Abstract
INTRODUCTION:
In patients with familial adenomatous polyposis, the Spigelman classification is recommended for staging and risk stratification of duodenal adenomatosis. Although the classification has been used for decades, it has never been formally validated.
METHODS:
We included consecutive FAP patients undergoing upper gastrointestinal endoscopic surveillance and evaluated the inter- and intrarater reliability of the Spigelman classification.
RESULTS:
The interrater reliability of the endoscopic parameters and the Spigelman classification was good and excellent, respectively. The intrarater reliability of the endoscopic parameters and the Spigelman classification was moderate and good, respectively.
DISCUSSION:
The results support continued use of the Spigelman classification as the primary end point for future studies and as key endoscopic performance measure.
INTRODUCTION
Familial adenomatous polyposis (FAP) is a hereditary disorder with a high prevalence of duodenal adenomatosis (1,2). In 1989, the Spigelman classification was introduced to assess the severity of duodenal polyposis and stratify patients according to their risk of duodenal cancer (3). While its correlation with cancer has been explored in several studies, a systematic validation of the reproducibility of the Spigelman classification has not been performed (4,5). Furthermore, the classification tends to underestimate the importance of adenomatous lesions at the major papilla (6). A conservative strategy is often recommended for small adenomatous lesions at the major papilla, with the threshold for endoscopic therapy often set at their being 10 mm in diameter, with high-grade dysplasia or villous morphology (7–9).
The primary aim of this study was to assess the interrater and intrarater reliability of the endoscopic assessment of the Spigelman classification conducted by experienced endoscopists. We also wanted to evaluate the endoscopic interrater and intrarater reliability in assessments of adenomatous transformation of the major papilla, including the size of the lesions.
METHODS AND MATERIALS
In this prospective study, we included consecutive patients with FAP from Hvidovre Hospital undergoing upper gastrointestinal endoscopic surveillance. The procedures were performed with state-of-the-art endoscopes (GIF-HQ190/GIF-1TH190/TJF-Q180V/TJF-Q190V, Olympus Medical Systems, Tokyo, Japan) without the use of caps and included biopsy sampling. The major papilla was, however, only biopsied if deemed necessary by the endoscopist. Choice of endoscopic modality was at the discretion of the endoscopists, but a sufficient visualization of the major papilla with white light was always included. To evaluate the interrater reliability (interclass correlation coefficient [ICC]) of the endoscopic assessment, all procedures were recorded, and the unedited videos were subsequently assessed according to the Spigelman classification by 5 endoscopists, all having long-term (>5 years) experience in treatment and surveillance of patients with FAP (Figure 1). To estimate the intrarater reliability, the assessments were repeated after 1 week. While evaluation of the Spigelman classification was defined before the study began, the separate study of the major papilla occurred afterward. The raters were asked, first, whether they could identify the major papilla and, if so, whether they found adenomatous tissue to be present and, if they did, whether the lesion was smaller or larger than 10 mm (Figure 1). The necessary ethical approval/clearance was obtained. The study was registered at clinicaltrial. gov (NCT03346980).
Figure 1.
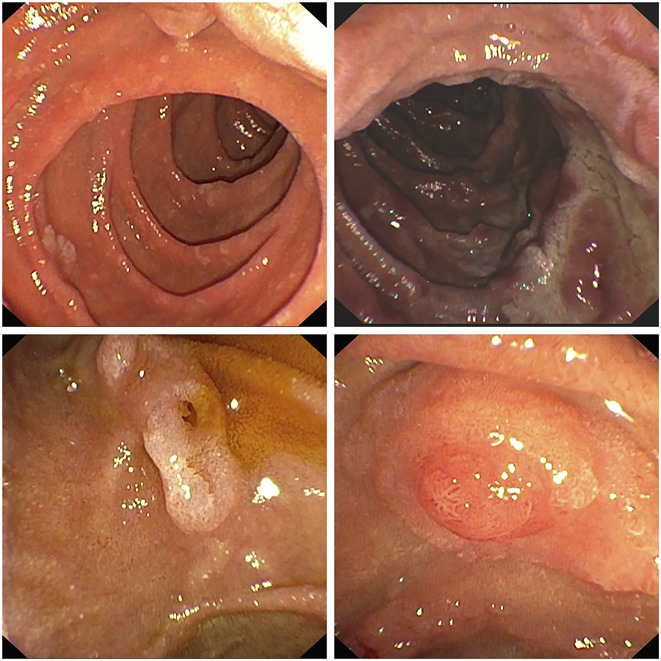
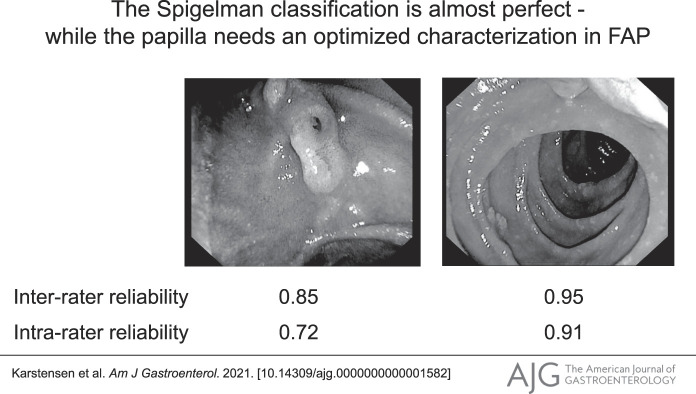
The upper images show the duodenum of 2 familial adenomatous polyposis (FAP) patients with duodenal polyposis of differing severity. The lower images demonstrate papillary adenomatous involvement, which is obvious on the left but could prove challenging to ascertain on the right.
Severity of duodenal adenomatosis was estimated using the Spigelman classification, which is a composite score of 2 endoscopic parameters (polyp number and maximum size of polyps) and 2 histopathological parameters (morphology and grade of dysplasia). The score ranges from 0 to 12, and it enables classification into 4 Spigelman stages (3).
Statistics
The sample size was calculated for the interrater reliability of the Spigelman classification according to there being 3 raters. To estimate an expected ICC of 0.9 being significantly higher than an ICC of 0.8, with power of 0.8 and a significance level of 0.05, 33 patients needed to be enrolled in the study (10). To estimate interrater reliability, the ICC (6) was applied using a 2-way random-effect model, absolute agreement, and average measures (11). For the intrarater reliability study, the mean of the ICC was estimated using 2-way mixed-effect model, absolute agreement, and average measures. ICC values were interpreted according to Portney and Watkins (12).
RESULTS
Between December 2017 and January 2020, 34 patients with FAP from 27 families were enrolled in the study. The mean age was 43.6 years (SD 17.7), and 11 (32%) patients were female individuals. The mean time of the procedures was 26.9 minutes (SD 9.6), and the endoscopic modality was side-viewing duodenoscope in most (91%) cases (see Supplementary Material, http://links.lww.com/AJG/C331).
Interrater and intrarater reliability of the Spigelman classification
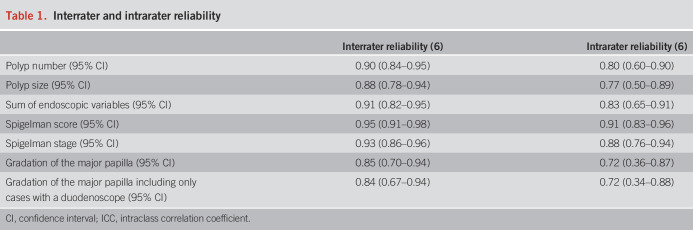
The interrater reliability of the Spigelman classification was determined to be excellent, with an ICC value of 0.95 (95% confidence interval 0.91–0.98) (Table 1). The Spigelman stages are used to stratify patients for the appropriate intervals between surveillances, and we found that the Spigelman stage had an excellent interrater reliability of 0.93 (0.86–0.96). The intrarater reliability of the Spigelman classification was estimated to be 0.91 (95% confidence interval 0.83–0.96), whereas the intrarater reliability of the Spigelman stages was good, with an ICC of 0.88 (0.76–0.94). The interrater reliability (6) of assessments of the presence and size of adenomatous tissue at the major papilla (no adenoma vs adenoma smaller than 10 mm vs adenoma 10 mm or larger) was 0.85 (0.70–0.94), which was indicative of moderate reliability. When assessing the intrarater reliability for the same parameter, the ICC was 0.72 (0.36–0.87). Excluding cases where the duodenoscope was omitted did not alter these results.
Table 1.
Interrater and intrarater reliability
DISCUSSION
In this prospective validation study of consecutive patients with FAP, we found the interrater reliability of the Spigelman classification was excellent, whereas the interrater reliability was good for the Spigelman stage. For 3 decades, the Spigelman classification has been used in patient care and in the international literature for grading duodenal polyposis in patients with FAP (13). The validation we present in this study supports its continued use for risk stratification of patients with FAP and duodenal adenomatosis and emphasize that in a multicenter setting, the Spigelman classification is appropriate as a primary outcome.
The Spigelman classification has often been criticized for underestimating the importance of adenomatous transformation of the major papilla. We conducted a simple endoscopic evaluation of the papilla and found that it had only a moderate interrater reliability and a poor intrarater reliability. Therefore, before an endoscopic evaluation of adenomatous tissue at the major papilla is included in any future modifications of the Spigelman classification, improved endoscopic reproducibility of the system ought to be demonstrated.
There are 2 limitations in this study. First, only a minority of the patients had histopathologically severe duodenal adenomatosis with high-grade dysplasia or villous morphology. However, the main focus of the study was the reproducibility of the endoscopic variables, and the distribution of these was representative of FAP in every phase. Second, the videos were completely unedited, which meant that the number and targets of the biopsy specimens were also included, which might have biased the endoscopists' evaluations, albeit slightly.
In conclusion, our evaluation of the Spigelman classification has shown it to have excellent reliability. Therefore, it should continue to serve as a primary end point for future studies and as a key performance measure during routine endoscopy in patients with FAP.
CONFLICTS OF INTEREST
Guarantor of the article: John Gásdal Karstensen, MD, PhD.
Specific author contributions: J.G.K., S.B., J.B., H.C.P. and P.N.S. designed and planned the study. J.G.K., S.B., M.B.E., M.A.O., and P.N.S. conducted a study. J.G.K. drafted the manuscript. All authors critically revised the manuscript and approved its final form.
Financial support: None to report.
Potential competing interests: None to report.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C331
Contributor Information
John Gásdal Karstensen, Email: john.gasdal.karstensen@regionh.dk.
Steffen Bülow, Email: steffen.bulow@gmail.com.
Johan Burisch, Email: burisch@gmail.com.
Mark Bremholm Ellebæk, Email: mark.ellebaek1@rsyd.dk.
Marcin Ostapiuk, Email: marost@rn.dk.
Hans Christian Pommergaard, Email: hcpommergaard@gmail.com.
Palle Nordblad Schmidt, Email: Palle.Nordblad.Schmidt@regionh.dk.
REFERENCES
- 1.Ghorbanoghli Z, Bastiaansen BA, Langers AM, et al. Extracolonic cancer risk in Dutch patients with APC (adenomatous polyposis coli)-associated polyposis. J Med Genet 2018;55:11–4. [DOI] [PubMed] [Google Scholar]
- 2.Karstensen JG, Burisch J, Pommergaard HC, et al. Colorectal cancer in individuals with familial adenomatous polyposis, based on analysis of the Danish polyposis registry. Clin Gastroenterol Hepatol 2019;17:2294–300.e1. [DOI] [PubMed] [Google Scholar]
- 3.Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;2:783–5. [DOI] [PubMed] [Google Scholar]
- 4.Groves CJ, Saunders BP, Spigelman AD, et al. Duodenal cancer in patients with familial adenomatous polyposis (FAP): Results of a 10 year prospective study. Gut 2002;50:636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bülow S, Björk J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut 2004;53:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Leerdam ME, Roos VH, van Hooft JE, et al. Endoscopic management of polyposis syndromes: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 2019;51:877–95. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwagi H, Spigelman AD, Debinski HS, et al. Surveillance of ampullary adenomas in familial adenomatous polyposis. Lancet 1994;344:1582. [DOI] [PubMed] [Google Scholar]
- 8.Thiruvengadam SS, Lopez R, O'Malley M, et al. Spigelman stage IV duodenal polyposis does not precede most duodenal cancer cases in patients with familial adenomatous polyposis. Gastrointest Endosc 2019;89:345–54.e2. [DOI] [PubMed] [Google Scholar]
- 9.Latchford AR, Neale KF, Spigelman AD, et al. Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 2009;7:659–63. [DOI] [PubMed] [Google Scholar]
- 10.Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Stat Med 2002;21:1331–5. [DOI] [PubMed] [Google Scholar]
- 11.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portney LGWM. Foundations of Clinical Research: Applications to Practice. New Jersey: Prentice Hall. 2000. [Google Scholar]
- 13.Bülow S. Results of national registration of familial adenomatous polyposis. Gut 2003;52:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.