Abstract
Rollouts of COVID-19 vaccines in the USA were opportunities to redress disparities that surfaced during the pandemic. Initial eligibility criteria, however, neglected geographic, racial/ethnic, and socioeconomic considerations. Marginalized populations may have faced barriers to then-scarce vaccines, reinforcing disparities. Inequalities may have subsided as eligibility expanded. Using spatial modeling, we investigate how strongly local vaccination levels were associated with socioeconomic and racial/ethnic composition as authorities first extended vaccine eligibility to all adults. We harmonize administrative, demographic, and geospatial data across postal codes in eight large US cities over 3 weeks in Spring 2021. We find that, although vaccines were free regardless of health insurance coverage, local vaccination levels in March and April were negatively associated with poverty, enrollment in means-tested public health insurance (e.g., Medicaid), and the uninsured population. By April, vaccination levels in Black and Hispanic communities were only beginning to reach those of Asian and White communities in March. Increases in vaccination were smaller in socioeconomically disadvantaged Black and Hispanic communities than in more affluent, Asian, and White communities. Our findings suggest vaccine rollouts contributed to cumulative disadvantage. Populations that were left most vulnerable to COVID-19 benefited least from early expansions in vaccine availability in large US cities.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11524-021-00589-0.
Keywords: COVID-19, Disparities, Inequality, Neighborhood, Pandemic, Race, Socioeconomic, Spatial, Urban, Vaccine
Introduction
Background: COVID-19 Vaccine Rollouts in the USA
In early 2021, state and local authorities in the USA vaccinated millions of people weekly against coronavirus disease 2019 (COVID-19).[1] Vaccination curbed viral infection and transmission and reduced illness, hospitalization, and death from COVID-19.[2, 3, 4, 5, 6, 7, 8] Vaccines were free countrywide regardless of health insurance coverage. Eligibility progressed in stages per state and local policy. Health care employees received first priority, followed by seniors, workers in designated occupations, and individuals with particular medical conditions.[9]
The most significant expansion in eligibility occurred from late March through April 2021. Doses remained scarce, but most jurisdictions allowed everyone age 16 and older to be vaccinated.[10, 11, 12, 13, 14, 15] On January 1, 2021, 5.5 million people had received at least one dose of a COVID-19 vaccine. That number increased to 30.3 million by February 1, 57.0 million by March 1, 111.1 million by April 1, and 153.7 million by May 1.[16] Growth plateaued in May. Over 206.6 million US residents were at least partially vaccinated by September 1; nearly half of them received their first dose in March or April. Eligibility expansion enabled rapid increases during these months.
Vaccine eligibility rules did not account for two key predictors of the burden of the COVID-19 pandemic in the USA: race/ethnicity and socioeconomic status (SES). At the community level, infection and mortality were more common where low-SES individuals and people of color (POC) comprised more of the population.[17, 18, 19, 20, 21, 22, 23, 9] At the individual level, Black and Hispanic people were disproportionately likely to experience infection, hospitalization, and death.[24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] Socioeconomic variables partially mediated racial/ethnic disparities.
Decision-makers might have opted against conditioning vaccine eligibility on racial/ethnic or socioeconomic factors to avoid legal challenges.[35,36] Still, there were viable ways to use vaccine eligibility policy to mitigate inequality.[37] For example, the Advisory Committee on Immunization Practices (ACIP) initially recommended prioritizing essential workers, which would have increased eligibility for low-SES people and POC.[38] Authorities ultimately hewed closer to ACIP’s final recommendations, giving greater weight to advanced age.[39]
Geographic allocation may have been the most promising indirect means of addressing disparities through the rollout of COVID-19 vaccines. SES, race/ethnicity, and geography are tightly linked in the reproduction of inequality in the USA; socioeconomic and racial/ethnic inequalities manifest in space, usually at hyperlocal scales.[40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51] Prioritizing local geographies in which residents had the highest risks of hardship from COVID-19 probably would have reduced mortality more than the age-based rollouts authorities chose.[52] Regardless of whether demographic targeting was constitutional, spatial targeting could have advanced vaccine equity.
Motivation: Early Vaccine Distribution and Cumulative Disadvantage
Authorities relied on individual initiative to distribute vaccines outside the health care workforce. This approach favored individuals with internet access, reliable transportation, and flexible schedules. States and localities used first-come, first-served online scheduling for scarce appointments at small numbers of sites. People with reliable internet access and white-collar jobs were better positioned to sign up. Limited locations and timed appointments were disadvantageous for people with restricted transit options and strict or uncertain work schedules, including the poor and many people with disabilities. Barriers to vaccination in March and April 2021 may have reinforced socioeconomic and racial/ethnic disparities.
Concern over vaccine hesitancy in the USA has abounded, but framing vaccination solely as a matter of individual choice obscures structural and material impediments. Researchers mostly attribute stagnant US vaccination rates to misinformation, mistrust in institutions, and political party affiliation [53, 54, 55, 56, 57, 58, 59, 60, 61,62, 63, 64]. At the same time, survey evidence suggests vaccination was linked to SES in Spring 2021[65]. Unvaccinated respondents reported three major economic concerns: taking time off work to get the vaccine, missing work due to side effects, and out-of-pocket costs. Plausible economic determinants of vaccine uptake as eligibility first expanded suggest racial/ethnic and socioeconomic disparities may have arisen.
If disparities persisted through April 2021, vaccine rollouts contributed to cumulative disadvantage [66, 67]. Advantages secure future advantages; inequality begets inequality—including at the neighborhood level.[43, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78] Vaccine rollouts may have propelled a circular process. POC and low-SES communities were most likely to experience serious illness or die from COVID-19. Equitable vaccine distribution would have mitigated racial/ethnic and socioeconomic gaps, but early vaccine distribution did not account for these inequalities. As a result, geographic clusters of unvaccinated people could have emerged, restarting the cycle by facilitating viral transmission [79, 80, 81, 82, 83, 84, 85]. Understanding racial/ethnic and socioeconomic vaccination disparities at the local level identifies harms that marginalized people experienced during the pandemic and helps explain the reproduction of urban spatial inequality in the USA [86, 87, 88, 89].
Overview
Our analysis provides a unique perspective on socioeconomic, racial/ethnic, and spatial disparities during the pandemic in the USA. Numerous studies have focused on geographic dimensions of COVID-19–related inequality,[90, 91, 92, 93, 94, 95, 96, 97] but few have examined spatial differences in vaccination below the state level.[59, 98, 99, 100] The temporal persistence of geographical vaccination disparities is particularly underexplored. We also contribute a novel dataset[101] that harmonizes initially incompatible sources. And unlike many studies of COVID-19 disparities—even analyses with a geographical focus—we modeled spatial dynamics.
We tested two hypotheses. First, we hypothesized that local areas in which POC and low-SES individuals comprised more of the population had lower vaccination levels in March and April 2021. Second, we hypothesized that, despite lower starting points, the same areas had smaller increases in vaccination between March and April.
We used spatial quantitative methods to test these hypotheses. We estimated associations between vaccination levels and racial/ethnic and socioeconomic composition, adjusting for populations with early eligibility due to age or employment. We collected administrative data on vaccination by postal code, covering eight of the 10 most populous US cities in March and April 2021. We combined these data with demographic estimates and geospatial data from the US Census Bureau. We used spatial interpolation to reconcile reporting irregularities.
We found that, although vaccines were free regardless of health insurance coverage, local vaccination levels in March and April were negatively associated with poverty, enrollment in means-tested public health insurance (e.g., Medicaid), and the uninsured population. By April, vaccination levels in Black and Hispanic communities were only beginning to reach those of Asian and White communities in March. Increases in vaccination were smaller in socioeconomically disadvantaged Black and Hispanic communities than in more affluent, Asian, and White communities. Our findings suggest vaccine rollouts contributed to cumulative disadvantage.
Data and Methods
Data
From online public databases maintained by state and local public health authorities, we gathered official counts of individuals with at least one dose of a COVID-19 vaccine in March and April 2021. Only geographically aggregated data were publicly available. We secured them for eight of the 10 most populous US cities: New York, Chicago, Houston, Phoenix, Philadelphia, San Antonio, San Diego, and Dallas (in descending order of population). The vaccination data capture a 3-week window during which eligibility expanded significantly. The number of individuals with at least one dose of a COVID-19 vaccine in the eight cities increased 34.7% from 4.6 to 7.1 million during this period. We present key details of the vaccination data in Table 1; we elaborate in Section e2.1 of the online supplement.
Table 1.
Vaccination data sources and coverage
| City | Source | Time 1 | Time 2 |
|---|---|---|---|
| New York | New York City Department of Health and Mental Hygiene | March 22, 2021 | April 13, 2021 |
| Chicago | Chicago Department of Public Health | March 22, 2021 | April 13, 2021 |
| Houston | Texas Department of State Health Services | March 22, 2021 | April 11, 2021 |
| Phoenix | Arizona Department of Health Services | March 22, 2021 | April 13, 2021 |
| Philadelphia | Philadelphia Department of Public Health | March 21, 2021 | April 12, 2021 |
| San Antonio | Texas Department of State Health Services | March 22, 2021 | April 11, 2021 |
| San Diego | County of San Diego Health and Human Services Agency | March 21, 2021 | April 12, 2021 |
| Dallas | Texas Department of State Health Services | March 22, 2021 | April 11, 2021 |
We used two datasets from the US Census Bureau. We collected demographic data from the 2015–2019 American Community Survey (ACS) Five-Year Estimates[102] and geospatial vector data from the 2019 TIGER/Line Shapefiles.[103] We provide further detail on these sources in Sections e2.2 and e2.3 of the online supplement.
Unit of Analysis
For brevity and interpretability, we refer to our units of analysis as ZIP Codes, the name for postal codes in the USA. The units of analysis were based on ZIP Codes, but reporting irregularities made ZIP Codes themselves inviable. Where necessary, we used overlay interpolation[104, 105] to exclude populations residing outside city limits. We provide extensive detail on the units of analysis and interpolation in Section e3 of the online supplement.
Independent Variables
Vaccination Priority Populations
We accounted for vaccination priority regulations by adjusting for populations of health care workers and seniors. Specific estimates were unavailable for health care workers, but ACS provided estimated counts of individuals employed in “health care and social assistance.” We also adjusted for the share of the population age 65 or older. These variables were the best available measures of the first groups prioritized for vaccination. We include more information on these variables in Section e2.2 of the online supplement.
Socioeconomic Composition
To examine the dependent variable’s association with socioeconomic composition, we included four indicators of SES. Two independent variables estimated health insurance status. Health insurance coverage was not universal in the USA as of the COVID-19 pandemic, and medical care remained expensive and stratified compared to other rich countries.[106, 107] We included variables estimating the share of the population enrolled in Medicaid or other means-tested public health insurance and the share without health insurance altogether. Together, these variables captured populations that were among the least integrated into the US health care system. We also included variables estimating the shares of the population under the federal poverty line and without internet access. We included the latter because making appointments online was usually the best way to secure a vaccine in early 2021. We include more information on our socioeconomic variables in Section e2.2 of the online supplement.
Racial/Ethnic Composition
We accounted for racial/ethnic composition because racism causes health inequity in the USA.[108, 109, 110, 111, 112, 113, 114, 115] Although race/ethnicity itself cannot cause anything, distributive systems that allocate resources according to racial/ethnic hierarchies create disparities among racial/ethnic groups.[116, 117, 118, 119, 120] These disparities often surface the net of SES. Including measures of racial/ethnic composition in our models enabled us to examine its direct association with vaccination, adjusting for SES.
Racism, however, is more than a conditional association between an outcome and racial/ethnic composition.[121, 122, 123, 124, 125, 126] It undergirds the gamut of US social, economic, and political processes. The distributions of socioeconomic covariates and unobserved mechanisms were racialized. We analyzed racism in the aggregate by considering direct and indirect pathways—mainly through simulations, described below and in Section e4.3 of the online supplement.
From ACS racial/ethnic categories, we created variables measuring the estimated populations of four mutually exclusive, non-exhaustive racial/ethnic groups: Asian, Black, Hispanic, and White. We defined Hispanic as Hispanic, Latino, or Spanish origin, of any race(s). We defined Black, Asian, and White as non-Hispanic and Black or African American alone, Asian alone, and White alone, respectively. This approach implies a fifth category comprised of non-Hispanic individuals of multiple races or any other race alone. The racial/ethnic variables did not sum to one (100%) unless the estimated population of the fifth category was zero.
We include more information on our framework for race/ethnicity and racial/ethnic variables in Section e2.2 of the online supplement.
Dependent Variable
The dependent variable approximated the share of each ZIP Code’s vaccine-eligible population that was partially or fully vaccinated against COVID-19. We calculated it by dividing the estimated number of residents with at least one dose of an approved vaccine by the estimated population age 15 and older. This denominator was the best available measure of the population to whom agencies were authorized to administer vaccines in March and April 2021. More information on the dependent variable is available in Section e2 of the online supplement.
Spatial-Statistical Analysis
We estimated population-weighted regressions with conventional adjustments for spatial clustering [127]. We report spatial error models (SEMs) estimated by maximum likelihood [127, 128, 129, 130, 131, 132]. Standard linear models (SLMs) are ill suited to estimate associations that vary across space. In this analysis, spatial heterogeneity could have arisen from unmeasurable factors such as COVID-19 exposure, hyperlocal idiosyncrasies in the effects or implementation of vaccination policies, and cultural influences. Standard tests[133, 134] strongly suggested SLMs exhibited spatial heterogeneity in our setting. We estimated SEMs with row-standardized nearest-neighbor weights () [135, 136, 137]. As the Moran’s test statistics in Table 3 [138] demonstrate, the SEMs eliminated the residual spatial clustering that emerged in the SLMs. The models incorporated city fixed effects to adjust for unmeasured variables that were constant among ZIP Codes within each city,[139] including elements of vaccination policies. Because multiple cities were in Texas, we calculated heteroskedasticity-robust standard errors clustered by state [140].
To illustrate the estimated associations, we simulated outcomes at representative values in the racial/ethnic and socioeconomic distributions of the sample. This approach resembled a marginal effects analysis but accounted for spatial clustering and yielded an overall average rather than a unit-level estimate.[141, 142, 143, 144] We present eight simulated scenarios: ZIP Codes with high Black populations and (1) low SES or (2) high SES; high Hispanic populations and (3) low SES or (4) high SES; high Asian populations and (5) low SES or (6) high SES; and high White populations and (7) low SES or (8) high SES. We defined low and high levels as below the 10th and above the 90th within-city percentiles, respectively.
We provide additional details on all aspects of our analytical approach, including the models and simulations, in Section e4 of the online supplement.
Results
Descriptive Findings
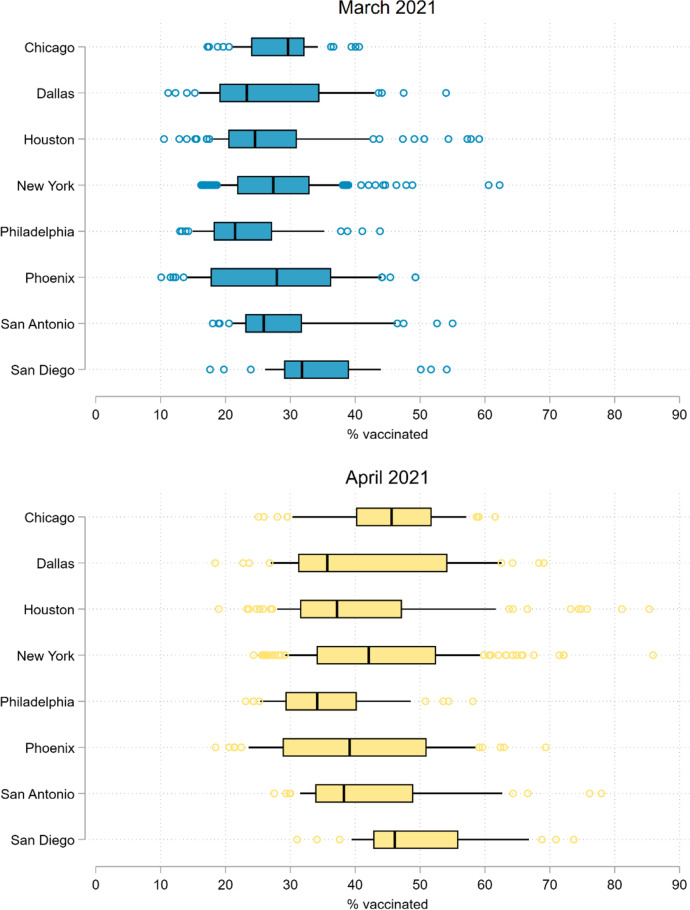
In Table 2, we present descriptive statistics at the ZIP Code level. On average across all 552 ZIP Codes, 28.0% of the population in March and 42.4% of the population in April had at least one dose of a COVID-19 vaccine, with a mean difference of 14.5 percentage points (p.p.) between March and April. Other than Philadelphia and San Diego, each city’s mean vaccination level fell within a two-point range (27–29%) in March and a five-point range (40–45%) in April. Although there was some variation between cities, vaccination levels varied considerably more across ZIP Codes within cities (see Fig. 1). In March, the standard deviation in vaccination levels was 3.0 p.p. between cities and 8.8 p.p. within cities; in April, it was 4.2 p.p. between cities and 11.9 p.p. within cities. The mean difference between the 10th and 90th percentiles of vaccination levels across cities was 21.6 p.p. in March and 31.0 p.p. in April.
Table 2.
Descriptive statistics on COVID-19 vaccination and population composition in ZIP Codes within and across eight large US cities, March and April 2021
| New York () | Chicago () | Houston () | ||||||
| % vaccinated, March | 28.18 | 7.94 | % vaccinated, March | 28.70 | 5.50 | % vaccinated, March | 27.23 | 10.03 |
| % vaccinated, April | 43.60 | 11.71 | % vaccinated, April | 45.08 | 9.46 | % vaccinated, April | 40.97 | 13.64 |
| % vaccinated, difference | 15.42 | 5.24 | % vaccinated, difference | 16.38 | 5.21 | % vaccinated, difference | 13.75 | 4.03 |
| % 65 + | 14.98 | 5.09 | % 65 + | 12.55 | 4.06 | % 65 + | 10.32 | 3.09 |
| % health care workers | 17.42 | 6.49 | % health care workers | 13.93 | 3.73 | % health care workers | 10.81 | 3.31 |
| % under poverty line | 15.91 | 9.42 | % under poverty line | 17.93 | 9.90 | % under poverty line | 18.33 | 9.36 |
| % w/ Medicaid, etc | 16.61 | 9.96 | % w/ Medicaid, etc | 12.98 | 9.48 | % w/ Medicaid, etc | 6.35 | 4.13 |
| % w/o health insurance | 8.16 | 4.40 | % w/o health insurance | 10.13 | 5.97 | % w/o health insurance | 25.09 | 12.31 |
| % w/o internet access | 14.62 | 6.47 | % w/o internet access | 16.34 | 9.10 | % w/o internet access | 16.51 | 10.85 |
| % Black | 19.82 | 23.39 | % Black | 29.67 | 33.53 | % Black | 22.73 | 18.63 |
| % Hispanic | 26.37 | 19.34 | % Hispanic | 22.43 | 21.94 | % Hispanic | 42.58 | 22.27 |
| % Asian | 14.77 | 13.96 | % Asian | 7.87 | 8.61 | % Asian | 6.56 | 6.23 |
| Phoenix () | Philadelphia () | San Antonio () | ||||||
| % vaccinated, March | 27.78 | 11.00 | % vaccinated, March | 23.30 | 7.65 | % vaccinated, March | 29.10 | 9.04 |
| % vaccinated, April | 40.17 | 13.48 | % vaccinated, April | 35.58 | 8.74 | % vaccinated, April | 42.21 | 11.90 |
| % vaccinated, difference | 12.38 | 3.19 | % vaccinated, difference | 12.28 | 2.01 | % vaccinated, difference | 13.11 | 3.33 |
| % 65 + | 11.32 | 4.35 | % 65 + | 14.18 | 4.61 | % 65 + | 11.90 | 3.24 |
| % health care workers | 12.08 | 2.16 | % health care workers | 20.63 | 4.38 | % health care workers | 14.06 | 2.31 |
| % under poverty line | 16.82 | 10.04 | % under poverty line | 22.25 | 11.17 | % under poverty line | 16.55 | 8.85 |
| % w/ Medicaid, etc | 12.38 | 7.14 | % w/ Medicaid, etc | 15.52 | 9.37 | % w/ Medicaid, etc | 4.99 | 3.01 |
| % w/o health insurance | 14.37 | 8.32 | % w/o health insurance | 8.74 | 3.58 | % w/o health insurance | 18.89 | 8.42 |
| % w/o internet access | 13.49 | 9.36 | % w/o internet access | 18.16 | 8.49 | % w/o internet access | 15.69 | 9.88 |
| % Black | 6.04 | 4.29 | % Black | 38.51 | 30.97 | % Black | 7.13 | 7.67 |
| % Hispanic | 37.30 | 23.95 | % Hispanic | 11.99 | 13.48 | % Hispanic | 61.79 | 20.90 |
| % Asian | 3.96 | 2.77 | % Asian | 6.96 | 5.82 | % Asian | 2.85 | 2.72 |
| San Diego () | Dallas () | Overall () | ||||||
| % vaccinated, March | 34.16 | 8.45 | % vaccinated, March | 27.02 | 10.40 | % vaccinated, March | 27.95 | 9.00 |
| % vaccinated, April | 50.30 | 10.81 | % vaccinated, April | 42.04 | 13.92 | % vaccinated, April | 42.44 | 12.34 |
| % vaccinated, difference | 16.13 | 3.45 | % vaccinated, difference | 15.02 | 4.72 | % vaccinated, difference | 14.48 | 4.56 |
| % 65 + | 13.18 | 4.36 | % 65 + | 10.55 | 5.72 | % 65 + | 12.75 | 4.81 |
| % health care workers | 12.87 | 2.00 | % health care workers | 11.22 | 2.42 | % health care workers | 14.58 | 5.45 |
| % under poverty line | 11.98 | 6.68 | % under poverty line | 17.11 | 9.02 | % under poverty line | 17.08 | 9.64 |
| % w/ Medicaid, etc | 9.63 | 7.17 | % w/ Medicaid, etc | 5.11 | 3.89 | % w/ Medicaid, etc | 11.52 | 9.04 |
| % w/o health insurance | 8.11 | 5.43 | % w/o health insurance | 24.26 | 11.89 | % w/o health insurance | 14.33 | 10.65 |
| % w/o internet access | 7.03 | 5.30 | % w/o internet access | 18.29 | 12.72 | % w/o internet access | 15.28 | 9.26 |
| % Black | 5.34 | 4.40 | % Black | 22.88 | 19.50 | % Black | 19.89 | 23.32 |
| % Hispanic | 26.84 | 21.38 | % Hispanic | 36.52 | 20.75 | % Hispanic | 32.68 | 23.95 |
| % Asian | 15.39 | 11.40 | % Asian | 5.23 | 9.70 | % Asian | 9.18 | 10.79 |
ZIP Codes across eight of the 10 most populous US cities. “Health care workers” refers to individuals employed in health care and social assistance. “Medicaid, etc.” refers to Medicaid or any other means-tested public health insurance. The “% vaccinated” is the percent of the population age 15 and older with at least one dose of a COVID-19 vaccine.
Fig. 1.
COVID-19 vaccination levels in the population age 15 and older of ZIP Codes in eight large US cities, March and April 2021. Note: Figures are box-and-whisker plots of vaccination levels in ZIP Codes across eight of the 10 most populous US cities. The boxes represent interquartile ranges. The vertical lines represent medians. The horizontal lines extend from the 10th to the 90th percentiles. Circles represent observations below the 10th and above the 90th percentiles. The “% vaccinated” is the percent of the population age 15 and older with at least one dose of a COVID-19 vaccine
Model Estimates
In Table 3, we summarize the results of the SEMs with all independent variables for three outcomes: March vaccination levels, April vaccination levels, and the difference between March and April vaccination levels. In both March and April, four variables were significantly associated with the dependent variable. The first, the percent of the population age 65 and older, reflects the policy choice to place older individuals among the earliest priority groups. The other three variables were measures of socioeconomic composition: the shares of the population under the poverty line, with means-tested public health insurance, and without health insurance. Adjusting for vaccination priority populations and racial/ethnic composition, markers of low SES were negatively associated with vaccination levels. In April, vaccination levels were positively associated with the Asian share of the population.
Table 3.
Spatial error model (SEM) estimates of COVID-19 vaccination levels in the population age 15 and older of ZIP Codes across eight large US cities, March and April 2021
| (1) | (2) | (3) | |
|---|---|---|---|
| % vaccinated, March | % vaccinated, April | Difference | |
| Vaccination priority populations | |||
| % 65 + | 0.593*** | 0.470*** | − 0.122* |
| (0.048) | (0.075) | (0.054) | |
| % health care workers | 0.147 | − 0.063 | − 0.201*** |
| (0.257) | (0.309) | (0.055) | |
| Socioeconomic composition | |||
| % under poverty line | − 0.102* | − 0.138** | − 0.039 |
| (0.051) | (0.051) | (0.023) | |
| % w/ Medicaid, etc | − 0.102*** | − 0.127** | − 0.021 |
| (0.024) | (0.046) | (0.029) | |
| % w/o health insurance | − 0.418*** | − 0.655*** | − 0.234*** |
| (0.039) | (0.053) | (0.023) | |
| % w/o internet access | − 0.040 | − 0.036 | 0.003 |
| (0.051) | (0.060) | (0.011) | |
| Racial/ethnic composition | |||
| % Black | − 0.111 | − 0.132 | − 0.021 |
| (0.061) | (0.084) | (0.025) | |
| % Hispanic | 0.041 | 0.076 | 0.036*** |
| (0.035) | (0.041) | (0.010) | |
| % Asian | 0.101 | 0.230* | 0.127*** |
| (0.067) | (0.103) | (0.037) | |
| Residual Moran’s | |||
| Standard linear model (SLM) | 0.250*** | 0.222*** | 0.202*** |
| Spatial error model (SEM) | 0.027 | 0.014 | − 0.015 |
SEMs estimated by maximum likelihood with row-standardized nearest-neighbor spatial weighting ().ZIP Codes across eight of the 10 most populous US cities. City fixed effects (reference: New York) and constant terms not shown. Percentages scaled from zero to one. All models weighted by estimated population age 15 and older. Heteroskedasticity-robust standard errors clustered by state in parentheses. ***; **; *. Moran’s-values calculated by permutation bootstrap (9999 iterations). “Health care workers” refers to individuals employed in health care and social assistance. “Medicaid, etc.” refers to Medicaid or any other means-tested public health insurance. The “% vaccinated” is the percent of the population age 15 and older with at least one dose of a COVID-19 vaccine.
Five variables were significantly associated with differences in vaccination between March and April. The shares of the population age 65 and older and employed in health care were associated with smaller increases. These associations probably reflect that these prioritized populations were widely vaccinated by the end of March. The Hispanic and Asian population shares were associated with larger increases in vaccination levels. The share of the population without health insurance was associated with smaller increases in vaccination levels.
As we detail in Tables e4.1 and e4.2 in the online supplement, we examined associations stepwise for socioeconomic and racial/ethnic composition. Racial/ethnic composition measures were often statistically significant in the absence of covariates measuring socioeconomic composition. When we included socioeconomic variables; however, the coefficients of the racial/ethnic variables were indistinguishable from zero. We further discuss implications below and in Section e4.3 of the online supplement.
Simulated Outcomes
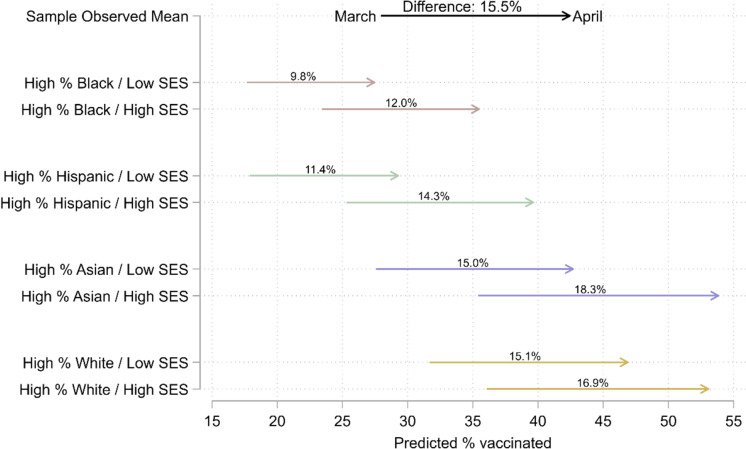
The simulations, illustrated in Fig. 2 and in Fig. e4.2 in the online supplement, contextualize relationships between racial/ethnic and socioeconomic composition. At both time points regardless of racial/ethnic composition, vaccination levels were higher where SES was higher. Socioeconomic disparities in vaccination were smaller where there was a high White population and larger where there were high Black, Hispanic, or Asian populations. In March, the highest vaccination levels (36.1%) were associated with high White populations and high SES; the lowest levels (17.7%) were associated with high Black populations and low SES. In April, the highest vaccination levels (53.8%) were associated with high Asian populations and high SES; the lowest levels (27.5%) were associated with high Black populations and low SES.
Fig. 2.
Simulated COVID-19 vaccination levels by racial/ethnic and socioeconomic composition in the population age 15 and older of ZIP Codes across eight large US cities, March and April 2021. Note: This figure illustrates simulated sample-wide means assuming each ZIP Code had a given socioeconomic and racial/ethnic composition. We defined low and high levels as below the 10th and above the 90th within-city percentiles, respectively. We defined SES levels by setting all four socioeconomic variables to the same within-city percentiles within each scenario. We set other independent variables to within-city averages in each scenario. We include the true (observed) sample-wide average values of the dependent variable on the top row for comparison. The “% vaccinated” is the percent of the population age 15 and older with at least one dose of a COVID-19 vaccine
Across racial compositions, the simulated change in vaccination levels between March and April was larger where SES was higher, as indicated by the numbers above each line in Fig. 2. Vaccination levels increased most (18.3 p.p.) where there were high Asian populations with high SES, followed by high White populations with high SES (16.9 p.p.). Vaccination levels increased least (9.8 p.p.) where there were high Black populations with low SES, followed by high Hispanic populations with low SES (11.4 p.p.).
Discussion
Key Findings
We examined COVID-19 vaccination in eight of the 10 most populous cities in the USA. In March and April 2021, vaccination levels varied more within cities—across ZIP Codes—than between cities. This finding suggests differences in state and local eligibility criteria contributed negligibly to disparities. Our models and simulations confirmed our hypotheses that ZIP Codes with higher shares of POC and low-SES individuals had lower vaccination levels and smaller increases over time. We now turn to three key findings.
Our finding that measures of racial/ethnic composition were statistically insignificant in the presence of socioeconomic covariates does not rule out racial/ethnic disparities. It suggests economic inequality and access to health insurance were fundamental mechanisms of local racial/ethnic gaps in vaccination. Furthermore, the relative magnitudes of the racial/ethnic variables’ coefficients were sometimes nearly as large as those of socioeconomic variables, albeit with slightly larger standard errors. Given the distribution of SES, ZIP Codes with high Black or Hispanic populations were associated with lower vaccination levels than those with high Asian or White populations.
Unlike internet access, measures of health insurance coverage were consistently associated with lower vaccination outcomes. This finding is surprising because internet access but not health insurance was directly tied to obtaining vaccine appointments. The insurance-related variables may capture multiple unobserved mechanisms: unfamiliarity with the medical system, perhaps due to reduced or discriminatory encounters with providers and insurers; incomplete or inaccurate information, including unawareness or skepticism that vaccines were free; and employment or other economic circumstances that impeded getting vaccinated or recovering from side effects. Survey or interview data may clarify individual-level mechanisms. Nonetheless, our results show that residents of large US cities who had tenuous connections to the health care system were less likely to benefit from an intervention that was free to all regardless of insurance coverage.
While several inequalities increased from March to April, one waned. ZIP Codes with high Hispanic populations were associated with larger increases in vaccinations, adjusting for other demographic and socioeconomic factors. Still, accounting for socioeconomic distributions, Hispanic communities were left behind overall as vaccination eligibility expanded.
Limitations
This study has several limitations. Authorities published vaccination data by ZIP Code only. Because ZIP Codes are suboptimal units for measuring inequality, disparities may be understated in this analysis. Representing ZIP Codes as areal polygons is distortive, potentially leading to measurement error.[145, 146, 147, 148, 149, 150, 151, 152] Furthermore, while they afford more local vantage points than states and counties, ZIP Codes cannot reveal finer, neighborhood-level dynamics. Our units of analysis averaged 38,123 residents, and one-quarter exceeded 50,000. At this scale, observations had substantial within-unit variation and relatively low between-unit variation, likely obscuring disparities.[52, 86, 89, 153, 154, 155, 156, 157, 158] We further discuss the analytical limitations of ZIP Codes in Section e3.1 of the online supplement.
The absence of individual-level data limited this analysis, but geographically aggregated data also presented advantages. It is difficult to determine how much our results reflected differential vaccine eligibility across ZIP Codes. We adjusted for key prioritized populations, however, and by mid-April, eligibility was approaching universal among US adults. In addition, the complete administrative data we used was more comprehensive than small surveys of self-reported behavior. Spatial analysis could also be optimal for guiding policy. Allocating resources geographically may be less resource-intensive than focusing on demographic subgroups. And, as we highlight above, spatial targeting is an effective tool for health equity.
Conclusion
Even as the number of vaccinated individuals increased by 7.1 million (34.7%) in the large US cities we studied, COVID-19 vaccination lagged in marginalized communities from late March to mid-April 2021. Vaccination gaps increased between low- and high-SES communities and between White or Asian and Black or Hispanic communities. The spatial clustering of unvaccinated individuals probably led to further public health issues.
Our findings suggest vaccination rollouts contributed to cumulative disadvantage at the community—and likely individual—level. Populations that experienced the highest burdens of infection and mortality from COVID-19 before vaccines were available had lower levels of vaccination during restricted vaccine eligibility. Gaps persisted or widened as eligibility first expanded. These disparities may have contributed to a bifurcated recovery in which advantaged communities began to move on from the COVID-19 pandemic while marginalized people continued to suffer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This project was supported by the California Center for Population Research at UCLA with training support (T32HD007545) and core support (P2CHD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. This work used computational and storage services associated with the Hoffman2 Shared Cluster, provided by the UCLA Institute for Digital Research and Education.
Thanks to Jonathan Daw, Ka-Yuet Liu, and Elizabeth Wrigley-Field for comments and suggestions. Additional thanks to Marc Caswell and Alexis Lantz.
Footnotes
Replication materials are available at 10.7910/DVN/O6EVCZ
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nicholas V. DiRago, Email: ndirago@g.ucla.edu
Meiying Li, Email: limeiyin@usc.edu.
Thalia Tom, Email: thaliato@usc.edu.
Will Schupmann, Email: wschupmann@g.ucla.edu.
Yvonne Carrillo, Email: yvonnecarrillo@ucla.edu.
Colleen M. Carey, Email: colleen.carey@cornell.edu
S. Michael Gaddis, Email: mgaddis@soc.ucla.edu.
References
- 1.Understanding vaccination progress. Johns Hopkins Coronavirus Resource Center. Accessed May 11, 2021. https://coronavirus.jhu.edu/vaccines/us-states
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson MG. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers — eight U.S. locations, December 2020–March 2021. Morb Mortal Wkly Rep. 2021;70(13):495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med. 2021;385(18):1718–1720. doi: 10.1056/NEJMc2106757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahly HME, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. Published online September 22, 2021. 10.1056/NEJMoa2113017 [DOI] [PMC free article] [PubMed]
- 7.Iheanacho CO, Eze UIH, Adida EA. A systematic review of effectiveness of BNT162b2 mRNA and ChAdOx1 adenoviral vector COVID-19 vaccines in the general population. Bull Natl Res Cent. 2021;45(1):150. doi: 10.1186/s42269-021-00607-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipsitch M, Kahn R. Interpreting vaccine efficacy trial results for infection and transmission. Vaccine. 2021;39(30):4082–4088. doi: 10.1016/j.vaccine.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gayle H, Foege W, Brown L, Kahn B, eds. Framework for Equitable Allocation of COVID-19 Vaccine. National Academies Press; 2020. [PubMed]
- 10.Tanne JH. Covid-19: biden buys 200 million doses of vaccines to tackle supply shortages across US. BMJ. 2021;372:n246. doi: 10.1136/bmj.n246. [DOI] [PubMed] [Google Scholar]
- 11.Money L, Lin RG. Waves of Californians will be newly eligible for the vaccine. But getting it will be tough. Los Angeles Times (online). Published March 11, 2021. https://www.proquest.com/latimes/docview/2499986329/. Accessed 3 Nov 2021.
- 12.Weise E. America could soon be swimming in COVID-19 vaccines: the shift from scarcity to surplus could bring its own problems. USA Today (online). Published March 9, 2021. Accessed November 3, 2021. https://www.usatoday.com/story/news/health/2021/03/09/covid-19-vaccine-us-surplus-april-coronavirus/4595458001/
- 13.Weekly COVID-19 vaccine allocations. Kaiser Family Foundation. Accessed November 3, 2021. https://www.kff.org/other/state-indicator/weekly-covid-19-vaccine-allocations/
- 14.Howard J. All 50 states now have expanded or will expand Covid vaccine eligibility to everyone 16 and up. CNN. Published April 5, 2021. Accessed April 23, 2021. https://www.cnn.com/2021/03/30/health/states-covid-19-vaccine-eligibility-bn/index.html
- 15.How to get a Covid-19 vaccine: a state-by-state guide. Wall Street Journal (online). Published April 15, 2021. Accessed September 5, 2021. https://www.wsj.com/articles/how-to-get-a-covid-19-vaccine-a-state-by-state-guide-11611703769
- 16.COVID data tracker. Centers for Disease Control and Prevention. Accessed September 5, 2021. https://covid.cdc.gov/covid-data-tracker
- 17.Abedi V, Olulana O, Avula V, et al. Racial, economic, and health inequality and COVID-19 infection in the United States. J Racial Ethn Health Disparities. 2021;8:732–742. doi: 10.1007/s40615-020-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouston SAP, Natale G, Link BG. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: a examination of the emergence of social inequalities. Soc Sci Med. 2021;268:113554. doi: 10.1016/j.socscimed.2020.113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do DP, Frank R. Unequal burdens: assessing the determinants of elevated COVID-19 case and death rates in New York City’s racial/ethnic minority neighbourhoods. J Epidemiol Community Health. 2021;75(4):321–326. doi: 10.1136/jech-2020-215280. [DOI] [PubMed] [Google Scholar]
- 20.Maroko AR, Nash D, Pavilonis BT. COVID-19 and inequity: a comparative spatial analysis of New York City and Chicago hot spots. J Urban Health. 2020;97(4):461–470. doi: 10.1007/s11524-020-00468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ransome Y, Ojikutu BO, Buchanan M, Johnston D, Kawachi I. Neighborhood social cohesion and inequalities in COVID-19 diagnosis rates by area-level Black/African American racial composition. J Urban Health. 2021;98(2):222–232. doi: 10.1007/s11524-021-00532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B, Rundle AG, Goodwin ATS, et al. COVID-19 testing, case, and death rates and spatial socio-demographics in New York City: an ecological analysis as of June 2020. Health Place. 2021;68:102539. doi: 10.1016/j.healthplace.2021.102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce JB, Harrington K, McCabe ME, et al. Racial/ethnic minority and neighborhood disadvantage leads to disproportionate mortality burden and years of potential life lost due to COVID-19 in Chicago Illinois. Health Place. 2021;68:102540. doi: 10.1016/j.healthplace.2021.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang TC, Kim S, Zhao Y, Choi S-wE. Examining spatial inequality in COVID-19 positivity rates across New York City ZIP codes. Health Place. 2021;69:102574. doi: 10.1016/j.healthplace.2021.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu T, Mack JA, Salvatore M, et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open. 2020;3(10):e2025197–e2025197. doi: 10.1001/jamanetworkopen.2020.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8):e2018039. doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrigley-Field E, Garcia S, Leider JP, Robertson C, Wurtz R. Racial disparities in COVID-19 and excess mortality in Minnesota. Socius. 2020;6:2378023120980918. doi: 10.1177/2378023120980918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths. Ann Intern Med. 2021;174(3):362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326–1334. doi: 10.1513/AnnalsATS.202011-1448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez L, Hart LH, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719–720. doi: 10.1001/jama.2020.26443. [DOI] [PubMed] [Google Scholar]
- 31.Shiels MS, Haque AT, Haozous EA, et al. Racial and ethnic disparities in excess deaths during the COVID-19 pandemic, March to December 2020. Ann Intern Med. Published online October 5, 2021. 10.7326/M21-2134 [DOI] [PMC free article] [PubMed]
- 32.Smith AR. Emergency department visits for COVID-19 by race and ethnicity — 13 states, October–December 2020. Morb Mortal Wkly Rep. 2021;70(15):566–569. doi: 10.15585/mmwr.mm7015e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang MH, Moonesinghe R, Truman BI. COVID-19 hospitalization by race and ethnicity: association with chronic conditions among Medicare beneficiaries, January 1–September 30, 2020. J Racial Ethn Health Disparities. Published online January 8, 2021. 10.1007/s40615-020-00960-y [DOI] [PMC free article] [PubMed]
- 34.Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161–1167. doi: 10.1007/s40615-020-00872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persad G, Peek ME, Emanuel EJ. Fairly prioritizing groups for access to COVID-19 vaccines. JAMA. 2020;324(16):1601–1602. doi: 10.1001/jama.2020.18513. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt H, Gostin LO, Williams MA. Is it lawful and ethical to prioritize racial minorities for COVID-19 vaccines? JAMA. 2020;324(20):2023. doi: 10.1001/jama.2020.20571. [DOI] [PubMed] [Google Scholar]
- 37.Persad G. Against exclusive survivalism: preventing lost life and protecting the disadvantaged in resource allocation. Hastings Cent Rep. 2021;51(5):47–51. doi: 10.1002/hast.1286. [DOI] [PubMed] [Google Scholar]
- 38.Dooling K. Phased allocation of COVID-19 vaccines. Presented at: Advisory Committee on Immunization Practices meeting; November 23, 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-11/COVID-04-Dooling.pdf
- 39.Goodnough A, Hoffman J. Up next: essential workers and people older than 74. New York Times. December 21, 2020:A.6.
- 40.Wilson WJ. The Truly Disadvantaged: The Inner City, the Underclass, and Public Policy. 2nd ed. Chicago: University of Chicago Press; 2012.
- 41.Massey DS, Denton NA. American Apartheid: Segregation and the Making of the Underclass. Cambridge: Harvard University Press; 1993.
- 42.Entwisle B. Putting people into place. Demography. 2007;44(4):687–703. doi: 10.1353/dem.2007.0045. [DOI] [PubMed] [Google Scholar]
- 43.Sampson RJ. Great American City: Chicago and the Enduring Neighborhood Effect. Chicago: University of Chicago Press; 2012.
- 44.Sharkey P. Stuck in Place: Urban Neighborhoods and the End of Progress toward Racial Equality. Chicago: University of Chicago Press; 2013.
- 45.Chetty R, Hendren N, Kline P, Saez E. Where is the land of opportunity?: the geography of intergenerational mobility in the United States. Q J Econ. 2014;129(4):1553–1623. doi: 10.1093/qje/qju022. [DOI] [Google Scholar]
- 46.Gaddis SM, Ghoshal R. Arab American housing discrimination, ethnic competition, and the contact hypothesis. Ann Am Acad Pol Soc Sci. 2015;660(1):282–299. doi: 10.1177/0002716215580095. [DOI] [Google Scholar]
- 47.Krysan M, Crowder K. Cycle of Segregation: Social Processes and Residential Stratification. New York: Russell Sage Foundation; 2017.
- 48.Rothstein R. The Color of Law: A Forgotten History of How Our Government Segregated America. New York: Liveright; 2017.
- 49.Galster G, Sharkey P. Spatial foundations of inequality: a conceptual model and empirical overview. RSF. 2017;3(2):1–33. doi: 10.7758/rsf.2017.3.2.01. [DOI] [Google Scholar]
- 50.Gaddis SM, Ghoshal R. Searching for a roommate: a correspondence audit examining racial/ethnic and immigrant discrimination among millennials. Socius. 2020;6:2378023120972287. doi: 10.1177/2378023120972287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaddis SM, DiRago N, Ghoshal R. Moving to the city: an audit study examining the consequences of racial/ethnic discrimination on residential segregation. SSRN Sch Pap. 2020;2605853. 10.2139/ssrn.2605853
- 52.Wrigley-Field E, Kiang MV, Riley AR, et al. Geographically targeted COVID-19 vaccination is more equitable and averts more deaths than age-based thresholds alone. Sci Adv. 7(40): 2099. 10.1126/sciadv.abj2099 [DOI] [PMC free article] [PubMed]
- 53.Kreps S, Prasad S, Brownstein JS, et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3(10):e2025594–e2025594. doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King WC, Rubinstein M, Reinhart A, Mejia R. COVID-19 vaccine hesitancy January-May 2021 among 18–64 year old US adults by employment and occupation. Prev Med Rep. 2021;24:101569. doi: 10.1016/j.pmedr.2021.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latkin CA, Dayton L, Yi G, Konstantopoulos A, Boodram B. Trust in a COVID-19 vaccine in the U.S.: a social-ecological perspective. Soc Sci Med. 2021;270:113684. doi: 10.1016/j.socscimed.2021.113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fridman A, Gershon R, Gneezy A. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS ONE. 2021;16(4):e0250123. doi: 10.1371/journal.pone.0250123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daly M, Jones A, Robinson E. Public trust and willingness to vaccinate against COVID-19 in the US from October 14, 2020, to March 29, 2021. JAMA. 2021;325(23):2397–2399. doi: 10.1001/jama.2021.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen JD, Feng W, Corlin L, et al. Why are some people reluctant to be vaccinated for COVID-19?: a cross-sectional survey among U.S. adults in May-June 2020. Prev Med Rep. 2021;24:101494. doi: 10.1016/j.pmedr.2021.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal R, Dugas M, Ramaprasad J, Luo J, Li G, Gao G. Socioeconomic privilege and political ideology are associated with racial disparity in COVID-19 vaccination. PNAS. 2021;118(33):e2107873118. doi: 10.1073/pnas.2107873118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guidry JPD, Laestadius LI, Vraga EK, et al. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control. 2021;49(2):137–142. doi: 10.1016/j.ajic.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J Community Health. 2021;46(2):270–277. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum Vaccines Immunother. Published online July 30, 2021. 10.1080/21645515.2021.1950504 [DOI] [PMC free article] [PubMed]
- 63.Willis DE, Andersen JA, Bryant-Moore K, et al. COVID-19 vaccine hesitancy: race/ethnicity, trust, and fear. Clin Transl Sci. Published online July 2, 2021. 10.1111/cts.13077 [DOI] [PMC free article] [PubMed]
- 64.Liu R, Li GM. Hesitancy in the time of coronavirus: temporal, spatial, and sociodemographic variations in COVID-19 vaccine hesitancy. SSM Popul Health. 2021;15:100896. doi: 10.1016/j.ssmph.2021.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamel L, Lopes L, Sparks G, Stokes M, Brodie M. KFF COVID-19 Vaccine Monitor – April 2021. Kaiser Family Foundation; 2021. Accessed May 12, 2021. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-april-2021/
- 66.Merton RK. The Matthew Effect in science. Science. 1968;159(3810):56–63. doi: 10.1126/science.159.3810.56. [DOI] [PubMed] [Google Scholar]
- 67.DiPrete TA, Eirich GM. Cumulative advantage as a mechanism for inequality: a review of theoretical and empirical developments. Annu Rev Sociol. 2006;32(1):271–297. doi: 10.1146/annurev.soc.32.061604.123127. [DOI] [Google Scholar]
- 68.Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing, “neighborhood effects:” social processes and new directions in research. Annu Rev Sociol. 2002;28(1):443–478. doi: 10.1146/annurev.soc.28.110601.141114. [DOI] [Google Scholar]
- 69.Sampson RJ, Sharkey P, Raudenbush SW. Durable effects of concentrated disadvantage on verbal ability among African-American children. PNAS. 2008;105(3):845–852. doi: 10.1073/pnas.0710189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wodtke GT, Harding DJ, Elwert F. Neighborhood effects in temporal perspective: the impact of long-term exposure to concentrated disadvantage on high school graduation. Am Sociol Rev. 2011;76(5):713–736. doi: 10.1177/0003122411420816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharkey P, Elwert F. The legacy of disadvantage: multigenerational neighborhood effects on cognitive ability. Am J Sociol. 2011;116(6):1934–1981. doi: 10.1086/660009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharkey P, Faber JW. Where, when, why, and for whom do residential contexts matter?: moving away from the dichotomous understanding of neighborhood effects. Annu Rev Sociol. 2014;40(1):559–579. doi: 10.1146/annurev-soc-071913-043350. [DOI] [Google Scholar]
- 73.Barber S, Hickson DA, Kawachi I, Subramanian SV, Earls F. Neighborhood disadvantage and cumulative biological risk among a socioeconomically diverse sample of African American adults: an examination in the Jackson Heart Study. J Racial Ethn Health Disparities. 2016;3(3):444–456. doi: 10.1007/s40615-015-0157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elías AS. Delayed disadvantage: neighborhood context and child development. Soc Forces. 2016;94(4):1847–1877. doi: 10.1093/sf/sow020. [DOI] [Google Scholar]
- 75.Chetty R, Hendren N. The impacts of neighborhoods on intergenerational mobility I: childhood exposure effects. Q J Econ. 2018;133(3):1107–1162. doi: 10.1093/qje/qjy007. [DOI] [Google Scholar]
- 76.Levy BL, Owens A, Sampson RJ. The varying effects of neighborhood disadvantage on college graduation: moderating and mediating mechanisms. Sociol Educ. 2019;92(3):269–292. doi: 10.1177/0038040719850146. [DOI] [Google Scholar]
- 77.Levy BL, Phillips NE, Sampson RJ. Triple disadvantage: neighborhood networks of everyday urban mobility and violence in U.S. cities. Am Sociol Rev. 2020;85(6):925–956. doi: 10.1177/0003122420972323. [DOI] [Google Scholar]
- 78.Momplaisir F, Haynes N, Nkwihoreze H, Nelson M, Werner RM, Jemmott J. Understanding drivers of coronavirus disease 2019 vaccine hesitancy among Blacks. Clin Infect Dis. 2021;ciab102. 10.1093/cid/ciab102 [DOI] [PMC free article] [PubMed]
- 79.Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–1396. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 80.Siddiqui M, Salmon DA, Omer SB. Epidemiology of vaccine hesitancy in the United States. Hum Vaccin Immunother. 2013;9(12):2643–2648. doi: 10.4161/hv.27243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33:D66–D71. doi: 10.1016/j.vaccine.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 82.Lieu TA, Ray GT, Klein NP, Chung C, Kulldorff M. Geographic clusters in underimmunization and vaccine refusal. Pediatrics. 2015;135(2):280–289. doi: 10.1542/peds.2014-2715. [DOI] [PubMed] [Google Scholar]
- 83.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315(11):1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin Infect Dis. 2021;ciab079. 10.1093/cid/ciab079 [DOI] [PMC free article] [PubMed]
- 85.Liang LL, Kuo HS, Ho HJ, Wu CY. COVID-19 vaccinations are associated with reduced fatality rates: evidence from cross-county quasi-experiments. J Glob Health. 2021;11:05019. doi: 10.7189/jogh.11.05019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masters NB, Eisenberg MC, Delamater PL, Kay M, Boulton ML, Zelner J. Fine-scale spatial clustering of measles nonvaccination that increases outbreak potential is obscured by aggregated reporting data. PNAS. 2020;117(45):28506–28514. doi: 10.1073/pnas.2011529117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Estep K, Greenberg P. Opting out: individualism and vaccine refusal in pockets of socioeconomic homogeneity. Am Sociol Rev. 2020;85(6):957–991. doi: 10.1177/0003122420960691. [DOI] [Google Scholar]
- 88.Gromis A, Liu KY. The emergence of spatial clustering in medical vaccine exemptions following California Senate Bill 277, 2015–2018. Am J Public Health. 2020;110(7):1084–1091. doi: 10.2105/AJPH.2020.305607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lei Y. Hyper focusing local geospatial data to improve COVID-19 vaccine equity and distribution. J Urban Health. 2021;98(4):453–458. doi: 10.1007/s11524-021-00552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen X, Zou Y, Gao H. Role of neighborhood social support in stress coping and psychological wellbeing during the COVID-19 pandemic: evidence from Hubei, China. Health Place. 2021;69:102532. doi: 10.1016/j.healthplace.2021.102532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glodeanu A, Gullón P, Bilal U. Social inequalities in mobility during and following the COVID-19 associated lockdown of the Madrid metropolitan area in Spain. Health Place. 2021;70:102580. doi: 10.1016/j.healthplace.2021.102580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo Y, Yu H, Zhang G, Ma DT. Exploring the impacts of travel-implied policy factors on COVID-19 spread within communities based on multi-source data interpretations. Health Place. 2021;69:102538. doi: 10.1016/j.healthplace.2021.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hao F, Shao W, Huang W. Understanding the influence of contextual factors and individual social capital on American public mask wearing in response to COVID–19. Health Place. 2021;68:102537. doi: 10.1016/j.healthplace.2021.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mayen Huerta C, Utomo A. Evaluating the association between urban green spaces and subjective well-being in Mexico city during the COVID-19 pandemic. Health Place. 2021;70:102606. doi: 10.1016/j.healthplace.2021.102606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robertson LS. Predictors of COVID-19-confirmed cases and fatalities in 883 US counties with a population of 50,000 or more: estimated effect of initial prevention policies. J Urban Health. 2021;98(2):205–210. doi: 10.1007/s11524-021-00514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slavik CE, Darlington JC, Buttle C, Sturrock SL, Yiannakoulias N. Has public health messaging during the COVID-19 pandemic reflected local risks to health?: a content analysis of tweeting practices across Canadian geographies. Health Place. 2021;69:102568. doi: 10.1016/j.healthplace.2021.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reitsma MB, Goldhaber-Fiebert JD, Salomon JA. Quantifying and benchmarking disparities in COVID-19 vaccination rates by race and ethnicity. JAMA Netw Open. 2021;4(10):e2130343. doi: 10.1001/jamanetworkopen.2021.30343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cardona S, Felipe N, Fischer K, Sehgal NJ, Schwartz BE. Vaccination disparity: quantifying racial inequity in COVID-19 vaccine administration in Maryland. J Urban Health. 2021;98(4):464–468. doi: 10.1007/s11524-021-00551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sacarny A, Daw JR. Inequities in COVID-19 vaccination rates in the 9 largest US cities. JAMA Health Forum. 2021;2(9):e212415. doi: 10.1001/jamahealthforum.2021.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siegel M, Critchfield-Jain I, Boykin M, et al. Racial/ethnic disparities in state-level COVID-19 vaccination rates and their association with structural racism. J Racial Ethn Health Disparities. Published online October 28, 2021. 10.1007/s40615-021-01173-7 [DOI] [PMC free article] [PubMed]
- 101.DiRago NV. Replication data for: “COVID-19 vaccine rollouts and the reproduction of urban spatial inequality: disparities within large U.S. cities in March and April 2021 by racial/ethnic and socioeconomic composition.” Harvard Dataverse. Published November 1, 2021. 10.7910/DVN/O6EVCZ
- 102.Jacobsen LA, Mather M, Jarosz B, et al. Understanding and Using American Community Survey Data: What All Data Users Need to Know. U.S. Census Bureau; 2020. https://www.census.gov/programs-surveys/acs/guidance/handbooks/general.html. Accessed 1 April 2021.
- 103.2019 TIGER/Line Shapefiles Technical Documentation. U.S. Census Bureau; 2019. https://www.census.gov/programs-surveys/geography/technical-documentation/complete-technical-documentation/tiger-geo-line.2019.html. Accessed 1 April 2021.
- 104.Lam NSN. Spatial interpolation methods: a review. Am Cartogr. 1983;10(2):129–150. doi: 10.1559/152304083783914958. [DOI] [Google Scholar]
- 105.Hawley K, Moellering H. A comparative analysis of areal interpolation methods. Cartogr Geogr Inf Sci. 2005;32(4):411–423. doi: 10.1559/152304005775194818. [DOI] [Google Scholar]
- 106.Reibling N, Ariaans M, Wendt C. Worlds of healthcare: a healthcare system typology of OECD countries. Health Policy. 2019;123(7):611–620. doi: 10.1016/j.healthpol.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 107.Lozano R, Fullman N, Mumford JE, et al. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1250–1284. doi: 10.1016/S0140-6736(20)30750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gee GC, Ford CL. Structural racism and health inequities: old issues, new directions. Du Bois Rev. 2011;8(1):115–132. doi: 10.1017/S1742058X11000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936–944. doi: 10.2105/AJPH.2011.300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol. 2015;41(1):311–330. doi: 10.1146/annurev-soc-073014-112305. [DOI] [Google Scholar]
- 111.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 112.Hicken MT, Kravitz-Wirtz N, Durkee M, Jackson JS. Racial inequalities in health: framing future research. Soc Sci Med. 2018;199:11–18. doi: 10.1016/j.socscimed.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cogburn CD. Culture, race, and health: implications for racial inequities and population health. Milbank Q. 2019;97(3):736–761. doi: 10.1111/1468-0009.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40(1):105–125. doi: 10.1146/annurev-publhealth-040218-043750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laster Pirtle WN. Racial capitalism: a fundamental cause of novel coronavirus (COVID-19) pandemic inequities in the United States. Health Educ Behav. 2020;47(4):504–508. doi: 10.1177/1090198120922942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bonilla-Silva E. Rethinking racism: toward a structural interpretation. Am Sociol Rev. 1997;62(3):465–480. doi: 10.2307/2657316. [DOI] [Google Scholar]
- 117.Zuberi T. Deracializing social statistics: problems in the quantification of race. Ann Am Acad Pol Soc Sci. 2000;568:172–185. doi: 10.1177/000271620056800113. [DOI] [Google Scholar]
- 118.Holland PW. Causation and race. In: Zuberi T, Bonilla-Silva E, editors. White Logic, White Methods. Rowman & Littlefield; 2008. pp. 93–109. [Google Scholar]
- 119.Gaddis SM. An introduction to audit studies in the social sciences. In: Gaddis SM, editor. Audit Studies: Behind the Scenes with Theory, Method, and Nuance. Springer; 2018. pp. 3–44. [Google Scholar]
- 120.Ray V. A theory of racialized organizations. Am Sociol Rev. 2019;84(1):26–53. doi: 10.1177/0003122418822335. [DOI] [Google Scholar]
- 121.Omi M, Winant H. Racial Formation in the United States. 3rd ed. New York: Routledge; 2015.
- 122.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;35:80–94. doi: 10.2307/2626958. [DOI] [PubMed] [Google Scholar]
- 123.Stewart QT, Sewell AA. Quantifying race: on methods for analyzing social inequality. In: Stanfield JH, editor. Rethinking Race and Ethnicity in Research Methods. Routledge; 2011. pp. 209–234. [Google Scholar]
- 124.Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17–35. doi: 10.1146/annurev-soc-071811-145508. [DOI] [Google Scholar]
- 125.Kohler-Hausmann I. Eddie Murphy and the dangers of counterfactual causal thinking about detecting racial discrimination. Northwest Univ Law Rev. 2019;113(5):1163–1228. https://scholarlycommons.law.northwestern.edu/nulr/vol113/iss5/6. Accessed 1 April 2021.
- 126.Rosen E, Garboden PME, Cossyleon JE. Racial discrimination in housing: how landlords use algorithms and home visits to screen tenants. Am Sociol Rev. 2021;86(5):787–822. doi: 10.1177/00031224211029618. [DOI] [Google Scholar]
- 127.Solon G, Haider SJ, Wooldridge JM. What are we weighting for? J Hum Resour. 2015;50(2):301–316. doi: 10.3368/jhr.50.2.301. [DOI] [Google Scholar]
- 128.Ord K. Estimation methods for models of spatial interaction. J Am Stat Assoc. 1975;70(349):120–126. doi: 10.1080/01621459.1975.10480272. [DOI] [Google Scholar]
- 129.Anselin L. Spatial Econometrics: Methods and Models. Springer; 1988.
- 130.LeSage JP, Pace RK. Introduction to Spatial Econometrics. Chapman & Hall/CRC; 2009.
- 131.Pace RK, LeSage JP. Omitted variable biases of OLS and spatial lag models. In: Páez A, Le Gallo J, Buliung RN, Dall’erba S, eds. Progress in Spatial Analysis: Methods and Applications. Springer; 2010:17–28.
- 132.Golgher AB, Voss PR. How to interpret the coefficients of spatial models: spillovers, direct and indirect effects. Spat Demogr. 2016;4(3):175–205. doi: 10.1007/s40980-015-0016-y. [DOI] [Google Scholar]
- 133.Rüttenauer T. Spatial regression models: a systematic comparison of different model specifications using Monte Carlo experiments. Sociol Methods Res. Published online November 29, 2019. 10.1177/0049124119882467
- 134.Schabenberger O, Gotway CA. Statistical Methods for Spatial Data Analysis. Chapman & Hall/CRC; 2005.
- 135.Anselin L, Bera AK, Florax R, Yoon MJ. Simple diagnostic tests for spatial dependence. Reg Sci Urban Econ. 1996;26(1):77–104. doi: 10.1016/0166-0462(95)02111-6. [DOI] [Google Scholar]
- 136.Tiefelsdorf M, Griffith DA, Boots B. A variance-stabilizing coding scheme for spatial link matrices. Environ Plan A. 1999;31(1):165–180. doi: 10.1068/a310165. [DOI] [Google Scholar]
- 137.Dubin R. Spatial weights. In: Fotheringham AS, Rogerson P, eds. The SAGE Handbook of Spatial Analysis. SAGE; 2009:125–157.
- 138.Smith TE. Notebook on Spatial Data Analysis. School of Engineering and Applied Science, University of Pennsylvania; 2014. https://www.seas.upenn.edu/~tesmith/NOTEBOOK/index.html. Accessed 1 April 2021.
- 139.Gormley TA, Matsa DA. Common errors: how to (and not to) control for unobserved heterogeneity. Rev Financ Stud. 2014;27(2):617–661. doi: 10.1093/rfs/hht047. [DOI] [Google Scholar]
- 140.Cameron AC, Miller DL. A practitioner’s guide to cluster-robust inference. J Hum Resour. 2015;50(2):317–372. doi: 10.3368/jhr.50.2.317. [DOI] [Google Scholar]
- 141.Brambor T, Clark WR, Golder M. Understanding interaction models: improving empirical analyses. Polit Anal. 2006;14(1):63–82. doi: 10.1093/pan/mpi014. [DOI] [Google Scholar]
- 142.Pepinsky TB. Visual heuristics for marginal effects plots. Res Polit. 2018;5(1). 10.1177/2053168018756668
- 143.Bivand R. Spatial econometrics functions in R: classes and methods. J Geogr Syst. 2002;4(4):405–421. doi: 10.1007/s101090300096. [DOI] [Google Scholar]
- 144.Goulard M, Laurent T, Thomas-Agnan C. About predictions in spatial autoregressive models: optimal and almost optimal strategies. Spat Econ Anal. 2017;12(2–3):304–325. doi: 10.1080/17421772.2017.1300679. [DOI] [Google Scholar]
- 145.Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. ZIP Code caveat: bias due to spatiotemporal mismatches between ZIP Codes and US Census–defined geographic areas. Am J Public Health. 2002;92(7):1100–1102. doi: 10.2105/AJPH.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grubesic TH, Matisziw TC. On the use of ZIP Codes and ZIP Code Tabulation Areas (ZCTAs) for the spatial analysis of epidemiological data. Int J Health Geogr. 2006;5(58). 10.1186/1476-072X-5-58 [DOI] [PMC free article] [PubMed]
- 147.Grubesic TH. ZIP Codes and spatial analysis: problems and prospects. Socioecon Plann Sci. 2008;42(2):129–149. doi: 10.1016/j.seps.2006.09.001. [DOI] [Google Scholar]
- 148.Beyer KMM, Schultz AF, Rushton G. Using ZIP Codes as geocodes in cancer research. In: Rushton G, Armstrong MP, Gittler J, et al., eds. Geocoding Health Data: The Use of Geographic Codes in Cancer Prevention and Control, Research, and Practice. CRC; 2008:37–68.
- 149.Liu K. Measurement error and its impact on partial correlation and multiple linear regression analyses. Am J Epidemiol. 1988;127(4):864–874. doi: 10.1093/oxfordjournals.aje.a114870. [DOI] [PubMed] [Google Scholar]
- 150.Hausman J. Mismeasured variables in econometric analysis: problems from the right and problems from the left. J Econ Perspect. 2001;15(4):57–67. doi: 10.1257/jep.15.4.57. [DOI] [Google Scholar]
- 151.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]
- 152.Loken E, Gelman A. Measurement error and the replication crisis. Science. 2017;355(6325):584–585. doi: 10.1126/science.aal3618. [DOI] [PubMed] [Google Scholar]
- 153.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? Am J Epidemiol. 2002;156(5):471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 154.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD. ZIP-Code-based versus tract-based income measures as long-term risk-adjusted mortality predictors. Am J Epidemiol. 2006;164(6):586–590. doi: 10.1093/aje/kwj234. [DOI] [PubMed] [Google Scholar]
- 155.Schuurman N, Bell N, Dunn JR, Oliver L. Deprivation indices, population health and geography: an evaluation of the spatial effectiveness of indices at multiple scales. J Urban Health. 2007;84(4):591–603. doi: 10.1007/s11524-007-9193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9(1):33. doi: 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parenteau MP, Sawada MC. The modifiable areal unit problem (MAUP) in the relationship between exposure to NO2 and respiratory health. Int J Health Geogr. 2011;10(1):58. doi: 10.1186/1476-072X-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Halonen JI, Vahtera J, Oksanen T, et al. Socioeconomic characteristics of residential areas and risk of death: is variation in spatial units for analysis a source of heterogeneity in observed associations? BMJ Open. 2013;3(4):e002474. doi: 10.1136/bmjopen-2012-002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.