis of pre‐clinical dementia (Sperling 2011), as this will be the subject of a separate review.
We included cross‐sectional studies that administered the index test and the reference standard(s) within a short time span (less than six months). We excluded case‐control studies because of the risk of bias (Whiting 2013). We did not include delayed verification studies, as these will be examined in a separate review, as described in the Background. We included studies where we anticipated 2 x 2 data would be available even if it was not reported in the original paper, and we contacted the authors to obtain it where necessary. Figure 1 outlines the process that we used for including articles in the review; further details are given in Selection of studies.
1.

Inclusion of studies
Participants
We included all participants who met the criteria for inclusion in community‐based or primary care described above. We defined primary care as non‐specialist, office‐based care with a first‐contact healthcare provider. We excluded studies where the MMSE was administered in a secondary care population, for example an emergency department, neurology ward or memory clinic.
We excluded studies of participants with previous or current substance abuse, central nervous system trauma (e.g. subdural haematoma), tumour or infection. Similarly, we excluded studies that recruited participants solely on the basis of disease state (for example; Parkinson's disease, multiple sclerosis, brain injury, motor neurone disease) or residence (for example; residential home, nursing home, prison), as they are not applicable to the general population. We considered that studies that recruited participants conditional on these criteria would have a different prevalence of dementia than the general population. We included studies that recruited participants from retirement communities (defined as non‐nursing elderly person independent facilitated living communities but not residential homes where multiple elderly people from different families lived in a single building with resident carers), as we considered that residents in these settings are likely to be similar to the population in terms of cognition and co‐morbidities.
Our intention was that the findings of this review would have relevance to clinicians in community health and primary care, and be applicable to people with 'usual dementia' (Brayne 2012). We therefore excluded studies that investigated specific clinical groups. For example, participants with a family history of Alzheimer's dementia may be more readily diagnosed with dementia, perhaps leading to verification bias. On this basis, we excluded studies that exclusively investigated people with a known genetic predisposition from this review and studies specifically investigating early‐onset dementia.
Examples of applicable studies for this review include the following.
Participants selected regardless of suspicion of cognitive disorder. This would be an unselected cross‐sectional survey that administered the MMSE to all participants and then evaluated all participants (or a random sample), regardless of MMSE result, with a full assessment for the presence or absence of the target disorder. These studies are analogous to screening and could be conducted in:
the community;
people attending primary care, as defined in Participants section, though we considered these studies would be uncommon as they would involve screening people for cognitive disorder in a doctor's office waiting room, and the ethics of this are not established.
-
Participants selected as being suspected of having cognitive disorder. These studies could be conducted in:
the community, though we anticipated these studies would be uncommon, as many people who are suspected of having cognitive disorder will seek evaluation in a healthcare setting;
primary care.
Thus, we anticipated that we might find studies using the MMSE in two different ways (evaluating people with and without suspicion of cognitive disorder) in two different clinical settings (community, before seeking diagnostic evaluation; and primary care, at the point of seeking diagnostic evaluation). We expected the diagnostic accuracy of the MMSE to differ between studies based in community and in primary care populations (and particularly in primary care participants selected on the basis of memory symptoms), and we planned to conduct separate analyses in these four potential study populations if appropriate.
Index tests
The index test is the 11‐item (maximum score 30) MMSE test (Folstein 1975). We recognised that other versions of the test exist (Grace 1995; Harrell 2000; Haubois 2012; Kabir 2000; Molloy 1991; Tschanz 2002), but we considered that these were best investigated in separate studies because of the substantial heterogeneity in the diagnostic test performance of these instruments. We also judged that including these index test variants would create an unfeasible workload for this review. However, we did not exclude studies on the basis of language, and we included studies that reported, for example, the diagnostic test accuracy of the Korean version of the 11‐item MMSE. Because education is associated with dementia (Fratiglioni 1991), some studies adjust the MMSE score for educational attainment (e.g. Liu 1996a).
Target conditions
The target condition was all‐cause dementia and any dementia subtype. We expected to find studies that focused on all‐cause dementia, Alzheimer disease dementia, vascular dementia, Lewy body dementia and frontotemporal dementia. We planned to appraise findings separately if we could extract included studies examining dementias of differing aetiologies or differing stages.
Reference standards
In this review, the target condition was dementia or its subtypes as defined by the clinical reference standards described in Davis 2013a and outlined below. We excluded studies that used neuropathological criteria as the only reference standard, as this review seeks to determine a cross‐sectional diagnosis of dementia. Clinical diagnosis included all‐cause (unspecified) dementia, as defined by any version of the DSM, which when conducting the review was most recently the fourth edition (APA 1994); any version of ICD, which when conducting the review was most recently ICD, 10th edition (WHO 1992) (see Appendix 1); or the Clinical Dementia Rating (Morris 1993). In studies in which a reference standard refers to different criteria for dementia (for example McKhann 1984: unlikely, possible, probable, definite), we considered people as having the disease if they were classified as having either probable or definite dementia.
Alzheimer's dementia
The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) have proposed the best ante‐mortem, clinical consensus 'reference standard' for Alzheimer's disease, defining three ante‐mortem groups: probable, possible, and unlikely Alzheimer's dementia (McKhann 1984). Newer criteria for Alzheimer's disease introduced in 2011 include the use of biomarkers (such as brain imaging and cerebrospinal fluid analysis) to contribute to diagnostic categories (McKhann 2011). We planned to present any studies that used these (biomarker) criteria in a separate category and to test the findings in a sensitivity analysis.
Lewy body dementia
The reference standard for Lewy body dementia is the McKeith criteria or their revision (McKeith 1996; McKeith 2005).
Frontotemporal dementia
The reference standard for frontotemporal dementia is the Lund criteria (Lund 1994).
Vascular dementia
The reference standard for vascular dementia is the National Institute of Neurological and Communicative Disorders and Stroke and the Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINCDS‐AIREN) criteria (Román 1993).
We recognised that different iterations of reference standards over time may not be directly comparable (e.g. DSM‐III‐R versus DSM‐IV, ICD‐9 versus ICD‐10) and that the validity of diagnoses may vary with the degree or manner in which the criteria have been applied (e.g. individual clinician versus algorithm versus consensus determination). We collected data on the method and application of the reference standard, and we planned to examine this as a source of heterogeneity if we considered it to be a source of bias. Although it is unlikely that a specific reference standard might favour particular index tests, there is the more general issue of incorporation bias, in which the reference standard is applied with knowledge of the index test because neuropsychological deficits are integral to the definition of dementia. This is less problematic in cross‐sectional studies because the index test and the reference standard may be administered completely independently.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group's specialised register (via the Cochrane Register of Studies); MEDLINE (OvidSP) (January 1946 to May 2014); EMBASE (OvidSP) (January 1972 to May 2014); BIOSIS previews (Thomson Reuters Web of Science) (January 1922 to May 2014); Web of Science Core Collection, including the Science Citation Index and the Conference Proceedings Citation Index (Thomson Reuters Web of Science) (January 1945 to May 2014); PsycINFO (OvidSP) (January 1806 to May 2014) and LILACS (BIREME). See Appendix 2 for the search strategies. Where appropriate, we used controlled vocabulary such as MeSH terms (in MEDLINE) and EMTREE (in EMBASE) and other controlled vocabulary in other databases, as appropriate. We did not use search filters designed to retrieve diagnostic test accuracy studies (collections of terms aimed at reducing the number needed to screen by filtering out irrelevant records and retaining only those that are relevant) as a method to restrict the search overall, because available filters have not yet proved sensitive enough for systematic review searches (Beynon 2013; Whiting 2011). We did not apply any language restriction to the electronic searches; we used translation services as necessary during the screening stages. A single researcher with extensive experience in systematic reviews performed the searches. We first performed the searches on 21 November 2012 and then again on 20 May 2014.
Searching other resources
We checked the reference lists of all relevant papers for additional studies. We also searched:
Meta‐analyses van Diagnostisch Onderzoek (MEDION database) (www.mediondatabase.nl);
Database of Abstracts of Reviews of Effects (DARE) (www.cochranelibrary.com);
Health Technology Assessments Database (HTA Database) in The Cochrane Library (www.cochranelibrary.com);
Aggressive Research Intelligence Facility (ARIF database) (www.arif.bham.ac.uk).
We attempted to contact authors where necessary to obtain details of unpublished studies.
Data collection and analysis
Selection of studies
Figure 1 shows a flowchart that we used when considering whether to include studies in the review.
The inclusion criteria were:
population is either community or primary care (see 'Types of studies');
reference standards as described above;
MMSE was used as an index test (alone or with other tests) and was administered to all study participants;
study design is cohort or nested case‐control.
Exclusion criteria were:
classic case‐control study design (subject to spectrum bias);
index test administered to only cases or controls, rather than to both groups (cannot calculate diagnostic accuracy).
We selected studies based on the title and abstract screening undertaken by a team of trained assessors. Two assessors independently reviewed all citations retrieved by the searches and classified them as relevant or not. Pairs of authors then assessed the full‐text papers of studies classified as possibly relevant, and we resolved any disagreements by discussion with a third, senior author. We show the process of study selection in a PRISMA flow diagram in Figure 2.
2.

PRISMA Flow diagram of included studies.
Data extraction and management
Two senior authors simultaneously extracted data on study characteristics and 2 x 2 data directly into Review Manager (RevMan 2014). We resolved disagreements by reaching consensus with a third, senior author.
Assessment of methodological quality
We assessed the risk of bias of each study using the QUADAS‐2 tool in duplicate (Whiting 2011), as recommended by Cochrane (see Appendix 4 for QUADAS 2 statements and Appendix 5 for anchoring statements). The 'Assessment of methodological quality table' helps the reader to evaluate the strength of evidence to support the diagnostic accuracy of the MMSE.
Statistical analysis and data synthesis
We expected the diagnostic accuracy of the MMSE to differ between studies based in community and in primary care settings (and particularly in primary care participants selected on the basis of memory symptoms), and we planned to conduct separate analyses in the four potential study populations (see Types of studies above). We also planned to conduct separate analyses as required for each subtype of dementia.
For all included studies, the data in the 2 x 2 tables (showing the binary test results cross‐classified with the binary reference standard) was used to calculate the sensitivities and specificities, with 95% confidence intervals. We present a summary of the included studies in Table 2 and Table 3. If studies reported more than one cut point, we presented the findings for all cut points reported.
1. Summary of included community studies.
| Study | Number of citationsa | Sample size | Number of people with dementia | Prevalence of dementia | Reference standard | Reported MMSE cut points indicating normal |
| Studies where 2 x 2 data was available | ||||||
| Aevarsson 2000 | 1 | 428 | 117 | 27.3% | DSM‐III | 24 |
| Baker 1993 | 1 | 55 | 1 | 1.8% | DSM‐III‐R | 24 |
| Burkart 2000 | 1 | 256 | 23 | 9.0% | DSM‐III‐R | 24, 25, 29 |
| Callahan 2002 | 1 | 344 | 15 | 4.6% | DSM‐III‐R | 22, 23, 24, 25, 26, 27, 28 |
| Correira 2001 | 1 | 109 | 15 | 13.8% | DSM‐IV | Adjusted for education |
| Eefsting 1997 | 1 | 2151 | 123 | 5.7% | DSM‐III‐R | 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 |
| Heun 1998 | 1 | 287 | 37 | 12.9% | DSM‐III‐R | 24, 25, 26 |
| Iavarone 2006 | 1 | 294 | 75 | 25.5% | DSM‐IV | Adjusted for education |
| Jacinto 2011 | 1 | 58 | 17 | 29.3% | DSM‐IV | Adjusted for education |
| Jeong 2004 | 1 | 235 | 46 | 19.6% | DSM‐IV | 19 |
| Kahle‐Wrobleski 2007 | 1 | 435 | 157 | 36.0% | DSM‐IV | 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 |
| Kathriarachchi 2005 | 1 | 37 | 17 | 38.0% | CDR | 17, 20, 23 |
| Kungsholmen Study 1992 | 7 | 668 | 314 | 47.0% | DSM‐III‐R | 24 |
| Macedo Montano 2005 | 1 | 156 | 34 | 21.8% | DSM‐IV‐R | 26 |
| Mackinnon 2003 | 1 | 646 | 36 | 5.6% | DSM‐III‐R | 24, 27 |
| Maki 2000 | 1 | 662 | 49 | 7.4% | DSM‐III‐R | 24 |
| MoVies Study 1993 | 5 | 1119 | 76 | 6.8% | DSM‐III‐R | 24, 25 |
| Lam 2008 | 1 | 5957 | 143 | 2.4% | DSM‐IV | Adjusted for education |
| Liu 1996a | 1 | 130 | 45 | 34.6% | DSM‐III | Adjusted for education |
| Pandav 2002 | 1 | 806 | 43 | 5.3% | DSM‐III‐R | 19, 20, 21, 22, 23, 24, 25, 26, 27 |
| PAQUID Study | 5 | 2730 | 101 | 3.7% | DSM‐III | 24 |
| Phantumchinda 1991 | 1 | 500 | 9 | 1.8% | DSM‐III | 10, 15, 17, 18, 21, 22 |
| Ramlall 2013 | 1 | 140 | 11 | 7.9% | DSM‐IV‐R | 24, 25 |
| Rosselli 2000 | 1 | 693 | 13 | 1.9% | DSM‐IV | Adjusted for education |
| Schultz‐Larsen 2007 | 1 | 242 | 69 | 28.5% | DSM‐IV | 24, 25, 26, 27 |
| Tang 1999 | 1 | 1201 | 29 | 2.4% | DSM‐III‐R | Adjusted for education |
| West Beijing Study 1989. | 2 | 99 | 10 | 10.1 | DSM‐III | 18 |
| Winblad 2010 | 1 | 114 | 24 | 21.1% | DSM‐IV | 25 |
| Studies where 2 x 2 data was not available | ||||||
| ADAMS Study 2007 | 3 | 509 | 129 | 25.3% | DSM‐IV | — |
| AMSTEL Study 1997 | 4 | 4123 | 261 | 6.3% | DSM‐III‐R | — |
| Fichter 1995 | 1 | 402 | 85 | 21.2% | DSM‐III‐R | — |
| Fillenbaum 1990 | 1 | 4164 | 26 | 0.6% | DSM‐III‐R | — |
| Frank 1996 | 1 | 380 | 56 | 14.7% | NINCDS‐ADRDA | — |
| Helsinki Aging Study 1994b | 2 | 656 | 93 | 14.2% | DSM‐III‐R | — |
| Keskinoglu 2009 | 1 | 490 | 63 | 12.9% | DSM‐III | — |
| Lee 2002 | 1 | 643 | 40 | 6.2% | DSM‐III | — |
| Li 2006 | 1 | 144 | 19 | 13.1% | DSM‐III | — |
| Lindesay 1997 | 1 | 297 | Not reported | Not reported | DSM‐III | — |
| Mingyuan 1998 | 1 | Not reported | Not reported | Not reported | DSM‐III‐R | — |
| Rummans 1996 | 1 | 201 | 21 | 10.4% | DSM‐III‐R | — |
| Scazufca 2009 | 1 | 1933 | 84 | 4.3% | DSM‐IV | — |
| Wilder 1995 | 1 | 795 | Not reported | — | DSM‐III | — |
CDR: clinical dementia rating; DSM: Diagnostic and Statistical Manual of Mental Disorders; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association. aRefers to the number of citations that we retrieved that refer to the same study and are listed in Included studies. bStudy reported sensitivity only and so could not be included in meta‐analysis in absence of paired data.
2. Summary of included primary care studies.
| Study | Number of citations a | Sample size | Number of people with dementia | Prevalence of dementia | Reference standard | Reported MMSE cut points indicating normal |
| Setting: asymptomatic primary care | ||||||
| Brodaty 2002 | 1 | 176 | 82 | 46.6% | DSM‐IV | 25 |
| Lavery 2007 | 1 | 313 | 28 | 8.9% | CDR | 23 |
| Lourenco 2006 | 1 | 303 | 78 | 25.7% | DSM‐IV | 18, 19, 20, 21, 22, 23, 24, 25, 26 |
| Pond 1994 | 1 | 367 | 57 | 15.5% | DSM‐III‐R | 24, 26 |
| Setting: symptomatic primary care | ||||||
| Carnero‐Pardo 2013 | 3 | 360 | 77 | 21.4% | DSM‐IV‐R | 17, 18, 19, 20, 21, 22, 23, 24, 25 |
| Cruz‐Orduna 2012 | 1 | 160 | 15 | 9.4% | DSM‐IV‐R | 19 |
CDR: clinical dementia rating; DSM: Diagnostic and Statistical Manual of Mental Disorders. aRefers to the number of citations that we retrieved that refer to the same study and are listed in Included studies.
In our main analysis, we performed meta‐analyses on pairs of sensitivity and specificity, stratified by setting (community and primary care) using the HSROC method, using only one estimate from each study (Macaskill 2010). Where reported, this was the standard MMSE cut point of 23/24, where 24 indicates normal cognition, and where unreported, we used either the only estimate that was reported, or the best estimate (from the top lefthand corner of the study ROC curve, acknowledging that this may overestimate diagnostic accuracy in that study). We used Stata software (Stata) to perform the analysis and used the data to plot the summary ROC curve. We then used a bivariate random‐effects model approach based on pairs of sensitivity and specificity (Chu 2006; Macaskill 2010; Reitsma 2005) to analyse the diagnostic accuracy at specific cut points in community‐based studies, where there appeared to be consensus that these were commonly reported cut points (24 and 25 indicating normal and MMSE adjusted for education).
Investigations of heterogeneity
We investigated heterogeneity in the first instance through visual examination of forest plots of sensitivities and specificities. We pre‐specified factors that would potentially contribute to heterogeneity and attempted to adjust for these in the meta‐analysis for average age of participants (in categories: 65 to 74 years, 75 to 84 years, 85 to 94 years, 95 years or more, unclear), sex, conduct of the test (in categories: specialist, trained non‐specialist, or unclear) and reference standard. We used likelihood ratio tests to compare the fit of candidate models when assessing the effect of a covariate on test performance (Macaskill 2010).
Sensitivity analyses
We planned to perform sensitivity analyses to determine the effect of excluding studies deemed to be at high risk of bias. However, as studies were generally at low risk of bias – no study had more than two of four QUADAS‐2 items assessed as having a high risk of bias – we did not do this as we did not pre‐specify a point at which we would deem a study to be at overall 'high risk of bias'.
Assessment of reporting bias
Quantitative methods for exploring reporting bias are not well established for studies of diagnostic test accuracy (DTA) (Bossuyt 2013)
Results
Results of the search
The search yielded 47,807 records, and 24,310 remained after removing duplicates (Figure 2). We reviewed the full text of 317 records (referring to 270 studies) and excluded 245 (referring to 222 studies), most commonly because of ineligible study design. We were unable to classify eight records because they were only available as abstracts and we could not obtain sufficient information about them despite attempting to contact authors (Gungen 2002; Jianbo 2013; Kornsey; Kvitting 2013; Orsi; Shaaban 2013; Upadhyaya 2010; Yu 2012). We found one ongoing study that had no results on diagnostic accuracy (Guiata 2012). We attempted to contact the authors of 17 articles, received replies from the authors of 10 and were able to include additional unpublished data from one study (Carnero‐Pardo 2013). We included 70 articles, referring to 48 studies, in our synthesis. Table 2 and Table 3 give details of the studies in the community and primary care, respectively. Of the 48 studies that we reviewed, we were able to perform meta‐analysis on 28 community‐based studies (44 articles) and 6 studies in primary care (8 articles). Of the 28 community studies that we included in the meta‐analysis, 7 reported accuracy estimates for level of education (referred to as 'education adjusted') and 21 reported accuracy estimates at various cut points. We could not include the remaining 14 community studies in the meta‐analysis because paired 2 x 2 data was not available despite contacting authors. However, we include them in this report for transparent reporting, because we believe 2 x 2 data should exist based on the study design and characteristics. Two of the six studies in primary care were conducted in symptomatic people, selected to the study on the basis of a reported concern about cognition(Carnero‐Pardo 2013; Cruz‐Orduna 2012), whereas the other four primary care studies and all of the community studies were in asymptomatic people, for whom reported cognitive difficulty was not a criterion for inclusion in the study. Studies reported the target condition as all‐cause dementia syndrome, so we could not analyse diagnostic accuracy by subtype.
In the meta‐analysis there were 12,110 participants in community studies and 1681 participants in primary care studies. For community‐based studies we were able to perform meta‐analysis using the bivariate method at cut points of 24 and 25 and in studies that adjusted accuracy estimates for level of education (referred to as 'education adjusted'). The Table 1 presents these results. For studies in primary care, we could not perform meta‐analysis using the bivariate method due to heterogeneity in the cut points reported, and so we cannot report a summary sensitivity and specificity. We include further details of the studies, including the design, sampling, reference standard and population, in the Characteristics of included studies.
We found one prior systematic review on the same topic, which included five studies that were excluded by our methods (Mitchell 2009). Table 4 presents the details of these studies. Cullen 2005 and Huppert 2005, two of the five studies that we excluded, used AGECAT as the reference standard (Copeland 1986), and the other three studies used CAMDEX (Roth 1986). Two of the studies used a cut point of 22 to determine normal cognition (Brayne 1989; Clarke 1991), one used a cut point of 23 (Huppert 2005), and two used a cut point of 24 (Cullen 2005; O'Connor 1989).
3. Papers included in Mitchell 2009 review but not in this review.
| Citation | Setting and sample | Prevalence of dementia | Index Test | Cut point indicating normal | Reference Standard | Sensitivity | Specificity | Reason for exclusion from this review |
| Belle 2000 |
MoVies Study 1993 Community age‐stratified random sample |
68 dementia and 1110 no dementia | MMSE | 27 | Short and Sweet Screening Instrument | 96% | 78% | No mention of MMSE in abstract and wrong reference standard in this paper but data from study (from another paper) included in our review |
| Brayne 1989 | Community Stratified random sample of 365 women aged 70‐79 years |
29 dementia 336 no dementia | MMSE | 22 | CAMDEX | 83% | 87% | No mention of MMSE in abstract and wrong reference standard |
| Clarke 1991 | Community Total sample of all people aged over 75 years on 1 GP list |
265 dementia, 150 no dementia | MMSE | 22 | CAMDEX | 77% | 71% | Wrong reference standard |
| Cullen 2005 | Community Sample of people aged over 65 years from GP lists |
44 dementia 1071 no dementia | MMSE | 24 | AGECAT | 91% | 87% | Wrong reference standard |
| Hooijer 1992 |
AMSTEL Study 1997 All elderly patients of 1 Amsterdam GP |
13 dementia 345 no dementia | MMSE | 24 | CAMDEX | 77% | 97% | Wrong reference standard in this paper but data from study (from another paper) included in our review |
| O'Connor 1989 | Community Total sample of all people aged over 75 on 5 GP lists |
196 dementia 285 no dementia | MMSE | 24 | CAMDEX | 86% | 92% | Wrong reference standard |
| Wind 1997 |
AMSTEL Study 1997 Age stratified sample of participating practices in AMSTEL |
114 dementia 419 no dementia | MMSE | 24 | AGECAT | 69% | 89% | Wrong reference standard in this paper but data from study (from another paper) included in our review |
| Huppert 2005 | Community MRC‐CFAS study Random, age‐stratified sampling of people aged over 65 years |
795 dementia 11,885 no dementia | MMSE | 23 | AGECAT | 88% | 92% | Wrong reference standard, abstract makes no mention of diagnostic accuracy and diagnostic accuracy terms such as sensitivity and specificity do not appear in the paper. |
Methodological quality of included studies
We used QUADAS‐2 to help determine the risk of bias for each study in order to determine the confidence that patients and clinicians can have in the results of each study (Appendix 4; Appendix 5). We summarise the main results below and in Figure 3 and Figure 4.
3.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
4.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
We judged Maki 2000 and Winblad 2010 to be at high risk of bias in the patient selection domain because Maki 2000 excluded people who lived alone and Winblad 2010 appeared to exclude people who were known to have dementia. We considered three studies to be at high risk of bias in the index test domain because it appeared that investigators did not specify the cut point before the analysis (Lindesay 1997; Lourenco 2006; Macedo Montano 2005). We identified nine studies that we considered to be at high risk of bias regarding flow and timing because we had concerns about partial verification of the index test: Eefsting 1997 administered the reference test to a proportion of each scoring band on the MMSE; Fillenbaum 1990 administered the reference standard to a sample of participants; Helsinki Aging Study 1994 only administered a reference standard to people who were diagnosed with possible dementia on the basis of an assessment by a GP; Jacinto 2011 did not describe the flow and timing; Kathriarachchi 2005, Lam 2008 and Macedo Montano 2005 partially verified the diagnosis with a sample of participants, but it was not clear how they selected the sample; Scazufca 2009 appeared to exclude people who were unable to answer items in the MMSE and said that 81 people were not approached but did not explain why. Finally, Maki 2000 administered the reference standard to a sample of people, but we could not reconcile the figures that were stated in the paper.
We assessed most studies as being applicable to the review question, though we had concerns that the selection of participants in six might reduce their applicability (Helsinki Aging Study 1994; Jacinto 2011; Li 2006; Maki 2000; Rosselli 2000; Winblad 2010). We had high concern that Li 2006 used a target condition (mild cognitive impairment and dementia) that was not applicable to our review question and were unable to include data on diagnostic accuracy from this study. As studies were generally at low risk of bias – no study had more than two of four QUADAS‐2 items that were assessed as high risk of bias – we did not exclude studies from the meta‐analysis based on the risk of bias as we did not pre‐specify a point at which we would deem a study to be at overall 'high risk of bias'.
Findings
Table 2 and Table 3 show that the accuracy of the MMSE for diagnosing dementia was reported at 18 cut points (MMSE score 10, 14 to 30 inclusive) in the community and 10 cut points (MMSE score 17 to 26 inclusive) in primary care studies. Table 1 presents the summary diagnostic accuracy in community studies: sensitivity 0.85 (95% confidence interval (CI) 0.74 to 0.92), specificity 0.90 (95% CI 0.8 to 0.95) at a cut point of 24 (15 studies); sensitivity 0.87 (95% CI 0.78 to 0.93), specificity 0.82 (95% CI 0.65 to 0.92) at a cut point of 25 (10 studies); and sensitivity 0.97 (95% CI 0.83 to 1.00), specificity 0.70 (95% CI 0.50 to 0.85) when adjusted for education (7 studies). In primary care studies, each cut point was reported by a maximum of three studies, and we could not provide a summary of sensitivity and specificity.
Community studies
In community studies the accuracy of the MMSE for the diagnosis of dementia was available for 18 cut points (10, 14 to 30 inclusive) and also adjusted for education. At a cut point of 10, accuracy was sensitivity 0.11 (95% CI 0.00 to 0.48), specificity 0.95 (95% CI 0.93 to 0.97) (Phantumchinda 1991), and at a cut point of 30 accuracy was sensitivity 1.00 (95% CI 0.98 to 1.00), specificity 0.00 (95% CI 0.00 to 0.01) (Kahle‐Wrobleski 2007). Figure 5 presents the summary ROC curve for community studies in the main analysis, including all 21 studies that reported 2 x 2 data, and Figure 6 presents the linked forest plot.
5.
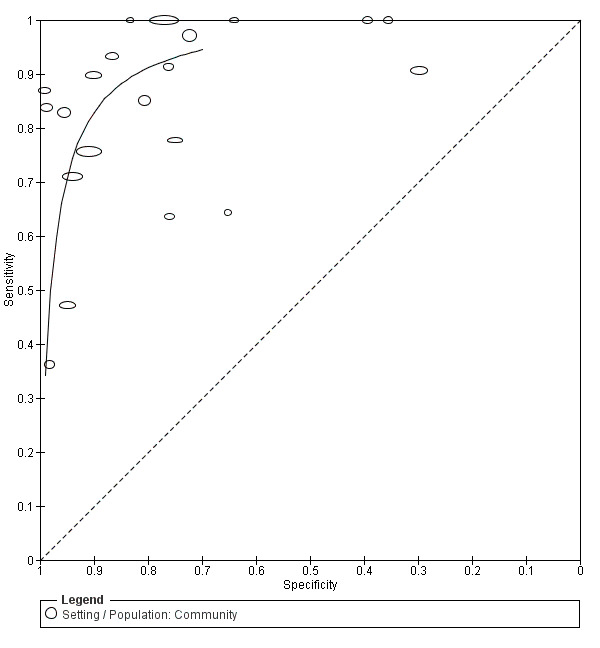
Summary ROC Plot of analysis 2 main community
6.

Forest plot of analysis 2 Main community
We were able to include seven community studies in the meta‐analysis of diagnostic accuracy in studies whose original investigators adjusted the MMSE for education. We present the summary ROC in Figure 7 and the forest plot in Figure 8. The pooled estimate for the diagnostic accuracy was sensitivity 0.97 (95% CI 0.83 to 1.00), specificity 0.70 (95% CI 0.50 to 0.85). We were able to include 15 studies in the meta‐analysis of diagnostic accuracy at a cut point of 24; Figure 9 presents the summary ROC curve and Figure 10, the forest plot; the pooled estimate for the diagnostic accuracy was sensitivity 0.85 (95% CI 0.74 to 0.92), specificity 0.90 (95% CI 0.82 to 0.95). We were able to include 10 studies in the meta‐analysis of diagnostic accuracy at a cut point of 25; Figure 11 presents the summary ROC and Figure 12, the forest plot. The pooled estimate for the diagnostic accuracy was sensitivity 0.87 (95% CI 0.78 to 0.93), specificity 0.82 (95% CI 0.65 to 0.92).
7.

Summary ROC plot of analysis 3 community, education adjusted. The black filled dot indicates the summary point estimate of diagnostic accuracy, the smaller dotted bubble indicates the 95% confidence interval around the summary point (containing the 'true value' within that region 95% of the time on the basis of the available data) and the larger dashed bubble indicates the 95% prediction region (containing results from a new future study 95% of the time).
8.

Forest plot of analysis 3 MMSE community, education adjusted
9.

Summary ROC plot of analysis 4 MMSE at 24 normality (23/24). The black filled dot indicates the summary point estimate of diagnostic accuracy, the smaller dotted bubble indicates the 95% confidence interval around the summary point (containing the 'true value' within that region 95% of the time on the basis of the available data) and the larger dashed bubble indicates the 95% prediction region (containing results from a new future study 95% of the time).
10.

Forest plot of analysis 4 MMSE at 24 normality (23/24).
11.

Summary ROC plot of analysis 5 MMSE at 25 normality. The black filled dot indicates the summary point estimate of diagnostic accuracy, the smaller dotted bubble indicates the 95% confidence interval around the summary point (containing the 'true value' within that region 95% of the time on the basis of the available data) and the larger dashed bubble indicates the 95% prediction region (containing results from a new future study 95% of the time).
12.

Forest plot of analysis 5 MMSE at 25 normality.
Primary care studies
In symptomatic people the accuracy of the MMSE for the diagnosis of dementia was available for 9 cut points (17 to 25 inclusive). At a cut point of 19, accuracy was sensitivity 0.80 (95% CI 0.52 to 0.96), specificity 0.86 (95% CI 0.80 to 0.91) in Cruz‐Orduna 2012 and sensitivity 0.88 (95% CI 0.79 to 0.95), specificity 0.87 (95% CI 0.82 to 0.91) in Carnero‐Pardo 2013 .Carnero‐Pardo 2013 reported accuracy for cut points from 17 (sensitivity 0.70 (95% CI 0.59 to 0.80), specificity 0.93 (95% CI 0.89 to 0.96)), to 25 (sensitivity 1.00 (95% CI 0.95 to 1.00), specificity 0.38 (95% CI 0.32 to 0.44)). At the traditional cut point of 24, the accuracy was sensitivity 1.00 (95% CI 0.95 to 1.00), specificity 0.46 (95% CI 0.40 to 0.52).
In asymptomatic people the accuracy of the MMSE for the diagnosis of dementia was available for 9 cut points (18 to 26 inclusive). Lourenco 2006 reported that at a cut point of 18, accuracy was sensitivity 0.35 (95% CI 0.24 to 0.46), specificity 0.94 (95% CI 0.90 to 0.97), and at a cut point of 26 the accuracy was sensitivity 0.90 (95% CI 0.81 to 0.95), specificity 0.50 (95% CI 0.43 to 0.56). At the traditional cut point of 24, Lourenco 2006 found a sensitivity of 0.65 (95% CI 0.59 to 0.72) and specificity of 0.65 (95% CI 0.59 to 0.72), while Pond 1994 reported sensitivity 0.95 (95% CI 0.92 to 0.97) and specificity 0.95 (95% CI 0.92 to 0.97).
Figure 13 presents the summary ROC curve for primary care studies and Figure 14, the forest plot.
13.

Summary ROC plot of analysis 1 Main analysis primary care
14.

Forest plot of analysis 1 Main analysis primary care
Heterogeneity
We used additional models to explore potential heterogeneity in the diagnostic accuracy of the main analysis by age, sex, conduct and reference standard. Additionally we attempted to evaluate heterogeneity by education and mean MMSE score in the sample, but this was not possible because these data were poorly reported in the original studies. We found no evidence against the null hypothesis of no heterogeneity for age (P = 1.00 likelihood ratio (LR) Chi2(4) = − 9.49), sex (P = 1.00 LR Chi2(4) = − 20.68) or conduct (P = 0.0647 LR Chi2(4) = 8.85). There was some evidence against the null hypothesis of no heterogeneity by reference standard (P = 0.0342 LR Chi2(8) = 16.63).
Sensitivity analyses
We did not perform any sensitivity analyses.
Discussion
Summary of main results
In community studies of asymptomatic people, with a cut point of 24 indicating normal cognition, the MMSE had pooled diagnostic accuracy of sensitivity 0.85 (95% CI 0.74 to 0.92), specificity 0.90 (95% CI 0.82 to 0.95). The pooled diagnostic accuracy at a cut point of 25 was similar, whereas the MMSE adjusted for education was better for screening, at the cost of more false positives, with sensitivity 0.97 (95% CI 0.83 to 1.00) and specificity 0.70 (95% CI 0.50 to 0.85). In primary care, a single study of symptomatic people reported that a cut point of 17 had higher specificity (0.93 95% CI 0.89 to 0.96) than a cut point of 24 (0.46 95% CI 0.40, 0.52), with some additional false negatives as the sensitivity fell from 1.00 (95% CI 0.95 to 1.00) to 0.70 (95% CI 0.59 to 0.80) (Carnero‐Pardo 2013).
The risk of bias in the included studies is summarised in Figure 3. We generally had low concern about bias in the included studies. There were some differences between studies, but we found no evidence against the null hypothesis of no statistical heterogeneity by age, sex, or conduct of the index test. We found some evidence of heterogeneity by reference standard, but most of the studies used the clinical DSM definition for the target condition of all‐cause dementia. Some of the confidence intervals around the point estimates are wide, which indicates some uncertainty in our results because of the limitations of the underlying data.
Strengths and weaknesses of the review
We used a comprehensive and sensitive search strategy that yielded substantially more results than a similar, earlier review that found only 775 articles and included a total of 21 studies in any setting (Mitchell 2009). We followed our pre‐specified peer‐reviewed protocol, which did not specify CAMDEX, CERAD or AGECAT as appropriate reference standards. Consequently we excluded studies that used these reference standards. We found few studies in primary care that used the references standards we specified and even fewer in participants selected on the basis of symptoms (symptomatic primary care). We found sufficient studies to perform meta‐analysis of test accuracy and to explore heterogeneity, but our evaluation was limited by poorly reported factors (education and mean MMSE score). On the other hand, assessing heterogeneity by factors that are not measured at the study level (e.g. education, severity of diagnosis or subtype diagnosis) is generally not recommended (Bossuyt 2013). We did not use a separate form (as we stated in the protocol), but we extracted data directly into RevMan after we set up the file using a set of pilot studies, as we pre‐specified (RevMan 2014). This was because we were concerned about introducing errors when copying data from an Access database into RevMan given the large number of studies. At least two authors, and usually three, performed data extraction and quality assessment, checking the accuracy of the extracted data in real time.
Applicability of findings to the review question
We consider that our findings are likely to be applicable to our review question. We had aimed to evaluate the strength of evidence for the accuracy of the MMSE for diagnosing Alzheimer's disease dementia and other dementias, but studies only reported all‐cause dementia, so we were not able to evaluate the diagnostic accuracy by subtype. Despite this, for clinicians and patients in primary care and community settings, the most important clinical question determining intervention and follow‐up is often, 'Does this person have a dementia now?', and our review addresses this.
Authors' conclusions
Implications for practice.
In this section we present results using natural frequencies, but these do not take account of the confidence intervals and uncertainty in our results and so should be interpreted with caution.
If clinicians would like to use the MMSE in primary care to rule in a diagnosis of dementia in a symptomatic person, there is evidence from one study that a cut point of 17 to indicate normal cognition would have higher specificity than a traditional cut point of 24, with a slightly lower sensitivity: the median prevalence of dementia in primary care studies was 18.5%, so – rounding up to 20% for the sake of convenience – of 1000 people in this setting, with 200 expected to have dementia at a cut point of 17 indicating normal, clinicians could expect 196 to test positive, of whom 140 (71%) would truly have dementia; 804 would test negative, of whom 744 (93%) would not have dementia (Carnero‐Pardo 2013). If the test were being used to identify anybody who might have dementia regardless of concern about cognition, then there is evidence from seven community‐based studies that the accuracy of the MMSE adjusted for education has a higher sensitivity than when a cut point of 24 or 25 is used (based on the meta‐analytical estimates of 15 studies and 10 studies, respectively), although the specificity is lower and consequently there are more false positives, and this might mean unnecessary further evaluation for some people. Clinicians and patients can be confident that the MMSE is likely to be of some diagnostic value at cut points of 24, 25 and adjusted for education, though the uncertainty in the estimates does not allow us to confidently choose between these three approaches.
We consider that the evidence we present supports the use of the MMSE as part of a diagnostic evaluation for dementia, but it should not be used in isolation to confirm or exclude disease. Our review allowed for a diagnosis of dementia at any stage of disease; we did not explore heterogeneity by disease spectrum, and to do so would have been challenging and not recommended (Bossuyt 2013). However, we would advocate considering carefully how our results apply in the clinical context. When considering the studies that reported the highest cut point in asymptomatic people in the community and the lowest cut point in symptomatic people in primary care, then based on Burkart 2000 at a cut point of 29 indicating normal cognition in a sample of 1000 asymptomatic people in the community where 65 have dementia, we would expect that 367 people would have a normal result, of whom 2 would have dementia. Conversely, based on Carnero‐Pardo 2013, at a cut point of 17 indicating normal cognition in a sample of 1000 people in primary care, where 200 have dementia, we would expect that 196 test positive, of whom 56 would not have dementia. Thus, a diagnosis of dementia may still be possible even with high (normal) scores on MMSE, and people may not have dementia even with low (abnormal) MMSE scores. The use of the MMSE in the community will usually be followed up by further clinical evaluation of people who have abnormal scores.
Implications for research.
The MMSE is now protected by copyright and is likely to be used less in clinical practice in the future than it has been in the past, but these data may be useful for research studies. We were surprised to find so few studies in symptomatic people. Original study authors did not optimally report factors that might affect performance of the test.
Many of the studies that we included, particularly those in the community, are screening studies (see Clinical pathway). An ageing population is prompting policymakers to consider changes to the traditional clinical pathway, and in the future non‐specialist clinicians in primary care may be responsible for diagnosing straightforward cases of dementia (Barrett 2014). However, we found very few studies to inform policymaking in this area.
We recommend that future work evaluate the diagnostic accuracy of tests in the context of the diagnostic pathway experienced by the patient: that is, allowing for the presence or absence of subjective memory problems, symptoms and the view of caregivers and clinicians. We also suggest that in addition to testing accuracy alone,investigators report how undergoing the MMSE (or other similar cognitive test) changes outcomes that are relevant to patients and clinicians, such as time to diagnosis, initiation of treatment or care package, subsequent additional testing and place of care. We advocate the use of STARDEM reporting criteria to aid transparent reporting of future diagnostic test accuracy studies with dementia as the target condition (Noel‐Storr 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2016 | Amended | Error in plain language summary corrected |
Acknowledgements
We thank the authors of original manuscripts who replied to correspondence and provided additional data.
Appendices
Appendix 1. Classification of dementia
World Health Organization International Classification of Diseases‐10
G1. Evidence of each of the following:
A decline in memory, which is most evident in the learning of new information, although in more severe cases, the recall of previously learned information also may be affected. The impairment applies to both verbal and nonverbal material. The decline should be objectively verified by obtaining a reliable history from an informant, supplemented, if possible, by neuropsychological tests or quantified cognitive assessments.
A decline in other cognitive abilities characterised by deterioration in judgement and thinking, such as planning and organising, and in the general processing of information. Evidence for this should be obtained when possible by interviewing an informant, supplemented, if possible, by neuropsychological tests or quantified objective assessments. Deterioration from a previously higher level of performance should be established.
G2. Preserved awareness of the environment during a period long enough to enable the unequivocal demonstration of G1. When episodes of delirium are superimposed, the diagnosis of dementia should be deferred.
G3. A decline in emotional control or motivation, or a change in social behaviour, manifest as at least one of the following.
Emotional liability.
Irritability.
Apathy.
Coarsening of social behaviour.
G4. For a confident clinical diagnosis, G1 should have been present for at least six months; if the period since the manifest onset is shorter, the diagnosis can only be tentative.
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision
A. The development of multiple cognitive deficits manifested by both:
memory impairment (impaired ability to learn new information or to recall previously learned information;
one (or more) of the following cognitive disturbances.
Aphasia (language disturbance).
Apraxia (impaired ability to carry out motor activities despite intact motor function).
Agnosia (failure to recognise or identify objects despite intact sensory function).
Disturbance in executive functioning (i.e. planning, organizing, sequencing, abstracting).
B. Each of the cognitive deficits in Criteria A1 and A2
Causes significant impairment in social or occupational functioning.
Represents a significant decline from a previous level of functioning.
C. The deficits do not occur exclusively during the course of a delirium.
D. The disturbance is not better accounted for by another Axis I disorder (e.g. major depressive disorder, schizophrenia).
Appendix 2. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) Most recent search: 20 May 2014 |
1. MMSE*.ti,ab. 2. sMMSE.ti,ab. 3. Folstein*.ti,ab. 4. MiniMental.ti,ab. 5. "mini mental stat*".ti,ab. 6. or/1‐5 |
Nov 2012: 10048 May 2014: 1657 |
| 2. EMBASE 1980‐2012 November 16 (Ovid SP) Most recent search: 20 May 2014 |
1. MMSE*.ti,ab. 2. sMMSE.ti,ab. 3. Folstein*.ti,ab. 4. MiniMental.ti,ab. 5. "mini mental stat*".ti,ab. 6. 3MS.ti,ab. 7. *mini mental state examination/ 8. or/1‐7 9. dement*.ti,ab. 10. alzheimer*.ti,ab. 11. exp *dementia/ 12. "vascular cognitive impair*".ti,ab. 13. ("lewy bod*" or DLB or LBD).ti,ab. 14. (AD or VaD or FTLD or FTD or DLB or LDB).ti,ab. 15. delirium/ 16. deliri*.ti,ab. 17. or/9‐16 18. exp *mild cognitive impairment/ 19. "cognit* impair*".ti,ab. 20. (forgetful* or confused or confusion).ti,ab. 21. MCI.ti,ab. 22. ACMI.ti,ab. 23. ARCD.ti,ab. 24. SMC.ti,ab. 25. CIND.ti,ab. 26. BSF.ti,ab. 27. AAMI.ti,ab. 28. LCD.ti,ab. 29. QD.ti,ab. 30. AACD.ti,ab. 31. MNCD.ti,ab. 32. MCD.ti,ab. 33. (nMCI or aMCI or mMCI).ti,ab. 34. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 35. "Petersen criteria".ab. 36. ((CDR adj2 "0.5") or ("clinical dementia rating" adj3 "0.5")).ab. 37. "cognit* declin*".ti,ab. 38. "cognit* deficit*".ti,ab. 39. or/18‐38 40. 17 or 39 41. 8 and 40 |
Nov 2012: 11675 May 2014: 2774 |
| 3. PsycINFO 1806‐November week 2 2012 (Ovid SP) Most recent search: 20 May 2014 |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. "cognit* impair*".mp. 26. exp Cognitive Impairment/ 27. MCI.ti,ab. 28. ACMI.ti,ab. 29. ARCD.ti,ab. 30. SMC.ti,ab. 31. CIND.ti,ab. 32. BSF.ti,ab. 33. AAMI.ti,ab. 34. MD.ti,ab. 35. LCD.ti,ab. 36. QD.ti,ab. 37. AACD.ti,ab. 38. MNCD.ti,ab. 39. MCD.ti,ab. 40. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42. "preclinical AD".mp. 43. "pre‐clinical AD".mp. 44. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45. (aMCI or MCIa).ti,ab. 46. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47. ("GDS 3" or "stage 3 GDS").ti,ab. 48. ("global deterioration scale" and "stage 3").mp. 49. "Benign senescent forgetfulness".ti,ab. 50. "mild neurocognit* disorder*".ti,ab. 51. (prodrom* adj2 dement*).ti,ab. 52. "age‐related symptom*".mp. 53. (episodic adj2 memory).mp. 54. ("pre‐clinical dementia" or "preclinical dementia").mp. 55. or/25‐54 56. 24 or 55 57. mini mental state examination/ 58. "mini mental stat*".ti,ab. 59. MiniMental.ti,ab. 60. Folstein*.ti,ab. 61. sMMSE.ti,ab. 62. MMSE*.ti,ab. 63. or/57‐62 64. 56 and 63 |
Nov 2012: 5740 May 2014: 728 |
| 4. Biosis previews 1926 to present (Thomson Reuters Web of Science) Most recent search: 20 May 2014 |
Topic=(MMSE OR sMMSE OR "mini mental stat*" OR folstein* OR MiniMental) AND Topic=(detect* OR diagnos* OR predict* OR identify OR validity OR validation OR validate OR utility OR sensitivity OR specificity OR screen* OR preval* OR incidence) AND Topic=(dement* OR alzheimer* OR cognitive OR cognition OR memory OR MCI OR petersen) Timespan=All Years. Databases=BIOSIS Previews. Lemmatization=On |
Nov 2012: 5713 May 2014: 609 |
| 5. Web of Science Core Collection, including the Science Citation Index and the Conference Proceedings Citation Index (Thomson Reuters Web of Science) Most recent search: 20 May 2014 |
Topic=(MMSE OR sMMSE OR "mini mental stat*" OR folstein* OR MiniMental) AND Topic=(detect* OR diagnos* OR predict* OR identify OR validity OR validation OR validate OR utility OR sensitivity OR specificity OR screen* OR preval* OR incidence) AND Topic=(dement* OR alzheimer* OR cognitive OR cognition OR memory OR MCI OR petersen) Timespan=1975‐01‐01 ‐ 2012‐11‐20. Databases=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH. Lemmatization=On |
Nov 2012: 7337 May 2014: 992 |
| 6. LILACS (BIREME) Most recent search: 20 May 2014 |
MMSE OR folstein OR "mini mental stat$" OR sMMSE OR MiniMental [Words] | Nov 2012: 224 May 2014: 28 |
| 7. ALOIS (CDCIG specialized register searched via the Cochrane Register of Studies) Most recent search: 20 May 2014 |
Index test field: MMSE The Dementia group register is based on a regular search of MEDLINE for diagnostic test accuracy studies using the strategy below. Relevant citations identified are then looked at in full and the healthcare condition of interest and index test/s are extracted and entered into the register. 1. "word recall".ti,ab. 2. ("7‐minute screen" OR “seven‐minute screen”).ti,ab. 3. ("6 item cognitive impairment test" OR “six‐item cognitive impairment test”).ti,ab. 4. "6 CIT".ti,ab. 5. "AB cognitive screen".ti,ab. 6. "abbreviated mental test".ti,ab. 7. "ADAS‐cog".ti,ab. 8. AD8.ti,ab. 9. "inform* interview".ti,ab. 10. "animal fluency test".ti,ab. 11. "brief alzheimer* screen".ti,ab. 12. "brief cognitive scale".ti,ab. 13. "clinical dementia rating scale".ti,ab. 14. "clinical dementia test".ti,ab. 15. "community screening interview for dementia".ti,ab. 16. "cognitive abilities screening instrument".ti,ab. 17. "cognitive assessment screening test".ti,ab. 18. "cognitive capacity screening examination".ti,ab. 19. "clock drawing test".ti,ab. 20. "deterioration cognitive observee".ti,ab. 21. ("Dem Tect" OR DemTect).ti,ab. 22. "object memory evaluation".ti,ab. 23. "IQCODE".ti,ab. 24. "mattis dementia rating scale".ti,ab. 25. "memory impairment screen".ti,ab. 26. "minnesota cognitive acuity screen".ti,ab. 27. "mini‐cog".ti,ab. 28. "mini‐mental state exam*".ti,ab. 29. "mmse".ti,ab. 30. "modified mini‐mental state exam".ti,ab. 31. "3MS".ti,ab. 32. “neurobehavio?ral cognitive status exam*”.ti,ab. 33. "cognistat".ti,ab. 34. "quick cognitive screening test".ti,ab. 35. "QCST".ti,ab. 36. "rapid dementia screening test".ti,ab. 37. "RDST".ti,ab. 38. "repeatable battery for the assessment of neuropsychological status".ti,ab. 39. "RBANS".ti,ab. 40. "rowland universal dementia assessment scale".ti,ab. 41. "rudas".ti,ab. 42. "self‐administered gerocognitive exam*".ti,ab. 43. ("self‐administered" and "SAGE").ti,ab 44. "self‐administered computerized screening test for dementia".ti,ab. 45. "short and sweet screening instrument".ti,ab. 46. "sassi".ti,ab. 47. "short cognitive performance test".ti,ab. 48. "syndrome kurztest".ti,ab. 49. ("six item screener" OR “6‐item screener”).ti,ab. 50. "short memory questionnaire".ti,ab. 51. ("short memory questionnaire" and "SMQ").ti,ab. 52. "short orientation memory concentration test".ti,ab. 53. "s‐omc".ti,ab. 54. "short blessed test".ti,ab. 55. "short portable mental status questionnaire".ti,ab. 56. "spmsq".ti,ab. 57. "short test of mental status".ti,ab. 58. "telephone interview of cognitive status modified".ti,ab. 59. "tics‐m".ti,ab. 60. "trail making test".ti,ab. 61. "verbal fluency categories".ti,ab. 62. "WORLD test".ti,ab. 63. "general practitioner assessment of cognition".ti,ab. 64. "GPCOG".ti,ab. 65. "Hopkins verbal learning test".ti,ab. 66. "HVLT".ti,ab. 67. "time and change test".ti,ab. 68. "modified world test".ti,ab. 69. "symptoms of dementia screener".ti,ab. 70. "dementia questionnaire".ti,ab. 71. "7MS".ti,ab. 72. ("concord informant dementia scale" or CIDS).ti,ab. 73. (SAPH or "dementia screening and perceived harm*").ti,ab. 74. or/1‐73 75. exp Dementia/ 76. Delirium, Dementia, Amnestic, Cognitive Disorders/ 77. dement*.ti,ab. 78. alzheimer*.ti,ab. 79. AD.ti,ab. 80. ("lewy bod*" or DLB or LBD or FTD or FTLD or “frontotemporal lobar degeneration” or “frontaltemporal dement*).ti,ab. 81. "cognit* impair*".ti,ab. 82. (cognit* adj4 (disorder* or declin* or fail* or function* or degenerat* or deteriorat*)).ti,ab. 83. (memory adj3 (complain* or declin* or function* or disorder*)).ti,ab. 84. or/75‐83 85. exp "sensitivity and specificity"/ 86. "reproducibility of results"/ 87. (predict* adj3 (dement* or AD or alzheimer*)).ti,ab. 88. (identif* adj3 (dement* or AD or alzheimer*)).ti,ab. 89. (discriminat* adj3 (dement* or AD or alzheimer*)).ti,ab. 90. (distinguish* adj3 (dement* or AD or alzheimer*)).ti,ab. 91. (differenti* adj3 (dement* or AD or alzheimer*)).ti,ab. 92. diagnos*.ti. 93. di.fs. 94. sensitivit*.ab. 95. specificit*.ab. 96. (ROC or "receiver operat*").ab. 97. Area under curve/ 98. ("Area under curve" or AUC).ab. 99. (detect* adj3 (dement* or AD or alzheimer*)).ti,ab. 100. sROC.ab. 101. accura*.ti,ab. 102. (likelihood adj3 (ratio* or function*)).ab. 103. (conver* adj3 (dement* or AD or alzheimer*)).ti,ab. 104. ((true or false) adj3 (positive* or negative*)).ab. 105. ((positive* or negative* or false or true) adj3 rate*).ti,ab. 106. or/85‐105 107. exp dementia/di 108. Cognition Disorders/di [Diagnosis] 109. Memory Disorders/di 110. or/107‐109 111. *Neuropsychological Tests/ 112. *Questionnaires/ 113. Geriatric Assessment/mt 114. *Geriatric Assessment/ 115. Neuropsychological Tests/mt, st 116. "neuropsychological test*".ti,ab. 117. (neuropsychological adj (assess* or evaluat* or test*)).ti,ab. 118. (neuropsychological adj (assess* or evaluat* or test* or exam* or battery)).ti,ab. 119. Self report/ 120. self‐assessment/ or diagnostic self evaluation/ 121. Mass Screening/ 122. early diagnosis/ 123. or/111‐122 124. 74 or 123 125. 110 and 124 126. 74 or 123 127. 84 and 106 and 126 128. 74 and 106 129. 125 or 127 or 128 130. exp Animals/ not Humans.sh. 131. 129 not 130 |
Nov 2012: 251 May 2014: 31 |
| TOTAL before de‐duplication | Nov 2012: 40993 May 2014: 6818 TOTAL: 47812 |
|
| TOTAL after de‐dupe | 24310 | |
Appendix 3. Information for extraction to proforma
Bibliographic details of primary paper: Author, title of study, year
Details of index test
Language of test
Was any translation of MMSE validated? (yes/no)
MMSE Diagnostic Threshold
Was the threshold pre‐specified? (yes/no)
Who administered the MMSE?
Was index test conducted without knowledge of reference standard results?
Could the conduct or interpretation of the index test have introduced bias?
Notes on conduct of index test
Reference Standard
Target condition
What was the prevalence of dementia in the sample population?
Who administered the reference standard?
Reference Standard
Was any attempt made to subtype dementia categories?
Was reference standard interpreted without knowledge of index test results?
Study population
Country of study
Number of participants
Number of participants in analysis
Patient sampling
Consecutive/random sampling (yes/no)
Did the study avoid inappropriate exclusions? (yes/no)
Could the selection process have introduced bias? (yes/no)
Comments on sampling, inclusions and exclusions
-
What is the patient population?
Unselected community
Community with possible memory problem
Unselected primary care
Primary care with possible memory problem
Age
Gender (% female participants)
Years of education
Social class
Comorbidity
Patient flow and timing
What was the interval between index test and reference standard?
Did all participants receive a reference standard?
Did all participants receive the same reference standard?
Notes of reference standard procedure.
Were all participants included in the analysis?
Were those not included in the analysis fully accounted for?
Notes on patient flow and timing
Other characteristics (e.g. ApoE status)
Attrition and missing data
Appendix 4. Assessment of methodological quality QUADAS‐2
| Domain | Patient selection | Index test | Reference standard | Flow and timing |
| Description | Describe methods of patient selection: Describe included patients (prior testing, presentation, intended use of index test and setting). | Describe the index test and how it was conducted and interpreted | Describe the reference standard and how it was conducted and interpreted. | Describe any patients who did not receive the index test(s) or reference standard or who were excluded from the 2 x 2 table (refer to flow diagram): Describe the time interval and any interventions between index test(s) and reference standard. |
| Signalling questions (yes/no/unclear) | Was a consecutive or random sample of patients enrolled? | Were the index test results interpreted without knowledge of the results of the reference standard? | Is the reference standard likely to correctly classify the target condition? | Was there an appropriate interval between index test(s) and reference standard? |
| Was a case‐control design avoided? | If a threshold was used, was it pre‐specified? | Were the reference standard results interpreted without knowledge of the results of the index test? | Did all patients receive a reference standard? | |
| Did the study avoid inappropriate exclusions? | Did all patients receive the same reference standard? | |||
| Were all patients included in the analysis? | ||||
| Risk of bias: (High/low/ unclear) |
Could the selection of patients have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the patient flow have introduced bias? |
| Concerns regarding applicability: (High/low/ unclear) |
Are there concerns that the included patients do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? | — |
Appendix 5. Anchoring statements for quality assessment of Mini Mental State Examination (MMSE) diagnostic studies
We provide some core anchoring statements for quality assessment of diagnostic test accuracy reviews of the MMSE in dementia. These statements are designed for use with the QUADAS‐2 tool and were derived during a two day, multidisciplinary focus group in 2010. If a QUADAS‐2 signalling question for a specific domain is answered 'yes' then the risk of bias can be judged to be 'low'. If a question is answered 'no' this indicates a risk of potential bias. The focus group was tasked with judging the extent of the bias for each domain. During this process it became clear that certain issues were key to assessing quality, whilst others were important to record but less important for assessing overall quality. To assist, we describe a 'weighting' system. Where an item is weighted 'high risk' then that section of the QUADAS‐2 results table is judged to have a high potential for bias if a signalling question is answered 'no'. For example in dementia diagnostic test accuracy studies, ensuring that clinicians performing dementia assessment are blinded to results of index test is fundamental. If this blinding was not present then the item on reference standard should be scored 'high risk of bias', regardless of the other contributory elements. Where an item is weighted 'low risk' then it is judged to have a low potential for bias if a signalling question for that section of the QUADAS‐2 results table is answered 'no'. Overall bias will be judged on whether other signalling questions (with a high risk of bias) for the same domain are also answered 'no'.
In assessing individual items, the score of unclear should only be given if there is genuine uncertainty. In these situations review authors will contact the relevant study teams for additional information.
Anchoring statements to assist with assessment for risk of bias
Domain 1: Patient selection
Risk of bias: could the selection of patients have introduced bias? (high/low/unclear)
Was a consecutive or random sample of patients enrolled?
Where sampling is used, the methods least likely to cause bias are consecutive sampling or random sampling, which should be stated, described or both. Non‐random sampling or sampling based on volunteers is more likely to be at high risk of bias.
Weighting: high risk of bias
Was a case‐control design avoided?
Case‐control study designs have a high risk of bias, but sometimes they are the only studies available especially if the index test is expensive or invasive. Nested case‐control designs (systematically selected from a defined population cohort) are less prone to bias but they will still narrow the spectrum of patients that receive the index test. Study designs (both cohort and case‐control) that may also increase bias are those designs where the study team deliberately increases or decreases the proportion of subjects with the target condition, for example a population study may be enriched with extra dementia subjects from a secondary care setting.
Weighting: High risk of bias
Did the study avoid inappropriate exclusions?
The study will be automatically graded as unclear if exclusions are not detailed (pending contact with study authors). Where exclusions are detailed, the study will be graded as 'low risk' if exclusions are felt to be appropriate by the review authors. Certain exclusions common to many studies of dementia are: medical instability; terminal disease; alcohol/substance misuse; concomitant psychiatric diagnosis; other neurodegenerative condition. However if 'difficult to diagnose' groups are excluded this may introduce bias, so exclusion criteria must be justified. For a community sample we would expect relatively few exclusions. Post hoc exclusions will be labelled 'high risk' of bias.
Weighting: high risk of bias
Applicability: are there concerns that the included patients do not match the review question? (high/low/unclear)
The included patients should match the intended population as described in the review question. If not already specified in the review inclusion criteria, setting will be particularly important – the review authors should consider population in terms of symptoms; pre‐testing; potential disease prevalence. Studies that use very selected subjects or subgroups will be classified as low applicability, unless they are intended to represent a defined target population, for example, people with memory problems referred to a specialist and investigated by lumbar puncture.
Domain 2: Index Test
Risk of bias: could the conduct or interpretation of the MMSE have introduced bias? (high/low/unclear)
Were the MMSE results interpreted without knowledge of the reference standard?
Terms such as 'blinded' or 'independently and without knowledge of' are sufficient and full details of the blinding procedure are not required. This item may be scored as 'low risk' if explicitly described or if there is a clear temporal pattern to the order of testing that precludes the need for formal blinding i.e. all MMSE assessments were performed before the dementia assessment. As most neuropsychological tests are administered by a third party, knowledge of dementia diagnosis may influence their ratings; tests that are self‐administered, for example using a computerised version, may have less risk of bias.
Weighting: High risk
Were the MMSE cut points pre‐specified?
For neuropsychological scales there is usually a cut point above which subjects are classified as 'test positive'; this may also be referred to as threshold; clinical cut‐off or dichotomisation point. Different cut points are used in different populations. A study is classified at higher risk of bias if the authors define the optimal cut‐off post hoc based on their own study data. Certain papers may use an alternative methodology for analysis that does not use thresholds, and these papers should be classified as not applicable.
Weighting: low risk
Were sufficient data on MMSE application given for the test to be repeated in an independent study?
Particular points of interest include method of administration (for example self‐completed questionnaire versus direct questioning interview); nature of informant; language of assessment. If a novel form of the index test is used, for example a translated questionnaire, details of the scale should be included and a reference given to an appropriate descriptive text, and there should be evidence of validation.
Weighting: low risk
Applicability: are there concerns that the MMSE, its conduct, or interpretation differ from the review question? (high/low/unclear)
Variations in the length, structure, language and administration of the index test may all affect applicability if they vary from those specified in the review question.
Domain 3: Reference Standard
Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias? (high/low/unclear)
Is the reference standard likely to correctly classify the target condition?
Commonly used international criteria to assist with clinical diagnosis of dementia include those detailed in DSM‐IV and ICD‐10. Criteria specific to dementia subtypes include but are not limited to NINCDS‐ADRDA criteria for Alzheimer’s dementia; McKeith criteria for Lewy Body dementia; Lund criteria for frontotemporal dementias; and the NINDS‐AIREN criteria for vascular dementia. Where the criteria used for assessment is not familiar to the review authors and the Cochrane Dementia and Cognitive Improvement group this item should be classified as 'high risk of bias'.
Weighting: high risk
Were the reference standard results interpreted without knowledge of the results of the MMSE?
Terms such as 'blinded' or 'independent' are sufficient, and full details of the blinding procedure are not required. This may be scored as 'low risk' if explicitly described or if there is a clear temporal pattern to order of testing i.e. all dementia assessments performed before [neuropsychological test] testing.
Informant rating scales and direct cognitive tests present certain problems. It is accepted that informant interview and cognitive testing is a usual component of clinical assessment for dementia, however specific use of the scale under review in the clinical dementia assessment should be scored as high risk of bias.
Weighting: high risk
Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study?
Particular points of interest for dementia assessment include the training/expertise of the assessor; and whether additional information was available to inform the diagnosis (e.g. neuroimaging; other neuropsychological test results), and whether this was available for all participants.
Weighting: variable risk, but high risk if method of dementia assessment not described
Applicability: are there concerns that the target condition as defined by the reference standard does not match the review question? (high/low/unclear)
There is the possibility that some methods of dementia assessment, although valid, may diagnose a far smaller or larger proportion of subjects with disease than in usual clinical practice. In this instance the item should be rated poor applicability.
Domain 4: Patient flow and timing (n.b. refer to, or construct, a flow diagram)
Risk of bias: could the patient flow have introduced bias? (high/low/unclear) Was there an appropriate interval between the MMSE and reference standard?
For a cross‐sectional study design, there is potential for the subject to change between assessments, however dementia is a slowly progressive disease, which is not reversible. The ideal scenario would be a same day assessment, but longer periods of time (for example, several weeks or months; and up to six months) are unlikely to lead to a high risk of bias.
Weighting: low risk
Did all subjects receive the same reference standard?
There may be scenarios where subjects who score 'test positive' on the index test have a more detailed assessment for the target condition. Where dementia assessment (or reference standard) differs between subjects this should be classified as high risk of bias.
Weighting: high risk
Were all subjects included in the final analysis?
Drop outs (and missing data) should be accounted for. Attrition that is higher than expected (compared to other similar studies) should be treated as a high risk of bias.
Weighting: high risk
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 MMSE at 14 normality | 1 | 435 |
| 2 MMSE at 15 normality | 2 | 935 |
| 3 MMSE at 16 normality | 1 | 435 |
| 4 MMSE at 17 normality | 4 | 1332 |
| 5 MMSE at 18 normality | 9 | 3848 |
| 6 MMSE at 19 normality | 9 | 4450 |
| 7 MMSE at 20 normality | 9 | 4092 |
| 8 MMSE at 21 normality | 8 | 4555 |
| 9 MMSE at 22 normality | 9 | 4899 |
| 10 MMSE at 23 normality | 11 | 4750 |
| 11 MMSE at 24 normality (23/24) | 25 | 12092 |
| 12 MMSE at 25 normality | 16 | 6744 |
| 13 MMSE at 26 normality | 9 | 5093 |
| 14 MMSE at 27 normality | 6 | 4624 |
| 15 MMSE at 28 normality | 3 | 2930 |
| 16 MMSE at 29 normality | 2 | 691 |
| 17 MMSE at 30 normality | 1 | 435 |
| 18 MMSE adjusted for education | 8 | 8630 |
| 22 MMSE at 10 normality | 1 | 500 |
| 23 Main analysis | 27 | 13790 |
1. Test.

MMSE at 14 normality.
2. Test.

MMSE at 15 normality.
3. Test.

MMSE at 16 normality.
4. Test.

MMSE at 17 normality.
5. Test.

MMSE at 18 normality.
6. Test.

MMSE at 19 normality.
7. Test.

MMSE at 20 normality.
8. Test.

MMSE at 21 normality.
9. Test.

MMSE at 22 normality.
10. Test.

MMSE at 23 normality.
11. Test.

MMSE at 24 normality (23/24).
12. Test.

MMSE at 25 normality.
13. Test.

MMSE at 26 normality.
14. Test.

MMSE at 27 normality.
15. Test.

MMSE at 28 normality.
16. Test.

MMSE at 29 normality.
17. Test.

MMSE at 30 normality.
18. Test.

MMSE adjusted for education.
22. Test.

MMSE at 10 normality.
23. Test.

Main analysis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ADAMS Study 2007.
| Study characteristics | |||
| Patient sampling | The ADAMS study sample came from the nationally representative Health and Retirement Study (HRS) as sample frame. From the larger HRS sample, a random subsample of 1770 individuals aged 70 or over was selected for participation in the ADAMS, with the goal of obtaining clinical assessments on about 850 individuals. In order to ensure a sufficient number of respondents across the full range of cognitive ability, investigators stratified the sample based on cognitive status. 5 cognitive strata (ranging from 'low functioning' to 'high normal') were defined based on respondents' performance on the cognitive measures in the most recent HRS interview (either 2000 or 2002, depending on the timing of recruitment into the ADAMS). Investigators used scores on the full set of HRS cognitive tests (ranging from 0 to 35 points) to classify self‐respondents, and scores on the IQCODE were used to classify proxy respondents. The cognitively normal group was further stratified by age (70–79 versus ≥ 80) and sex in order to ensure adequate numbers in each of these subgroups. The original HRS sample consisted of individuals born between 1931 and 1941, inclusive. This sample came from a screening of 69,336 households that was conducted in 1992. That sample of households was generated using a multi‐stage, clustered area probability frame. The second sample was generated for what began as a separate study: Asset and Health Dynamics among the Oldest Old (AHEAD). This sample consists of individuals born in 1923 or before. Those born between 1914 and 1923, and about half of those born in 1913 or before, were identified through the same household screening used to identify the original HRS sample. The other half of those born in 1913 or before were identified using the Medicare enrolment files maintained by the Health Care Financing Administration (HCFA, since renamed the Centres for Medicare & Medicaid Services, or CMS). In 1998, the HRS and AHEAD studies were merged, with a single interview schedule. 856 adults were sampled from ADAMS, however 155 of these were excluded due to not completing the MMSE. |
||
| Patient characteristics and setting | Participants included 509 white, 124 African Americans, and 68 Latinos (> 70 years old) from the Aging, Demographics, and Memory Study who completed the MMSE and FOME. There were 314 men, average age 80.5 years. Average education was 9.2 years. Average MMSE score was 23. | ||
| Index tests | MMSE, non‐validated Spanish versions where necessary. | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐IV. Participants consented to a 3‐4 h structured assessment conducted in‐home, including a medical examination with a nurse and a neuropsychological battery with a trained psychometrician. A panel of 3 expert scientists, including a neurologist, cognitive neuroscientist, and geropsychiatrist determined the participants' initial DSM‐IV cognitive status based on the in‐home diagnostic evaluation, which assessed several cognitive domains. The final cognitive status was made by a consensus panel of experts based on a review of the information collected through the neuropsychological, medical, and neurological assessment measures. We assess this as meaning that not all 701 participants were clinically evaluated by a specialist. |
||
| Flow and timing | All participants who received MMSE also received the reference standard. Note that this was due to the way that the sample was selected and we have accounted for this in the QUADAS‐2 item regarding inappropriate exclusions. | ||
| Comparative | |||
| Notes | Participants were excluded if they couldn't/hadn't completed the MMSE. We contacted the authors to ask them to provide 2 x 2 data, unstratified by ethnicity, but they were unable to do this. The diagnostic accuracy stratified by ethnicity was: For white participants, normal vs. dementia: sensitivity 0.87, specificity 0.98 (no confidence intervals; cut point 24 indicating normal; 509 participants, 129 with dementia) For African American participants, normal vs. dementia: sensitivity 0.92, specificity 0.84 (no confidence intervals; cut point 21 indicating normal; 124 participants, 37 with dementia) For Latino participants, normal vs. dementia: sensitivity 0.92, specificity 0.84 (no confidence intervals, cut point 21 indicating normal; 68 participants 13 with dementia) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Aevarsson 2000.
| Study characteristics | |||
| Patient sampling | Community sample of adults on census | ||
| Patient characteristics and setting | All 85‐year olds, in Goteborg, Sweden, who were registered for census, were invited for survey and a systematic subsample (N = 494) were clinically evaluated and comprise this diagnostic test accuracy study. 1986, 1987 | ||
| Index tests | Swedish MMSE | ||
| Target condition and reference standard(s) | Dementia. DSM‐III‐R criteria and subtypes ‐ possibility of incorporation bias as MMSE formed a part of the reference standard | ||
| Flow and timing | Information collected at 1 interview, but informant interview considered separately to examination | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
AMSTEL Study 1997.
| Study characteristics | |||
| Patient sampling | Subjects were participants in AMSTEL 2 phase population study. 4051 participants aged 65‐84 were recruited. The population base for the AMSTEL study included all individuals aged 65‐84 who lived in Amsterdam and were registered with a GP. Within each practice a fixed proportion of respondents was randomly selected from each of four 5‐year age strata (65‐69, . . . 80‐84). | ||
| Patient characteristics and setting | 64.2% women, average age 75.4, 8.2 years of education, mean MMSE score 26.9 | ||
| Index tests | Non‐validated Dutch translation of MMSE | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R using CAMDEX as a framework | ||
| Flow and timing | 4051 participants had MMSE, in phase II everybody with an MMSE of < 22, an age stratified sample of people with MMSE scores > 22 were invited (N = 511). Those people were seen and assessed a median of 7 weeks (range 1‐22) after the index test. | ||
| Comparative | |||
| Notes | We were unable to include this in the meta‐analysis. The marginal totals were 261 disease positives, 3790 disease negatives, 72 test positives. After several lots of correspondence with the authors, we were provided with some sensitivity and specificity data (for cut point 24 indicating normality this was sensitivity 0.69, specificity 0.47); however, it was not possible to reconcile the 2 sets of figures. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Baker 1993.
| Study characteristics | |||
| Patient sampling | The sample was drawn from 2 sites in San Antonio, Texas: a senior citizen housing complex and a senior centre. The senior housing complex was comprised of 72 apartments in 2‐storey dwellings located in a section of the city that was predominantly African American. The senior citizen centre was located in the same area. In addition to providing hot meals, health screening programmes, and various community activities, it also provided the opportunity for volunteer activities. | ||
| Patient characteristics and setting | The sample was African American and comprised 41 females and 14 men (75% female), mean age was 79. Mean MMSE 26. | ||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | Reference standard was applied to all 55 participants within an average of 7 days (3 interviews were completed outside of this time‐frame due to conflicting schedules). | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Brodaty 2002.
| Study characteristics | |||
| Patient sampling | "Of 380 community‐dwelling patients recruited by their GPs, 283 completed the study. Patients were included if they were aged 75 years or more regardless of cognitive status. To imitate usual practice, subjects aged 50 to 74 suspected of having a memory problem were also included. Patients were excluded if they resided in a nursing home; if they had a diagnosis of depression or delirium; or if poor English language abilities, sight, or hearing precluded testing. "GPs were asked to administer the GPCOG (before subsequent refinement) and AMT to consecutive eligible patients and to contact an informant (by telephone or in person) who had known the patient for at least 5 years. Approximately 5 weeks later, a research psychologist visited the patient at home, administered the various instruments, including the CAMDEX and the GPCOG again, and, where possible, interviewed an informant." |
||
| Patient characteristics and setting | "There were no differences between participants diagnosed with dementia and those without dementia as regards gender (40.6% of the sample were male), relationship with informant (40.6% were spouses), living arrangements (87.6% lived in a private home and the others in retirement villages or hostels), or education (mean 9.4 years). Patients' overall mean age was 79.6 years (range 56–94); 32 patients (11.3%) were aged 50 to 75. Those diagnosed with dementia were older (mean 80.7 years) than those without dementia (79.1 years) and less likely to be living with an informant (71.1% and 82.9%, respectively)." | ||
| Index tests | "Psychologist‐administered MMSE" | ||
| Target condition and reference standard(s) | Dementia as diagnosed according to DSM‐IV | ||
| Flow and timing | "[D]iagnoses of dementia and delirium were established according to DSM‐IV criteria on all 156 subjects suspected to be cognitively impaired (CAMCOG score < 85) and a random sample of 20 cognitively intact individuals (CAMCOG score > 84) (62.2% of all cases reviewed). No case was found to meet criteria for delirium." | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Burkart 2000.
| Study characteristics | |||
| Patient sampling | Age‐stratified random sample of 1305 subjects with over‐sampling of advanced age, invited by letters and telephone | ||
| Patient characteristics and setting | Community: 291 consented but only 256 completed tests n = 256 for analysis | ||
| Index tests | MMSE (as part of SIDAM = Structured Interview for the Diagnosis of Dementia of the Alzheimer Type, Multi‐infarct dementia and dementias of other aetiologies). SIDAM takes 28 minutes rather than MMSE 7 minutes (Zaudig 1991) Given by trained medical students |
||
| Target condition and reference standard(s) | Dementia. ICD‐10 and DSM‐III‐R | ||
| Flow and timing | Medical students performed personal interviews. Psychiatrists made formal diagnoses. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Callahan 2002.
| Study characteristics | |||
| Patient sampling | "For the community‐based sample, the geographic target area consisted of 29 contiguous census tracts with a total population of 82,387 and total households of 32,954 in the 1990 US Census. Black persons comprised 86% of this population, which also represents more than 2/3 of Indianapolis' elderly black population. A random sample of 60% of residential addresses was constructed by the IndianapolisWater Company using all residential addresses in the target area, and identified homes were then visited by interviewers from May 1, 1992‐April 30, 1993. Patients residing in nursing homes are not included in this sample. Eligible subjects had to be (1) a resident at a sampled address, (2) black, and (3) age 65 years or older. A total of 7590 households were approached, 4915 of which did not have an eligible resident. Of the 2582 eligible persons, 2212 (85.7%) agreed to participate. These subjects were screened with the Community Screening Instrument for Dementia (CSI‐D) . . . "Items for the CSI‐D were selected from several widely used screening instruments including . . . the Mini‐Mental State Examination." |
||
| Patient characteristics and setting | Community‐based sample only 344 participants Mean age 74.4 years (range 65‐99) 59.4% women 100% black 10.4 years of education 4.3% dementia 26.4% cognitively impaired Mean MMSE 26.1 |
||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Dementia, DSM‐III‐R AND ICD‐10 (Both necessary). NINCDS/ADRDA for possible or probable Alzheimer's disease |
||
| Flow and timing | "A stratified sample of the community‐based subjects was selected for full clinical assessments based on their performance on the CSI‐D. All subjects who scored poorly on the CSI‐D were invited for clinical assessments and we also selected a 50% sample of those with intermediate performance, and a 5% sample of those with good performance. Patients aged 75 and older were over‐sampled in the 5% sample so that 75% of the patients with good performance on the CSI‐D would be 75 years of age or older . . . "There were 351 patients selected for full clinical assessments but seven were too severely impaired to complete the standardized questionnaires. Data for the remaining 344 (98%) subjects are included here" |
||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Carnero‐Pardo 2013.
| Study characteristics | |||
| Patient sampling | Sampled from Granada and Madrid, Spain. Sampling was prospective and consecutive and included all those subjects who had memory loss complaints from the patient, the family or the person accompanying them, or that was suspected by the doctor on the basis of general observations. | ||
| Patient characteristics and setting | 255 (70.8%) women and 105 men, average age 72.6 years, 30 people (8.3%) were illiterate, 180 had less than primary education, 180 had more than primary education. Average MMSE score was 21.1 | ||
| Index tests | MMSE The MMSE was carried out in primary care, validated in the NORMACODEM study (Blesa 2001) "doing without the spelling of world backwards" ‐ this is one of the items in the MMSE, and an alternative question of serial subtractions of 7 from 100 may be used instead. | ||
| Target condition and reference standard(s) | Dementia, DSM‐IV‐TR The reference standard was conducted in specialised care by an expert neurologist, without knowing the results of the MMSE. |
||
| Flow and timing | All of the selected subjects independent of the results of the screening test underwent the reference standard (complete verification) In the study from Granada, as it is explained in the primary study record, the maximum time interval between assessments in the Primary Care Center and Cognitive Behavioral Neurology Unit was 2 weeks. In the study from Madrid, the interval between both evaluations was 31.05 ± 49.2 days (range 0‐329) The study in Granada was conducted in 4 health centres during 1 year (1 Feb 2008 to 31 Jan 2009); whereas the sample from Madrid was largely (174 subjects) from 1 health centre and was selected between 1 April 2000 and 31 October 2002. |
||
| Comparative | |||
| Notes | Translated by Mr William Eustace on 31 October 2014. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Correira 2001.
| Study characteristics | |||
| Patient sampling | City of Recife, Brazil. The elderly were identified and invited to participate in a survey by a trained census or community health agent working in the area who scheduled an interview with the researcher. | ||
| Patient characteristics and setting | Community‐dwelling elderly aged 65 or more, had to have an informant who had known them for at least 10 years prior to the study. | ||
| Index tests | MMSE conducted in Portuguese. The MMSE cut point scores for cognitive impairment according to education were set as follows: Illiterate = 20, 1–4 years of education = 25, 5–8 years of education = 26, 9–11 years of education = 28, and 29 for those with more than 11 years of schooling. | ||
| Target condition and reference standard(s) | Dementia. DSM‐IV | ||
| Flow and timing | The data were collected from October 2008 to January 2009 | ||
| Comparative | |||
| Notes | The education adjusted thresholds, particularly for those with more than 11 years of schooling were very high (29 out of 30 for this group,) and we are unsure how this would be applicable. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Cruz‐Orduna 2012.
| Study characteristics | |||
| Patient sampling | Systematically included all individuals who attended the 7 medical clinics of the Pena Prieta Primary Care Centre (Health District 1, Autonomous Community of Madrid) between 1 April 2000 and 31 October 2002. | ||
| Patient characteristics and setting | Age 50‐88 inclusive
Any complaint or suspicion, raised by the patient, an informant or the PCP, related to cognition, cognition‐
related functions (i.e. performance of activities of daily living, ADLs) or behaviour, of unknown aetiology
Those with no informant were excluded 70.9% women In half of the cases (49.5%), patient and informant lived together. |
||
| Index tests | MMSE (validated Spanish version) | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐IV‐R | ||
| Flow and timing | If the patient and the informant gave their consent, the detection instruments were applied and an appointment was made to carry out a formal neuropsychological workup some days later. Patients who did not present with an informant and wished to undergo the formal neuropsychological evaluation and assessment by a neurologist were permitted to do so, although they were not included in the present study. | ||
| Comparative | |||
| Notes | 27% of the 15 people with dementia were illiterate, compared with 7% of the people with MCI and 0 of those with normal cognition. 9% of those with normal cognition had a superior education compared with 4% of those with MCI and 0 of those with dementia. The mean MMSE score we quote in the covariate section is taken from the related Olazarán paper which includes those people with no informant (16 people who weren't included in the analysis): 25.1 = NCI 20.8 = MCI 16.1 = dementia |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Eefsting 1997.
| Study characteristics | |||
| Patient sampling | Multi‐stage stratified randomised sampling | ||
| Patient characteristics and setting | Community based population | ||
| Index tests | MMSE Dutch validated version | ||
| Target condition and reference standard(s) | Dementia | ||
| Flow and timing | A proportion of each scoring band was given the reference standard within 6 weeks of index test. | ||
| Comparative | |||
| Notes | The diagnostic utility was weighted to the whole population (2151) based on 390 people who were clinically assessed. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Fichter 1995.
| Study characteristics | |||
| Patient sampling | Random sample of 402 people were invited, of whom 358 participated | ||
| Patient characteristics and setting | Over 85 in Munich in 1990. Sample included residents of homes for the elderly | ||
| Index tests | MMSE included in SIDAM | ||
| Target condition and reference standard(s) | All dementias according to DSM‐III‐R | ||
| Flow and timing | Index test and reference standard were done concurrently | ||
| Comparative | |||
| Notes | There may be some incorporation bias due to same person doing both tests concurrently. No information on diagnostic accuracy is available despite attempting to contact the authors |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Fillenbaum 1990.
| Study characteristics | |||
| Patient sampling | Stratified from larger sample of 4164 residents. They sampled based on the SPMSQ score and assessed 164 people with MMSE, all of whom underwent a reference standard. | ||
| Patient characteristics and setting | Half of the sample lives in a primarily urban county, half in rural counties. All levels of education and socioeconomic status represented. Mixed ethnicity. 54% black. > 65 years old. | ||
| Index tests | MMSE administered as part of a battery of tests | ||
| Target condition and reference standard(s) | Dementia according to DSM‐III | ||
| Flow and timing | We have concerns because although 164 people underwent both index test and reference standard, the diagnostic utility is presented as representative of 4164 even though the vast majority of them didn't undergo either of the tests. | ||
| Comparative | |||
| Notes | The diagnostic utility was weighted to the whole population (4164) based on 164 people who were clinically assessed. There was no raw data for the 164 people who actually received the test. Specificity was presented for black people and white people respectively as 58% and 94% and sensitivity was reported as 100% in both groups, though as stated we have concerns because the figures are "weighted to represent the total five county black and white community". No raw data is available despite attempting to contact authors. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Frank 1996.
| Study characteristics | |||
| Patient sampling | 1692 community‐dwelling people aged 55 and older from the Rancho Bernardo study. | ||
| Patient characteristics and setting | Of the sample, 380 people were in this analysis aged 65‐94. 75% of men and 64% women attended college. There were 167 men and 213 women. | ||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Alzheimer's disease diagnosed according to NINCS‐ARDRA | ||
| Flow and timing | No information given on timing. It would appear that everyone who underwent the index test also received the reference standard. | ||
| Comparative | |||
| Notes | No information on diagnostic accuracy was available despite attempting to contact the authors | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Helsinki Aging Study 1994.
| Study characteristics | |||
| Patient sampling | A random sample was identified from the community register which included all citizens of Helsinki: 300 75‐year olds, 300 80‐year olds and 300 85‐year olds living in Helsinki on 1 January 1989 ‐ a total 656 participants, which was 83% of the sample (240 aged 75, 214 aged 80 and 202 aged 85). | ||
| Patient characteristics and setting | Community sample of people > 75 in Helsinki, Finland. Urban population. Reference standard was given only to those with a CDR score of ≥ 0.5 (i.e. those with MCI or dementia) | ||
| Index tests | MMSE (presumably Finnish or Russian but not stated) | ||
| Target condition and reference standard(s) | All dementia as diagnosed by DMS‐III‐R | ||
| Flow and timing | 17 subjects were unable to complete the MMSE due to severe dementia that was diagnosed at reference standard. Subjects were given a CDR as assessed by a GP. On the basis of this, screen positives (people scoring 0.5 or more) were given neurological evaluation (N = 174). | ||
| Comparative | |||
| Notes | Because of partial verification it is not possible to fully complete the 2 x 2 table as only information on people who had dementia is available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Heun 1998.
| Study characteristics | |||
| Patient sampling | Subjects were a stratified sample with an over‐representation of older and male subjects for invitation. Interview rate was 291 of 1193. Participants were younger, more often male and cognitively normal than invited population. | ||
| Patient characteristics and setting | Community setting. Patients aged between 60‐100 | ||
| Index tests | MMSE as part of SIDAM | ||
| Target condition and reference standard(s) | Dementia according to DSM‐III‐R | ||
| Flow and timing | MMSE was conducted as part of the SIDAM and formal diagnosis was made with the information gathered concurrently at a later point. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Iavarone 2006.
| Study characteristics | |||
| Patient sampling | A random sample of 300 residents out of 1089 residents > 60 years of age living in San Marcinello (Campania) received door‐to‐door visit by a specialised team including a geriatrician and a 5th year psychology student. | ||
| Patient characteristics and setting | Residents of a rural community in Southern Italy (San Marcinello, province of Caserta). Average age was 71.9, average 3.2 years of education, average MMSE was 19.7, 58% women. | ||
| Index tests | Validated version of the Italian MMSE, corrected for education and age | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐IV. Due to the fact that the reference standard was performed at the same time as the index tests, we have some concerns that the outcome of the reference standard may have been affected by the index test scores. | ||
| Flow and timing | Index test and reference standard information was collected in the same interview for all participants. Due to this process there may be some risk of incorporation bias. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Jacinto 2011.
| Study characteristics | |||
| Patient sampling | This study was carried out at the Albert Einstein long‐term care institutions (LTCI), a Brazilian facility for elderly that includes a nursing home service, an assisted living facility, and an outpatient geriatric clinic. It was a cross‐sectional study with 86 elders being invited to participate; they were independent and semi‐dependent residents living in the LTCI. 58 agreed to participate in the study; the remaining 28 who refused had no statistical difference in relation to gender, age and dependency level (P > 0.05 ‐ actual p value not given) when compared to the included ones. |
||
| Patient characteristics and setting | As population ages, LTCIs play a crucial role in the elderly care. Although LTCIs are historically characterised as places where care‐demanding people live, more frequently healthy elderly have decided to live in these facilities for many different reasons (more intense social contact or even enjoying what is offered at LTCIs such as balanced and proper food, recreational and physical activity and specialised medical care). The participants had 60 or more years and mean educational level of 10 years. | ||
| Index tests | MMSE (we assume a validated version of the Portuguese translation). "The Mini Mental State Examination (MMSE) has been widely studied in different populations, including in Brazil. Its performance is closely related to schooling and cutoff scores for the Brazilian population have been well established." The MMSE cut points were adjusted according to level of education (as from Bertolucci et al 1994): 13 for illiterate, 18 for elementary and middle school educated and 26 for high school education. |
||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐IV | ||
| Flow and timing | There is no description of the flow and timing of this paper. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Jeong 2004.
| Study characteristics | |||
| Patient sampling | Participants > 65 were recruited in Noam‐dong, Namwon City, South Korea. Of 522 eligible, 235 completed tests. | ||
| Patient characteristics and setting | General community population, small South Korean city of 6883 population | ||
| Index tests | Korean version of MMSE | ||
| Target condition and reference standard(s) | DSM‐IV | ||
| Flow and timing | No information given on timing of reference standard. All participants received the reference standard. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Kahle‐Wrobleski 2007.
| Study characteristics | |||
| Patient sampling | At the beginning of 2001, 1150 of the original Southern California Leisure World 90 + study cohort were invited to join. N = 524 | ||
| Patient characteristics and setting | 90+ LeisureWorld (retirement community) inhabitants | ||
| Index tests | MMSE as part of 3MS | ||
| Target condition and reference standard(s) | Dementia according to DSM‐IV | ||
| Flow and timing | Concurrent index test and reference standard. 86 participants failed to complete evaluation and were documented. 3 additional participants did not report their educational status and were excluded. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Kathriarachchi 2005.
| Study characteristics | |||
| Patient sampling | All households with individuals > 65 were selected from a database of households maintained by Colombo District University of Sri Jayewardenepura and from that, a stratified computer‐generated random sample of 400 people was selected as the study population. Of those, they evaluated only 363. | ||
| Patient characteristics and setting | Semi‐urban community in Sri Lanka | ||
| Index tests | MMSE Sinhalese version, previously validated by Da Silva 2002 | ||
| Target condition and reference standard(s) | Dementia diagnosed according to CDR | ||
| Flow and timing | 400 participants were initially identified; 363 were evaluated at phase I. The rest were difficult to assess due to unavailability of the selected individuals or their caregivers after repeated attempts to contact them. Of the 363, a sample of 40 individuals was selected for phase II. This was a concentrated sample of individuals who had scores ranging from normal to severe dementia on the rating scales (MMSE, IQCODE). Of the 40 who were selected for phase II, only 37 were evaluated. 3 were not evaluated due to death or moving. Of the 37 evaluated on phase II, 14 had dementia. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Keskinoglu 2009.
| Study characteristics | |||
| Patient sampling | Population of the elderly were 4012 subjects living in Narlıdere, and cluster sampling method was used for sample size. Using the Epi‐Info 2000 package, the minimum sample size based on sensitivity of 95%, 2% precision confidence interval (CI) of 95% was calculated as 407 elderly subjects. Given the design effect (1.2), the calculated sample size was 488 elderly. The number of houses including this sample size was requested from Turkish Statistical Institute (TUIK). TUIK reported 2601 houses, including 50 clusters of houses for sample size of district, and 517 elderly persons were determined to reside in a total of 2401 visited houses. Participation rate was 94.8% (490 elderly). | ||
| Patient characteristics and setting | This cross‐sectional study was conducted among elderly individuals aged ≥ 65 in the town of Narlýdere (an urban area) in the Turkish province of Izmir. Mean age of the total 490 elderly subjects was 71.8 years (range 65–114 years; SD 6.5). Of the elderly population, 59.2% were females, 70.8% fell in the younger age group (65–74 years) and 62.7% were married. The mean schooling year was 1.6 years (SD 0.07), 34.7% were illiterate and 50.4% did not graduate from primary school. In our study population, 9.8% of the subjects were socially uninsured, and 27.2% did not have any personal income. | ||
| Index tests | rMMSE‐T. Researchers exposed some problems regarding some items of the previously validated Turkish version of MMSE (MMSE‐T). The index test in this study is an attempt to make a Turkish version that is more like the original Folstein MMSE. | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐IV‐R | ||
| Flow and timing | All subjects who screened positive and negative were transported to Dokuz Eylul University Hospital, Department of Neurology in same week, and clinical diagnosis of dementia were made by the senior neurologist using DSM‐IV‐R. | ||
| Comparative | |||
| Notes | Data on diagnostic accuracy were only available stratified by educational level, and not adjusted for education. We attempted to contact the authors to obtain non‐stratified data but had no reply. Because it is not clear what level of education would meet the definition of 'educated' we were unable to combine the stratified tables. Total 490 participants (170 illiterate, 77 literate, 128 primary school, 29 middle school, 53 high school, 3 university) For educated elderly (definition and numbers unclear, no confidence intervals) Cut points 19 indicating normal sensitivity 45.5%, specificity 97.8% 20 indicating normal sensitivity 63.6%, specificity 97.8% 21 indicating normal sensitivity 72.7%, specificity 97.0% 22 indicating normal sensitivity 81.8%, specificity 97.0% 23 indicating normal sensitivity 90.9%, specificity 97.0% 24 indicating normal sensitivity 90.9%, specificity 89.7% 25 indicating normal sensitivity 90.9%, specificity 78.4% For educated elderly (definition unclear and numbers unclear, no confidence intervals) Cut points 18 indicating normal sensitivity 76.9%, specificity 92.8% 19 indicating normal sensitivity 82.7%, specificity 92.3% 20 indicating normal sensitivity 86.5%, specificity 85.1% 21 indicating normal sensitivity 88.5%, specificity 77.9% 22 indicating normal sensitivity 92.3%, specificity 73.3% 23 indicating normal sensitivity 92.3%, specificity 65.6% |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kungsholmen Study 1992.
| Study characteristics | |||
| Patient sampling | All inhabitants > 74 years of age in an area of Stockholm (Kungsholmen) in October 1987 (2368 individuals) living at home or in institutions. 1810 participated. | ||
| Patient characteristics and setting | Community setting over 74 in an area of Stockholm, Sweden. Relatively well‐educated (mean 8.77 years of education) | ||
| Index tests | MMSE Swedish version | ||
| Target condition and reference standard(s) | All dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | The nurses saw the patients to do MMSE an average of 2 months before the clinical diagnosis. In phase 1 the MMSE was used as a screening test with 24 or above indicating normality. In phase 2 all of those scoring 23 or less (385) plus a gender‐ and age‐matched sample (354) of the screen negatives (total 1425) were given a clinical diagnosis. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Lam 2008.
| Study characteristics | |||
| Patient sampling | Households were randomly selected from census records in Hong Kong. 6891 > 60 were identified and 6100 received MMSE. Nested case‐control. | ||
| Patient characteristics and setting | Community‐based sample of people aged > 60 in Hong Kong | ||
| Index tests | Validated version of Chinese MMSE | ||
| Target condition and reference standard(s) | Dementia according to DSM‐IV | ||
| Flow and timing | 6891 invited, 6100 participated MMSE of those 2073 screened positive (CMMSE < 19 for illiterate participants; < 21, 1‐2 years education; < 23, 2 + years education). Of those, 1336 refused to participate in reference standard and 737 went on to reference standard, of those 143 had dementia. 194 of the 4027 who screened negative were clinically assessed, of whom none had dementia. Not clear what the timing of the reference standard was in relation to index test. |
||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Lavery 2007.
| Study characteristics | |||
| Patient sampling | "The Steel Valley Seniors Survey was a clinical epidemiologic study of dementia in primary care patients aged 65 years and older in a small‐town community in southwestern Pennsylvania. From 1999 to 2001, participants were recruited from the offices of 15 physicians who provided care to older adults and agreed to provide access to patients and to medical records of consenting patients. Participants were designated as 'symptomatic' if self‐reported memory complaints were documented in their charts. Reports by family members, if any, were excluded because many participants were unaccompanied by relatives when they visited their physicians." | ||
| Patient characteristics and setting | "Among the 642 participants selected for comprehensive assessment, those who did and did not undergo the assessment were similar (P > 0.05 ‐ actual p value not given) with regard to age (77.5 vs 77.7 years), sex, (68.7% vs 64.0% women), education (66.8% vs 63.3% with ≥ 12 years), and race (92.5% vs 93.5% white). However, those assessed had a marginally higher mean (SD) MMSE score than those who were not (24.5 (SD 3.4) vs 24.0 (SD 3.2), P = 0.047)." | ||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Dementia according to CDR ("A standard algorithm is used to generate a summary score ranging from 0 (no dementia) through 0.5, 1, 2, and 3, reflecting questionable, mild, moderate, and severe dementia. A CDR rating ≥1 was treated as a diagnosis of dementia." | ||
| Flow and timing | "All participants who scored ≤ 24 on the MMSE, and a randomly selected comparison group of participants who scored ≥ 25, were offered a comprehensive assessment at home. Of 1107 primary care patients aged 65 + years screened with the MMSE in the physicians' offices, the comprehensive assessment was offered to 642 participants: 343 with MMSE scores ≤ 24 and a comparison group of 299 randomly selected from those with scores ≥ 25. 3 of the 642 (0.5%) moved away, 3 (0.5%) died before the home visit, and 358 (55.8%) underwent the comprehensive assessments." | ||
| Comparative | |||
| Notes | No information on diagnostic accuracy is available for the total sample, despite attempting to contact the authors. We have used data on the people without memory complaints. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Lee 2002.
| Study characteristics | |||
| Patient sampling | The study was conducted from June 1999 to April 2000 in Kwanak district, Seoul, South Korea. A disproportionate age‐stratified random sample of 953 ≥ 65 was drawn, with a deliberate over‐sampling of those over 80. | ||
| Patient characteristics and setting | The total population of Kwanak district was 533,577, of whom 4% of the population was ≥ 65. 67.5% of the invited population participated ( N = 643). Of these, 66% were women. 32% were 65‐69 (the largest age band). 43.4% had no education, 35.3% had 1‐6 years of education and 21.3% had ≥ 7 years of education. The mean MMSE score in from the participants was 21.5. | ||
| Index tests | Korean MMSE (validated) | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐IV | ||
| Flow and timing | A stratified sample of those in phase I (index test stage) were invited to phase II (reference standard) by a neurologist. 307 were drawn from across the scores on the index test to have the reference standard. | ||
| Comparative | |||
| Notes | No 2 x 2 data were presented. We contacted the authors for this information but received no response. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Li 2006.
| Study characteristics | |||
| Patient sampling | Subjects were randomly selected elderly Chinese Singaporeans. Exclusions were for head trauma, stroke, evidence of cerebrovascular disease, neurological disease, systemic illness or unstable medical conditions that were judged as having a possible effect on the tested aspects. Also excluded were those using, or with a history of using benzodiazepines or barbituates. | ||
| Patient characteristics and setting | 2/3 subjects were drawn from the community and 1/3 from secondary care. Subjects were 65‐90 with normal hearing and vision. 50.7% female with the majority of subjects between 70‐75. | ||
| Index tests | MMSE offered in both Chinese (validated) and English | ||
| Target condition and reference standard(s) | Alzheimer's disease diagnosed according to NINCDS‐ADRDA and MCI as diagnosed by CDR. | ||
| Flow and timing | The test battery was performed all at once, including the reference standard. | ||
| Comparative | |||
| Notes | Data were presented of diagnostic accuracy for MCI and MCI and AD together, but not for AD separately. We wrote to the authors for this data but received no response. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Lindesay 1997.
| Study characteristics | |||
| Patient sampling | Random sample of age ≥ 65 registered with GP in Leicester | ||
| Patient characteristics and setting | Community‐based sample of people aged > 65 in Leicester, UK | ||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | All dementia. ICD‐10 | ||
| Flow and timing | The time between 1st and 2nd interviews was median 9 weeks. Full psychiatric assessment and reference standard was restricted to those who scored less than 22, or who had a CARE‐D score (depression rating) of > 7 on first screen and a random third of the rest. | ||
| Comparative | |||
| Notes | 2 x 2 data were only available stratified by ethnic origin. We wrote to the authors to ask for un‐stratified data but received no response. The data that were presented were "weighted according to the sampling fraction at second stage diagnostic interview": 74 of total 207 Gujarati people sampled (149 participating) and 59 of total 185 white people sampled (148 participating) Optimal cut point of MMSE to detect
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Liu 1996a.
| Study characteristics | |||
| Patient sampling | A representative population of San‐Ming district in Kaohshiung City (an industrial port in Southern Taiwan) ‐ 20 of 86 administrative areas were chosen and then 60 participants randomly from each area. This was a sample of 1200 people [of whom 1016 were interviewed] representing 12,356 people aged ≥ 65 years. | ||
| Patient characteristics and setting | 535 men, 481 (47%) women, 72.3% were aged 65‐74. 42.6% were illiterate. 29.4% had been to elementary school. 28% had been to high school. Overall prevalence of dementia 4.4%. | ||
| Index tests | Validated version of Chinese MMSE. "Scores were below 17 in the illiterate group, below 21 in the elementary school educated group, and below 25 in the high school educated group." The review authors take the previous sentence to be the cut points for the 3 groups; however, due to ambiguity in the language this is not entirely clear. | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III | ||
| Flow and timing | Of the 1200 sampled, 1016 were interviewed: 50 refused, 91 couldn't be contacted, 30 had changed address, 9 were sick and 4 were deceased. All those screening positive on CMMSE went on to receive full clinical diagnosis along with a 5% sample of those screening negative. If participants performed poorly on the CMMSE (lower than cut point + 2), a proxy (usually a close relative or caregiver) was interviewed for the Blessed Dementia Rating Scale and questionnaires. |
||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Lourenco 2006.
| Study characteristics | |||
| Patient sampling | A convenience sample of those over 65 who presented at outpatient primary care clinic. We have confirmed that this meets the definition of primary care in the protocol. | ||
| Patient characteristics and setting | Primary care patients aged ≥ 65, poorly educated [only 4.3% had more than 8 years of schooling]. Low socioeconomic status. 26.4% illiterate. | ||
| Index tests | MMSE, validated and modified Portuguese version | ||
| Target condition and reference standard(s) | All dementia. Diagnosed according to DSM‐IV and ICD‐10 | ||
| Flow and timing | A research assistant trained in conducting MMSE gave index test. The geriatricians and neuropsychologists made a formal diagnosis according to ICD‐10 and DSM‐IV | ||
| Comparative | |||
| Notes | 20 (6.6%) of participants had suffered a stroke and 57 (18.8%) had depression. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Macedo Montano 2005.
| Study characteristics | |||
| Patient sampling | Participant survivors of a cohort who were ≥ 65 years and residing in Sao Paolo, Brazil when recruited 7 years previously. | ||
| Patient characteristics and setting | Community setting, > 72 years old in Sao Paolo, Brazil | ||
| Index tests | MMSE, presumably translated into Portuguese (not stated) | ||
| Target condition and reference standard(s) | Dementia according to DSM‐IV and NINCDS‐ADRDA | ||
| Flow and timing | Timing unclear. All elderly scoring < 26 received reference standard along with a sample of the rest. | ||
| Comparative | |||
| Notes | The quality of reporting in this paper made it difficult to make judgement on the risk of bias. We have concerns about the quality of conduct. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Mackinnon 2003.
| Study characteristics | |||
| Patient sampling | Probability sample of persons aged ≥ 70 years drawn from the electoral roll for Canberra and the neighbouring town of Queanbeyan and stratified to recruit equal numbers of male and female. | ||
| Patient characteristics and setting | Asymptomatic community ≥ 70 in Australia | ||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | Participants were interviewed in their homes by lay interviewers. MMSE was administered as part of the interview. Diagnosis according to DSM‐III‐R was made using information from interviews after completion of all interviews. | ||
| Comparative | |||
| Notes | Of 945 participants, interviews including the IQCODE could be obtained for 694 participants. The MMSE scores were not available for 48 participants. Diagnoses of dementia could not be made for 19 persons. The analyses presented here were undertaken on 646 participants for whom all data was available. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Maki 2000.
| Study characteristics | |||
| Patient sampling | 1438 participants were invited (the entire > 65 population of the town), of whom 1255 agreed to take part. Those who lived alone were excluded due to lack of informant, leaving 818. The data were presented for 662 subjects. | ||
| Patient characteristics and setting | Elderly general population > 65 in in rural community of Nakayama town in Ehime, Japan | ||
| Index tests | MMSE (we presume a Japanese version ‐ not reported) | ||
| Target condition and reference standard(s) | All dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | The paper says that "we analysed data from 818 subjects for this paper" but in fact, data is only presented for 662 people, and no reason is given for this discrepancy. 258 subjects were selected for the clinical evaluation using at least 1 of the following criteria 1) MMSE score of ≤ 23, 2) SMQ (validated short memory questionnaire) score of ≤ 39/46, 3) Karasawa scale score of ≥ 1 and, 4) 0 score on 3‐word recall. Additionally, they reselected 50 of the remaining 560 at random to undergo reference standard. This totals 308. We cannot see where the total of 662 has come from. Timing of tests is not clear. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Mingyuan 1998.
| Study characteristics | |||
| Patient sampling | The study population was based on a study conducted in 1987. In 1987, a total of 5055 people were included and according to the DSM‐III‐R , 159 of the 5055 had dementia, 4896 of the 5055 were normal. In 1992, the 4896 people were investigated again, and 792 died, 1080 were lost to follow‐up, so 3024 people were involved in the present study. |
||
| Patient characteristics and setting | 57.34% female | ||
| Index tests | MMSE (we presume CMMSE, but no information about validation is given) | ||
| Target condition and reference standard(s) | Dementia according to DSM‐III‐R | ||
| Flow and timing | Reference standard was given to 711 participants whose MMSE were positive (the total score was lower than the cut point which was based on the education level) and 321 participants whose MMSE were negative (stratified sampling). | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
MoVies Study 1993.
| Study characteristics | |||
| Patient sampling | The validation sample for this study was an age‐stratified random sample drawn from a population of more than 17,000 older adults in 23 communities of the mid‐Monongahela Valley of Southwestern Pennsylvania (the MoVIES sample, initiated in the early 1990s). | ||
| Patient characteristics and setting | Participants were English speakers aged ≥ 65 (mean age 73.1), with at least 6 years of formal education (median education was high school graduate), living in the community (southwestern Pennsylvania). 96.6% white, 54.6% female, 56.9% married and 31.1% living alone. | ||
| Index tests | Standard MMSE | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R. There is concern about the conduct of the reference standard. There were 2 stages, in the first instance, trained non‐specialists gathered information and a psychiatrist made a tentative diagnosis. Those who were identified as not demented in this initial diagnosis were not evaluated further. People who were identified as possibly or probably demented were evaluated by a specialist. Some cases of dementia may not have been identified. | ||
| Flow and timing | It is unclear how many of the entire group received the reference standard. The reference standard comprised 2 stages: in the first instance, trained non‐specialists gathered information and a psychiatrist made a tentative diagnosis. Those who were identified as not demented in this initial diagnosis were not evaluated further. People who were identified as possibly or probably demented were evaluated by a specialist. Some cases of dementia may not have been identified. From the way that the data is presented, we can work out patient flow through index test and reference standard. However, we are confident that all test positives and some test negatives received the reference standard. | ||
| Comparative | |||
| Notes | Data presented are drawn from multiple papers relating to the MoVies study, largely Borson 2003 and Ganguli 1993. We have contacted the authors to try to reconcile some differences in data presented, particularly regarding sample size and flow of participants, but unfortunately, they were unable to clarify. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Pandav 2002.
| Study characteristics | |||
| Patient sampling | Population‐based, largely illiterate sample of 5126 individuals aged ≥ 55 in 28 villages in the rural community of Ballabgarh in northern India out of a total population of 5134. | ||
| Patient characteristics and setting | Population‐based, largely illiterate (73.3%) sample of 5126 individuals aged ≥ 55 in 28 villages in the rural community of Ballabgarh in northern India. 95.4% of women were illiterate, as were 53.8% of men. Illiteracy was defined by inability to read a local newspaper and write a sentence. | ||
| Index tests | HMSE (Hindi Mental State Examination) being validated in this study | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | Of the 5126 subjects who were thus cognitively or functionally screened, 536 (10.5%) were selected as screen‐positives for standardised clinical diagnosis and a random sample of 270 (5.3%) were selected as screen negatives to also undergo standardised clinical diagnostic evaluation. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
PAQUID Study.
| Study characteristics | |||
| Patient sampling | A random sample of 4050 was drawn according to a 3‐stage sampling design. The first 2 stages resulted in the selection of 37 parishes distributed across Gironde, France. Subjects were then randomly selected from the electoral lists after stratification by age and sex. | ||
| Patient characteristics and setting | 2792 subjects 65 years and older living independently in Gironde, France. The age and sex distribution for the sample was similar to the overall target population. 22.2% of men (619) were aged 65‐74, 14.9% of men (417) were aged 75‐84, 3% (84) were over 84 (total 1120 men). 28.1% (784) were aged 65‐74, 23.9% (667) were aged 75‐84, 7.9% (221) were aged > 84 (total 1672 women). | ||
| Index tests | We assume that the MMSE was conducted in French. There was no data given as to a validated translation. | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III | ||
| Flow and timing | All 2792 subjects were administered psychometric tests (including MMSE) at home by a psychologist and applied the DSM‐III diagnostic criteria. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Phantumchinda 1991.
| Study characteristics | |||
| Patient sampling | A random sample of 500 people > 60, resident in a large urban slum, Klong Toey, Bangkok. Had to have lived in the slum for over a year and willing to take part. Interviewed the elderly subjects and their close relatives. Started in 1989. | ||
| Patient characteristics and setting | Low socioeconomic status. 41% are aged 60‐64; 26% are aged 65‐69; 17% aged 70‐74. Only 6.2% were > 80. Low education: 87.4% had < 4 years of education. | ||
| Index tests | Field survey version of the MMSE, translated into Thai (non‐validated version) | ||
| Target condition and reference standard(s) | Dementia according to DSM‐III‐R | ||
| Flow and timing | Everyone was seen by a physician. Those thought to have dementia were then seen by a neurologist for confirmation. More confident in the determination of true positives. 500 out of 588 agreed to take part. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Pond 1994.
| Study characteristics | |||
| Patient sampling | "All GPs conducting clinics at a large retirement village complex in Sydney, Australia, were approached to take part in this study, provided that they had at least 10 patients aged 70 or over who were living either independently or in hostel accommodation . . . "During the pre‐ and post‐intervention sampling phases each GP completed a 1 page questionnaire for each patient attending and recorded his/her opinion on the dementia status of each of these patients, as well as an opinion on whether the patient was depressed or not. The reasons for attendance were recorded as were major chronic illnesses . . . "Of the 258 patients approached in the pre‐intervention sample, 45 refused and 13 were excluded due to illness (12 physical illness; 1 probable depression), while 200 had a home interview, of which 105 had the additional CIE assessment. In the post‐intervention sample, 218 patients were approached. Of these, 50 refused, 1 was excluded due to physical illness, and 167 had a home interview, including 69 who completed the CIE." |
||
| Patient characteristics and setting | Pre‐intervention (academic training) Age 82.5 years (SD 5.9) Mean MMSE 26 (SD 3.7) 86% female 66% Hostel Post‐intervention Age 82.9 years (SD 6.1) MMSE 25.9 (SD 3.6) 88% female 73% Hostel Hostels are "low level residential care" |
||
| Index tests | MMSE administered in the patient's own home by a trained nurse | ||
| Target condition and reference standard(s) | Dementia, DSM‐III‐R and ICD‐10 'probable dementia' diagnoses | ||
| Flow and timing | "Patients who agreed to join the study were then, within a week of their consultation, interviewed in their own home by a registered nurse . . . A 1 in 2 subsample received an abridged version of the Canberra Interview for the Elderly (CIE),19 a structured interview with an informant component. The CIE enables the generation of a set of diagnoses for each patient based upon the DSM‐III‐R classification system and the draft International Classification of Diseases, version 10 (ICD‐10). Selection for the CIE subsample was random, taking every second consecutive case." | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Ramlall 2013.
| Study characteristics | |||
| Patient sampling | A convenience sample of 302 residents in a non‐governmental home for the elderly housing in Durban, South Africa. A total of 1371 residents and those receiving frail care in assisted and independent living for people aged ≥ 60 years | ||
| Patient characteristics and setting | Inclusion criteria were residents who were aged ≥ 60 years, had a minimum of 8 years of formal schooling, were able to speak, read and write in English and gave written informed consent. Exclusion criteria were residents with severe physical, mental or sensory handicap that precluded their engagement with the assessment. Given these inclusion (informed written consent) and exclusion criteria, we have concerns that those most severely affected by dementia will be excluded from the study. We anticipate that this would result in an underestimation of the diagnostic utility of the test. |
||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Dementia diagnosed according DSM‐IV‐R | ||
| Flow and timing | 302 were convenience sampled. Of those, 38 screen positives and 102 randomly selected screen negatives had a clinical diagnostic evaluation. | ||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Rosselli 2000.
| Study characteristics | |||
| Patient sampling | The sample was obtained from the 5 principal urban conglomerates of Colombia (Bogota, Medellin, Cali, Barranquilla, Bucaramanga) according to the 1993 census. In each of these regions, sampling was carried out as follows.
The sample was representative of Colombia as a whole. |
||
| Patient characteristics and setting | Visited 2560 homes and interviewed 9328 participants, of those, 1949 were > 50 years old. 1686 individuals responded to MMSE, but 75 were excluded because they died or only partially completed 3 or more questions. In total 1611 were included. 613 were male = 38.1% 998 were women = 61.9% Average 3 years education Mean age = 62.9 years Given that the review is looking at the utility of the test in adults over the age of 65, we have marked the applicability of the population as 'of high concern'. |
||
| Index tests | Translated version of the MMSE. The cut points were adjusted for education as follows: ≤ 21 for < 6 years schooling; < 24 for 7‐12 years of schooling; < 27 for those with > 12 years of schooling. For individuals older than 65 years of age an additional point was added to the total mark. For those older than 75, 2 additional points were added to the total mark. Participants with visual limitation also received 2 additional points. |
||
| Target condition and reference standard(s) | Dementia, DSM‐IV | ||
| Flow and timing | People who scored below a cut point were evaluated by a clinician, together with a 5% random sample of 'healthy' participants. Of the 1611 participants, 536 who scored below education adjusted cut point (33.3%), 209 of these individuals suspected of possible dementia did not attend, so 327 completed evaluation. The average MMSE score of people who did not attend (19.05 ± 3.72) was statistically higher than the MMSE score of people who did attend (16.38 ± 4.54) P < 0.001. Of the 327 who completed the reference standard, 12 had dementia. Of the 1075 participants with satisfactory scores on MMSE, 366 (34%) were seen by a neurologist for other reasons, and 1 of these was found to have dementia. |
||
| Comparative | |||
| Notes | Translated by Mr William Eustace on 30 October 2014 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Rummans 1996.
| Study characteristics | |||
| Patient sampling | A population‐based, age‐stratified, random sample of residents of Rochester, Minnesota, USA aged ≥ 65 | ||
| Patient characteristics and setting | 406 people were sampled and contacted, of whom 201 agreed to participate. 65% were female. The mean age was 78.3 for men and 80.2 for women. | ||
| Index tests | MMSE with standard cut‐off of 24 indicating normality, combined with AVLT. | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | Patients scoring 23 or below on MMSE or failing the other screens (22 in total) went on to receive reference standard, along with 15 who passed all screens. | ||
| Comparative | |||
| Notes | Sensitivity and specificity is presented for MMSE and AVLT combined (92.3% and 100% respectively) in the paper. We contacted the authors for the separate data for MMSE only and received no response. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Scazufca 2009.
| Study characteristics | |||
| Patient sampling | Participants were those enrolled in the baseline assessment of the Sao Paulo Ageing and Health Study (SPAH). The investigation was carried out in the borough of Butantã, located on the west side of the city. Between May 2003 and April 2005 all residents aged ≥ 65 years living in 66 census sectors of the Butantã borough, covering a population of approximately 63,000 residents, were invited to participate in the SPAH. A total of 2072 persons (91.4% of those invited) were recruited through systematic door knocking. Interviews for the assessment of dementia took place at participants' residences approximately 1 week after recruitment. | ||
| Patient characteristics and setting | Population based. 60.6% female, 43% aged 65‐69, 27% aged 70‐74, 38.5% had 0 years of education. 50% had a personal income of < USD 127 per month. | ||
| Index tests | Validated version of the Brazilian version of the MMSE | ||
| Target condition and reference standard(s) | Dementia according to DSM‐IV | ||
| Flow and timing | A total of 2072 persons were recruited. Included in the analysis were 1933 participants (93.3% of those assessed for the prevalence study). Among the 139 participants excluded, 48 were unable to answer ≥ 5 items of the questionnaire due to severe physical or mental impairment (18 cases of dementia, 20 subjects with sensory impairments – eye or hearing problems, 10 with other physical incapacities), 10 were approached but refused to answer the MMSE questions, and 81 were not approached by the research team for the battery of assessments that included the MMSE. | ||
| Comparative | |||
| Notes | Reference standard was DSM‐IV applied by algorithm rather than by a psychiatrist or neurologist. Data is only presented for the diagnostic accuracy of the MMSE stratified by educational level (none, 744 participants; ≥ 1 year, 1189 participants). There were 84 people with dementia out of a total of 1933. Because we do not know the number of people with dementia in these 2 groups, despite attempting to contact the authors, it is not possible to enter the data separately and so the study cannot be included in the meta analysis. No education cut points 14 indicating normal sensitivity 72.3% specificity 84.9% 15 indicating normal sensitivity 78.7% specificity 77.8% 16 indicating normal sensitivity 87.2% specificity 70.4% 17 indicating normal sensitivity 93.6% specificity 61.3% 18 indicating normal sensitivity 93.6% specificity 49.1% 19 indicating normal sensitivity 95.7% specificity 40.2% 20 indicating normal sensitivity 97.9% specificity 29.7% ≥ 1 year(s) of education cut points 18 indicating normal sensitivity 91.9%, specificity 89.5% 19 indicating normal sensitivity 91.9%, specificity 85.4% 20 indicating normal sensitivity 94.6%, specificity 78.8% 21 indicating normal sensitivity 94.6%, specificity 69.9% 22 indicating normal sensitivity 94.6%, specificity 59.9% 23 indicating normal sensitivity 94.6%, specificity 50.6% 24 indicating normal sensitivity 100%, specificity 37.6% 25 indicating normal sensitivity 100%, specificity 27.8% |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Schultz‐Larsen 2007.
| Study characteristics | |||
| Patient sampling | The study sample was drawn from respondents to the so‐called Brønshøj‐Husum Study, a 3‐stage population study of dementia and functional ability among all community‐dwelling elderly women (1147) and men (635) born between 1913 and 1918 and living in a district of Copenhagen in January 1994. | ||
| Patient characteristics and setting | 65% had ≤ 7 years education, 64% were women in phase 1 sample and 53.5% women at stage 2. Average age was 79 in whole sample and 80.2 in the phase 2 sample. 56% lived alone. | ||
| Index tests | Standardised Danish version of the original MMSE | ||
| Target condition and reference standard(s) | Dementia according to DSM‐IV | ||
| Flow and timing | In the first stage of the study, 67% (759 women and 430 men) of the population underwent a baseline
and cognitive assessment in their homes by a trained research nurse using standard instruments including the
MMSE. In the second stage, a subsample of initial respondents was asked to undergo a clinical neuropsychological examination. The subsample included all subjects who screened positive (MMSE < 26 among subjects with 7 years of schooling, < 27 for subjects with longer education) at the baseline assessment (N = 156) besides a random sample of individuals stratified by age and sex (N = 164) among subjects who screened negative at the baseline assessment. |
||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Tang 1999.
| Study characteristics | |||
| Patient sampling | Participants were cluster sampled by draw from different districts of Chengdu, China. | ||
| Patient characteristics and setting | Participants were 55.79% female and had a mean age of 67.12. 53.96% (648) of the participants were from downtown; 26.56% (319) were from rural‐urban continuum; 19.48% (234) were from rural settings. The education level of the participants who lived downtown were the highest, followed by the participants who lived in the rural areas. The participants living in the rural‐urban continuum had the lowest educational levels. | ||
| Index tests | Chinese version of MMSE but no validation information given | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | Not all participants received the reference standard. 1. Those whose score of the CMMSE below the criteria (illiterate group: CMMSE ≤17,primary school group: CMMSE ≤20, middle school and above CMMSE ≤24) had the reference standard. 2. Those whose score of the CMMSE was normal, but the doctor or family member suspected dementia had the reference standard 3. To avoid the missed diagnoses, 20% of the participants in the illiterate group whose CMMSE score were 18 or 19 were randomly selected to have the reference standard. 4. 4% of the participants whose CMMSE score were normal were also randomly selected to have the reference standard. |
||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
West Beijing Study 1989.
| Study characteristics | |||
| Patient sampling | All the households with residents aged ≥ 60 years in the West district of Beijing, specifically 4 communities in the west district of Beijing. | ||
| Patient characteristics and setting | 585 females, 505 males. 33.7% illiterate. Illiteracy in women 53.8%. 60.8% of those who had been employed were workers in service industry. 36.2% were professional or administrative workers. 51.2% of women and 17.1% of men were widowed. 70% lived with their children. | ||
| Index tests | The MMSE was translated into Chinese from English, with each item being discussed with a neuroepidemiologist from the United States, the late Professor B. Schoenberg. | ||
| Target condition and reference standard(s) | Dementia according to DSM‐III (slightly modified). Criterion A was thus extended to be "loss of intellectual ability of sufficient severity to interfere with daily living, social and occupational functioning of a sufficient degree that help is needed on a day‐to‐day basis either part or all of the time". | ||
| Flow and timing | N = 1090. Only 1072 received MMSE, of those, all those who scored ≤ 17 on MMSE (n = 42) plus a randomised sample of 5.5% (57 participants) of the rest received the full clinical examination. The 18 people who couldn't undergo an MMSE were screened using a different test, the Crichton Scale. | ||
| Comparative | |||
| Notes | The overall prevalence of dementia was calculated as 1.3% | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Wilder 1995.
| Study characteristics | |||
| Patient sampling | Patients were recruited from the North Manhattan Aging Project by census. | ||
| Patient characteristics and setting | Urban setting, Manhattan, USA. 790 people in total. 355 Latino, 299 African American and 136 white. 39% were aged 78‐84, 46% had 5‐11 years of education. | ||
| Index tests | MMSE with some translations in real‐time for non‐native speakers of English. | ||
| Target condition and reference standard(s) | Dementia diagnosed according to DSM‐III‐R | ||
| Flow and timing | No information about timing given. All screen positives and a portion of screen negatives were referred for reference standard. | ||
| Comparative | |||
| Notes | We contacted the authors for 2 x 2 data and received no reply so are unable to include this study in the meta‐analysis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
Winblad 2010.
| Study characteristics | |||
| Patient sampling | A total population based clinico‐epidemiological study was carried out covering all of the 1680 inhabitants aged ≥ 60 in the rural municipality of Haapajarvi in Northern Finland. Registers of health and social welfare and population interviews were used. | ||
| Patient characteristics and setting | Aged ≥ 60 in the rural municipality of Haapajarvi in Northern Finland. We have concerns about the applicability due to the exclusion of previously diagnosed dementia patients. | ||
| Index tests | MMSE assumed to be in Finnish. No information about validation of the translation. There is no reference at all to the index test, including the original Folstein paper. | ||
| Target condition and reference standard(s) | All‐cause dementia diagnosed according to DSM‐IV | ||
| Flow and timing | The total ≥ 60 population was 1680, they did a first stage dementia awareness campaign. They took a random sample of 840, excluding those who they had seen at the campaign, which left 757, of whom 490 proceeded to index test. Of the 490 who received the index test, they performed reference standard on 114 people. The reference standard was performed on 82 out of 110 (75%) of those who scored < 25 on MMSE. The reference standard was performed on 32 out of 380 (8.4%) of those who scored 25 or more on MMSE. |
||
| Comparative | |||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on MMSE application given for the test to be repeated in an independent study? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Was sufficient information on the method of dementia assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
3MS: Modified Mini‐Mental State Examination; AD: Alzheimer's disease; ADL: activities of daily living; ADRDA: Alzheimer's Disease and Related Disorders Association; AMT: Abbreviated Mental Test; AVLT: Auditory Verbal Learning Test; CDR: clinical dementia rating; CIE: Canberra Interview for the Elderly; CMMSE: Chinese Mini‐Mental State Examination; CSI‐D: Community Screening Instrument for Dementia; DSM: Diagnostic and Statistical Manual of Mental Disorders; GPCOG: General Practitioner Assessment of Cognition; ICD: International Classification of Diseases; IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly; LTCI: long‐term care institution; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; NCI: no cognitive impairment; NINCDS: National Institute of Neurological and Communicative Disorders and Stroke; PCP: primary care practitioner; SIDAM: Structured Interview for Diagnosis of Dementia of the Alzheimer Type; SMQ: short memory questionnaire; SPMSQ: Short Portable Mental Status Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐rajeh 1999 | Wrong setting (secondary care) |
| Almeida 1998 | Wrong setting (secondary care) |
| Basic 2009 | Wrong setting (secondary care) |
| Bastide 2012 | Wrong setting (secondary care) |
| Belmin 2007 | Wrong setting (secondary care) |
| Bermejo 1999 | Wrong setting (secondary care) |
| Bermejo‐Pareja 2009 | Wrong index test (37‐item MMSE) |
| Bland 2001 | Wrong index test (3MS, not MMSE) |
| Borson 1999 | Wrong study design (case‐control) |
| Borson 2000 | Wrong study design (case‐control) |
| Borson 2005 | Wrong study design (case‐control) |
| Braekhus 1995 | Wrong study design (case‐control; all participants pre‐diagnosed as non‐dementia patients) |
| Brooke 1999 | Wrong setting (secondary care) |
| Burnham 2012 | Wrong setting (secondary care) |
| Cacho 2010 | Wrong setting (secondary care) |
| Canadian Study of Health and Aging | Wrong index test (3MS) |
| Cao 2012 | Wrong setting (secondary care) |
| Carpenter 2011 | Wrong setting (secondary care) |
| Cercy 2012 | Wrong study design (case‐control) |
| Cerveira 2009 | Wrong setting (secondary care) |
| Cervilla 2004 | Wrong study design (only those scoring 25 or less received reference standard ‐ partial verification). |
| Chaves 2007 | Wrong setting (secondary care) |
| Chaves 2009 | Wrong reference standard (none; prevalence study only) |
| Chester 2011 | Wrong reference standard (none) |
| Chong 2010 | Wrong study design (case‐control) |
| Clark 1999 | Wrong study design (case‐control; all patients had pre‐diagnosed cognitive impairment) |
| Clarke 1991 | Wrong reference standard (CAMDEX with no reference to DSM) |
| Cossa 1997 | Wrong study design (only index test positives received the reference standard) |
| Cossa 1999 | Wrong study design (partial verification ‐ reference standard applied only to those with positive index test result) |
| Costa 2012 | Wrong study design (not diagnostic test accuracy study) |
| Cullen 2005 | Wrong reference standard |
| Dahl 2007 | Wrong study design (partial verification ‐ only index test positives received reference standard) |
| Damian 2011 | Wrong setting (secondary care) |
| Dash 2006 | Wrong setting (secondary care) |
| Davous 1988 | Wrong reference standard (None) |
| De Beaman | Wrong setting (secondary care) |
| De Jager 2009 | Wrong study design |
| De Silva 2002 | Wrong reference standard (CAMCOG) |
| Del‐Ser 1997 | Wrong setting (secondary care) |
| Derrer 2001 | Wrong study design (case‐control) |
| Dierckx 2011 | Wrong study design |
| Diniz 2007 | Wrong setting (secondary care) |
| Dong 2012 | Wrong setting (secondary care) |
| Donnelly 2008 | Wrong reference standard |
| Drachmann 1996 | Wrong study design (case‐control; drawn from partially secondary care population) |
| Duron | Wrong target condition (dementia not key outcome) |
| Fabrigoule 1995 | Wrong study design |
| Feher 1992 | Wrong setting (secondary care) |
| Fernandez‐Martinez 2008 | Wrong study design (partial verification ‐ only index test positives received reference standard) |
| Fernandez‐Martinez 2010 | Wrong setting (secondary care) |
| Ferrero‐Arias 2001 | Wrong setting (secondary care) |
| Ferruci 1998 | Wrong setting (secondary care) |
| Fong 2010 | Wrong setting (secondary care) |
| Forlani | Wrong index test (not looking at accuracy of MMSE) |
| Fountoulakis 1998 | Wrong study design (case‐control) |
| Fountoulakis 2000 | Wrong study design (case‐control) |
| Fratiglioni 1993 | Wrong index test (not looking at accuracy of MMSE) |
| Fratiglioni 1994 | Wrong index test (not looking at accuracy of MMSE) |
| Fujiawara 2003 | Wrong study design (case‐control; all patients had cognitive decline) |
| Gabryelewicz 2002 | Wrong study design (partial verification ‐ only index test positives received reference standard) |
| Galvin 2010 | Wrong setting (prevalence of dementia over 60%. Secondary or tertiary care setting.) |
| Ganguli 2004b | Wrong study design (partial verification ‐ only index test positives received reference standard) |
| Ganguli 2010a | Wrong study design (partial verification ‐ only index test positives received reference standard) |
| Ganguli 2010b | Wrong study design (partial verification ‐ only index test positives received reference standard) |
| Ganzer 2003 | Wrong setting (secondary care) |
| Garcia | Wrong study design (case‐control; all healthy participants at baseline) |
| Garcia 1993 | Wrong reference standard (MMSE is the reference standard) |
| Geerlings 1999 | Wrong study design (no DTA data) |
| Gibbons | Wrong setting (secondary care) |
| Goldman 2001 | Wrong index test (no MMSE in study) |
| Gondo 2006 | Wrong reference standard (none) |
| Grigoletto 1999 | Wrong study design (case‐control; excluded those with symptoms) |
| Grober 2008 | Wrong study design (case‐control; excluded those with MMSE score < 18) |
| Harder 1995 | Wrong study design (case‐control) |
| Hartmann 2002 | Wrong setting (secondary care) |
| Hashizume 2004 | Wrong setting (secondary care) |
| Helkala 2002 | Wrong study design (partial verification ‐ only those scoring < 24 on MMSE received reference standard) |
| Hensel 2009 | Wrong study design (case‐control; excludes participants with dementia symptoms) |
| Hogervorst | Wrong study design |
| Holsinger 2012 | Wrong index test (3MS data presented ‐ no MMSE extracted) |
| Huppert 2005 | Wrong study design (no test accuracy data) |
| Ibrahim 2009 | Wrong study design |
| Ideno 2012 | Wrong study design |
| Ihl 2005 | Wrong study design (case‐control) |
| Jagger 1992 | Wrong reference standard |
| Jeong 2007 | Wrong study design (case‐control; excludes cognitively impaired) |
| Jervis 2007 | Wrong study design (not a test accuracy study) |
| Jeste 1992 | Wrong study design (case‐control) |
| Jones 2010 | Wrong setting (secondary care) |
| Jonsson 2010 | Wrong study design (not a test accuracy study) |
| Jorm 1996 | Wrong reference standard |
| Jorm 1997 | Wrong study design (not a test accuracy study) |
| Kal'bus | Wrong study design (participant ages 35‐65) |
| Kamenski 2009 | Wrong reference standard (none) |
| Kanegae 2008 | Wrong setting (secondary care) |
| Kaufer 2008 | Wrong setting (secondary care) |
| Khachaturian 2000 | Wrong index test (3MS) |
| Kirby 2001 | Wrong reference standard (AGECAT with no reference to DSM) |
| Kliegel 2004 | Wrong reference standard (none) |
| Kochhann 2010 | Wrong study design |
| Koski 2011 | Wrong setting (secondary care) |
| Koson | Wrong study design (patients scoring < 24 on MMSE excluded) |
| Krigbaum 2012 | Wrong study design (case‐control) |
| Kukull 1994 | Wrong study design (case‐control; only dementia patients) |
| Kuslansky 2004 | Wrong study design (case‐control) |
| Lam | Wrong study design (looking at change in MCI) |
| Lam 2005a | Wrong reference standard (only CDR 0.5) |
| Lam 2005b | Wrong study design (no DTA data) |
| Larner 2012 | Wrong setting (secondary care) |
| Larson 1984 | Wrong setting (secondary care) |
| Lautenschlager 1986 | Wrong setting (secondary care) |
| Law 1995 | Wrong index test (3MS) |
| Lee | Wrong study design (prevalence of cognitive impairment) |
| Lee 1997 | Wrong study design (partial verification; only index test positives received reference standard) |
| Lee 2009 | Wrong study design (partial verification; only index test positives received reference standard) |
| Leoutsakos 2012 | Wrong study design (case‐control; all participants had AD) |
| Li 2009 | Wrong study design (partial verification; only index test positives received reference standard) |
| Li 2013 | Wrong setting (secondary care) |
| Limpawattana 2012 | Wrong setting (secondary care) |
| Lin 1998 | Wrong study design (partial verification; only index test positives received reference standard) |
| Liu 1994 | Wrong index test (CASI) |
| Liu 1995 | Wrong study design (partial verification; only index test positives received reference standard) |
| Liu 1996b | Wrong study design |
| Liu 1998 | Wrong study design (partial verification; only index test positives received reference standard) |
| Llibre 2009 | Wrong study design (prevalence study) |
| Lobo 2008 | Wrong study design |
| Lopes 2010 | Wrong study design (case‐control; only those with cognitive impairment) |
| Lopez‐Pousa 1995 | Wrong reference standard |
| Luis 2009 | Wrong study design |
| MacKenzie 1996 | Wrong study design |
| MacKnight 1999 | Wrong index test (3MS) |
| MacNeill 2000 | Wrong setting (secondary care) |
| Marcos de Vega | Wrong target condition (MCI) |
| Medina | Wrong study design (not DTA; no MMSE accuracy data) |
| Meguro 2007 | Wrong study design (case‐control; wrong patient population) |
| Molloy 1997 | Wrong study design (not a DTA study) |
| Moretti | Wrong target condition (MCI) |
| Mungas 1996 | Wrong study design |
| Murden 1991 | Wrong study design |
| Murden 1997 | Wrong study design (case‐control; wrong participant population) |
| Nadler 1995 | Wrong setting (secondary care) |
| Narasimhalu 2008 | Wrong setting (secondary care) |
| Neri 2001 | Wrong study design (partial verification; only index test positives received the reference standard) |
| Ng 2007 | Wrong reference standard |
| Nishiwaki 2004 | Wrong reference standard |
| Noale 2006 | Wrong study design (delayed verification) |
| Nourhashemi 2008 | Wrong study design (case‐control; wrong population) |
| O'Bryant 2008 | Wrong study design |
| O'Connor 1989 | Wrong study design |
| Olazaran 2004 | Wrong target condition (MCI) |
| Onishi 2006 | Wrong setting (secondary care) |
| Ostrosky‐Solis 1999 | Wrong study design (case‐control) |
| Pachet 2010 | Wrong study design |
| Pardo 1990 | Wrong study design (case‐control) |
| Perneczky 2006 | Wrong setting (secondary care) |
| Pezzotti 2008 | Wrong study design (case‐control; participants with cognitive impairment only) |
| Pouretemad 2009 | Wrong study design |
| Qu 2005 | Wrong study design (partial verification. Only index test positives received reference standard.) |
| Quiroga 2004 | Wrong study design (case‐control) |
| Rabins | Wrong study design |
| Rai 1998 | Wrong setting (secondary care) |
| Rai 2008 | Wrong setting (secondary care) |
| Raina 2013 | Wrong study design (case‐control) |
| Rait 2000 | Wrong reference standard |
| Raskind 1999 | Wrong study design (No MMSE test accuracy data) |
| Riedel‐Heller 1999 | Wrong study design (case‐control; only healthy participants) |
| Roelands 1992 | Wrong study design (only index test positives received the reference standard) |
| Schrijnemaekers 2006 | Wrong study design (case‐control) |
| Sikkes 2013 | Wrong study design (case‐control) |
| Spering 2012 | Wrong study design |
| Stewart 2002 | Wrong study design (normative study) |
| Stoppe | Wrong study design |
| Storey 2002 | Wrong setting (tertiary care) |
| Storey 2004 | Wrong study design |
| Subra 2012 | Wrong target condition |
| Sugishita | Wrong index test (short MMSE) |
| Tae 2010 | Wrong study design |
| Taillandier 2002 | Wrong setting (secondary care) |
| Tamura | Wrong study design |
| Tang‐Wai 2003 | Wrong setting (secondary care) |
| Tappen 2012 | Wrong setting (mixed recruitment, largely from secondary care) |
| Tariq 2006 | Wrong setting (secondary care) |
| Tariska 2003 | Wrong setting (secondary care) |
| Thibodeau 2011 | Wrong study design (longitudinal change) |
| Thiele | Wrong study design (case‐control; all subjects had MCI at baseline) |
| Tian | Wrong index test |
| Tierney 2000 | Wrong setting (secondary care) |
| Timpano 2013 | Wrong setting (secondary care; and case‐control) |
| Tombaugh 1996 | Wrong index test (3MS) |
| Tombaugh 2005 | Wrong study design (case‐control; all participants had MCI at baseline) |
| Travers 2013 | Wrong setting (secondary care) |
| Trenkle 2007 | Wrong target condition (MCI) |
| Tschanz 2004 | Wrong study design |
| Tuokko 1995 | Wrong index test (3MS) |
| Uhlmann 1991 | Wrong study design (case‐control) |
| Unger 1999 | Wrong study design |
| Van der Cammen 1992 | Wrong setting (secondary care) |
| Van Exel 2003 | No reference standard |
| Van Sanden 2012 | Wrong study design |
| Vantaa 85+ | Wrong study design (case‐control; all demented subjects were excluded) |
| Vas 2001 | Wrong study design (case‐control; selected population) |
| Vercambre 2010 | Wrong reference test (MMSE is part of the reference standard) |
| Vigliecca 2012 | Wrong study design |
| Waite 2001 | Wrong target condition (cognitive impairment) |
| Watfa 2001 | Wrong study design (case‐control; subjects at baseline all in good cognitive health) |
| Weston 1987 | Wrong reference standard (none) |
| White 2002 | Wrong study design (partial verification; only those with positive index tests received reference standard) |
| Whitney 2012 | Wrong setting (secondary care) |
| Wolf Klein 1989 | Wrong setting (secondary care) |
| Wrobel 2007 | Wrong reference standard |
| Wu 2002 | Wrong study design (partial verification; only index test positives received reference standard) |
| Wu 2003 | Wrong study design (no DTA data presented) |
| Wu 2006 | Wrong study design |
| Yang 2006 | Wrong study design (partial verification; only those with positive index test received reference standard) |
| Yavorsky | Wrong setting (secondary care) |
| Ylikoski 1992 | Wrong study design |
| Yuseph 1997 | Wrong study design (not a DTA study) |
| Zaragoza study | Wrong reference standard |
| Zaudig 1992 | Wrong study design (case‐control) |
| Zhang 1998 | Wrong study design |
| Zhang 2012 | Wrong study design |
3MS: Modified Mini‐Mental State Examination; AD: Alzheimer's disease; CAMCOG: Cambridge cognition examination; CASI: Cognitive Abilities Screening Instrument; CDR: clinical dementia rating; DSM: Diagnostic and Statistical Manual of Mental Disorders; DTA: diagnostic test accuracy; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination.
Characteristics of studies awaiting classification [ordered by study ID]
Gungen 2002.
| Study characteristics | |||
| Patient sampling | No information available | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | — | ||
Jianbo 2013.
| Study characteristics | |||
| Patient sampling | No information available | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | — | ||
Kornsey.
| Study characteristics | |||
| Patient sampling | No information available | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | — | ||
Kvitting 2013.
| Study characteristics | |||
| Patient sampling | A total of 81 participants (≥ 65 years old and living at home) were recruited from 4 primary health care centres in Sweden between December 2007 and May 2009. Of these patients, 52 exhibited possible cognitive impairment and 29 were presumed cognitively healthy and visiting primary care for some other medical problem. All 81 patients were asked to participate in the study during an appointment with a general practitioner. 1 patient declined. | ||
| Patient characteristics and setting | 48 women (59%) and 33 men, 77 of the 81 were native Swedish speakers, average age 77.2 years, average education 10.4 years, duration of cognitive symptoms 1.5 years. | ||
| Index tests | MMSE (we presume a Swedish translation. No reference was given to any validation of the translation.) | ||
| Target condition and reference standard(s) | Dementia diagnosed according to ICD‐10 | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | — | ||
Orsi.
| Study characteristics | |||
| Patient sampling | No information available | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | — | ||
Shaaban 2013.
| Study characteristics | |||
| Patient sampling | "This was a cross‐sectional study involving 49 Community dwelling elderly age 65 years and above who attended primary care in Keylantan from January to February 2010. Those who were diagnosed to have Parkinson's disease, mental retardation, psychiatric illness and physical handicaps were excluded." | ||
| Patient characteristics and setting | "A total of 49 respondents were involved in this study. Majority of the subjects were Malay (98%) and married (88%)." 64% were female and 61% were educated to primary level. Median age was 68 years. "According to DSM‐IV criteria 79.6% were normal and 20.4% had dementia." | ||
| Index tests | The Malay version of the MMSE (previously validated) | ||
| Target condition and reference standard(s) | Dementia, DSM‐IV‐TR | ||
| Flow and timing | Information on timing is not clearly stated but we assume that the index test and reference standard were conducted on the same day. | ||
| Comparative | — | ||
| Notes | — | ||
Upadhyaya 2010.
| Study characteristics | |||
| Patient sampling | No information available | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | — | ||
Yu 2012.
| Study characteristics | |||
| Patient sampling | "Three communities from ChaoYang District, 1 community from XiCheng District, and 2 villages from Chang Ping District were then conveniently selected to recruit the participants from. Residents listed in the census of the community registration that were aged 60 and above were contacted for participation. 1056 participants participated in the present study, and 1001 participants were included in the final data analyses based on the following inclusion and exclusion criteria. Inclusion criteria were individuals (1) who were 60 years old or older and registered as permanent residents in their residing district in Beijing (N = 1056), and (2) who completed both the MoCA‐BJ and the MMSE (N = 1036). Exclusion criteria were individuals (1) who had missing clinical diagnoses (N = 25), and (2) who had received a clinical diagnosis of depression (N = 10)." | ||
| Patient characteristics and setting | From table 1. Average age 70.66 years, average years of education 10.1 years, average MMSE 25.9. 57.1% female. | ||
| Index tests | MMSE | ||
| Target condition and reference standard(s) | Dementia, DSM IV | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | email sent 24 October to clarify missing information on sampling, case‐control, flow and timing | ||
DSM: Diagnostic and Statistical Manual of Mental Disorders; ICD: International Classification of Diseases; MMSE: Mini‐Mental State Examination.
Characteristics of ongoing studies [ordered by study ID]
Guiata 2012.
| Trial name or title | InveCe.Ab study |
| Target condition and reference standard(s) | Comparative |
| Index and comparator tests | Comparative |
| Starting date | Comparative |
| Contact information | Antonio Guaita MD; GolgiCenci Foundation |
| Notes | Prevalence study, no diagnostic test accuracy data published yet |
Differences between protocol and review
Data extraction
We planned to extract data on study characteristics and quality to a study‐specific pro forma. However, we finally entered the data directly into RevMan because the number of studies that were eligible for inclusion was so large that we wanted to avoid any possibility of introducing errors in transcribing data from one file or format to another (RevMan 2014). At least two senior authors worked together in real time to perform data entry and check the transcription to avoid human error.
Analysis
We anticipated that the target condition would comprise two categories: (1) dementia (all‐cause) and (2) dementia subtypes (Alzheimer's, vascular, Lewy body, frontotemporal), but our included studies only reported the all‐cause dementia outcome, so we only performed meta‐analysis with this as the target condition.
Sensitivity analyses
We planned to perform sensitivity analysis to determine the effect of excluding studies that we deemed to be at high risk of bias. However, as studies were generally at low risk of bias ‐ no study had more than two of four QUADAS‐2 items assessed as having a high risk of bias ‐ we did not do this as we did not pre‐specify a point at which we would deem a study to be at overall 'high risk of bias'.
Heterogeneity
We did not investigate heterogeneity in test accuracy by education because this was poorly reported. We could not examine heterogeneity by mean MMSE score because the models were unstable.
Contributions of authors
Sam Creavin wrote and revised the protocol, screened citations, reviewed full text articles, extracted data, assessed quality of studies, performed meta‐analysis and wrote the manuscript.
Susanna Wisniewski helped draft the protocol, screened citations, reviewed full text articles, extracted data, assessed quality of studies and helped to draft the manuscript.
Anna H Noel‐Storr helped draft the protocol, screened citations, reviewed full text articles, extracted data and assessed quality of studies and helped to draft the manuscript.
Sarah Cullum helped draft the protocol, screened citations, reviewed full text articles and helped to draft the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research, UK.
Sam Creavin is an NIHR Academic Clinical Fellow.
-
NIHR, UK.
This review was supported by the National Institute for Health Research, via a Cochrane Programme Grant to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
The authors declare no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
ADAMS Study 2007 {published data only (unpublished sought but not used)}
- Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology 2005;25(4):181‐91. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29(1‐2):125‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideaux T, Beaudreau SA, Fernandez S, O'Hara R. Utility of the abbreviated Fuld Object Memory Evaluation and MMSE for detection of dementia and cognitive impairment not dementia in diverse ethnic groups. Journal of Alzheimer's Disease 2012;31(2):371‐86. [CRS: 99900001000000007; PUBMED: 22555374] [DOI] [PubMed] [Google Scholar]
Aevarsson 2000 {published data only}
- Aevarsson O, Skoog I. A longitudinal population study of the mini‐mental state examination in the very old: relation to dementia and education. Dementia and Geriatric Cognitive Disorders 2000;11(3):166‐75. [CRS: 99900001000000030; PUBMED: 10765048] [DOI] [PubMed] [Google Scholar]
AMSTEL Study 1997 {published data only (unpublished sought but not used)}
- Hooijer C, Dinkgreve M, Jonker C, Lindeboom J, Kay DWK. Short screening tests for dementia in the elderly population. I. A comparison between AMTS, MMSE, MSQ and SPMSQ. International Journal of Geriatric Psychiatry 1992;7(8):559‐71. [Google Scholar]
- Launer LJ, Wind AW, Deeg DJ. Nonresponse pattern and bias in a community‐based cross‐sectional study of cognitive functioning among the elderly. American Journal of Epidemiology 1993;139(8):803‐12. [DOI] [PubMed] [Google Scholar]
- Schmand B, Lindeboom J, Hooijer C, Jonker C. Relation between education and dementia: the role of test bias revisited. Journal of Neurology, Neurosurgery & Psychiatry 1995;59(2):170‐4. [CRS: 99900001000000038; 99900001000000038; PUBMED: 7629532] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind AW, Schellevis FG, Staveren G, Scholten RP, Jonker C, Eijk JT. Limitations of the Mini‐Mental State Examination in diagnosing dementia in general practice. International Journal of Geriatric Psychiatry 1997;12(1):101‐8. [CRS: 99900001000000034; PUBMED: 9050431] [DOI] [PubMed] [Google Scholar]
Baker 1993 {published data only}
- Baker FM, Robinson BH, Stewart B. Use of the mini‐mental state examination in African American elders. Clinical Gerontologist: The Journal of Aging and Mental Health 1993;14(1):5‐13. [Google Scholar]
Brodaty 2002 {published data only}
- Brodaty H, Pond D, Kemp NM, Luscombe G, Harding L, Berman K, et al. The GPCOG: a new screening test for dementia designed for general practice. Journal of the American Geriatrics Society 2002;50(3):530‐4. [DOI] [PubMed] [Google Scholar]
Burkart 2000 {published data only}
- Burkart M, Heun R. Psychometric analysis of the selective reminding procedure in a sample from the general elderly population. Dementia and Geriatric Cognitive Disorders 2000;11(2):74‐80. [CRS: 99900001000000031; PUBMED: 10705164] [DOI] [PubMed] [Google Scholar]
Callahan 2002 {published data only}
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six‐item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care 2002;40(9):771‐81. [PUBMED: 12218768] [DOI] [PubMed] [Google Scholar]
Carnero‐Pardo 2013 {published and unpublished data}
- Carnero‐Pardo C, Espejo‐Martinez B, Lopez‐Alcalde S, Espinosa‐Garcia M, Saez‐Zea C, Vilchez‐Carrillo R, et al. Effectiveness and costs of phototest in dementia and cognitive impairment screening. BMC Neurology 2011;11:92. [CRS: 99900001000000010; 99900001000000010; PUBMED: 21801419] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creavin S. Solicitud de información para la revisión Cochrane : Efectividad del Mini‐Mental en la detección del deterioro cognitivo en Atención Primaria [personal communication]. Email to: C Carnero‐Pardo 31 October 2014.
- Olazaran J, Torreroc P, Cruz I, Aparicio E, Sanz A, Mula N, et al. Mild cognitive impairment and dementia in primary care: the value of medical history. Family Practice 2011;28:385–392. [DOI] [PubMed] [Google Scholar]
Correira 2001 {published data only}
- Correia CC, Lima F, Junqueira F, Campos MS, Bastos O, Petribu K, et al. AD8‐Brazil: cross‐cultural validation of the ascertaining dementia interview in Portuguese. Journal of Alzheimer's Disease 2011;27(1):177‐85. [DOI] [PubMed] [Google Scholar]
Cruz‐Orduna 2012 {published data only}
- Cruz‐Orduna I, Bellon JM, Torrero P, Aparicio E, Sanz A, Mula N, et al. Detecting MCI and dementia in primary care: effectiveness of the MMS, the FAQ and the IQCODE. Family Practice 2012;29(4):401‐6. Erratum in: Family Practice 2013;30(4):481. [CENTRAL: 99900001000000008; PUBMED: 22121012] [DOI] [PubMed] [Google Scholar]
Eefsting 1997 {published data only}
- Eefsting JA, Boersma F, Tilburg W, Brink W. Usefulness of the 'Mini‐mental state examination' (MMSE) for the diagnosis of dementia; a study of the criterion validity in a Dutch rural population [Bruikbaarheid van de 'Mini‐mental state examination' voor het vaststellen van dementie; onderzoek naar de criteriumvaliditeit in een Nederlandse plattelandspopulatie]. Nederlands Tijdschrift voor Geneeskunde 1997;141(43):2066‐70. [PubMed] [Google Scholar]
Fichter 1995 {published data only}
- Fichter MM, Meller I, Schroppel H, Steinkirchner R. Dementia and cognitive impairment in the oldest old in the community. Prevalence and comorbidity. British Journal of Psychiatry 1995;166(5):621‐9. [CRS: 99900001000000039; PUBMED: 7620747] [DOI] [PubMed] [Google Scholar]
Fillenbaum 1990 {published data only}
- Fillenbaum G, Heyman A, Williams K, Prosnitz B, Burchett B. Sensitivity and specificity of standardized screens of cognitive impairment and dementia among elderly black and white community residents. Journal of Clinical Epidemiology 1990;43(7):651‐60. [CRS: 99900001000000051; PUBMED: 2370572] [DOI] [PubMed] [Google Scholar]
Frank 1996 {published data only (unpublished sought but not used)}
- Frank R, Wiederholt WC, Kritz‐Silverstein DK, Salmon DP, Barrett‐Connor E. Effects of sequential neuropsychological testing of an elderly community‐based sample. Neuroepidemiology 1996;15(5):257‐68. [CRS: 99900001000000035; PUBMED: 8878078] [DOI] [PubMed] [Google Scholar]
Helsinki Aging Study 1994 {published data only}
- Juva K, Sulkava R, Erkinjuntti T, Ylikoski R, Valvanne J, Tilvis R. Staging the severity of dementia: comparison of clinical (CDR, DSM‐III‐R), functional (ADL, IADL) and cognitive (MMSE) scales. Acta Neurologica Scandinavica 1994;90(4):293‐8. [CRS: 99900001000000042; PUBMED: 7839817] [DOI] [PubMed] [Google Scholar]
- Juva K, Sulkava R, Erkinjuntti T, Ylikoski R, Valvanne J, Tilvis R. Usefulness of the clinical dementia rating scale in screening for dementia. International Psychogeriatrics 1995;7(1):17‐24. [DOI] [PubMed] [Google Scholar]
Heun 1998 {published data only}
- Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. International Journal of Geriatric Psychiatry 1998;13(6):368‐80. [CRS: 99900001000000033; PUBMED: 9658272] [DOI] [PubMed] [Google Scholar]
Iavarone 2006 {published data only}
- Iavarone A, Milan G, Vargas G, Lamenza F, Falco C, Gallotta G, et al. Role of functional performance in diagnosis of dementia in elderly people with low educational level living in Southern Italy. Aging Clinical and Experimental Research 2007;19(2):104‐9. [CRS: 99900001000000017; PUBMED: 17446720] [DOI] [PubMed] [Google Scholar]
Jacinto 2011 {published data only}
- Jacinto AF, Aguiar AC, Franco FG, Ribeiro MI, Citero Vde A. Dementia Rating Scale psychometric study and its applicability in long term care institutions in Brazil. Einstein 2011;10(3):318‐22. English, Portuguese. [DOI] [PubMed] [Google Scholar]
Jeong 2004 {published data only}
- Jeong SK, Cho KH, Kim JM. The usefulness of the Korean version of modified Mini‐Mental State Examination (K‐mMMSE) for dementia screening in community dwelling elderly people. BMC Public Health 2004;4:31. [CRS: 99900001000000023; 99900001000000023; PUBMED: 15283869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahle‐Wrobleski 2007 {published data only}
- Kahle‐Wrobleski K, Corrada MM, Li B, Kawas CH. Sensitivity and specificity of the mini‐mental state examination for identifying dementia in the oldest‐old: the 90+ study. Journal of the American Geriatrics Society 2007;55(2):284‐9. [CRS: 99900001000000018; 99900001000000018; PUBMED: 17302668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kathriarachchi 2005 {published data only}
- Kathriarachchi ST, Sivayogan S, Jayaratna SD, Dharmasena SR. Comparison of three instruments used in the assessment of dementia in Sri Lanka. Indian Journal of Psychiatry 2005;47(2):109‐12. [CRS: 99900001000000002; 99900001000000002; PUBMED: 20711293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keskinoglu 2009 {published data only}
- Keskinoglu P, Ucku R, Yener G, Yaka E, Kurt P, Tunca Z. Reliability and validity of revised Turkish version of Mini Mental State Examination (rMMSE‐T) in community‐dwelling educated and uneducated elderly. International Journal of Geriatric Psychiatry 2009;24(11):1242‐50. [CRS: 99900001000000012; PUBMED: 19337986] [DOI] [PubMed] [Google Scholar]
Kungsholmen Study 1992 {published data only}
- Fratiglioni L, Grut M, Forsell Y, Viitanen M, Winblad B. Clinical diagnosis of Alzheimer's disease and other dementias in a population survey. Agreement and causes of disagreement in applying Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition, Criteria. Archives of Neurology 1992;49(9):927‐32. [CRS: 99900001000000046; PUBMED: 1520083] [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Jorm AF, Grut M, Viitanen M, Holmen K, Ahlbom A, et al. Predicting dementia from the Mini‐Mental State Examination in an elderly population: the role of education. Journal of Clinical Epidemiology 1993;46(3):281‐7. [CRS: 99900001000000045; PUBMED: 8455053] [DOI] [PubMed] [Google Scholar]
- Grut M, Fratiglioni L, Viitanen M, Winblad B. Accuracy of the Mini‐Mental Status Examination as a screening test for dementia in a Swedish elderly population. Acta Neurologica Scandinavica 1993;87(4):312‐7. [CRS: 99900001000000044; PUBMED: 8503262] [DOI] [PubMed] [Google Scholar]
- Palmer K, Backman L, Winblad B, Fratiglioni L. Detection of Alzheimer's disease and dementia in the preclinical phase: population based cohort study. BMJ (Clinical Research Ed.) 2003;326(7383):245. [CRS: 99900001000000026; 99900001000000026; PUBMED: 12560271] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer's disease: a growth mixture modeling analysis. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior 2007;43(7):826‐34. [CRS: 99900001000000016; PUBMED: 17941341] [DOI] [PubMed] [Google Scholar]
- Small BJ, Viitanen M, Backman L. Mini‐Mental State Examination item scores as predictors of Alzheimer's disease: incidence data from the Kungsholmen Project, Stockholm. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 1997;52(5):M299‐304. [DOI] [PubMed] [Google Scholar]
- Wang HX, Ericsson K, Winblad B, Fratiglioni L. The Human Figure Drawing test as a screen for dementia in the elderly: a community‐based study. Archives of Gerontology and Geriatrics 1998;27(1):25‐34. [CRS: 99900001000000003; PUBMED: 18653148] [DOI] [PubMed] [Google Scholar]
Lam 2008 {published data only}
- Lam LC, Tam CW, Lui VW, Chan WC, Chan SS, Wong S, et al. Prevalence of very mild and mild dementia in community‐dwelling older Chinese people in Hong Kong. International Psychogeriatrics 2008;20(1):135‐48. [CRS: 99900001000000015; PUBMED: 17892609] [DOI] [PubMed] [Google Scholar]
Lavery 2007 {published data only (unpublished sought but not used)}
- Lavery LL, Lu SY, Chang CC, Saxton J, Ganguli M. Cognitive assessment of older primary care patients with and without memory complaints. Journal of General Internal Medicine 2007;22(7):949‐54. [PUBMED: 17453265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2002 {published data only (unpublished sought but not used)}
- Lee DY, Lee JH, Ju Y‐S, Lee KU, Kim KW, Jhoo JH, et al. The prevalence of dementia in older people in an urban population of Korea: the Seoul study. Journal of the American Geriatrics Society 2002;50(7):1233‐9. [DOI] [PubMed] [Google Scholar]
Li 2006 {published data only (unpublished sought but not used)}
- Li M, Ng TP, Kua EH, Ko SM. Brief informant screening test for mild cognitive impairment and early Alzheimer's disease. Dementia & Geriatric Cognitive Disorders 2006;21(5‐6):392‐402. [DOI] [PubMed] [Google Scholar]
Lindesay 1997 {published data only}
- Lindesay J, Jagger C, Mlynik‐Szmid A, Sinorwala A, Peet S, Moledina F. The Mini‐Metal State Examination (MMSE) in an elderly immigrant Gujarati population in the United Kingdom. International Journal of Geriatric Psychiatry 1997;12(12):1155‐67. [PubMed] [Google Scholar]
Liu 1996a {published data only (unpublished sought but not used)}
- Liu HC, Lin KN, Teng EL, Wang SJ, Fuh JL, Guo NW, et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. Journal of the American Geriatrics Society 1995;43(2):144‐9. [DOI] [PubMed] [Google Scholar]
Lourenco 2006 {published data only}
- Lourenco RA, Veras RP. Mini‐Mental State Examination: psychometric characteristics in elderly outpatients [Mini‐Exame do Estado Mental: caracteristicas psicometricas em idosos ambulatoriais]. Revista de Saude Publica 2006;40(4):712‐9. [CRS: 99900001000000021; PUBMED: 16906312] [DOI] [PubMed] [Google Scholar]
Macedo Montano 2005 {published data only}
- Montano MB, Ramos LR. Validity of the Portuguese version of Clinical Dementia Rating [Validade da versao em portugues da Clinical Dementia Rating]. Revista de Saude Publica 2005;39(6):912‐7. [CRS: 99900001000000022; PUBMED: 16341400] [DOI] [PubMed] [Google Scholar]
Mackinnon 2003 {published data only}
- Mackinnon A, Khalilian A, Jorm AF, Korten AE, Christensen H, Mulligan R. Improving screening accuracy for dementia in a community sample by augmenting cognitive testing with informant report. Journal of Clinical Epidemiology 2003;56(4):358‐66. [CRS: 99900001000000025; PUBMED: 12767413] [DOI] [PubMed] [Google Scholar]
Maki 2000 {published data only}
- Maki N, Ikeda M, Hokoishi K, Nebu A, Komori K, Hirono N, et al. The validity of the MMSE and SMQ as screening tests for dementia in the elderly general population‐‐ a study of one rural community in Japan. Dementia and Geriatric Cognitive Disorders 2000;11(4):193‐6. [CRS: 99900001000000029; PUBMED: 10867444] [DOI] [PubMed] [Google Scholar]
Mingyuan 1998 {published data only}
- Mingyuan Z, Katzman R, Peljun C. Incidence of dementia and Alzheimer's disease. Zhonghua Jingshenke Zazhi [Chinese Journal of Psychiatry] 1998;31(4):195‐98. Chinese. [Google Scholar]
MoVies Study 1993 {published data only}
- Borson S, Scanlan JM, Chen P, Ganguli M. The Mini‐Cog as a screen for dementia: validation in a population‐based sample. Journal of the American Geriatrics Society 2003;51(10):1451‐4. [CRS: 99900001000000024; PUBMED: 14511167] [DOI] [PubMed] [Google Scholar]
- Ganguli M, Belle S, Ratcliff G, Seaberg E, Huff FJ, Porten K, et al. Sensitivity and specificity for dementia of population‐based criteria for cognitive impairment: the MoVIES project. Journal of Gerontology 1993;48(4):M152‐61. [CRS: 99900001000000043; PUBMED: 8315228] [DOI] [PubMed] [Google Scholar]
- Ganguli M, Ratcliff G, Dekosky ST. Cognitive test scores in community‐based older adults with and without dementia. Aging & Mental Health 1997;1(2):176‐80. [Google Scholar]
- Ganguli M, Ratcliff G, Huff FJ, Belle S, Kancel MJ, Fischer L, et al. Effects of age, gender, and education on cognitive tests in a rural elderly community sample: norms from the Monongahela Valley Independent Elders Survey. Neuroepidemiology 1991;10(1):42‐52. [CRS: 99900001000000049; PUBMED: 2062416] [DOI] [PubMed] [Google Scholar]
- Ganguli M, Seaberg EC, Ratcliff GG, Belle SH, DeKosky ST. Cognitive stability over 2 years in a rural elderly population: the MoVIES project. Neuroepidemiology 1996;15(1):42‐50. [CRS: 99900001000000036; PUBMED: 8719048] [DOI] [PubMed] [Google Scholar]
Pandav 2002 {published data only}
- Pandav R, Fillenbaum G, Ratcliff G, Dodge H, Ganguli M. Sensitivity and specificity of cognitive and functional screening instruments for dementia: the Indo‐U.S. Dementia Epidemiology Study. Journal of the American Geriatrics Society 2002;50(3):554‐61. [CRS: 99900001000000028; PUBMED: 11943056] [DOI] [PubMed] [Google Scholar]
PAQUID Study {published data only}
- Commenges D, Gagnon M, Letenneur L, Dartigues JF, Barberger‐Gateau P, Salamon R. Improving screening for dementia in the elderly using, Mini‐Mental State Examination subscores, Benton's Visual Retention Test, and Isaacs' Set Test. Epidemiology (Cambridge, Mass.) 1992;3(2):185‐8. [CRS: 99900001000000047; PUBMED: 1576226] [DOI] [PubMed] [Google Scholar]
- Commenges D, Gagnon M, Letenneur L, Dartigues JF, Barberger‐Gateau P, Salamon R. Statistical description of the Mini‐Mental State Examination for French elderly community residents. Paquid Study Group. Journal of Nervous and Mental Disease 1992;180(1):28‐32. [CRS: 99900001000000048; PUBMED: 1538203] [DOI] [PubMed] [Google Scholar]
- Gagnon M, Letenneur L, Dartigues JF, Commenges D, Orgogozo JM, Barberger‐Gateau P, et al. Validity of the Mini‐Mental State examination as a screening instrument for cognitive impairment and dementia in French elderly community residents. Neuroepidemiology 1990;9(3):143‐50. [CRS: 99900001000000050; PUBMED: 2402325] [DOI] [PubMed] [Google Scholar]
- Letenneur L, Commenges D, Dartigues JF, Barberger‐Gateau P. Incidence of dementia and Alzheimer's disease in elderly community residents of south‐western France. International Journal of Epidemiology 1994;23(6):1256‐61. [CRS: 99900001000000041; PUBMED: 7721529] [DOI] [PubMed] [Google Scholar]
- Proust‐Lima C, Amieva H, Dartigues JF, Jacqmin‐Gadda H. Sensitivity of four psychometric tests to measure cognitive changes in brain aging‐population‐based studies. American Journal of Epidemiology 2007;165(3):344‐50. [CRS: 99900001000000020; 99900001000000020; PUBMED: 17105962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phantumchinda 1991 {published data only}
- Phanthumchinda K, Jitapunkul S, Sitthi‐Amorn C, Bunnag SC, Ebr‐Him S. Prevalence of dementia in an urban slum population in Thailand: validity of screening methods. International Journal of Geriatric Psychiatry 1991;6(9):639‐46. [Google Scholar]
Pond 1994 {published data only}
- Pond CD, Mant A, Kehoe L, Hewitt H, Brodaty H. General practitioner diagnosis of depression and dementia in the elderly: can academic detailing make a difference?. Family Practice 1994;11(2):141‐7. [PUBMED: 7958576] [DOI] [PubMed] [Google Scholar]
Ramlall 2013 {published data only}
- Ramlall S, Chipps J, Bhigjee AI, Pillay BJ. The sensitivity and specificity of subjective memory complaints and the subjective memory rating scale, deterioration cognitive observee, mini‐mental state examination, six‐item screener and clock drawing test in dementia screening. Dementia and Geriatric Cognitive Disorders 2013;36(1‐2):119‐35. [CRS: 99900001000000005; PUBMED: 23860433] [DOI] [PubMed] [Google Scholar]
Rosselli 2000 {published data only}
- Rosselli D. The mini‐mental state examination as a diagnostic selection test for dementia: a Colombian population study [El examen mental abreviado (mini‐mental state examination) como prueba de seleccion para el diagnostico de demencia: estudio poblacional colombiano]. Revista de Neurologia 2000;30(5):428‐32. [PubMed] [Google Scholar]
Rummans 1996 {published data only (unpublished sought but not used)}
- Rummans TA, Smith GE, Lin SC, Waring SC, Kokmen E. Comorbidity of dementia and psychiatric disorders in older persons. American Journal of Geriatric Psychiatry 1997;5(3):261‐7. [DOI] [PubMed] [Google Scholar]
Scazufca 2009 {published data only}
- Scazufca M, Almeida OP, Vallada HP, Tasse WA, Menezes PR. Limitations of the Mini‐Mental State Examination for screening dementia in a community with low socioeconomic status: results from the Sao Paulo Ageing & Health Study. European Archives of Psychiatry and Clinical Neuroscience 2009;259(1):8‐15. [CRS: 99900001000000013; PUBMED: 18560791] [DOI] [PubMed] [Google Scholar]
Schultz‐Larsen 2007 {published data only}
- Schultz‐Larsen K, Lomholt RK, Kreiner S. Mini‐Mental Status Examination: a short form of MMSE was as accurate as the original MMSE in predicting dementia. Journal of Clinical Epidemiology 2007;60(3):260‐7. [CRS: 99900001000000019; PUBMED: 17292020] [DOI] [PubMed] [Google Scholar]
Tang 1999 {published data only}
- Tang M, Zou X, Han H, Wang Y, Zhang L, Tang M, et al. Application of the Chinese version of the Mini‐Mental State Exam (MMSE) in 55 year‐olds and above from districts of Chengdu City, China. Zhongguo Xinli Weisheng Zazh [Chinese Mental Health Journal] 1999;13(4):200‐2. Chinese. [Google Scholar]
West Beijing Study 1989 {published data only}
- Li G, Shen YC, Chen CH, Zhao YW, Li SR, Lu M. An epidemiological survey of age‐related dementia in an urban area of Beijing, China. Acta Psychiatrica Scandinavica 1989;79(6):557‐63. [DOI] [PubMed] [Google Scholar]
- Li G, Shen YC, Chen CH, Zhau YW, Li SR, Lu M. A 3‐year follow‐up study of age‐related dementia in an urban area of Beijing. Acta Psychiatrica Scandinavica 1991;83(2):99‐104. [DOI] [PubMed] [Google Scholar]
Wilder 1995 {published data only (unpublished sought but not used)}
- Wilder D, Cross P, Chen J, Gurland B, Lantigua RA, Teresi J, et al. Operating characteristics of brief screens for dementia in a multicultural population. American Journal of Geriatric Psychiatry 1995;3(2):96‐107. [DOI] [PubMed] [Google Scholar]
Winblad 2010 {published data only}
- Winblad I, Viramo P, Remes A, Manninen M, Jokelainen J. Prevalence of dementia ‐ a rising challenge among ageing populations. European Geriatric Medicine 2010;1(6):330‐3. [Google Scholar]
References to studies excluded from this review
Almeida 1998 {published data only}
- Almeida OP. Mini mental state examination and the diagnosis of dementia in Brazil [Mini exame do estado mental e o diagnostico de demencia no Brasil]. Arquivos de Neuro‐psiquiatria 1998;56(3B):605‐12. [CRS: 99900001000000156; PUBMED: 9850757] [DOI] [PubMed] [Google Scholar]
Al‐rajeh 1999 {published data only}
- Al‐Rajeh S, Ogunniyi A, Awada A, Daif A, Zaidan R. Preliminary assessment of an Arabic version of the Mini‐Mental state examination. Annals of Saudi Medicine 1999;19(2):150‐2. [CRS: 99900001000000008; PUBMED: 17337959] [DOI] [PubMed] [Google Scholar]
Basic 2009 {published data only}
- Basic D, Khoo A, Conforti D, Rowland J, Vrantsidis F, LoGiudice D, et al. Rowland universal dementia assessment scale, mini‐mental state examination and general practitioner assessment of cognition in a multicultural cohort of community‐dwelling older persons with early dementia. Australian Psychologist 2009;44(1):40‐53. [Google Scholar]
Bastide 2012 {published data only}
- Bastide L, Breucker S, Berge M, Fery P, Pepersack T, Bier JC. The Addenbrooke's Cognitive Examination Revised is as effective as the original to detect dementia in a French‐speaking population. Dementia and Geriatric Cognitive Disorders 2012;34(5‐6):337‐43. [CRS: 99900001000000014; PUBMED: 23222058] [DOI] [PubMed] [Google Scholar]
Belmin 2007 {published data only}
- Belmin J, Pariel‐Madjlessi S, Surun P, Bentot C, Feteanu D, Lefebvre des Noettes V, et al. The cognitive disorders examination (Codex) is a reliable 3‐minute test for detection of dementia in the elderly (validation study on 323 subjects). Presse Medicale 2007;36(9 Pt 1):1183‐90. [CRS: 99900001000000086; PUBMED: 17433613] [DOI] [PubMed] [Google Scholar]
Bermejo 1999 {published data only}
- Bermejo F, Morales JM, Valerga C, Ser T, Artolazabal J, Gabriel R. Comparison between two abbreviated Spanish versions of mental status assessment for the diagnosis of dementia. Data of study in the dwelling‐community elderly people [Comparación entre dos versiones Españolas abreviadas de evaluación del estado mental en el diagnóstico de demencia. Datos de un estudio en ancianos residentes en la comunidad]. Medicina Clinica 1999;112(9):330‐4. [PubMed] [Google Scholar]
Bermejo‐Pareja 2009 {published data only}
- Bermejo‐Pareja F, Benito‐Leon J, Vega S, Olazaran J, Toledo M, Diaz‐Guzman J, et al. Consistency of clinical diagnosis of dementia in NEDICES: a population‐based longitudinal study in Spain. Journal of Geriatric Psychiatry and Neurology 2009;22(4):246‐55. [CRS: 99900001000000058; PUBMED: 19417217] [DOI] [PubMed] [Google Scholar]
- Prieto G, Contador I, Tapias‐Merino E, Mitchell AJ, Bermejo‐Pareja F. The Mini‐Mental‐37 test for dementia screening in the Spanish population: an analysis using the Rasch Model. Clinical Neuropsychologist 2012;26(6):1003‐18. [DOI] [PubMed] [Google Scholar]
Bland 2001 {published data only}
- Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini‐Mental State examination (3MS) as a screen for dementia. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 2001;46(6):506‐10. [CRS: 99900001000000137; PUBMED: 11526806] [DOI] [PubMed] [Google Scholar]
Borson 1999 {published data only}
- Borson S, Brush M, Gil E, Scanlan J, Vitaliano P, Chen J, et al. The Clock Drawing Test: utility for dementia detection in multiethnic elders. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 1999;54(11):M534‐40. [CRS: 99900001000000152; PUBMED: 10619314] [DOI] [PubMed] [Google Scholar]
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive 'vital signs' measure for dementia screening in multi‐lingual elderly. 2000; Vol. 15, issue 11:1021‐7. [DOI] [PubMed]
- Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. Journal of the American Geriatrics Society 2005;53(5):871‐4. [DOI] [PubMed] [Google Scholar]
Borson 2000 {published data only}
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive 'vital signs' measure for dementia screening in multi‐lingual elderly. International Journal of Geriatric Psychiatry 2000;15(11):1021‐7. [CRS: 99900001000000141; PUBMED: 11113982] [DOI] [PubMed] [Google Scholar]
Borson 2005 {published data only}
- Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini‐Cog and Mini‐Mental State Examination in a multiethnic sample. Journal of the American Geriatrics Society 2005;53(5):871‐4. [CRS: 99900001000000111; PUBMED: 15877567] [DOI] [PubMed] [Google Scholar]
Braekhus 1995 {published data only}
- Braekhus A, Laake K, Engedal K. A low, 'normal' score on the Mini‐Mental State Examination predicts development of dementia after three years. Journal of the American Geriatrics Society 1995;43(6):656‐61. [CRS: 99900001000000180; PUBMED: 7775725] [DOI] [PubMed] [Google Scholar]
Brooke 1999 {published data only}
- Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. International Journal of Geriatric Psychiatry 1999;14(11):936‐40. [CRS: 99900001000000154; PUBMED: 10556864] [PubMed] [Google Scholar]
Burnham 2012 {published data only}
- Burnham S, Graham P, Wilson B, Ames D, MacAulay L, Martins R, et al. Intensity of dementia through latent variable modelling (I‐DELV) in the AIBL cohort. Proceedings of the Alzheimer's Association International Conference; 2012 Jul 14‐19; Vancouver, BC Canada. Alzheimer's and Dementia 2012;8(4 Suppl):P131. [Google Scholar]
Cacho 2010 {published data only}
- Cacho J, Benito‐Leon J, Garcia‐Garcia R, Fernandez‐Calvo B, Vicente‐Villardon JL, Mitchell AJ. Does the combination of the MMSE and clock drawing test (mini‐clock) improve the detection of mild Alzheimer's disease and mild cognitive impairment?. Journal of Alzheimer's Disease 2010;22(3):889‐96. [CRS: 99900001000000042; PUBMED: 20858951] [DOI] [PubMed] [Google Scholar]
Canadian Study of Health and Aging {published data only}
- Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini‐Mental State examination (3MS) as a screen for dementia. Canadian Journal of Psychiatry ‐ Revue Canadienne de Psychiatrie 2001;46(6):506‐10. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Sykes E, Mcdowell I, Verreault R, Laurin D. More Than the Epidemiology of Alzheimer's Disease: Contributions of the Canadian Study of Health and Aging. Canadian Journal of Psychiatry‐Revue Canadienne De Psychiatrie 2004;49(2):83‐91. [DOI] [PubMed] [Google Scholar]
- McDowell I, Hill G, Lindsay J, Helliwell B, Costa L, Beattie BL, et al. Canadian Study of Health and Aging: Study methods and prevalence of dementia. Canadian Medical Association Journal 1994;150(6):899‐912. [PMC free article] [PubMed] [Google Scholar]
- McDowell I, Kristjansson B, Hill GB, Herbert R. Community screening for dementia: the Mini‐Mental State Exam (MMSE) and Modified Mini‐Mental State Exam (3MS) compared. Journal of Clinical Epidemiology 1997;50(4):377‐83. [DOI] [PubMed] [Google Scholar]
- O'Connell ME, Tuokko H, Graves RE, Kadlec H. Correcting the 3MS for bias does not improve accuracy when screening for cognitive impairment or dementia. Journal of Clinical and Experimental Neuropsychology 2004;26(7):970‐80. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Test‐retest reliable coefficients and 5‐year change scores for the MMSE and 3MS. Archives of Clinical Neuropsychology 2005;20(4):485‐503. [DOI] [PubMed] [Google Scholar]
Cao 2012 {published data only}
- Cao L, Hai S, Lin X, Shu D, Wang S, Yue J, et al. Comparison of the Saint Louis University Mental Status Examination, the Mini‐Mental State Examination, and the Montreal Cognitive Assessment in detection of cognitive impairment in Chinese elderly from the geriatric department. Journal of the American Medical Directors Association 2012;13(7):626‐9. [CRS: 99900001000000016; PUBMED: 22698956] [DOI] [PubMed] [Google Scholar]
Carpenter 2011 {published data only}
- Carpenter CR, Bassett ER, Fischer GM, Shirshekan J, Galvin JE, Morris JC. Four sensitive screening tools to detect cognitive dysfunction in geriatric emergency department patients: brief Alzheimer's Screen, Short Blessed Test, Ottawa 3DY, and the caregiver‐completed. Academic Emergency Medicine 2011;18(4):374‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cercy 2012 {published data only}
- Cercy SP. Diagnostic accuracy of a new instrument for detecting cognitive dysfunction. International Journal of Geriatric Psychiatry 2012;27(9):914‐23. [CRS: 99900001000000019; PUBMED: 22020766] [DOI] [PubMed] [Google Scholar]
- Cercy SP, Simakhodskaya Z, Elliott A. Diagnostic accuracy of a new instrument for detecting cognitive dysfunction in an emergent psychiatric population: the Brief Cognitive Screen. Academic Emergency Medicine 2010;17(3):307‐15. [CRS: 99900001000000053; PUBMED: 20370764] [DOI] [PubMed] [Google Scholar]
Cerveira 2009 {published data only}
- Cerveira MO, Heisler A, Fonseca M, Rech L, Silva MB, Camozzato AL, et al. Cognitive impairment screening in a Southern Brazilian elderly outpatient sample. Alzheimer's and Dementia 2009;5(4):274. [Google Scholar]
Cervilla 2004 {published data only}
- Cervilla J, Prince M, Joels S, Lovestone S, Mann A. Premorbid cognitive testing predicts the onset of dementia and Alzheimer's disease better than and independently of APOE genotype. Journal of Neurology, Neurosurgery & Psychiatry 2004;75(8):1100‐6. [CRS: 99900001000000120; 99900001000000120; PUBMED: 15258208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaves 2007 {published data only}
- Chaves ML, Camozzato AL, Godinho C, Kochhann R, Schuh A, Almeida VL, et al. Validity of the clinical dementia rating scale for the detection and staging of dementia in Brazilian patients. Alzheimer Disease and Associated Disorders 2007;21(3):210‐7. [PUBMED: 17804953] [DOI] [PubMed] [Google Scholar]
Chaves 2009 {published data only}
- Chaves ML, Camozzato AL, Godinho C, Piazenski I, Kaye J. Incidence of mild cognitive impairment and Alzheimer disease in Southern Brazil. Journal of Geriatric Psychiatry and Neurology 2009;22(3):181‐7. [CRS: 99900001000000060; PUBMED: 19307320] [DOI] [PubMed] [Google Scholar]
Chester 2011 {published data only}
- Chester JG, Grande LJ, Milberg WP, McGlinchey RE, Lipsitz LA, Rudolph JL. Cognitive screening in community‐dwelling elders: performance on the clock‐in‐the‐box. American Journal of Medicine 2011;124(7):662‐9. [CRS: 99900001000000035; 99900001000000035; PUBMED: 21592451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2010 {published data only}
- Chong MS, Lim WS, Chan SP, Feng L, Niti M, Yap P, et al. Diagnostic performance of the Chinese Frontal Assessment Battery in early cognitive impairment in an Asian population. Dementia and Geriatric Cognitive Disorders 2010;30(6):525‐32. [CRS: 99900001000000041; PUBMED: 21252547] [DOI] [PubMed] [Google Scholar]
Clark 1999 {published data only}
- Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, et al. Variability in annual mini‐mental state examination score in patients with probable alzheimer disease: a clinical perspective of data from the consortium to establish a registry for alzheimer disease. Archives of Neurology 1999;56(7):857‐62. [DOI] [PubMed] [Google Scholar]
Clarke 1991 {published data only}
- Clarke M, Jagger C, Anderson J, Battcock T, Kelly F, Stern MC. The prevalence of dementia in a total population: a comparison of two screening instruments. Age and Ageing 1991;20(6):396‐403. [CRS: 99900001000000202; PUBMED: 1776585] [DOI] [PubMed] [Google Scholar]
- Collerton D, Collerton J, Jagger J, Bond J, Saxby BK, Brooker H, McKeith IG, Kirkwood T. Cognitive performance in a UK cohort of 85 year olds: The Newcastle 85+ study. Journal of Psychophysiology: International Conference “Aging & Cognition”. 2011; Vol. 25 (s1):9.
- Jagger C, Clarke M, Anderson J. Screening for dementia ‐ a comparison of 2 tests using receiver operating characteristic (Roc) analysis. International Journal of Geriatric Psychiatry 1992;7(9):659‐65. [Google Scholar]
Cossa 1997 {published data only}
- Cossa FM, Della Sala S, Musicco M, Spinnler H, Ubezio MC. Comparison of two scoring systems of the Mini‐Mental State Examination as a screening test for dementia. Journal of Clinical Epidemiology 1997;50(8):961‐5. [DOI] [PubMed] [Google Scholar]
Cossa 1999 {published data only}
- Cossa FM, Sala SD, Musicco M, Spinnler H, Ubezio MC. The Milan overall dementia assessment and the mini‐mental state examination compared: an epidemiological investigation of dementia. European Journal of Neurology 1999;6(3):289‐94. [DOI] [PubMed] [Google Scholar]
Costa 2012 {published data only}
- Costa D, Severo M, Fraga S, Barros H. Mini‐Cog and Mini‐Mental State Examination: agreement in a cross‐sectional study with an elderly sample. Dementia and Geriatric Cognitive Disorders 2012;33(2‐3):118‐24. [DOI] [PubMed] [Google Scholar]
Cullen 2005 {published data only}
- Cullen B, Fahy S, Cunningham CJ, Coen RF, Bruce I, Greene E, et al. Screening for dementia in an Irish community sample using MMSE: a comparison of norm‐adjusted versus fixed cut‐points. International Journal of Geriatric Psychiatry 2005;20(4):371‐6. [CRS: 99900001000000112; PUBMED: 15799072] [DOI] [PubMed] [Google Scholar]
Dahl 2007 {published data only}
- Dahl A, Berg S, Nilsson SE. Identification of dementia in epidemiological research: a study on the usefulness of various data sources. Aging Clinical and Experimental Research 2007;19(5):381‐9. [CRS: 99900001000000082; PUBMED: 18007116] [DOI] [PubMed] [Google Scholar]
Damian 2011 {published data only}
- Damian AM, Jacobson SA, Hentz JG, Belden CM, Shill HA, Sabbagh MN, et al. The Montreal Cognitive Assessment and the mini‐mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dementia and Geriatric Cognitive Disorders 2011;31(2):126‐31. [CRS: 99900001000000037; PUBMED: 21282950] [DOI] [PubMed] [Google Scholar]
Dash 2006 {published data only}
- Dash P, Troupin A, Thomson J, Knowlton M. The Q&E in the detection of mild Alzheimer's disease. Research and Practice in Alzheimer's Disease 2006;11:191‐5. [Google Scholar]
Davous 1988 {published data only}
- Davous P, Lamour Y. Elementary test of concentration, orientation and memory. Application to the detection of dementia states in daily practice [Le test elementaire de concentration, orientation et memoire. Application au depistage d'un etat dementiel en pratique quotidienne]. Presse Medicale 1988;17(11):513‐5. [PubMed] [Google Scholar]
De Beaman {published data only}
- Beaman SR, Beaman PE, Garcia‐Pena C̃, Villa A, Heres J, Córdova A, et al. Validation of a modified version of the Mini‐Mental State Examination (MMSE) in Spanish. Aging Neuropsychology and Cognition 2004;11(1):1‐11. [Google Scholar]
De Jager 2009 {published data only}
- Jager CA, Schrijnemaekers AC, Honey TE, Budge MM. Detection of MCI in the clinic: evaluation of the sensitivity and specificity of a computerised test battery, the Hopkins Verbal Learning Test and the MMSE. Age and Ageing 2009;38(4):455‐60. [CRS: 99900001000000061; PUBMED: 19454402] [DOI] [PubMed] [Google Scholar]
Del‐Ser 1997 {published data only}
- Del‐Ser T, Morales JM, Barquero MS, Canton R, Bermejo F. Application of a Spanish version of the "Informant Questionnaire on Cognitive Decline in the Elderly" in the clinical assessment of dementia. Alzheimer Disease and Associated Disorders 1997;11(1):3‐8. [CRS: 99900001000000167; PUBMED: 9071438] [DOI] [PubMed] [Google Scholar]
Derrer 2001 {published data only}
- Derrer DS, Howieson DB, Mueller EA, Camicioli RM, Sexton G, Kaye JA. Memory testing in dementia: how much is enough?. Journal of Geriatric Psychiatry and Neurology 2001;14(1):1‐6. [CRS: 99900001000000140; PUBMED: 11281309] [DOI] [PubMed] [Google Scholar]
De Silva 2002 {published data only}
- Silva HA, Gunatilake SB. Mini Mental State Examination in Sinhalese: a sensitive test to screen for dementia in Sri Lanka. International Journal of Geriatric Psychiatry 2002;17(2):134‐9. [CRS: 99900001000000133; PUBMED: 11813275] [DOI] [PubMed] [Google Scholar]
Dierckx 2011 {published data only}
- Dierckx E, Carlon M, Engelborghs S, Raedt R, Deyn P, Ponjaert‐Kristoffersen I. Early detection of MCI: is the MOCA more sensitive for cognitive decline than the MMSE (a pilot study)?. Alzheimer's and dementia 2011;7(4):S535 (P3‐071). [Google Scholar]
Diniz 2007 {published data only}
- Diniz BS, Nunes PV, Yassuda MS, Pereira FS, Flaks MK, Viola LF, et al. Mild cognitive impairment: cognitive screening or neuropsychological assessment?. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil : 1999) 2008;30(4):316‐21. [CRS: 99900001000000068; PUBMED: 19142405] [DOI] [PubMed] [Google Scholar]
Dong 2012 {published data only}
- Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, et al. The Montreal Cognitive Assessment is superior to the Mini‐Mental State Examination in detecting patients at higher risk of dementia. International Psychogeriatrics 2012;24(11):1749‐55. [CRS: 99900001000000015; PUBMED: 22687278] [DOI] [PubMed] [Google Scholar]
Donnelly 2008 {published data only}
- Donnelly K, Donnelly JP, Cory E. Primary care screening for cognitive impairment in elderly veterans. American Journal of Alzheimer's Disease and Other Dementias 2008;23(3):218‐26. [CRS: 99900001000000075; PUBMED: 18375531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drachmann 1996 {published data only}
- Drachman DA, Swearer JM, Kane K, Osgood D, O'Toole C, Moonis M. The cognitive assessment screening test (CAST) for dementia. Journal of Geriatric Psychiatry and Neurology 1996;9(4):200‐8. [CRS: 99900001000000168; PUBMED: 8970013] [DOI] [PubMed] [Google Scholar]
Duron {published data only}
- Duron E, Plichart M, Rigaud AS, Laboure F, Hanon O. Metabolic syndrome and cognitive function in an elderly cohort with memory complaints: A transversal study. Alzheimer's and Dementia 2011;7(4):S351. [Google Scholar]
Fabrigoule 1995 {published data only}
- Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger‐Gateau P. Social and leisure activities and risk of dementia: a prospective longitudinal study. Journal of the American Geriatrics Society 1995;43(5):485‐90. [CRS: 99900001000000182; PUBMED: 7730528] [DOI] [PubMed] [Google Scholar]
Feher 1992 {published data only}
- Feher EP, Mahurin RK, Doody RS, Cooke N, Sims J, Pirozzolo FJ. Establishing the limits of the Mini‐Mental State. Examination of 'subtests'. Archives of Neurology 1992;49(1):87‐92. [CRS: 99900001000000201; PUBMED: 1728269] [DOI] [PubMed] [Google Scholar]
Fernandez‐Martinez 2008 {published data only}
- Fernandez‐Martinez M, Castro‐Flores J, Perez de Las Heras S, Mandaluniz‐Lekumberri A, Gordejuela‐Menocal M, Zarranz‐Imirizaldu JJ. Risk factors for dementia in the epidemiological study of Munguialde County (Basque Country‐Spain). BMC Neurology 2008;8:39. [CRS: 99900001000000072; 99900001000000072; PUBMED: 18922150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez‐Martinez 2010 {published data only}
- Fernandez‐Martinez M, Molano A, Castro J, Zarranz JJ. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's disease, and its relationship with cognitive impairment. Current Alzheimer Research 2010;7(6):515‐26. [DOI] [PubMed] [Google Scholar]
Ferrero‐Arias 2001 {published data only}
- Ferrero‐Arias J, Sanchez‐Saudinos M, Lamet‐Gil I. Five by five test. A brief instrument for the detection of cognitive impediment in clinical settings [El test "cinco por cinco". Un instrumento breve para la detección de impedimento cognitivo en contextos clínicos]. Neurologia (Barcelona, Spain) 2001;16(6):254‐61. [CRS: 99900001000000138; PUBMED: 11423042] [PubMed] [Google Scholar]
Ferruci 1998 {published data only}
- Ferrucci L, Lungo I, Guralnik JM, Bandinelli S, Benvenuti E, Salani B, et al. Is the telephone interview for cognitive status a valid alternative in persons who cannot be evaluated by the Mini Mental State Examination?. Aging Clinical and Experimental Research 1998;10(4):332‐8. [CRS: 99900001000000157; PUBMED: 9825025] [DOI] [PubMed] [Google Scholar]
Fong 2010 {published data only}
- Fong TG, Jones RN, Rudolph JL, Yang FM, Tommet D, Habtemariam D, et al. Development and validation of a brief cognitive assessment tool: the sweet 16. Archives of Internal Medicine 2011;171(5):432‐7. [CRS: 99900001000000038; PUBMED: 21059967] [DOI] [PubMed] [Google Scholar]
Forlani {published data only}
- Forlani C, Morri M, Ferrari B, Dalmonte E, Ronchi D, Atti AR. Cognitive status in 75 years and older subjects. A population‐based study. European Psychiatry 2010;25(1):1493. [Google Scholar]
Fountoulakis 1998 {published data only}
- Fountoulakis KN, Tsolaki M, Mohs RC, Kazis A. Epidemiological dementia index: a screening instrument for Alzheimer's disease and other types of dementia suitable for use in populations with low education level. Dementia and Geriatric Cognitive Disorders 1998;9(6):329‐38. [CRS: 99900001000000159; PUBMED: 9769446] [DOI] [PubMed] [Google Scholar]
Fountoulakis 2000 {published data only}
- Fountoulakis KN, Tsolaki M, Paulopoulos H, Kazis A. Validation of the epidemiological dementia index in geriatric outpatients. International Psychogeriatrics 2000;12(2):195‐208. [CRS: 99900001000000142; PUBMED: 10937540] [DOI] [PubMed] [Google Scholar]
Fratiglioni 1993 {published data only}
- Fratiglioni L. Epidemiology of Alzheimer's disease. Issues of etiology and validity. Acta Neurologica Scandinavica 1993;145 Supplementum:1‐70. [CRS: 99900001000000191; PUBMED: 8333250] [PubMed] [Google Scholar]
Fratiglioni 1994 {published data only}
- Fratiglioni L, Forsell Y, Aguero Torres H, Winblad B. Severity of dementia and institutionalization in the elderly: prevalence data from an urban area in Sweden. Neuroepidemiology 1994;13(3):79‐88. [CRS: 99900001000000189; PUBMED: 8015667] [DOI] [PubMed] [Google Scholar]
Fujiawara 2003 {published data only}
- Fujiwara Y, Amano H, Mori S, Watanabe S, Kumagai S, Yoshida Y, et al. Toward constructing a system for detecting and coping with senile dementia in early stages among community‐dwelling older people. Nippon Koshu Eisei Zasshi [Japanese Journal of Public Health] 2003;50(8):739‐48. Japanese. [CRS: 99900001000000125; PUBMED: 14515751] [PubMed] [Google Scholar]
Gabryelewicz 2002 {published data only}
- Gabryelewicz T, Parnowski T, Szafranska A, Matuszewska E, Jarkiewicz E, Kotapka‐Minc S, et al. The prevalence of dementia in Poland: a population‐based, door‐to‐door survey in an urban community. Archives of Psychiatry and Psychotherapy 2002;4(1):17‐26. [Google Scholar]
Galvin 2010 {published data only}
- Galvin JE, Fagan AM, Holtzman DM, Mintun MA, Morris JC. Relationship of dementia screening tests with biomarkers of Alzheimer's disease. Brain 2010;133(11):3290‐300. [CRS: 99900001000000044; 99900001000000044; PUBMED: 20823087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganguli 2004b {published data only}
- Ganguli M, Ratcliff G, Huff FJ, Belle S, Kancel MJ, Fischer L, et al. Effects of age, gender, and education on cognitive tests in a rural elderly community sample: norms from the Monongahela Valley Independent Elders Survey. Neuroepidemiology 1991;10(1):42‐52. [CRS: 99900001000000203; PUBMED: 2062416] [DOI] [PubMed] [Google Scholar]
Ganguli 2010a {published data only}
- Ganguli M, Bilt JV, Lee CW, Snitz BE, Chang CC, Loewenstein DA, et al. Cognitive test performance predicts change in functional status at the population level: the MYHAT Project. Journal of the International Neuropsychological Society 2010;16(5):761‐70. [CRS: 99900001000000045; 99900001000000045; PUBMED: 20609270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganguli 2010b {published data only}
- Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela‐Youghiogheny Healthy Aging Team (MYHAT) project. American Journal of Geriatric Psychiatry 2010;18(8):674‐83. [CRS: 99900001000000048; 99900001000000048; PUBMED: 20220597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganzer 2003 {published data only}
- Ganzer S, Arlt S, Schoder V, Buhmann C, Mandelkow E‐M, Finckh U, et al. CSF‐tau, CSF‐Aß1‐42, ApoE‐genotype and clinical parameters in the diagnosis of Alzheimer’s disease: combination of CSF‐tau and MMSE yields highest sensitivity and specificity. Journal of Neural Transmission 2003;110(10):1149‐60. [DOI] [PubMed] [Google Scholar]
Garcia {published data only}
- Garcia A, Hemraj A, Klar S, Chestney T, Khoja L, Day A. Homocysteine and cognitive tests as predictors of cognitive decline: a cohort study.
Garcia 1993 {published data only}
- Garcia CE, Loyola JC, Armstrong TC, Muhienbrock F, Blake PE. A simple method to assess cognitive function: the clock drawing test [Prueba del reloj: un método simple para evaluar demencia]. Revista Médica de Chile 1983;121(11):1284‐8. [PubMed] [Google Scholar]
Geerlings 1999 {published data only}
- Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. American Journal of Psychiatry 1999;156(4):531‐7. [DOI] [PubMed] [Google Scholar]
Gibbons {published data only}
- Gibbons L, Carle A, Insel P, MacKin S, Mukherjee S, McKay Curtis S, et al. Composite scores for memory out performed other approaches to the neuropsychological data collected by the Alzheimer's disease neuroimaging initiative (ADNI). Alzheimer's and Dementia.
Goldman 2001 {published data only}
- Goldman WP, Morris JC. Evidence that age‐associated memory impairment is not a normal variant of aging. Alzheimer Disease and Associated Disorders 2001;15(2):72‐79. [DOI] [PubMed] [Google Scholar]
Gondo 2006 {published data only}
- Gondo Y, Hirose N, Arai Y, Inagaki H, Masui Y, Yamamura K, Shimizu K‐I, Takayama M, Ebihara Y, Nakazawa S, Kitagawa K. Functional status of centurians in Tokyo, Japan: developing better phentypes of exceptional longevity. Journal of Gerontology 2006;61A(3):305‐10. [DOI] [PubMed] [Google Scholar]
Grigoletto 1999 {published data only}
- Grigoletto F, Zappala G, Anderson DW, Lebowitz BD. Norms for the Mini‐Mental State Examination in a healthy population. Neurology 1999;53(2):315‐20. [DOI] [PubMed] [Google Scholar]
Grober 2008 {published data only}
- Grober E, Hall C, Lipton RB, Teresi JA. Primary care screen for early dementia. Journal of the American Geriatrics Society 2008;56(2):206‐13. [DOI] [PubMed] [Google Scholar]
Harder 1995 {published data only}
- Harder H, Lanser JBK, Haan EHF, Roos RAC. The Mini‐mental state is insufficient as a screening test for cognitive deterioration in a neurological setting [De 'Mini‐mental state' ‐ test ontoereikend als screeningtest voor cognitieve deterioratie op een afdeling Neurologie]. Ned Tijdschr Geneeskd 1995;139:1742‐5. [PubMed] [Google Scholar]
Hartmann 2002 {published data only}
Hashizume 2004 {published data only}
- Hashizume T. Severity of Alzheimer disease and the significance of a dementia test battery. Jikeikai Ika Daigaku zasshi [ Tokyo Jikeikai Medical Journal] 2004;119(1):41‐50. [Google Scholar]
Helkala 2002 {published data only}
- Helkala EL, Kivipelto M, Hallikainen M, Alhainen K, Heinonen H, Tuomilehto J, et al. Usefulness of repeated presentation of Mini‐Mental State Examination as a diagnostic procedure‐‐a population‐based study. Acta Neurologica Scandinavica 2002;106(6):341‐6. [CRS: 99900001000000129; PUBMED: 12460138] [DOI] [PubMed] [Google Scholar]
Hensel 2009 {published data only}
- Hensel A, Luck T, Luppa M, Glaesmer H, Angermeyer MC, Riedel‐Heller SG. Does a reliable decline in Mini Mental State Examination total score predict dementia? Diagnostic accuracy of two reliable change indices. Dementia and Geriatric Cognitive Disorders 2009;27(1):50‐8. [CRS: 99900001000000066; PUBMED: 19129701] [DOI] [PubMed] [Google Scholar]
Hogervorst {published data only}
- Hogervorst E, Rahardjo TB, Bandelow S. Cross cultural validation of a dementia screening test. Alzheimer's and Dementia 2011;7(4):S160‐S161. [Google Scholar]
Holsinger 2012 {published data only}
- Holsinger T, Plassman BL, Stechuchak KM, Burke JR, Coffman CJ, Williams JWJ. Screening for cognitive impairment: comparing the performance of four instruments in primary care. Journal of the American Geriatrics Society 2012;60(6):1027‐36. [CRS: 99900001000000025; PUBMED: 22646750] [DOI] [PubMed] [Google Scholar]
Huppert 2005 {published data only}
- Huppert FA, Cabelli ST, Matthews FE, MRC Cognitive Function and Ageing Study. Brief cognitive assessment in a UK population sample ‐‐ distributional properties and the relationship between the MMSE and an extended mental state examination. BMC Geriatrics 2005;5:7. [CRS: 99900001000000110; 99900001000000110; PUBMED: 15869717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2009 {published data only}
- Ibrahim NM, Shohaimi S, Chong HT, Rahman AH, Razali R, Esther E, et al. Validation study of the Mini‐Mental State Examination in a Malay‐speaking elderly population in Malaysia. Dementia and Geriatric Cognitive Disorders 2009;27(3):247‐53. [CRS: 99900001000000063; PUBMED: 19246909] [DOI] [PubMed] [Google Scholar]
Ideno 2012 {published data only}
- Ideno Y, Takayama M, Hayashi K, Takagi H, Sugai Y. Evaluation of a Japanese version of the Mini‐Mental State Examination in elderly persons. Geriatrics & Gerontology International 2012;12(2):310‐6. [CRS: 99900001000000028; PUBMED: 22122408] [DOI] [PubMed] [Google Scholar]
Ihl 2005 {published data only}
- Ihl R, Biesenbach A, Brieber S, Grass‐Kapanke B, Salamon T. A head‐to‐head comparison of the sensitivity of two screening tests for dementia: mini‐mental‐state‐examination (mmse) and the test for the early detection of dementia with discrimination from depression (te4d). Polish Psychogeriatria Polska 2005;2(3):263‐271. [Google Scholar]
Jagger 1992 {published data only}
- Jagger C, Clarke M, Anderson J, Battcock T. Misclassification of dementia by the mini‐mental state examination‐‐are education and social class the only factors?. Age and Ageing 1992;21(6):404‐11. [CRS: 99900001000000194; PUBMED: 1471577] [DOI] [PubMed] [Google Scholar]
Jeong 2007 {published data only}
- Jeong JW, Kim KW, Lee DY, Lee SB, Park JH, Choi EA, et al. A normative study of the Revised Hasegawa Dementia Scale: comparison of demographic influences between the Revised Hasegawa Dementia Scale and the Mini‐Mental Status Examination. Dementia and Geriatric Cognitive Disorders 2007;24(4):288‐93. [CRS: 99900001000000083; PUBMED: 17717415] [DOI] [PubMed] [Google Scholar]
Jervis 2007 {published data only}
- Jervis LL, Beals J, Fickenscher A, Arciniegas DB. Performance on the Mini‐Mental State Examination and Mattis Dementia Rating Scale among older American Indians. Journal of Neuropsychiatry and Clinical Neurosciences 2007;19(2):173‐8. [CRS: 99900001000000089; PUBMED: 17431064] [DOI] [PubMed] [Google Scholar]
Jeste 1992 {published data only}
- Jeste DV, Wragg RE, Salmon DP, Harris MJ, Thal LJ. Cognitive deficits of patients with Alzheimer's disease with and without delusions. American Journal of Psychiatry 1992;149(2):184‐9. [CRS: 99900001000000200; PUBMED: 1734737] [DOI] [PubMed] [Google Scholar]
Jones 2010 {published data only}
- Jones K, Perlman CM, Hirdes JP, Scott T. Screening cognitive performance with the Resident Assessment Instrument for Mental Health Cognitive Performance Scale. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 2010;55(11):736‐40. [CRS: 99900001000000043; PUBMED: 21070702] [DOI] [PubMed] [Google Scholar]
Jonsson 2010 {published data only}
- Jonsson M, Zetterberg H, Straaten E, Lind K, Syversen S, Edman A, et al. Cerebrospinal fluid biomarkers of white matter lesions ‐ cross‐sectional results from the LADIS. European Journal of Neurology 2010;17(3):377‐82. [DOI] [PubMed] [Google Scholar]
Jorm 1996 {published data only}
- Jorm AF, Broe GA, Creasey H, Sulway MR, Dent O, Fairley MJ, et al. Further data on the validity of the informant questionnaire on cognitive decline in the elderly (IQCODE). International Journal of Geriatric Psychiatry 1996;11(2):131‐9. [Google Scholar]
Jorm 1997 {published data only}
- Jorm AF, Mackinnon AJ, Christensen H, Henderson AS, Jacomb PA, Korten AE. The psychogeriatric assessment scales (PAS): further data on psychometric properties and validity from a longitudinal study. International Journal of Geriatric Psychiatry 1997;12(1):93‐100. [DOI] [PubMed] [Google Scholar]
Kal'bus {published data only}
- Kal'bus O. Diabetes mellitus and cognitive decline: Screening scales are not sensitive enough in early stages of disease. European Journal of Neurology 2012;64:616. [Google Scholar]
Kamenski 2009 {published data only}
- Kamenski G, Dorner T, Lawrence K, Psota G, Rieder A, Schwarz F, et al. Detection of dementia in primary care: comparison of the original and a modified Mini‐Cog Assessment with the Mini‐Mental State Examination. Mental Health in Family Medicine 2009;6(4):209‐17. [CRS: 99900001000000004; 99900001000000004; PUBMED: 22477912] [PMC free article] [PubMed] [Google Scholar]
Kanegae 2008 {published data only}
- Kanegae S, Ichimaru N, Fleming R, Koizumi S. Development of the Japanese version Care planning assessment Tool (J‐CPAT): reliability and validity studies. Nippon Ronen Igakkai Zasshi [Japanese Journal of Geriatrics] 2008;45(3):323‐9. Japanese. [DOI] [PubMed] [Google Scholar]
Kaufer 2008 {published data only}
- Kaufer DI, Williams CS, Braaten AJ, Gill K, Zimmerman S, Sloane PD. Cognitive screening for dementia and mild cognitive impairment in assisted living: comparison of 3 tests. Journal of the American Medical Directors Association 2008;9(8):586‐93. [CRS: 99900001000000071; PUBMED: 19083293] [DOI] [PubMed] [Google Scholar]
Khachaturian 2000 {published data only}
- Khachaturian AS, Gallo JJ, Breitner JC. Performance characteristics of a two‐stage dementia screen in a population sample. Journal of Clinical Epidemiology 2000;53(5):531‐40. [CRS: 99900001000000144; PUBMED: 10812327] [DOI] [PubMed] [Google Scholar]
Kirby 2001 {published data only}
- Fahy S, Lawlor BA. The clock drawing test in primary care: Sensitivity in dementia detection and specificity against normal and depressed elderly. International Journal of Geriatric Psychiatry 2001;16(10):935‐940. [DOI] [PubMed] [Google Scholar]
- Kirby M, Denihan A, Bruce I, Coakley D, Lawlor BA. The clock drawing test in primary care: sensitivity in dementia detection and specificity against normal and depressed elderly. International Journal of Geriatric Psychiatry 2001;16(10):935‐40. [CRS: 99900001000000134; PUBMED: 11607936] [DOI] [PubMed] [Google Scholar]
Kliegel 2004 {published data only}
- Kliegel M, Sliwinski M. MMSE cross‐domain variability predicts cognitive decline in centenarians. Gerontology 2004;50(1):39‐43. [CRS: 99900001000000123; PUBMED: 14654726] [DOI] [PubMed] [Google Scholar]
Kochhann 2010 {published data only}
- Kochhann R, Varela J, Santos de M, Lisboa C, Saraiva Chaves M, Lorena F. The mini mental state examination: review of cutoff points adjusted to schooling in a large southern brazilian sample. 2010; Vol. 4, issue 1:35‐41. [DOI] [PMC free article] [PubMed]
Koski 2011 {published data only}
- Koski L, Xie H, Konsztowicz S. Improving precision in the quantification of cognition using the Montreal Cognitive Assessment and the Mini‐Mental State Examination. International Psychogeriatrics 2011;23(7):1107‐15. [CRS: 99900001000000033; PUBMED: 21281555] [DOI] [PubMed] [Google Scholar]
Koson {published data only}
- Koson P, Cunderlikova M, Blahova M, Varsanyiova O, Vrazda L, Gogolak I. Different sensitivity of neuropsychological screening tests ace‐r and mmse in patients with mci and dementia. Alzheimer's and Dementia 2012;8(4):369. [Google Scholar]
Krigbaum 2012 {published data only}
- Krigbaum G, Amin K, Virden TB, Baca L, Uribe A. A pilot study of the sensitivity and specificity analysis of the standard‐Spanish version of the Culture‐Fair Assessment of Neurocognitive Abilities and the Examen Cognoscitivo Mini‐Mental in the Dominican Republic. Applied Neuropsychology 2012;19(1):53‐60. [CRS: 99900001000000029; PUBMED: 22385380] [DOI] [PubMed] [Google Scholar]
Kukull 1994 {published data only}
- Kukull WA, Larson EB, Teri L, Bowen J, McCormick W, Pfanschmidt ML. The Mini‐Mental State Examination score and the clinical diagnosis of dementia. Journal of Clinical Epidemiology 1994;47(9):1061‐7. [CRS: 99900001000000184; PUBMED: 7730909] [DOI] [PubMed] [Google Scholar]
Kuslansky 2004 {published data only}
- Kuslansky G, Katz M, Verghese J, Hall CB, Lapuerta P, LaRuffa G, et al. Detecting dementia with the Hopkins Verbal Learning Test and the Mini‐Mental State Examination. Archives of Clinical Neuropsychology 2004;19(1):89‐104. [CRS: 99900001000000122; PUBMED: 14670382] [PubMed] [Google Scholar]
Lam {published data only}
- Lam LC, Tam CW, Yeung GT, Lui VW, Fung AW. Predictors of improvement in cognitive ability in Chinese persons with mild cognitive impairment: A 2‐year follow up. Alzheimer's and Dementia 2012;8:365. [Google Scholar]
Lam 2005a {published data only}
- Lam LC, Lui VW, Tam CW, Chiu HF. Subjective memory complaints in Chinese subjects with mild cognitive impairment and early Alzheimer's disease. International Journal of Geriatric Psychiatry 2005;20(9):876‐82. [CRS: 99900001000000107; PUBMED: 16116581] [DOI] [PubMed] [Google Scholar]
Lam 2005b {published data only}
- Lam LC, Lui VW, Chiu HF, Chan SS, Tam CW. Executive function impairment in community elderly subjects with questionable dementia. Dementia and Geriatric Cognitive Disorders 2005;19(2‐3):86‐90. [CRS: 99900001000000113; PUBMED: 15572877] [DOI] [PubMed] [Google Scholar]
Larner 2012 {published data only}
- Larner AJ. An audit of the Addenbrooke's Cognitive Examination (ACE) in clinical practice. International Journal of Geriatric Psychiatry 2005;20(6):593‐4. [CRS: 99900001000000108; PUBMED: 15962353] [DOI] [PubMed] [Google Scholar]
- Larner AJ. Mini‐mental Parkinson (MMP) as a dementia screening test: comparison with the Mini‐Mental State Examination (MMSE). Current Aging Science 2012;5(2):136‐9. [CRS: 99900001000000018; PUBMED: 21834788] [DOI] [PubMed] [Google Scholar]
Larson 1984 {published data only}
- Larson EB, Reifler BV, Canfield C, Cohen GD. Evaluating elderly outpatients with symptoms of dementia. Hospital & Community Psychiatry 1984;35(5):425‐8. [CRS: 99900001000000209; PUBMED: 6724537] [DOI] [PubMed] [Google Scholar]
Lautenschlager 1986 {published data only}
- Lautenschlaeger E, Meier HR, Donnelly M. Folstein Vs. Goldfarb Mental Status Examinations. Clinical gerontologist 1986;4(4). [Google Scholar]
Law 1995 {published data only}
- Law S, Wolfson C. Validation of a French version of an informant‐based questionnaire as a screening test for Alzheimer's disease. British Journal of Psychiatry 1995;167(4):541‐4. [CRS: 99900001000000173; PUBMED: 8829727] [DOI] [PubMed] [Google Scholar]
Lee {published data only}
- Lee DY, Lee JH, Ju YS, Lee KU, Kim KW, Jhoo JH, et al. The prevalence of dementia in older people in an urban population of Korea: the Seoul study. Journal of the American Geriatrics Society 2002;50(7):1233‐9. [CRS: 99900001000000130; PUBMED: 12133018] [DOI] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee CS, Chang SF, Su CL, Chen ZY, Chen RC. Neuroepidemiological study in Ilan, Taiwan (NESIT): (3) An epidemiological survey of dementia in a rural area. Acta Neurologica Taiwanica 1997;6(1):27‐35. [Google Scholar]
Lee 2009 {published data only}
- Lee JG, Shin BS, You YS, Kim JE, Yoon SW, Jeon DW, et al. Decreased serum brain‐derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investigation 2009;6(4):299‐305. [CRS: 99900001000000006; 99900001000000006; PUBMED: 20140129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leoutsakos 2012 {published data only}
- Leoutsakos JM, Han D, Mielke MM, Forrester SN, Tschanz JT, Corcoran CD, et al. Effects of general medical health on Alzheimer's progression: the Cache County Dementia Progression Study. International Psychogeriatrics 2012;24(10):1561‐70. [CRS: 99900001000000017; 99900001000000017; PUBMED: 22687143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li H, Zhang HH, Huang H, Wang YZ, Huang HL. Prevalence of dementia among rural elderly in Gushan township, Fuzhou. Chung‐Hua Liu Hsing Ping Hsueh Tsa Chih [Chinese Journal of Epidemiology] 2009;30(8):772‐5. Chinese. [CRS: 99900001000000056; PUBMED: 20193195] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li X, Xiao S, Fang Y, Zhu M, Wang T, Seeher K, et al. Validation of the General Practitioner Assessment of Cognition ‐ Chinese version (GPCOG‐C) in China. International Psychogeriatrics 2013;25(10):1649‐57. [CRS: 99900001000000010; PUBMED: 23835084] [DOI] [PubMed] [Google Scholar]
Limpawattana 2012 {published data only}
- Limpawattana P, Tiamkao S, Sawanyawisuth K. The performance of the Rowland Universal Dementia Assessment Scale (RUDAS) for cognitive screening in a geriatric outpatient setting. Aging Clinical and Experimental Research 2012;24(5):495‐500. [CRS: 99900001000000012; PUBMED: 22395236] [DOI] [PubMed] [Google Scholar]
- Limpawattana P, Tiamkao S, Sawanyawisuth K, Thinkhamrop B. Can Rowland Universal Dementia Assessment Scale (RUDAS) replace Mini‐mental State Examination (MMSE) for dementia screening in a Thai geriatric outpatient setting?. American Journal of Alzheimer's Disease and Other Dementias 2012;27(4):254‐9. [CRS: 99900001000000023; PUBMED: 22615482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 1998 {published data only}
- Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. Journal of the Neurological Sciences 1998;160(1):67‐75. [CRS: 99900001000000158; PUBMED: 9804120] [DOI] [PubMed] [Google Scholar]
Liu 1994 {published data only}
- Liu HC, Chou P, Lin KN, Wang SJ, Fuh JL, Lin HC, et al. Assessing cognitive abilities and dementia in a predominantly illiterate population of older individuals in Kinmen. Psychological Medicine 1994;24(3):763‐70. [CRS: 99900001000000187; PUBMED: 7991758] [DOI] [PubMed] [Google Scholar]
Liu 1995 {published data only (unpublished sought but not used)}
- Liu HC, Lin KN, Teng EL, Wang SJ, Fuh JL, Guo NW, et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. Journal of the American Geriatrics Society 1995;43(2):144‐9. [CRS: 99900001000000183; PUBMED: 7836638] [DOI] [PubMed] [Google Scholar]
Liu 1996b {published data only}
- Liu CK, Lin RT, Chen YF, Tai CT, Yen YY, Howng SL. Prevalence of dementia in an urban area in Taiwan. JTaiwan Yi Zhi [Journal of the Formosan Medical Association] 1996;95(10):762‐8. [CRS: 99900001000000169; PUBMED: 8961673] [PubMed] [Google Scholar]
Liu 1998 {published data only}
- Liu CK, Lai CL, Tai CT, Lin RT, Yen YY, Howng SL. Incidence and subtypes of dementia in southern Taiwan: impact of socio‐demographic factors. Neurology 1998;50(6):1572‐9. [CRS: 99900001000000162; PUBMED: 9633696] [DOI] [PubMed] [Google Scholar]
Llibre 2009 {published data only}
- Llibre Jde J, Fernandez Y, Marcheco B, Contreras N, Lopez AM, Otero M, et al. Prevalence of dementia and Alzheimer's disease in a Havana municipality: a community‐based study among elderly residents. MEDICC Review 2009;11(2):29‐35. [CRS: 99900001000000005; PUBMED: 21483315] [DOI] [PubMed] [Google Scholar]
Lobo 2008 {published data only}
- Lobo A, Lopez‐Anton R, Camara C, Quintanilla MA, Campayo A, Saz P, et al. Non‐cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, Alzheimer's type. Neurotoxicity Research 2008;14(2‐3):263‐72. [CRS: 99900001000000070; PUBMED: 19073431] [DOI] [PubMed] [Google Scholar]
Lopes 2010 {published data only}
- Lopes MA, Furtado EF, Ferrioli E, Litvoc J, Bottino CM. Prevalence of alcohol‐related problems in an elderly population and their association with cognitive impairment and dementia. Alcoholism, Clinical and Experimental Research 2010;34(4):726‐33. [CRS: 99900001000000051; PUBMED: 20102571] [DOI] [PubMed] [Google Scholar]
Lopez‐Pousa 1995 {published data only}
- Lopez Pousa S, Llinas Regla J, Vilalta Franch J, Fernandez De Pinedo RL. The prevalence of dementia in Girona. Neurologia 1995;10(5):189‐193. [PubMed] [Google Scholar]
Luis 2009 {published data only}
- Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. International Journal of Geriatric Psychiatry 2009;24(2):197‐201. [CRS: 99900001000000067; PUBMED: 18850670] [DOI] [PubMed] [Google Scholar]
MacKenzie 1996 {published data only}
- MacKenzie DM, Copp P, Shaw RJ, Goodwin GM. Brief cognitive screening of the elderly: a comparison of the Mini‐Mental State Examination (MMSE), Abbreviated Mental Test (AMT) and Mental Status Questionnaire (MSQ). Psychological Medicine 1996;26(2):427‐30. [CRS: 99900001000000171; PUBMED: 8685299] [DOI] [PubMed] [Google Scholar]
MacKnight 1999 {published data only}
- MacKnight C, Graham J, Rockwood K. Factors associated with inconsistent diagnosis of dementia between physicians and neuropsychologists. Journal of the American Geriatrics Society 1999;47(11):1294‐9. [DOI] [PubMed] [Google Scholar]
MacNeill 2000 {published data only}
- MacNeill SE, Lichtenberg PA. The MacNeill‐Lichtenberg Decision Tree: a unique method of triaging mental health problems in older medical rehabilitation patients. Archives of Physical Medicine and Rehabilitation 2000;81(5):618‐22. [CRS: 99900001000000145; PUBMED: 10807102] [DOI] [PubMed] [Google Scholar]
Marcos de Vega {published data only}
- Marcos De Vega A, Zea Sevilla M, Gomez Vicente L, Parejo Carbonel B, Manzano Palomo S. Amnestic mild cognitive impairment (aMCI) or pre‐dementia Alzheimer's disease. A longitudinal follow‐up of aMCI cases in the Behavior and Cognition Unit of Clinico Hospital (BCU‐CH), Madrid, Spain. European Journal of Neurology. [Google Scholar]
Medina {published data only}
- Medina P. Mild Cognitive Impairment prevalence in the population older than 65 years old. Neurodegenerative Diseases 2011:Poster presentation. [Google Scholar]
Meguro 2007 {published data only}
- Meguro K, Ishii H, Kasuya M, Akanuma K, Meguro M, Kasai M, et al. Incidence of dementia and associated risk factors in Japan: The Osaki‐Tajiri Project. Journal of the Neurological Sciences 2007;260(1‐2):175‐82. [CRS: 99900001000000087; PUBMED: 17553526] [DOI] [PubMed] [Google Scholar]
Molloy 1997 {published data only}
- Molloy DW, Standish TI. A guide to the standardized Mini‐Mental State Examination. International Psychogeriatrics 1997;9(Suppl 1):87‐94; discussion 143‐50. [CRS: 99900001000000163; PUBMED: 9447431] [DOI] [PubMed] [Google Scholar]
Moretti {published data only}
- Moretti F, Atti AR, Cesano S, Morini V, Forlani C, Bernabei V, Modenese A, Dalmonte E, Ronchi D. The use of mmse to identify mild cognitive impairment (MCI). European Psychiatry 2009;24(Suppl 1):S853. [Google Scholar]
Mungas 1996 {published data only}
- Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini‐Mental State Examination for English and Spanish‐speaking elderly. Neurology 1996;46(3):700‐6. [CRS: 99900001000000172; PUBMED: 8618670] [DOI] [PubMed] [Google Scholar]
Murden 1991 {published data only}
- Murden RA, McRae TD, Kaner S, Bucknam ME. Mini‐mental state exam scores vary with education in black and whites. Journal of the American Geriatrics Society 1991;39(2):149‐155. [DOI] [PubMed] [Google Scholar]
Murden 1997 {published data only}
- Murden RA, Galbraith J. A modified mini‐mental state exam for use in the poorly educated. Clinical Gerontologist: The Journal of Aging and Mental Health 1997;17(4):23‐33. [Google Scholar]
Nadler 1995 {published data only}
- Nadler JD, Relkin NR, Cohen MS, Hodder RA, Reingold J, Plum F. Mental status testing in the elderly nursing home population. Journal of Geriatric Psychiatry and Neurology 1995;8(3):177‐83. [CRS: 99900001000000174; PUBMED: 7576043] [DOI] [PubMed] [Google Scholar]
Narasimhalu 2008 {published data only}
- Narasimhalu K, Lee J, Auchus AP, Chen CP. Improving detection of dementia in Asian patients with low education: combining the Mini‐Mental State Examination and the Informant Questionnaire on Cognitive Decline in the Elderly. Dementia and Geriatric Cognitive Disorders 2008;25(1):17‐22. [CRS: 99900001000000080; PUBMED: 18025825] [DOI] [PubMed] [Google Scholar]
Neri 2001 {published data only}
- Neri M, Roth M, Rubichi S, DeVreese LP, Bolzani R, Cipolli C. The validity of informant report for grading the severity of Alzheimer's dementia. Aging Clinical and Experimental Research 2001;13(1):22‐9. [CRS: 99900001000000139; PUBMED: 11292148] [DOI] [PubMed] [Google Scholar]
Ng 2007 {published data only}
- Ng TP, Niti M, Chiam PC, Kua EH. Ethnic and educational differences in cognitive test performance on mini‐mental state examination in Asians. American Journal of Geriatric Psychiatry 2007;15(2):130‐9. [CRS: 99900001000000093; PUBMED: 17272733] [DOI] [PubMed] [Google Scholar]
Nishiwaki 2004 {published data only}
- Nishiwaki Y, Breeze E, Smeeth L, Bulpitt CJ, Peters R, Fletcher AE. Validity of the Clock‐Drawing Test as a screening tool for cognitive impairment in the elderly. American Journal of Epidemiology 2004;160(8):797‐807. [CRS: 99900001000000117; PUBMED: 15466502] [DOI] [PubMed] [Google Scholar]
Noale 2006 {published data only}
- Noale M, Limongi F, Minicuci N. Identification of factorial structure of MMSE based on elderly cognitive destiny: the Italian Longitudinal Study on Aging. Dementia and Geriatric Cognitive Disorders 2006;21(4):233‐41. [CRS: 99900001000000103; PUBMED: 16465051] [DOI] [PubMed] [Google Scholar]
Nourhashemi 2008 {published data only}
- Nourhashemi F, Ousset PJ, Gillette‐Guyonnet S, Cantet C, Andrieu S, Vellas B, et al. A 2‐year follow‐up of 233 very mild (CDR 0.5) Alzheimer's disease patients (REAL.FR cohort). International Journal of Geriatric Psychiatry 2008;23(5):460‐5. [CRS: 99900001000000078; PUBMED: 17894422] [DOI] [PubMed] [Google Scholar]
O'Bryant 2008 {published data only}
- O'Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff‐Radford NR, Petersen RC, et al. Detecting dementia with the mini‐mental state examination in highly educated individuals. Archives of Neurology 2008;65(7):963‐7. [CRS: 99900001000000074; 99900001000000074; PUBMED: 18625866] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 1989 {published data only}
- O'Connor DW, Pollitt PA, Hyde JB, Fellows JL, Miller ND, Brook CP, et al. The reliability and validity of the Mini‐Mental State in a British community survey. Journal of Psychiatric Research 1989;23(1):87‐96. [CRS: 99900001000000207; PUBMED: 2666647] [DOI] [PubMed] [Google Scholar]
Olazaran 2004 {published data only}
- Olazaran J, Trincado R, Bermejo F, Benito‐Leon J, Diaz J, Vega S. Selective memory impairment on an adapted Mini‐Mental State Examination increases risk of future dementia. International Journal of Geriatric Psychiatry 2004;19(12):1173‐80. [CRS: 99900001000000116; PUBMED: 15526309] [DOI] [PubMed] [Google Scholar]
Onishi 2006 {published data only}
- Onishi J, Suzuki Y, Umegaki H, Kawamura T, Imaizumi M, Iguchi A. Which two questions of Mini‐Mental State Examination (MMSE) should we start from?. Archives of Gerontology and Geriatrics 2007;44(1):43‐8. [CRS: 99900001000000096; PUBMED: 16687183] [DOI] [PubMed] [Google Scholar]
Ostrosky‐Solis 1999 {published data only}
- Ostrosky‐Solis F, Lopez‐Arango G, Ardila A. Influences of age and education in the Mini Mental State Examination in a Spanish speaking population. Salud mental (Mexico City, Mexico) 1999;22. [DOI] [PubMed] [Google Scholar]
Pachet 2010 {published data only}
- Pachet A, Astner K, Brown L. Clinical utility of the mini‐mental status examination when assessing decision‐making capacity. Journal of Geriatric Psychiatry and Neurology 2010;23(1):3‐8. [CRS: 99900001000000055; PUBMED: 19661490] [DOI] [PubMed] [Google Scholar]
Pardo 1990 {published data only}
- Pardo C, Joya C. Spanish Version of Mini‐Mental State Examination S‐Mmse in Diagnosis of Dementia a Study in Colombia. Neurobiology of aging 1990;11. [Google Scholar]
Perneczky 2006 {published data only}
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini‐mental state examination and clinical dementia rating. American Journal of Geriatric Psychiatry 2006;14(2):139‐44. [CRS: 99900001000000105; PUBMED: 16473978] [DOI] [PubMed] [Google Scholar]
Pezzotti 2008 {published data only}
- Pezzotti P, Scalmana S, Mastromattei A, Lallo D, Progetto AWG. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: a prospective observational study. BMC Family Practice 2008;9:29. [CRS: 99900001000000076; 99900001000000076; PUBMED: 18477390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pouretemad 2009 {published data only}
- Pouretemad HR, Khatibi A, Ganjavi A, Shams J, Zarei M. Validation of Addenbrooke's cognitive examination (ACE) in a Persian‐speaking population. Dementia and Geriatric Cognitive Disorders 2009;28(4):343‐7. [CRS: 99900001000000057; PUBMED: 19864908] [DOI] [PubMed] [Google Scholar]
Qu 2005 {published data only}
- Qu Q‐M, Qiao J, Guo F. Influence of screening dementia with mmse combining with delay memory test. Chinese Chinese Journal of Clinical Psychology 2005;13(1):83‐85. [Google Scholar]
Quiroga 2004 {published data only}
- Quiroga P, Albala C, Klaasen G. Validation of a screening test for age associated cognitive impairment, in Chile [Validacion de un test de tamizaje para el diagnostico de demencia asociada a edad, en Chile]. Revista Medica de Chile 2004;132(4):467‐78. [CRS: 99900001000000118; PUBMED: 15382519] [DOI] [PubMed] [Google Scholar]
Rabins {published data only}
- Rabins P, Schwartz S, Tschanz J, Corcoran C, Black B, Fauth E, Mielke M, Lyketsos C. Neuropsychiatric symptoms at baseline predict shorter time to severe dementia in a population‐based sample of incident Alzheimer's disease: The Cache county dementia progression study. Alzheimer's and Dementia 2012;8(4):126‐127. [Google Scholar]
Rai 1998 {published data only}
- Rai GS, Blackman I. Usefulness of Mini Mental State Examination (Mmse) and Clock Drawing Test (Cdt) in Early Diagnosis of Dementia. Journal of the American Geriatrics Society 1998;46. [Google Scholar]
Rai 2008 {published data only}
Raina 2013 {published data only}
- Raina SK, Raina S, Chander V, Grover A, Singh S, Bhardwaj A. Development of a cognitive screening instrument for tribal elderly population of Himalayan region in northern India. Journal of neurosciences in rural practice 2013;4:147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rait 2000 {published data only}
- Rait G, Burns A, Baldwin R, Morley M, Chew‐Graham C, Leger AS. Validating screening instruments for cognitive impairment in older South Asians in the United Kingdom. International Journal of Geriatric Psychiatry 2000;15(1):54‐62. [DOI] [PubMed] [Google Scholar]
Raskind 1999 {published data only}
- Raskind MA, Cyrus PA, Ruzicka BB, Gulanski BI. The effects of metrifonate on the cognitive, behavioral, and functional performance of Alzheimer's disease patients. Metrifonate Study Group. Journal of Clinical Psychiatry 1999;60(5):318‐25. [CRS: 99900001000000150; PUBMED: 10362441] [DOI] [PubMed] [Google Scholar]
Riedel‐Heller 1999 {published data only}
- Riedel‐Heller SG, Matschinger H, Schork A, Angermeyer MC. Do memory complaints indicate the presence of cognitive impairment? Results of a field study. European Archives of Psychiatry and Clinical Neuroscience 1999;249(4):197‐204. [CRS: 99900001000000149; PUBMED: 10449595] [DOI] [PubMed] [Google Scholar]
Roelands 1992 {published data only}
- Roelands M, Baro F, Dom H, Wostyn P. Epidemiology research on dementia in Antwerp, Belgium. Neuroepidemiology 1992;11(Suppl 1):48‐51. [DOI] [PubMed] [Google Scholar]
- Roelands M, Wostyn P, Dom H, Baro F. The prevalence of dementia in Belgium: A population‐based door‐to‐door survey in a rural community. Neuroepidemiology 1994;13(4):155‐161. [DOI] [PubMed] [Google Scholar]
Schrijnemaekers 2006 {published data only}
- Schrijnemaekers AM, Jager CA, Hogervorst E, Budge MM. Cases with mild cognitive impairment and Alzheimer's disease fail to benefit from repeated exposure to episodic memory tests as compared with controls. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society 2006;28(3):438‐55. [CRS: 99900001000000102; PUBMED: 16618630] [DOI] [PubMed] [Google Scholar]
Sikkes 2013 {published data only}
- Sikkes SAM, Pijnenburg YAL, Knol DL, Lange‐De Klerk ESM, Scheltens P, Uitdehaag BMJ. Assessment of instrumental activities of daily living in dementia: diagnostic value of the Amsterdam Instrumental Activities of Daily Living Questionnaire. Journal of geriatric psychiatry and neurology 2013;26:244‐250. [DOI] [PubMed] [Google Scholar]
Spering 2012 {published data only}
- Spering CC, Hobson V, Lucas JA, Menon CV, Hall JR, O'Bryant SE. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer's disease in ethnically diverse highly educated individuals: an analysis of the NACC database. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 2012;67(8):890‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2002 {published data only}
- Stewart R, Johnson J, Richards M, Brayne C, Mann A, Medical Council Cognitive Function Ageing Study. The distribution of Mini‐Mental State Examination scores in an older UK African‐Caribbean population compared to MRC CFA study norms. International Journal of Geriatric Psychiatry 2002;17(8):745‐51. [DOI] [PubMed] [Google Scholar]
Stoppe {published data only}
- Stoppe G, Buss K, Wolf S, Stiens G, Maeck L. Screening for dementia in very old age in primary care. European Psychiatry 2010;25(Suppl 1):589. [Google Scholar]
Storey 2002 {published data only}
- Storey JE, Rowland JT, Basic D, Conforti DA. A comparison of five clock scoring methods using ROC (receiver operating characteristic) curve analysis. International Journal of Geriatric Psychiatry 2001;16(4):394‐9. [DOI] [PubMed] [Google Scholar]
Storey 2004 {published data only}
- Storey JE, Rowland JTJ, Conforti DA, Dickson HG. The Rowland Universal Dementia Assessment Scale (Rudas): a Multicultural Cognitive Assessment Scale. International Psychogeriatrics 2004;16(1):13‐31. [DOI] [PubMed] [Google Scholar]
Subra 2012 {published data only}
- Subra J, Gillette‐Guyonnet S, Cesari M, Oustric S, Vellas B. The integration of frailty into clinical practice: preliminary results from the gerontopole. Journal of Nutrition, Health & Aging 2012;16(8):714‐20. [DOI] [PubMed] [Google Scholar]
Sugishita {published data only}
- Sugishita M. A short form of MMSE (7 categories) and a short form of cdr (memory category only) can classify subjects into normal, MCI subjects and mild Alzheimer's. Alzheimer's and Dementia 2009;5(4). [Google Scholar]
Tae 2010 {published data only}
- Tae HK, Jin HJ, Joon HP, Jeong LK, Seung HR, Seok WM, Il HC, Dong WL, Jong CY, Yeon JD, Seok BL, Moon DK, Ki WK. Korean version of mini mental status examination for dementia screening and its short form. Psychiatry Investigation 2010;7(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Taillandier 2002 {published data only}
- Taillandier S, Ducorail MA, Gerbaud L, Jalenques I. Hospital screening for cognitive disorders in patients over 60‐years old [Le depistage hospitalier des troubles cognitifs chez les patients de plus de 60 ans]. Annales Medico‐Psychologiques 2002;160(5‐6):435‐43. [Google Scholar]
Tamura {published data only}
- Tamura T, Tshji M, Higashi Y, Sekine M, Kohdabashi A, Fujimoto T, Mitsuyama M. New computer‐based cognitive function test for the elderly. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine & Biology Society 2006;1:692‐4. [DOI] [PubMed] [Google Scholar]
Tang‐Wai 2003 {published data only}
- Tang‐Wai DF, Knopman DS, Geda YE, Edland SD, Smith GE, Ivnik RJ, et al. Comparison of the Short Test of Mental Status and the Mini‐Mental State Examination in mild cognitive impairment. Archives of Neurology 2003;60(12):1777‐81. [DOI] [PubMed] [Google Scholar]
Tappen 2012 {published data only}
- Tappen RM, Rosselli M, Engstrom G. Use of the MC‐FAQ and the MMSE‐FAQ in cognitive screening of older African Americans, Hispanic Americans, and European Americans. American Journal of Geriatric Psychiatry 2012;20(11):955‐62. [DOI] [PubMed] [Google Scholar]
Tariq 2006 {published data only}
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University Mental Status Examination and the Mini‐Mental Status Examination for the detecting dementia and mild neurocognitive disorder ‐ a pilot study. American Journal of Geriatric Psychiatry 2006;14(11):900‐10. [DOI] [PubMed] [Google Scholar]
Tariska 2003 {published data only}
- Tariska P, Paksy A. Mini‐Cog: a simple method for very brief screening of mental decline [Mini‐Cog: a mentalis hanyatlas "ultrarovid" es egyszeru szuresenek lehetosege]. Orvosi Hetilap 2003;144(17):803‐9. [PubMed] [Google Scholar]
Thibodeau 2011 {published data only}
- Thibodeau MP, Yaffe K. 20 year trajectories of cognition among elderly women who develop cognitive impairmentAlzheimer's and Dementia. Alzheimer's and Dementia. 2011; Vol. 7, issue 4:10.1016/j.jalz.2011.05.1053.
Thiele {published data only}
- Thiele F, Young S, Wenzel F, Buchert R. Combining predictive markers of cognitive decline in MCI: Cost‐benefit analysis. Alzheimer's and Dementia. 2011; Vol. 7, issue 4:S546‐S547.
Tian {published data only}
- Tian J, Shi J, Wei M, Miao Y, Wang Y. The scale of delayed story recall: A brief screening tool for amnestic mild cognitive impairment in chinese elderly. Alzheimer's and Dementia. 2012; Vol. 8, issue 4:P361.
Tierney 2000 {published data only}
- Tierney MC, Herrmann N, Geslani DM, Szalai JP. Contribution of informant and patient ratings to the accuracy of the Mini‐Mental State Examination in predicting probable Alzheimer's disease. Journal of the American Geriatric Society 2003;51(6):813‐8. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Szalai JP, Dunn E, Geslani D, McDowell I. Prediction of probable Alzheimer disease in patients with symptoms suggestive of memory impairment. Archives of Family Medicine 2000;9(6):527‐32. [DOI] [PubMed] [Google Scholar]
Timpano 2013 {published data only}
- Timpano F, Bonanno L, Bramanti P. Videoconference‐based Mini Mental State Examination: a validation study. Telemedicine and e‐Health 2013;19(12):931‐7. [DOI] [PubMed] [Google Scholar]
Tombaugh 1996 {published data only}
- Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini‐Mental State Examination (MMSE) and the Modified MMSE (3MS): a pyschometric comparison and normative data. Psychological Assessment 1996;8(1):48‐59. [Google Scholar]
Tombaugh 2005 {published data only}
- Tombaugh TN. Test‐retest reliable coefficients and 5‐year change scores for the MMSE and 3MS. Archives of Clinical Neuropsychology 2005;20(4):485‐503. [DOI] [PubMed] [Google Scholar]
Travers 2013 {published data only}
- Travers C, Byrne GJ, Pachana NA, Gray KK. Validation of the interRAI cognitive performance scale against independent clinical diagnosis and the Mini‐Mental State Examination in older hospitalized patients. The Journal of Nutrition, Health and Aging 2013;17(5):435‐9. [DOI] [PubMed] [Google Scholar]
Trenkle 2007 {published data only}
- Trenkle DL, Shankle WR, Azen SP. Detecting cognitive impairment in primary care: performance assessment of three screening instruments. Journal of Alzheimer's Disease 2007;11(13):323‐35. [DOI] [PubMed] [Google Scholar]
Tschanz 2004 {published data only}
- Tschanz J, Klein E, Treiber K, Corcoran C, Norton M, Toone L, et al. Neuropsychiatric symptoms in mild cognitive impairment and dementia: prevalence and relationship to cognitive and functional impairment. The Cache County Study. [Abstract P2‐288]. Epidemiology and Risk Factors of Alzheimer's Disease 2004;25(Suppl 2):S314. [Google Scholar]
Tuokko 1995 {published data only}
- Tuokko H, Kristjansson E, Miller J. Neuropsychological detection of dementia: an overview of the neuropsychological component of the Canadian Study of Health and Aging. Journal of Clinical and Experimental Neuropsychology 1995;17(3):352‐73. [DOI] [PubMed] [Google Scholar]
Uhlmann 1991 {published data only}
- Uhlmann RF, Larson EB. Effect of education on the Mini‐Mental State Examination as a screening test for dementia. Journal of the American Geriatrics Society 1991;39(9):876‐80. [DOI] [PubMed] [Google Scholar]
Unger 1999 {published data only}
- Unger JM, Belle G, Heyman A. Cross‐sectional versus longitudinal estimates of cognitive change in nondemented older people: a CERAD study. Journal of the American Geriatrics Society 1999;47(5):559‐63. [DOI] [PubMed] [Google Scholar]
Van der Cammen 1992 {published data only}
- Cammen TJ, Harskamp F, Stronks DL, Passchier J, Schudel WJ. Value of the Mini‐Mental State Examination and informants' data for the detection of dementia in geriatric outpatients. Psychological Reports 1992;71(3 Pt 1):1003‐9. [DOI] [PubMed] [Google Scholar]
Van Exel 2003 {published data only}
- Exel E, Craen AJ, Remarque EJ, Gussekloo J, Houx P, Bootsma‐van der Wiel A, Frolich M, Macfarlane PW, Blauw GJ, Westendorp RG. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology 2003;61(12):1695‐701. [DOI] [PubMed] [Google Scholar]
Van Sanden 2012 {published data only}
- Sanden S, Diels J, Gaudig M, Spencer M, Thompson G, Arrighi HM. Faster cognitive decline is associated with decreasing survival in patients with Alzheimer's disease. Value in Health 2012;15(7):A547. [Google Scholar]
Vantaa 85+ {published data only}
- Juva K, Sulkava R, Verkkoniemi A, Niinisto L. Sex differences in cognitive performance among the very old‐mini‐mental state examination in a population aged 85. Journal of Clinical Geropsychology 2001;7(1):39‐45. [Google Scholar]
Vas 2001 {published data only}
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of dementia in an urban Indian population. International Psychogeriatrics 2001;13(4):439‐50. [DOI] [PubMed] [Google Scholar]
Vercambre 2010 {published data only}
- Vercambre MN, Cuvelier H, Gayon YA, Hardy‐Leger I, Berr C, Trivalle C, Boutron‐Ruault MC, Clavel‐Chapelon F. Validation study of a French version of the modified telephone interview for cognitive status (F‐TICS‐m) in elderly women. International Journal of Geriatric Psychiatry 2010;25(11):1142‐9. [DOI] [PubMed] [Google Scholar]
Vigliecca 2012 {published data only}
- Vigliecca N, Silvana Penalva Marisa Carola, Molina Silvia Cristina, Voos Javier Alfredo V, Marcelo R. Is the folstein's mini‐mental test an aphasia test?. Applied Neuropsychology 2012;19(3):221‐228. [DOI] [PubMed] [Google Scholar]
Waite 2001 {published data only}
- Waite LM, Broe GA, Grayson DA, Creasey H. Preclinical syndromes predict dementia: the Sydney older persons study. Journal of Neurology, Neurosurgery & Psychiatry 2001;71(3):296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watfa 2001 {published data only}
- Watfa G, Husson N, Buatois S, Laurain MC, Miget P, Benetos A. Study of Mini‐Mental State Exam evolution in community‐dwelling subjects aged over 60 years without dementia. Journal of Nutrition, Health & Aging 2011;15(10):901‐4. [DOI] [PubMed] [Google Scholar]
Weston 1987 {published data only}
- Weston WW. Screening for dementia in family practice. Canadian Family Physician 1987;33:2495‐2500. [PMC free article] [PubMed] [Google Scholar]
White 2002 {published data only}
- White N, Scott A, Woods RT, Wenger GC, Keady JD, Devakumar M. The limited utility of the Mini‐Mental State Examination in screening people over the age of 75 years for dementia in primary care. British Journal of General Practice 2002;52(485):1002‐3. [PMC free article] [PubMed] [Google Scholar]
Whitney 2012 {published data only}
- Whitney KA, Mossbarger B, Herman SM, Ibarra SL. Is the montreal cognitive assessment superior to the mini‐mental state examination in detecting subtle cognitive impairment among middle‐aged outpatient U.S. Military veterans?. Archives of clinical neuropsychology 2012;27:742‐748. [DOI] [PubMed] [Google Scholar]
Wolf Klein 1989 {published data only}
- Wolf‐Klein GP//Silverstone FA//Levy AP//Brod MS//et al. Screening for alzheimer's disease by clock drawing. Journal of the American Geriatrics Society 1989;37(8):730‐734. [DOI] [PubMed] [Google Scholar]
Wrobel 2007 {published data only}
- Wrobel NH, Farrag MF. Preliminary Validation of an Arabic Version of the Mmse in the Elderly. Clinical Gerontologist 2007;31(3):75‐93. [Google Scholar]
Wu 2002 {published data only}
- Wu C, Zhou D, Como P, Fan J, Qiao Y. Applicability of the chinese version of the mini‐mental state examination in the screening of alzheimer's disease in rural areas of china. [Chinese]. Chinese Mental Health Journal 2002;16(4):242‐245. [Google Scholar]
Wu 2003 {published data only}
- Wu C, Zhou D, Wen C, Zhang L, Como P, Qiao Y. Relationship between blood pressure and Alzheimer's disease in Linxian County, China. Life Sciences 2003;72(10):1125‐33. [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu Y‐G, Zhang GB, Zhang CL, Li ZB, Wei CH, Chen JC, Huang DL, Zhao R, Huang JR, Wei B, Zhang XM. Cognitive function of 320 people over 65 years from longevous areas in Guangxi Zhuang Autonomous Region: Feasibility of the mini‐mental state examination. Neural Regeneration Research 2006;1(9):797‐800. [Google Scholar]
Yang 2006 {published data only}
- Yang YH, Lai CL, Lin RT, Tai CT, Liu CK. Cut‐off values of blessed dementia rating scale and its clinical application in elderly Taiwanese. Kaohsiung Journal of Medical Sciences 2006;22(8):377‐84. [DOI] [PubMed] [Google Scholar]
Yavorsky {published data only}
- Yavorsky C, DiClemente G, Opler M, Khan A, Jovic S, Rothman B. Establishing threshold scores and profiles of cognitive impairment for the Alzheimer's disease assessment scale‐cognitive subscale (ADAS‐COG) for patients with higher dementia (MMSE<12), Alzheimer's disease and probable mci. Alzheimer's and Dementia 2012;8(4 Suppl):415‐416. [Google Scholar]
Ylikoski 1992 {published data only}
- Ylikoski R, Erkinjuntti T, Sulkava R, Juva K, Tilvis R, Valvanne J. Correction for age, education and other demographic variables in the use of the Mini Mental State Examination in Finland. Acta Neurologica Scandinavica 1992;85(6):391‐6. [DOI] [PubMed] [Google Scholar]
Yuseph 1997 {published data only}
- Yuseph RL, Dupree LW, Vanderploeg RD, Cohen D. Predictive Validity of the Mini‐Mental State Exam in the Diagnosis of Alzheimer's Disease. Archives of clinical neuropsychology 1997;12. [Google Scholar]
Zaragoza study {published data only}
- Lobo A, Saz P, Dia JL. The AGECAT 'organic' section as a screening instrument for minor cognitive deficits. Psychiatric Journal of the University of Ottawa 1990;15(4):212‐5. [PubMed] [Google Scholar]
- Lobo A, Saz P, Marcos G, Dia JL, Camara C, Ventura T, Asin FM, Pascual LF, Montanes JA, Aznar S. Re‐Validation of the Mini‐Examen Cognoscitivo (First Spanish Version of the Mini‐Mental Status Examination) in the Elderly People. Medicina Clinica 1999;112(20):767‐774. [PubMed] [Google Scholar]
- Lobo A, Saz P, Marcos G, Dia JL, De‐la‐Camara C. The prevalence of dementia and depression in the elderly community in a southern European population. The Zaragoza study. Archives of General Psychiatry 1995;52(6):497‐506. [DOI] [PubMed] [Google Scholar]
- Saz P, Dia JL, Camara C, Carreras S, Marcos G, Lobo A. Reliability and validity of the Spanish version of the GMS‐AGECATE package for the assessment of dementia and cognitive disturbances. International Journal of Geriatric Psychiatry 1996;11(8):721‐728. [Google Scholar]
Zaudig 1992 {published data only}
- Zaudig M. A new systematic method of measurement and diagnosis of 'mild cognitive impairment' and dementia according to ICD‐10 and DSM‐III‐R criteria. International Psychogeriatrics 1992;4(Suppl 2):203‐19. [DOI] [PubMed] [Google Scholar]
Zhang 1998 {published data only}
- Zhang Z, Hong X, Li H. The Mini‐Mental State Examination in the Chinese residents population aged 55 years and over in the urban and rural areas of Beijing. Chinese Journal of Neurology 1999;32(3):149‐153. [Google Scholar]
Zhang 2012 {published data only}
- Zhang D, Shen D. Predicting future clinical changes of MCI patients using longitudinal and multimodal biomarkers. PLoS ONE 2012;7(3):e33182. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gungen 2002 {published data only}
- Gungen C, Ertan T, Eker E, Yasar R, Engin F. [Reliability and validity of the standardized Mini Mental State Examination in the diagnosis of mild dementia in Turkish population]. [Turkish]. Turk Psikiyatri Dergisi 2002;13(4):273‐81. [PubMed] [Google Scholar]
Jianbo 2013 {published data only}
- Jianbo H, Weihua Z, Shaohua H, Ning W, Manli H, Yi X. Brief screening tool for mild cognitive impairment in elder chinese: Validation of the chinese version of the montreal cognitive assessment. American Journal of Geriatric Psychiatry 2011. [Google Scholar]
Kornsey {published data only (unpublished sought but not used)}
- Kornsey E, Rovner B, Casten R. Racial disparities in a community‐based sample of a novel memory screening protocol. Alzheimer's and Dementia 2011;7(4 Suppl):S613. [Google Scholar]
Kvitting 2013 {published data only (unpublished sought but not used)}
- Johansson MM, Kvitting AS, Wressle E, Marcusson J. Clinical utility of cognistat in multiprofessional team evaluations of patients with cognitive impairment in Swedish primary care. International Journal of Family Medicine 2014;2014:649253. [DOI: 10.1155/2014/649253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orsi {published data only (unpublished sought but not used)}
- Orsi E, Xavier AJ, Sigulem D, Kupek E, Ramos LR. Temporal orientation is the best discriminative item of the folstein's mini mental status examination. Alzheimer's and Dementia. 2009; Vol. 5, issue 4 Suppl:275.
Shaaban 2013 {published data only (unpublished sought but not used)}
- Shaaban J, Aziz AA, Abdullah Z, Razak AA. Validation of the malay version of rowland universal dementia assessment scale (m‐rudas) among elderly attending primary care clinic. International Medical Journal 2013;20:555‐558. [Google Scholar]
Upadhyaya 2010 {published data only}
- Upadhyaya AK, Rajagopal M, Gale TM. The six item cognitive impairment test (6‐CIT) as a screening test for 150 dementia: Comparison with Mini‐Mental State Examination (MMSE). Current Aging Science 2010;3(2):138‐142. [DOI] [PubMed] [Google Scholar]
Yu 2012 {published data only (unpublished sought but not used)}
- Yu P, Dean RA, Hall SD, Qib Y, Sethuramana G, Willis BA, Siemers ER, Martenyi F, Tauscher JT, Schwarz AJ. Enriching Amnestic Mild Cogitive Impairment Populations for Clinical Trials: Optimal Combination of Biomarkers to Predict Conversion to Dementia. Journal of Alzheimer’s Disease 2012;32:373‐385. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Guiata 2012 {published data only (unpublished sought but not used)}
- Guaita A, Abbondanza S, Colombo M, Davin A, Forloni G, Polito L, Vaccaro R, Valle E, Vitali S, Ferretti VV, Villani S. Cognitive impairment, dementia and "good performers": Prevalence in the population study "InveCe.AB". Journal of nutrition, health & aging 2012;16(9):827‐828. [Google Scholar]
Additional references
Ahmad 2010
- Ahmad S, Orrell M, Iliffe S, Gracie A. GPs' attitudes, awareness, and practice regarding early diagnosis of dementia. British Journal of General Practice 2010;60(578):e360–e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1994
- American Psychological Association. Diagnostic and statistical manual of mental disorders (DSM‐IV). 4th Edition. Washington, D.C.: American Psychological Association, 1994. [Google Scholar]
Arevalo‐Rodriguez 2013
- Arevalo‐Rodriguez I, Smailagic N, Ciapponi A, Sanchez‐Perez E, Giannakou A, Roqué i Figuls M, et al. Mini‐Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2007
- Banerjee S, Willis R, Matthews D, Contell F, Chan J, Murray J. Improving the quality of care for mild to moderate dementia: an evaluation of the Croydon Memory Service Model. International Journal of Geriatric Psychiatry 2007;22(8):782‐8. [PUBMED: 17243196] [DOI] [PubMed] [Google Scholar]
Barrett 2014
- Barrett E, Burns A. Dementia Revealed. What Primary Care Needs to Know. London: Department of Health, 2014. [http://www.england.nhs.uk/wp‐content/uploads/2014/09/dementia‐revealed‐toolkit.pdf] [Google Scholar]
Belle 2000
- Belle SH, Mendelsohn AB, Seaberg EC, Ratcliff G. A brief cognitive screening battery for dementia in the community.. Neuroepidemiology. 2000;19(1):43‐50. [PUBMED: 10654287] [DOI] [PubMed] [Google Scholar]
Bertolucci 1994
- Bertolucci PHF, Brucki S, Campacci SR, Juliano Y. [The Mini‐Mental State Examination in an outpatient population: influence of literacy]. Arq Neuropsiquiatr 1994;52(1):1‐7. [PubMed] [Google Scholar]
Beynon 2013
- Beynon R, Leeflang MM, McDonald S, Eisinga A, Mitchell RL, Whiting P, et al. Search strategies to identify diagnostic accuracy studies in MEDLINE and EMBASE. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.MR000022.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Birks 2006
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blesa 2001
- Blesa R, Pujol M, Aguilar M, Santacruz P, Bertran‐Serra I, Hernandez G, et al. Clinical validity of the 'mini‐mental state' for Spanish speaking communities. Neuropsychologia 2001;39:1150‐1157. [DOI] [PubMed] [Google Scholar]
Borson 2000
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini‐cog: a cognitive 'vital signs' measure for dementia screening in multi‐lingual elderly. International Journal of Geriatric Psychiatry 2000;15:1021‐7. [PUBMED: 11113982] [DOI] [PubMed] [Google Scholar]
Bossuyt 2013
- Bossuyt P, Davenport C, Deeks J, Hyde C, Leeflang M, Scholten R. Chapter 11: Interpreting results and drawing conclusions. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The Cochrane Collaboration, 2013. Available from: http://srdta.cochrane.org/. Version 0.9. The Cochrane Collaboration.
Bourne 2007
- Bourne J. Improving Services and Support for People with Dementia. London: National Audit Office, 2007. [Google Scholar]
Boustani 2003
- Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2003;138(11):927‐37. [DOI] [PubMed] [Google Scholar]
Brayne 1989
- Brayne C, Calloway P. An epidemiological study of dementia in a rural population of elderly women. British Journal of Psychiatry 1989;155:214‐9. [PUBMED: 2597917] [DOI] [PubMed] [Google Scholar]
Brayne 2012
- Brayne C, Davis D. Making Alzheimer's and dementia research fit for populations. The Lancet 2012;380(9851):1441‐3. [DOI: 10.1016/S0140-6736(12)61803-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunet 2012
- Brunet MD, McCartney M, Heath I, Tomlinson J, Gordon P, Cosgrove J, et al. Open letter to the prime minister and chief medical officer for England: There is no evidence base for proposed dementia screening. BMJ 2012;345:e8588. [DOI] [PubMed] [Google Scholar]
CFAS 2001
- Neuropathology Group, Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late‐onset dementia in a multicentre, community‐based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). The Lancet 2001;357(9251):169‐75. [PUBMED: 11213093] [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [17098577] [DOI] [PubMed] [Google Scholar]
Clare 2003
- Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early‐stage Alzheimer's disease and vascular dementia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003260] [DOI] [PubMed] [Google Scholar]
Copeland 1986
- Copeland JR, Dewey ME, Griffiths‐Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychologial Medicine 1986;16(1):89‐99. [PUBMED: 3515380] [DOI] [PubMed] [Google Scholar]
Cordell 2013
- Cordell CB, Borson S, Boustani M, Chodosh J, Reuben D, Verghese J, et al. Medicare Detection of Cognitive Impairment Workgroup. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers & Dementia 2013;9(2):141‐50. [DOI: 10.1016/j.jalz.2012.09.011] [DOI] [PubMed] [Google Scholar]
Crum 1993
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA: The Journal of the American Medical Association 1993;269(18):2386‐91. [PUBMED: 8479064] [PubMed] [Google Scholar]
Cullen 2005
- Cullen B, Fahy S, Cunningham CJ, Coen RF, Bruce I, Greene E, et al. Screening for dementia in an Irish community sample using MMSE: a comparison of norm‐adjusted versus fixed cut‐points. International Journal of Geriatric Psychiatry 2005;20(4):371‐6. [PUBMED: 15799072] [DOI] [PubMed] [Google Scholar]
Davis 2013a
- Davis DHJ, Creavin ST, Noel‐Storr A, Quinn TJ, Smailagic N, Hyde C, et al. Neuropsychological tests for the diagnosis of Alzheimer’s disease dementia and other dementias: a generic protocol for cross‐sectional and delayed‐verification studies. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2013b
- Davis DHJ, Creavin ST, Yip JLY, Noel‐Storr AH, Brayne C, Cullum S. The Montreal Cognitive Assessment for the diagnosis of Alzheimer’s disease and other dementia disorders. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010775] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Silva 2002
- Silva HA, Gunathilake SB. Mini‐Mental State Examination in Sinhalese: a sensitive test to screen for dementia in Sri Lanka. International Journal of Geriatric Psychiatry 2002;17:134‐139. [DOI] [PubMed] [Google Scholar]
De Silva 2010
- Silva V, Hanwella R. Why are we copyrighting science?. BMJ 2010;341:c4738. [PUBMED: 20847026] [DOI] [PubMed] [Google Scholar]
Dementia CQUIN
- Department of Health. Using the Commissioning for Quality and Innovation (CQUIN) payment framework. Guidance on new national goals for 2012‐13. Department of Health, 2012. Available from: https://www.gov.uk/government/publications/using‐the‐commissioning‐for‐quality‐and‐innovation‐cquin‐payment‐framework‐guidance‐on‐new‐national‐goals‐for‐2012‐13.
DOH recommendations
- Department of Health. General medical services—contractual changes 2013‐2014 [Letter to chairman of BMA General Practitioners Committee]. Available from: https://www.wp.dh.gov.uk/publications/files/2012/12/GMS‐Contract‐letter.pdf 6 December 2012.
Fage 2013
- Fage BA, Seitz DP, Gill SS, Herrmann N, Smailagic N, Chan CCH, Nikolaou V. Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a community setting. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010860] [DOI] [PubMed] [Google Scholar]
Ferri 2005
- Ferri C P, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M, Alzheimer's Disease International. Global prevalence of dementia: a Delphi consensus study. The Lancet 2005;366(9503):2112‐7. [PUBMED: 16360788] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [PUBMED: 1202204] [DOI] [PubMed] [Google Scholar]
Fratiglioni 1991
- Fratiglioni L, Grut M, Forsell Y, Viitanen M, Grafström M, Holmén K, et al. Prevalence of Alzheimer's disease and other dementias in an elderly urban population: relationship with age, sex, and education.. Neurology 1991;41(12):1886‐92. [DOI: ; PUBMED: 1745343] [DOI] [PubMed] [Google Scholar]
Glanville 2010
- Glanville J, Cikalo M, Crawford F, Dozier M, McIntosh H. Handsearching did not yield additional unique FDG‐PET diagnostic test accuracy studies compared with electronic searches: a preliminary investigation. Research Synthesis Methods 2010;3(3):202‐13. [DOI: 10.1002/jrsm.1046] [DOI] [PubMed] [Google Scholar]
Grace 1995
- Grace J, Nadler JD, White DA, Guilmette TJ, Giuliano AJ, Monsch AU, et al. Folstein vs modified Mini‐Mental State Examination in geriatric stroke. Stability, validity, and screening utility. Archives of Neurology 1995;52(5):477‐84. [PUBMED: 7733842] [DOI] [PubMed] [Google Scholar]
Harrell 2000
- Harrell LE, Marson D, Chatterjee A, Parrish JA. The Severe Mini‐Mental State Examination: a new neuropsychologic instrument for the bedside assessment of severely impaired patients with Alzheimer disease. Alzheimer Disease and Associated Disorders 2000;14(3):168‐75. [PUBMED: 10994658] [DOI] [PubMed] [Google Scholar]
Haubois 2012
- Haubois G, Decker L, Annweiler C, Launay C, Allali G, R Herrmann F, et al. Derivation and validation of a Short form of the Mini‐Mental State Examination for the screening of dementia in older adults with a memory complaint. European Journal of Neurology 2012;20(3):588‐90. [DOI: 10.1111/j.1468-1331.2012.03830.x] [DOI] [PubMed] [Google Scholar]
Hooijer 1992
- Hooijer C, Dinkgreve M, Jonker C, Lindeboom J, Kay DWK. Short screening tests for dementia in the elderly population. I. A comparison between AMTS, MMSE, MSQ and SPMSQ. International Journal of Geriatric Psychiatry 1992;7(8):559–71. [DOI: 10.1002/gps.930070805] [DOI] [Google Scholar]
Huppert 2005
- Huppert FA, Cabelli ST, Matthews FE, MRC Cognitive Function and Ageing Study. Brief cognitive assessment in a UK population sample ‐‐ distributional properties and the relationship between the MMSE and an extended mental state examination. BMC Geriatrics 2005;5:7. [PUBMED: 15869717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabir 2000
- Kabir ZN, Herlitz A. The Bangla adaptation of Mini‐Mental State Examination (BAMSE): an instrument to assess cognitive function in illiterate and literate individuals. International Journal of Geriatric Psychiatry 2000;15(5):441‐50. [PUBMED: 10822243] [DOI] [PubMed] [Google Scholar]
Kamenski 2009
- Kamenski G, Dorner T, Lawrence K, Psota G, Rieder A, Schwarz F, et al. Detection of dementia in primary care: comparison of the original and a modified Mini‐Cog Assessment with the Mini‐Mental State Examination. Mental Health in Family Medicine 2009;6(4):209‐17. [PUBMED: 22477912] [PMC free article] [PubMed] [Google Scholar]
Kukull 1994
- Kukull WA, Larson EB, Teri L, Bowen J, McCormick W, Pfanschmidt ML. The Mini‐Mental State Examination score and the clinical diagnosis of dementia. Journal of Clinical Epidemiology 1994;47(9):1061‐7. [PUBMED: 7730909] [DOI] [PubMed] [Google Scholar]
Le Couteur 2013
- Couteur DG, Doust J, Creasey H, Brayne C. Political drive to screen for pre‐dementia: not evidence based and ignores the harms of diagnosis. BMJ 2013;347:f5125. [DOI: 10.1136/bmj.f5125] [DOI] [PubMed] [Google Scholar]
Leeflang 2008
- Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data‐driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clinical Chemistry 2008;54(4):729‐37. [PUBMED: 18258670] [DOI] [PubMed] [Google Scholar]
Lund 1994
- Neary D, Brun A, Englund B, Gustafson L, Passant U, Mann DMA, Snowden JS. Clinical and neuropathological criteria for frontotemporal dementia: the Lund and Manchester Groups. Journal of Neurology, Neurosurgery, and Psychiatry 1994;57(4):416‐8. [PUBMED: 8163988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from: http://srdta.cochrane.org/. The Cochrane Collaboration.
Matthews 2009
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable‐risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Medicine 2009;6(11):e1000180. [PUBMED: 19901977] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2013
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. Medical Research Council Cognitive Function and Ageing Collaboration. A two‐decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. The Lancet 2013;382(9902):1405‐12. [DOI: 10.1016/S0140-6736(13)61570-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKeith 1996
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47(5):1113‐24. [PUBMED: 8909416] [DOI] [PubMed] [Google Scholar]
McKeith 2005
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65(12):1863‐72. [PUBMED: 16237129] [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34(7):939‐44. [PUBMED: 6610841] [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: Journal of the Alzheimer's Association 2011;7(3):263‐9. [PUBMED: 21514250] [DOI] [PMC free article] [PubMed] [Google Scholar]
McShane 2006
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003154.pub5] [DOI] [PubMed] [Google Scholar]
Mitchell 2009
- Mitchell AJ. A meta‐analysis of the accuracy of the mini‐mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43(4):411‐31. [DOI: 10.1016/j.jpsychires.2008.04.014] [DOI] [PubMed] [Google Scholar]
Molloy 1991
- Molloy DW, Alemayehu E, Roberts R. Reliability of a standardized Mini‐Mental State Examination compared with the traditional Mini‐Mental State Examination. The American Journal of Psychiatry 1991;148(1):102‐5. [PUBMED: 1984692] [DOI] [PubMed] [Google Scholar]
Morris 1993
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43(11):2412‐4. [8232972] [DOI] [PubMed] [Google Scholar]
NICE 2006
- National Institute for Health and Clinical Excellence Social Care Institute for Excellence. Dementia: supporting people with dementia and their carers in health and social care. The British Psychological Society and Gaskell, 2006. Available from: http://www.scie.org.uk/publications/misc/dementia/dementia‐fullguideline.pdf.
NICE 2013
- National Institute for Health and Clinical Excellence Social Care Institute for Excellence. Summary of recommendations for the NICE menu of indicators for the QOF. National Institute for Health and Clinical Excellence Social Care Institute for Excellence, 2013. Available from: http://www.nice.org.uk/aboutnice/qof/indicators.jsp. London: National Institute for Health and Care Excellence.
Noel‐Storr 2014
- Noel‐Storr AH, McCleery JM, Richard E, Ritchie CW, Flicker L, Cullum SJ, et al. Reporting standards for studies of diagnostic test accuracy in dementia: The STARDdem Initiative. Neurology 2014;83(4):364‐73. [PUBMED: 24944261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Porta 2008
- Porta M. A Dictionary of Epidemiology. 5th Edition. Oxford: Oxford University Press, 2008. [Google Scholar]
Quinn 2013
- Quinn TJ, Fearon P, Young C, Noel‐Storr AH, McShane R, Stott DJ. IQCODE for the diagnosis of Alzheimer’s disease dementia and other dementias within a general practice (primary care) setting. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010771] [DOI] [Google Scholar]
Rasmussen 2013
- Rasmussen J. Would doctors routinely asking older patients about their memory improve dementia outcomes? Yes. BMJ 2013;346:f1780. [DOI: 10.1136/bmj.f1780.] [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [PUBMED: 8094895] [DOI] [PubMed] [Google Scholar]
Roth 1986
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. British Journal of Psychiatry 1986;149:698‐709. [PUBMED: 3790869] [DOI] [PubMed] [Google Scholar]
Savva 2009
Scheltens 2011
- Scheltens P, Rockwood K. How golden is the gold standard of neuropathology in dementia?. Alzheimer's & Dementia: Journal of the Alzheimer's Association 2011;7(4):486‐9. [PUBMED: 21784357] [DOI] [PubMed] [Google Scholar]
Sperling 2011
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: Journal of the Alzheimer's Association 2011;7(3):280‐92. [PUBMED: 21514248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stata [Computer program]
- Stata Corp. Stata 13. College Station, Texas, USA: Stata Corp, 2013.
Su 2014
- Su YP, Chang CK, Hayes RD, Perera G, Broadbent M, To D, et al. Mini‐mental state examination as a predictor of mortality among older people referred to secondary mental healthcare. PLoS One 2014;3(9):e105312. [DOI: 10.1371/journal.pone.0105312; PUBMED: 25184819] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tschanz 2002
- Tschanz JT, Welsh‐Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC, Cache County Study Group. An adaptation of the modified mini‐mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 2002;15(1):28‐38. [PUBMED: 11877549] [PubMed] [Google Scholar]
Tsoi 2015
- Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive tests to detect dementia: a systematic review and meta‐analysis. JAMA Internal Medicine 2015;175(9):1450‐8. [PUBMED: 26052687] [DOI] [PubMed] [Google Scholar]
US Preventive Services 2003
- US Preventive Services Task Force. Screening for dementia. US Preventive Services Task Force, 2003. Available from: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdeme.htm.
Whiting 2011
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. Journal of Clinical Epidemiology 2011;64(6):602‐7. [PUBMED: 21075596] [DOI] [PubMed] [Google Scholar]
Whiting 2013
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, QUADAS‐2 Steering Group. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. Journal of Clinical Epidemiology 2013;66(10):1093‐104. [DOI: 10.1016/j.jclinepi.2013.05.014] [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization, 1992. [Google Scholar]
Wimo 2010
- Wimo A, Prince M. World Alzheimer's Report 2010: The Global Economic Impact of Dementia. London, UK: Alzheimer’s Disease International, 2010. [http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf] [Google Scholar]
Wind 1997
- Wind AW, Schellevis FG, Staveren G, Scholten RP, Jonker C, Eijk JT. Limitations of the Mini‐Mental State Examination in diagnosing dementia in general practice. International Journal of Geriatric Psychiatry 1997;12(1):101‐8. [PUBMED: 9050431 ] [DOI] [PubMed] [Google Scholar]
Zaudig 1991
- Zaudig M, Mittelhammer J, Hiller W. SIDAM‐‐A structured interview for the diagnosis of dementia of the Alzheimer type, multi‐infarct dementia and dementias of other aetiology according to ICD‐10 and DSM‐III‐R [SIDAM – Strukturiertes Interview für die Diagnose ei‐ ner Demenz vom Alzheimer Typ, der Multiin‐ farkt‐Demenz und Demenzen anderer Ätiolo‐ gie nach ICD‐10 and DSM‐III‐R]. Psychological Medicine 1991;21(1):225‐236. [DOI] [PubMed] [Google Scholar]


