Supplemental Digital Content is available in the text.
Keywords: aldosterone, chronic kidney disease, type 2 diabetes, finerenone, heart failure, mineralocorticoid receptor antagonist
Background:
Chronic kidney disease and type 2 diabetes are independently associated with heart failure (HF), a leading cause of morbidity and mortality. In the FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) and FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) trials, finerenone (a selective, nonsteroidal mineralocorticoid receptor antagonist) improved cardiovascular outcomes in patients with albuminuric chronic kidney disease and type 2 diabetes. These prespecified analyses from FIGARO-DKD assessed the effect of finerenone on clinically important HF outcomes.
Methods:
Patients with type 2 diabetes and albuminuric chronic kidney disease (urine albumin-to-creatinine ratio ≥30 to <300 mg/g and estimated glomerular filtration rate ≥25 to ≤90 mL per min per 1.73 m2, or urine albumin-to-creatinine ratio ≥300 to ≤5000 mg/g and estimated glomerular filtration rate ≥60 mL per min per 1.73 m2), without symptomatic HF with reduced ejection fraction, were randomized to finerenone or placebo. Time-to-first-event outcomes included new-onset HF (first hospitalization for HF [HHF] in patients without a history of HF at baseline); cardiovascular death or first HHF; HF-related death or first HHF; first HHF; cardiovascular death or total (first or recurrent) HHF; HF-related death or total HHF; and total HHF. Outcomes were evaluated in the overall population and in prespecified subgroups categorized by baseline HF history (as reported by the investigators).
Results:
Overall, 7352 patients were included in these analyses; 571 (7.8%) had a history of HF at baseline. New-onset HF was significantly reduced with finerenone versus placebo (1.9% versus 2.8%; hazard ratio [HR], 0.68 [95% CI, 0.50–0.93]; P=0.0162). In the overall population, the incidences of all HF outcomes analyzed were significantly lower with finerenone than placebo, including an 18% lower risk of cardiovascular death or first HHF (HR, 0.82 [95% CI, 0.70–0.95]; P=0.011), a 29% lower risk of first HHF (HR, 0.71 [95% CI, 0.56–0.90]; P=0.0043) and a 30% lower rate of total HHF (rate ratio, 0.70 [95% CI, 0.52–0.94]). The effects of finerenone on improving HF outcomes were not modified by a history of HF. The incidence of treatment-emergent adverse events was balanced between treatment groups.
Conclusions:
The results from these FIGARO-DKD analyses demonstrate that finerenone reduces new-onset HF and improves other HF outcomes in patients with chronic kidney disease and type 2 diabetes, irrespective of a history of HF.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02545049.
Clinical Perspective.
What Is New?
These prespecified analyses of the FIGARO-DKD trial (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) describe novel heart failure (HF)–related outcomes, not previously published in finerenone studies, including new-onset HF (hospitalization for HF in patients without a history of HF), and different outcomes containing first or total (first and recurrent) hospitalization for HF events in the overall population.
The results of these analyses indicate that, in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) on a maximum tolerated labeled dose of renin–angiotensin system inhibitor therapy, finerenone reduces new-onset HF and improves HF-related outcomes, irrespective of history of HF.
What Are the Clinical Implications?
This is the first indication that a nonsteroidal mineralocorticoid receptor antagonist may provide benefit in a population with CKD and T2D in which patients with HF with reduced ejection fraction and New York Heart Association class II to IV were excluded, indicating that patients with CKD in T2D at risk of HF or early-stage HF may benefit from finerenone treatment.
FIGARO-DKD included patients with less advanced CKD than studied in other trials in patients with CKD and T2D.
The results suggest screening for albuminuria and early treatment of patients with CKD and T2D are important to reduce HF burden in this patient population.
Heart failure (HF) is a leading cause of hospitalization and accounts for approximately 7% of cardiovascular deaths.1,2 In patients with chronic kidney disease (CKD) and type 2 diabetes (T2D), comorbid HF is a major health concern and an added economic burden on patients and health care systems.3 Both CKD and T2D are independently associated with HF.4–7 Patients with newly diagnosed HF with comorbid CKD and T2D have a greater risk of cardiovascular and noncardiovascular hospitalization and mortality compared with patients with 1 or none of these conditions.8 Furthermore, this higher risk was found to correlate with the severity of CKD.8
Management of this patient population is challenging because current treatment guidelines have limited applicability to patients with comorbid conditions, and many common glucose-lowering therapies, including metformin, sulfonylureas, sodium-glucose cotransporter-2 inhibitors (SGLT-2is), and most dipeptidyl peptidase-4 inhibitors, are more challenging to use in patients with CKD.9,10 According to current major global treatment guidelines for HF, mineralocorticoid receptor antagonists (MRAs) have a class IA recommendation in patients with HF with reduced ejection fraction (HFrEF).2,11,12 However, to date, there has been no prospective evaluation of the effect of MRAs on the prevention or worsening of HF in patients with CKD and/or T2D, and currently, they are infrequently prescribed to this population.13
Finerenone, a selective, nonsteroidal MRA, has shown beneficial effects on cardiovascular outcomes in patients with CKD and T2D. The phase III FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) and FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) trials showed that finerenone improved the cardiovascular composite outcome of time to cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF (HHF) compared with placebo and was well tolerated in patients with albuminuric CKD and T2D.14,15
The aims of these prespecified analyses of the FIGARO-DKD trial were to (1) assess the effects of finerenone on the incidence of new-onset HF (first HHF in patients without a history of HF) and with T2D and albuminuric CKD (stage 1–4 CKD with moderately to severely elevated albuminuria); (2) evaluate the effect of finerenone on important HF outcomes; and (3) assess the benefit of finerenone by baseline history of HF in this population, which excluded patients with symptomatic HFrEF (New York Heart Association class II–IV).
Methods
Study Design and Participants
The current analyses investigated time-to-event HF outcomes from the FIGARO-DKD trial. The study design and main efficacy and safety outcomes of this trial have been published previously.15,16 The study is registered with the European Union Clinical Trials Register (EudraCT 2015-000950-39) and ClinicalTrials.gov (NCT02545049). Anonymized data and materials will be made publicly available in the future.
In brief, FIGARO-DKD, an international, randomized, double-blind, placebo-controlled, multicenter, phase III trial, was designed to determine the effect of finerenone in reducing cardiovascular morbidity and mortality in addition to standard of care in patients with albuminuric CKD (stage 2–4 with moderately elevated albuminuria, or stage 1–2 with severely increased albuminuria) and T2D. The study was conducted in accordance with the principles of the Declaration of Helsinki. Protocol approvals were obtained from local regulatory authorities and ethics committees. Written informed consent was provided by all participants.
Patients were eligible if they were ≥18 years of age, clinically diagnosed with T2D and albuminuric CKD (defined as either [1] persistent, moderately increased urine albumin-to-creatinine ratio ≥30 to <300 mg/g and estimated glomerular filtration rate [eGFR] ≥25 to ≤90 mL per min per 1.73 m2, or [2] persistent severely increased urine albumin-to-creatinine ratio ≥300 to ≤5000 mg/g and eGFR ≥60 mL per min per 1.73 m2), treated with a maximum tolerated dose of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker in accordance with the manufacturer’s label before the screening visit, and with a serum potassium level of ≤4.8 mEq/L at screening. Patients excluded from this study included those with chronic symptomatic HFrEF (New York Heart Association class II–IV) at the run-in visit (a class IA recommendation for an MRA); known significant nondiabetic kidney disease; stroke, transient ischemic cerebral attack, acute coronary syndrome, or HHF in the 30 days before the screening visit; a recent history of dialysis for acute kidney failure; a kidney transplant; or uncontrolled hypertension.
Procedures and Outcomes
Patients were randomized 1:1 to receive once-daily oral treatment with finerenone (10 or 20 mg) or matching placebo. Patients randomized to the finerenone group received an initial daily dose of 10 mg or 20 mg if they had an eGFR of 25 to <60 mL per min per 1.73 m2 or ≥60 mL per min per 1.73 m2, respectively. Up-titration was encouraged after 1 month provided serum potassium was ≤4.8 mmol/L and eGFR was stable; down-titration was allowed any time after treatment initiation. The prespecified subgroups included in these analyses were categorized by the presence or absence of a documented medical history of HF at baseline, as reported by the investigators.
The prespecified outcomes for these analyses were (1) time to new-onset HF (first HHF in patients without a history of HF at baseline); (2) a composite of time to cardiovascular death or first HHF; (3) a composite of time to HF-related death or first HHF; (4) time to first HHF15; (5) a composite of time to cardiovascular death or total (first or recurrent) HHF; (6) a composite of time to HF-related death or total HHF; and (7) time to total HHF. Outcomes 2 to 7 were investigated in the full study population and by history of HF. A cardiovascular composite outcome including time to cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or HHF was also investigated in these analyses (see details in the Supplemental Material). All potential cardiovascular outcomes in the study, as well as all hospitalizations and deaths, were prospectively reviewed and adjudicated by an independent clinical event committee composed of cardiologists blinded to treatment assignment. The criteria used by the clinical event committee to adjudicate HHF events are provided in the Supplemental Material. The definition for new-onset HF (first HF in patients without a history of HF) required all criteria for an HHF event to be met, and in addition, the patient was required to have no previous documented history of HF (see details in the Supplemental Material).
Adverse events (AEs) that occurred or worsened during study drug treatment or up to 3 days after temporary or permanent interruption were considered as treatment-emergent AEs and were evaluated by history of HF. Hyperkalemia AEs were reported by the investigator on the basis of their clinical judgment without specification of a threshold for potassium using the Medical Dictionary for Regulatory Activities preferred terms “hyperkalemia” and “blood potassium increased.” Serum potassium elevations >5.5 mmol/L and >6.0 mmol/L detected on any central laboratory potassium measurements are also reported.
Statistical Analysis
Efficacy analyses were performed in the full analysis set composed of all randomized patients without critical Good Clinical Practice violations. Safety analyses were performed in the safety set composed of all patients of the full analysis set who had taken at least 1 dose of study drug. Baseline characteristics of patients grouped by history of HF were reported as mean (SD), median and interquartile range, or number and percentage. All efficacy outcomes were analyzed as time-to-event outcomes: (1) time to new-onset HF; (2) time to cardiovascular death or first HHF; (3) time to HF-related death or first HHF; (4) time to first HHF; (5) time to cardiovascular death or total (first or recurrent) HHF; (6) time to HF-related death or total HHF; and (7) time to total HHF. Efficacy events were reported from randomization up to the end-of-study visit, and patients were censored if there was no event at the date of their last contact with complete information on all components of their respective outcomes. Log-rank tests, stratified by region, eGFR, and albuminuria categories at screening and history of cardiovascular disease were used to test the null hypothesis of equal hazards of finerenone versus placebo for the cardiovascular and HF-associated time-to-first-event outcomes. Treatment effects were expressed as hazard ratios (HRs) with corresponding 95% CIs from a Cox proportional hazards model adjusted for stratification factors. In addition, medical history of HF and its interaction with treatment were included in the model to test the independence of the treatment effect from medical history of HF. Andersen-Gill models with robust standard errors (Lin Wei Yang and Ying proportional rates model), stratified by region, type of albuminuria at screening, eGFR category at screening, and cardiovascular disease history, were used to calculate the rate ratio with corresponding 95% CIs for first-and-recurrent-event outcomes. Treatment-emergent AEs were expressed as number and percentage, and vital signs were expressed as mean (SD) during the course of the study.
Analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
Patients
The FIGARO-DKD trial randomized 7437 patients. After the prospective exclusion of 85 patients from all analyses because of critical violations of Good Clinical Practice, 7352 patients were assessed in the full analysis set. The study concluded after a median follow-up of 3.4 years. Among the patients in the full analysis set, 571 (7.8%) had a history of HF at baseline (290 patients in the finerenone group and 281 patients in the placebo group).
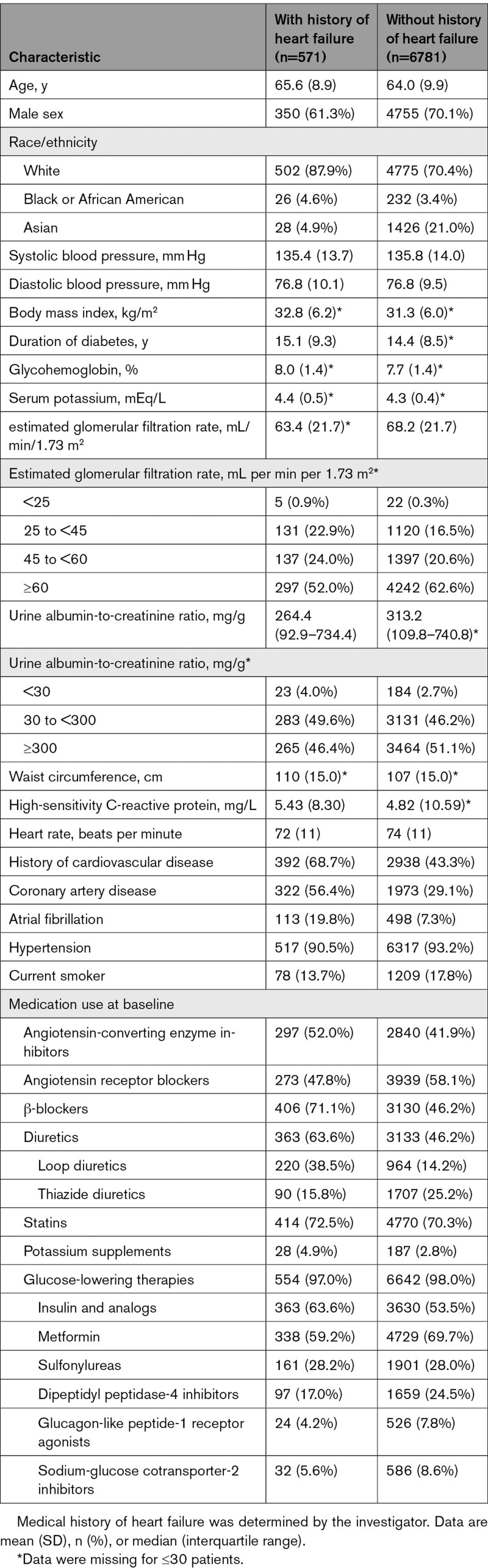
Overall, the baseline characteristics and medication for patients with and without a history of HF were balanced between treatment groups (Table, Table S1). Compared with patients without a history of HF, those with a history of HF were more likely to be female, be White (and less likely Asian), and have a higher body mass index and a greater waist circumference, higher level of high-sensitivity C-reactive protein, lower heart rate, and lower mean eGFR and median urine albumin-to-creatinine ratio. These patients also had a longer mean duration of diabetes, were more likely to have a history of atherosclerotic cardiovascular disease, and were more likely to be receiving β-blockers, diuretics, potassium supplements, and insulin. In contrast, fewer patients with a history of HF were treated with metformin and dipeptidyl peptidase-4 inhibitors than patients without HF.
Table 1.
Patient Baseline Characteristics, by History of Heart Failure at Baseline
Effect of Finerenone on the Time to New-Onset HF (First HHF in Patients Without a History of HF) Outcome
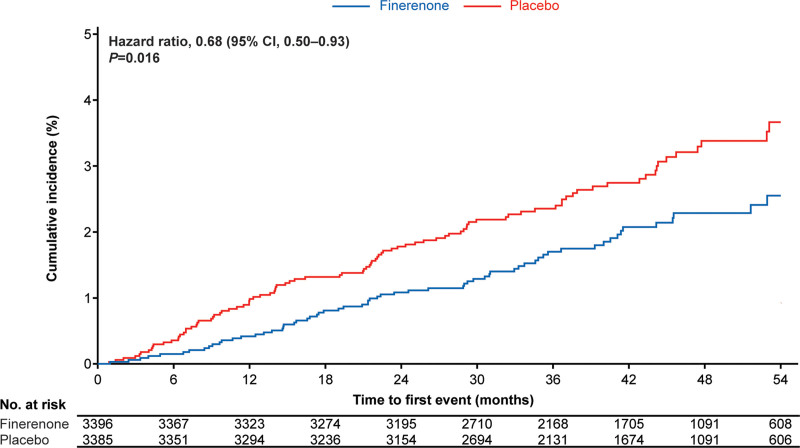
The incidence of new-onset HF (time to first adjudicated HHF in patients without a history of HF) was significantly lower with finerenone (65/3396 patients [1.9%]; 0.57 per 100 patient-years [95% CI, 0.44–0.72]) than placebo (95/3385 patients [2.8%]; 0.84 per 100 patient-years [95% CI, 0.68–1.02]; HR, 0.68 [95% CI, 0.50–0.93]; P=0.016; Figure 1, Table S2). The absolute risk reduction observed using Kaplan-Meier estimates at month 48 for new-onset HF was 1.1%, corresponding to a number needed to treat to prevent 1 such event of 91 (95% CI, 49–605).
Figure 1.
Kaplan-Meier estimate of time to new-onset HF (first hospitalization for HF in patients without a history of HF at baseline). HF indicates heart failure.
Effect of Finerenone on HF-Associated Outcomes
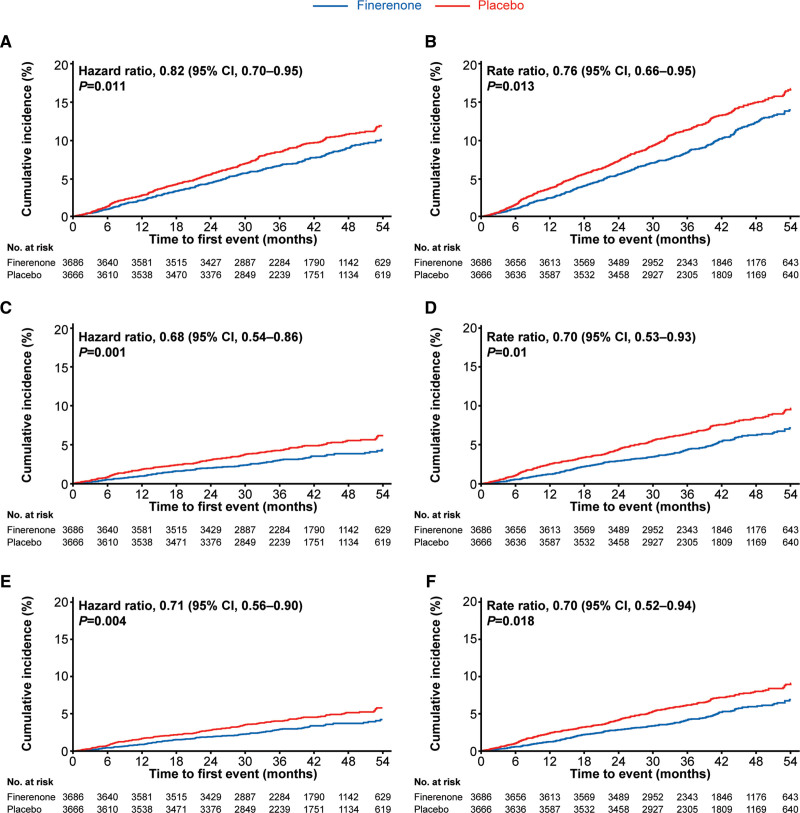
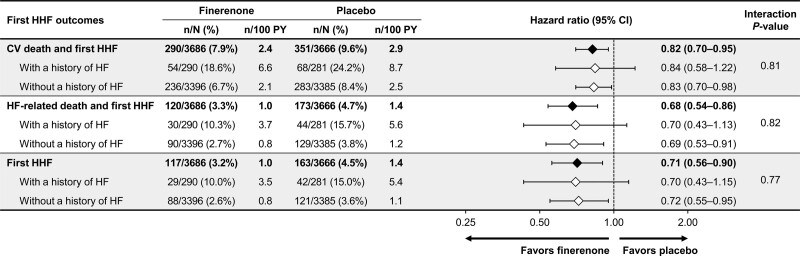
HF-associated time-to-event outcomes in the total study population were significantly reduced with finerenone compared with placebo. These included a reduction in the composite of time to cardiovascular death or first HHF (finerenone, 290/3686 [7.9%]; placebo, 351/3666 [9.6%]; HR, 0.82 [95% CI, 0.70–0.95]; P=0.011); the composite of time to HF-related death or first HHF (finerenone, 120/3686 [3.3%]; placebo, 173/3666 [4.7%]; HR, 0.68 [95% CI, 0.54–0.86]; P=0.0013); and time to first HHF (finerenone, 117/3686 [3.2%]; placebo, 163/3666 [4.4%]; HR, 0.71 [95% CI, 0.56–0.90]; P=0.0043; Figure 2, Table S2). The absolute risk reduction observed using Kaplan-Meier estimates at month 48 for the composite outcome of time to cardiovascular death or first HHF was 1.8%, corresponding to a number needed to treat to prevent 1 such event of 55 (95% CI, 29–393). For the composite outcome of time to HF-related death or first HHF, the absolute risk reduction at month 48 was 1.7%, with a number needed to treat of 60 (95% CI, 35–177). For first HHF, the absolute risk reduction at month 48 was 1.4%, with a number needed to treat of 70 (95% CI, 39–292).
Figure 2.
Time to HF-related outcomes. A, Kaplan-Meier estimates for time to cardiovascular death or first HHF in the overall population. B, Mean cumulative function estimates for time to cardiovascular death or total HHF in the overall population. C, Kaplan-Meier estimates for time to HF-related death or first HHF in the overall population. D, Mean cumulative function estimates for time to HF-related death or total HHF in the overall population. E, Kaplan-Meier estimates for time to first HHF in the overall population. F, Mean cumulative function estimates for time to total HHF in the overall population. HF indicates heart failure; and HHF, hospitalization for heart failure.
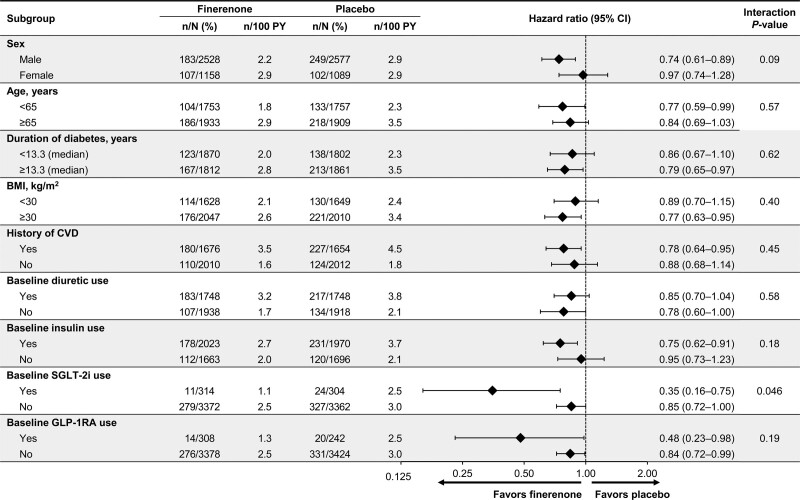
Results for the outcome of time to cardiovascular death or first HHF were not modified across key prespecified and post hoc subgroups, except for a nominally significant interaction for the SGLT-2i subgroup (Figure 3). Broadly similar results were observed for the time to HF-related death or first HHF and first HHF outcomes (Figures S1 and S2).
Figure 3.
Time to cardiovascular death or first hospitalization for heart failure in key prespecified and post hoc subgroups. BMI indicates body mass index; CVD, cardiovascular disease; GLP-1RA, glucagon-like peptide 1 receptor agonist; PY, patient-years; and SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
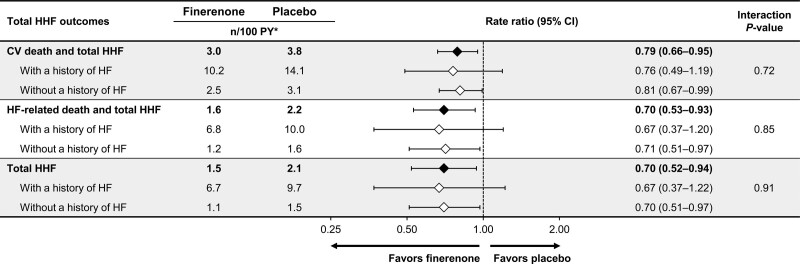
In addition, time to cardiovascular death or total HHF, time to HF-related death or total HHF, and time to total HHF were also significantly lower in the finerenone group compared with the placebo group (rate ratio, 0.79 [95% CI, 0.66–0.95]; P=0.013; rate ratio, 0.70 [95% CI, 0.53–0.93]; P=0.014; and rate ratio, 0.70 [95% CI, 0.52–0.94], respectively; Figure 2, Table S2). The absolute reduction in mean cumulative events observed at month 48 for the composite outcome of cardiovascular death and total HHF was 3.0%.
The incidence rate of all HF-associated outcomes was higher in patients with a history of HF than those without in both treatment groups. However, a history of HF did not modify the effect of finerenone on the composite of time to cardiovascular death or first HHF (54/290 [18.6%] patients with a history of HF, 236/3396 [6.7%] patients without a history of HF; P value for interaction, 0.81), the composite of time to HF-related death or first HHF (30/290 [10.3%] patients with a history of HF, 90/3396 [2.7%] patients without a history of HF; P value for interaction, 0.82) or time to first HHF (29/290 [10.0%] patients with a history of HF, 88/3396 [2.6%] patients without a history of HF; P value for interaction, 0.77; Figure 4). Because event rates were higher in patients with a history of HF, absolute risk reductions with finerenone in patients with a history of HF were larger than for those without a history of HF. The absolute risk difference observed using Kaplan-Meier estimates at month 48 for the composite outcome of time to cardiovascular death or first HHF was –4.8% (95% CI, –13.7 to 4.1) for patients with a history of HF and –1.6% (95% CI, –3.1 to 0.0) for patients without a history of HF. For the composite outcome of time to HF-related death and first HHF, the absolute risk difference at month 48 was –5.1% (95% CI, –12.6 to 2.3) and –1.4% (95% CI, –2.5 to –0.4) for patients with and without a history of HF at baseline, respectively. For the first HHF outcome, the absolute risk difference at month 48 was –4.1% (95% CI, –11.3 to 3.1) and –1.2% (95% CI, –2.2 to –0.2) for patients with and without a history of HF at baseline, respectively. Similar results were seen for the HF outcomes including total HHF instead of first HHF (Figure 5). Finerenone also consistently lowered the composite cardiovascular outcome of time to cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or HHF, irrespective of HF history at baseline (Figure S3).
Figure 4.
HF-associated outcomes including first HHF, overall, and by history of HF. CV indicates cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; and PY, patient-years.
Figure 5.
HF-associated outcomes including total HHF, overall, and by history of HF. CV indicates cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; and PY, patient-years. *Event rate (n/100 PY) is the sum of all events in all patients divided by overall time under risk for all patients.
Safety Outcomes and Vital Signs by History of HF
Among patients with and without a history of HF, the incidence of overall treatment-emergent AEs between the treatment groups was balanced (Table S3). In both the finerenone and placebo groups, the incidence of hyperkalemia was higher in patients without a history of HF than in those with a history of HF (Table S3). However, irrespective of history of HF, the incidence of treatment-emergent hyperkalemia was greater with finerenone than with placebo (8.3% versus 4.6%, respectively, in patients with a history of HF; and 11.0% versus 5.3%, respectively, in patients without a history of HF). However, few cases of hyperkalemia led to permanent discontinuation of study drug (finerenone, 0.3%, and placebo, 0.7%, in patients with a history of HF; and finerenone, 1.3%, and placebo, 0.3%, in patients without a history of HF). Finerenone led to a modest reduction in systolic blood pressure from baseline in both patients with a history of HF (–2.8 mm Hg at month 4 and –2.6 mm Hg at month 12 for finerenone versus 0.1 mm Hg at month 4 and 0.2 mm Hg at month 12 for placebo) and patients without a history HF (–3.2 mm Hg at month 4 and –2.9 mm Hg at month 12 for finerenone versus 0.3 mm Hg at month 4 and 0.1 mm Hg at month 12 for placebo; Figure S4). Finerenone had no effect on body weight in patients with and without a history of HF (Figure S5).
Discussion
In patients with T2D and CKD (stage 2–4 CKD with moderately elevated albuminuria or stage 1–2 CKD with severely elevated albuminuria) without a history of symptomatic HFrEF, finerenone led to a significant reduction in the risk of clinically important time-to-event HF outcomes, including a 29% reduction in the risk of first HHF and an 18% reduction in the risk of cardiovascular death or first HHF versus placebo. This effect was independent of a history of HF. The risk of new-onset HF was 32% lower with finerenone compared with placebo; this is the first study to show that an MRA, specifically the selective, nonsteroidal MRA finerenone, may prevent the development of HF in patients with CKD and T2D.
The benefits of finerenone on HF outcomes observed in this study, including the prevention of HF and the reduction in time-to-first-event and total HHF, can be expected to translate into meaningful improvements in patient outcomes. Patients with CKD, T2D, and HF are at high risk of further cardiovascular events.17 Compared with other comorbid cardiovascular and kidney diseases, the development of HF, both alone and in combination with other comorbidities such as CKD, has been shown to be associated with the highest risk of death and decrease in lifespan in patients newly diagnosed with T2D.18 A systematic review of medical costs associated with HF in the United States demonstrated that hospitalization is the major contributor to the overall direct costs for HF, constituting 65% of all medical HF costs over a 1-year period from the index hospitalization event. After initial HHF, readmission rates for any cause are high, with around 20% of patients being readmitted within 30 days, further exacerbating medical costs and patient outcomes.19
Kidney dysfunction is independently associated with adverse outcomes in patients with HF, irrespective of left ventricular ejection fraction.6,20 Common risk factors linking HF and kidney dysfunction include ageing, diabetes, and hypertension as well as shared pathogenic pathways.6 Because of established hemodynamic and neurohormonal cross-talk between the heart and kidneys, acute or chronic dysfunction in one organ can cause acute or chronic dysfunction in the other.21 Proinflammatory and profibrotic processes may provide a pathophysiological link between progressive kidney and heart dysfunction.22–25 Overactivation of the mineralocorticoid receptor, which is extensively expressed in the heart, kidneys, and vasculature, is linked to organ damage by inflammation and fibrosis.26 Thus, potent and selective blockade of the mineralocorticoid receptor with finerenone may be effective in reducing HF outcomes in CKD through amelioration of these processes. The entire population in the FIGARO-DKD trial had CKD and T2D; therefore, all patients in the study can be considered as having at least stage A HF (ie, at risk of developing HF), with a significant proportion of patients likely having stage B HF (ie, pre-HF with no signs or symptoms of HF but structural heart disease and/or asymptomatic cardiac dysfunction and/or elevated cardiac biomarkers27,28). Therefore, the results of these analyses suggest that finerenone slows progression from at-risk (stage A) or pre-HF (stage B) to symptomatic HF (stage C).
With the exclusion of patients with symptomatic HFrEF in FIGARO-DKD (because of the class IA recommendation in guidelines for MRA treatment12), the subgroup of patients with HF analyzed in this study are likely those with stage B (asymptomatic HFrEF or HF with preserved ejection fraction [HFpEF]) or stage C HF (ie, current or previous signs and symptoms of HF with mildly reduced ejection fraction or HFpEF). Indeed, the baseline characteristics of the patients with a history of HF in the present study—more likely female, obese with lower eGFR, and higher C-reactive protein—are more indicative of a HFpEF population. Data on the efficacy and safety of steroidal MRAs in patients with HFpEF are mixed.29–31 Data from the current analyses are limited to allow for any conclusions, but it is the first set of preliminary data evaluating a nonsteroidal MRA in patients with HFpEF or HF with mildly reduced ejection fraction. The ongoing FINEARTS-HF study (Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients With Heart Failure), which is evaluating the efficacy and safety of finerenone in patients with HF and a left ventricular ejection fraction ≥40%, will offer a robust insight into the effects of finerenone in this patient population.32
In the present study, finerenone led to a 29% reduction in the risk of first HHF compared with placebo,15 and furthermore, the rate of total HHF was reduced by 30%. The FIGARO-DKD trial included patients at high cardiovascular risk, with a broader spectrum of patients with CKD, yet overall, less advanced kidney disease than those included in FIDELIO-DKD.14–16 In the FIDELIO-DKD study, a reduction in the risk of cardiovascular outcomes was also observed with finerenone compared with placebo. This was reflected by lower incidence rates for HHF as well as cardiovascular death in the finerenone group; however, the magnitude of risk reduction for HHF was smaller than that observed in the present study, at 14%.14 In patients with CKD, T2D, and HF, mortality and hospitalization rates have been shown to increase with CKD severity.8 Therefore, initiating treatment at earlier stages of disease such as those represented in these analyses, 62% of whom had albuminuric CKD with an eGFR ≥60 mL per min per 1.73 m2, may be more beneficial for this patient population,8,15,16 and may explain in part the larger magnitude of treatment effect observed with finerenone on HF outcomes in the FIGARO-DKD trial.
Other therapies that have been proven to improve HF outcomes in patients with T2D with CKD include angiotensin receptor blockers. In the RENAAL trial (Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan) in patients with T2D and CKD, which excluded patients with HF before enrollment, losartan (an angiotensin receptor blocker) reduced the risk of first HHF by 32%. Comparable relative risk reductions in first HHF were seen with finerenone in this study—in patients already receiving a maximum tolerated labeled dose of a renin–angiotensin system inhibitor. More recently, SGLT-2is have been shown to improve HF outcomes in patients with T2D with and without CKD, and in patients with HFrEF and HFpEF with and without CKD.33–36 In patients with T2D, regardless of CKD, a meta-analysis showed a 22% and 32% lower risk of cardiovascular death or HHF and HHF, respectively, with an SGLT-2i versus placebo32; for comparison, in this study, in which all patients had CKD, finerenone reduced the risk of these outcomes by 18% and 29%, respectively. In the present study, in the subgroup of patients receiving an SGLT-2i at baseline, a greater effect was observed with finerenone in addition to an SGLT-2i compared with an SGLT-2i alone on the composite outcome of cardiovascular death and HHF. This may suggest an increasing treatment benefit when finerenone is given in addition to an SGLT-2i, but because of the small size of this subgroup, this result should be interpreted with caution.
In these analyses, finerenone was generally well tolerated, with overall balanced treatment-emergent AEs between treatment groups. As anticipated, the incidence of hyperkalemia was higher with finerenone than placebo, but few cases led to hospitalization or permanent drug discontinuation, reflecting the minimal clinical effect of this adverse effect. Studies investigating eplerenone and spironolactone in the treatment of HF have found an increased incidence of hyperkalemia compared with placebo.29,31,37,38 For example, in a post hoc analysis of the TOPCAT trials (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) investigating patients enrolled in the United States, Canada, Brazil, and Argentina, spironolactone led to a higher incidence of hyperkalemia (potassium ≥5.5 mmol/L) compared with placebo (25.2% and 8.9%, respectively; P<0.001).30 The phase IIa ARTS trial (Mineralocorticoid Receptor Antagonist Tolerability Study), which investigated the safety and tolerability of finerenone compared with spironolactone in patients with a clinical diagnosis of HFrEF (New York Heart Association class II–III and left ventricular ejection fraction <40%) and mild or moderate CKD (60 to <90 mL per min per 1.73 m2), showed that finerenone at doses up to 10 mg once daily achieved a similar reduction in N-terminal pro-B-type natriuretic peptide, but was associated with lower incidences of hyperkalemia compared with spironolactone at doses of 25 to 50 mg once daily (4.5% versus 11.1%, respectively).39
These analyses had a few limitations, including the definition of a medical history of HF, which was determined on the basis of investigator reports without a specification or consistent documentation of left ventricular ejection fraction or natriuretic peptide levels. The relatively small subgroup sample size of patients with a history of HF may preclude some of the comparisons between those with and without a history of HF. Therefore, these points should be considered when interpreting the results presented.
In summary, these secondary analyses demonstrated promising results on the benefits of finerenone on improving HHF in patients without a history of HF and other clinically important HF outcomes in patients with CKD and T2D, independent of a history of HF.
Article Information
This work was presented at the American Heart Association Scientific Sessions 2021, November 13–15, 2021.
Acknowledgments
The authors are indebted to the patients who have participated in this trial, the FIGARO-DKD trial investigators, and the study centers who supported the trial. The Executive Committee designed the study in conjunction with the sponsor. G.F. wrote the first draft of the report. All authors were involved in data analysis and interpretation, and in drafting and critically revising the report. All authors had access to study results, and the first and corresponding author assumes responsibility for the integrity and accuracy of the data reported. All authors reviewed and approved the final submitted version of the report. Medical writing assistance was provided by Ines Neves, MSc, and Connie Lam, PhD, of Chameleon Communications International, and was funded by Bayer AG.
Sources of Funding
The FIGARO-DKD trial was conducted and funded by Bayer AG. The funder participated in study design, data collection, data analysis, data interpretation, and approval of the article. Analyses were conducted by the sponsor, and all authors had access to and participated in the interpretation of the data.
Disclosures
G.F. reports that he is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor Pharma; he is a senior consulting editor for JACC Heart Failure; and has received research support from the European Union. S.D.A. has received research support from Abbott Vascular and Vifor International, and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier, and Vifor Pharma. R.A. reported personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals Inc during the conduct of the study; he also reported personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius, Janssen, Relypsa, Sanofi, and Vifor Pharma; he has received personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co, and Reata, and nonfinancial support from E.R. Squibb & Sons, Opko Pharmaceuticals, and Otsuka America Pharmaceutical. He is a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa; and adjudication committees for AbbVie, Bayer, Boehringer Ingelheim, and Janssen. He has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; and he has received research grants from the US Veterans Administration and the National Institutes of Health. L.M.R. reports receipt of consultancy fees from Bayer. P.R. reports personal fees from Bayer during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi, and Vifor; all fees are given to Steno Diabetes Center Copenhagen. G.L.B. reports research funding, paid to the University of Chicago Medicine, from Bayer during the conduct of the study; he also reported research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics; he acted as a consultant and received personal fees from for Alnylam, Merck, and Relypsa; he is an editor of the American Journal of Nephrology, Nephrology, and Hypertension, and section editor of UpToDate; and is an associate editor of Diabetes Care and Hypertension Research. P.K., C.T., A.J., and A.L. are full-time employees of Bayer AG, Division Pharmaceuticals, Germany. He is the coinventor of finerenone and holds US and European patents relating to finerenone (US8436180B2 and EP2132206B1). B.P. reports consultant fees for AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Proton Intel, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; he has stock options for KBP Biosciences, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, Proton Intel, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent no. 9931412) and a provisional patent for histone acetylation–modulating agents for the treatment and prevention of organ injury (provisional US patent no. 63/045,784).
Supplemental Material
Supplemental Methods
Criteria for Heart Failure Hospitalization Events and New-Onset Heart Failure
Additional Outcomes Analyzed
Supplemental Results
Effect of Finerenone on Cardiovascular Outcomes by History of Heart Failure
Tables S1–S3
Figures S1–S5
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AE
- adverse event
- eGFR
- estimated glomerular filtration rate
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- HFrEF
- heart failure with reduced ejection fraction
- HHF
- hospitalization for heart failure
- HR
- hazard ratio
- MRA
- mineralocorticoid receptor antagonist
- SGLT-2i
- sodium-glucose cotransporter-2 inhibitor
- T2D
- type 2 diabetes
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.057983.
For Sources of Funding and Disclosures, see page 446.
Contributor Information
Stefan D. Anker, Email: s.anker@cachexia.de.
Rajiv Agarwal, Email: ragarwal@iupui.edu.
Luis M. Ruilope, Email: ruilope@icloud.com.
Peter Rossing, Email: peter.kolkhof@bayer.com.
George L. Bakris, Email: gbakris@gmail.com.
Christoph Tasto, Email: christoph.tasto@bayer.com.
Amer Joseph, Email: amer.joseph@bayer.com.
Peter Kolkhof, Email: peter.kolkhof@bayer.com.
Andrea Lage, Email: andrea.lage@bayer.com.
Bertram Pitt, Email: bpitt@umich.edu.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, et al. Writing Committee Members. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System. Chapter 6: Healthcare expenditures for persons with CKD. USRDS Annual data report. Volume 1: Chronic kidney disease. 2020. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Available at: https://adr.usrds.org/2020/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd. Accessed July 1, 2021. [Google Scholar]
- 4.Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–872. doi: 10.1002/ejhf.1170 [DOI] [PubMed] [Google Scholar]
- 5.Triposkiadis F, Xanthopoulos A, Bargiota A, Kitai T, Katsiki N, Farmakis D, Skoularigis J, Starling RC, Iliodromitis E. Diabetes mellitus and heart failure. J Clin Med. 2021;10:3682. doi: 10.3390/jcm10163682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippatos G, Farmakis D, Parissis J. Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J. 2014;35:416–418. doi: 10.1093/eurheartj/eht515 [DOI] [PubMed] [Google Scholar]
- 7.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–1444. doi: 10.1093/eurheartj/ehv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson CA, Seidu S, Zaccardi F, McCann G, Kadam UT, Davies MJ, Lam CS, Heerspink HL, Khunti K. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine. 2021;32:100739. doi: 10.1016/j.eclinm.2021.100739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel PA, Liang L, Khazanie P, Hammill BG, Fonarow GC, Yancy CW, Bhatt DL, Curtis LH, Hernandez AF. Antihyperglycemic medication use among Medicare beneficiaries with heart failure, diabetes mellitus, and chronic kidney disease. Circ Heart Fail. 2016;9:e002638. doi: 10.1161/CIRCHEARTFAILURE.115.002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care. 2016;39(suppl 2):S165–S171. doi: 10.2337/dcS15-3006 [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. ; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. ; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 13.Chu L, Fuller M, Jervis K, Ciaccia A, Abitbol A. Prevalence of chronic kidney disease in type 2 diabetes: The Canadian Registry of Chronic Kidney Disease in Diabetes Outcomes (CREDO) study. Clin Ther. 2021. doi: 10.1016/j.clinthera.2021.07.015 [DOI] [PubMed] [Google Scholar]
- 14.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, et al. ; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, et al. ; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
- 16.Ruilope LM, Agarwal R, Anker SD, Bakris GL, Filippatos G, Nowack C, Kolkhof P, Joseph A, Mentenich N, Pitt B; FIGARO-DKD Study Investigators. Design and baseline characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial. Am J Nephrol. 2019;50:345–356. doi: 10.1159/000503712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, Banerjee A, Thuresson M, Okami S, Garal-Pantaler E, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: A large multinational cohort study. Diabetes Obes Metab. 2020;22:1607–1618. doi: 10.1111/dom.14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zareini B, Blanche P, D’Souza M, Elmegaard Malik M, Nørgaard CH, Selmer C, Gislason G, Kristensen SL, Køber L, Torp-Pedersen C, et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases: a nationwide study. Circ Cardiovasc Qual Outcomes. 2020;13:e006260. doi: 10.1161/CIRCOUTCOMES.119.006260 [DOI] [PubMed] [Google Scholar]
- 19.Urbich M, Globe G, Pantiri K, Heisen M, Bennison C, Wirtz HS, Di Tanna GL. A systematic review of medical costs associated with heart failure in the USA (2014–2020). Pharmacoeconomics. 2020;38:1219–1236. doi: 10.1007/s40273-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deswal A. Heart failure with reduced ejection fraction and renal dysfunction: beta-blockers do not disappoint. J Am Coll Cardiol. 2019;74:2905–2907. doi: 10.1016/j.jacc.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 21.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, et al. ; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 22.Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–944. doi: 10.1161/CIRCULATIONAHA.117.028814 [DOI] [PubMed] [Google Scholar]
- 25.Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–598. doi: 10.1002/ejhf.497 [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–161. doi: 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam CS. Diabetic cardiomyopathy: an expression of stage B heart failure with preserved ejection fraction. Diab Vasc Dis Res. 2015;12:234–238. doi: 10.1177/1479164115579006 [DOI] [PubMed] [Google Scholar]
- 28.Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27:387–412. doi: 10.1016/j.cardfail.2021.01.022 [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. ; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 31.Xiang Y, Shi W, Li Z, Yang Y, Wang SY, Xiang R, Feng P, Wen L, Huang W. Efficacy and safety of spironolactone in the heart failure with mid-range ejection fraction and heart failure with preserved ejection fraction: a meta-analysis of randomized clinical trials. Medicine (Baltimore). 2019;98:e14967. doi: 10.1097/MD.0000000000014967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayer. Study to evaluate the efficacy (effect on disease) and safety of finerenone on morbidity (events indicating disease worsening) and mortality (death rate) in participants with heart failure and left ventricular ejection fraction (proportion of blood expelled per heart stroke) greater or equal to 40% (FINEARTS-HF). 2020. Accessed November 5, 2022. https://clinicaltrials.gov/ct2/show/NCT04435626
- 33.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 35.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 36.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 37.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa100949221073363 [Google Scholar]
- 38.Cooper LB, Lippmann SJ, Greiner MA, Sharma A, Kelly JP, Fonarow GC, Yancy CW, Heidenreich PA, Hernandez AF. Use of mineralocorticoid receptor antagonists in patients with heart failure and comorbid diabetes mellitus or chronic kidney disease. J Am Heart Assoc. 2017;6:e006540. doi: 10.1161/JAHA.117.006540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.