Abstract
INTRODUCTION:
The disease burden of nonalcoholic fatty liver disease (NAFLD) increases rapidly, in line with the obesity pandemic. Physical activity has been linked to a lower risk of NAFLD. However, the impact of different intensities of activity and sedentary behavior and whether their effects on NAFLD are explained by metabolic health remain unclear.
METHODS:
We performed cross-sectional analyses within the population-based Rotterdam Study cohort. Abdominal ultrasound and accelerometry data were collected between 2009 and 2014. NAFLD was defined as hepatic steatosis diagnosed by ultrasound, in the absence of secondary causes for steatosis: viral hepatitis, steatogenic drugs, and excessive alcohol. We categorized accelerometry data into sedentary time and light, moderate, and vigorous physical activities.
RESULTS:
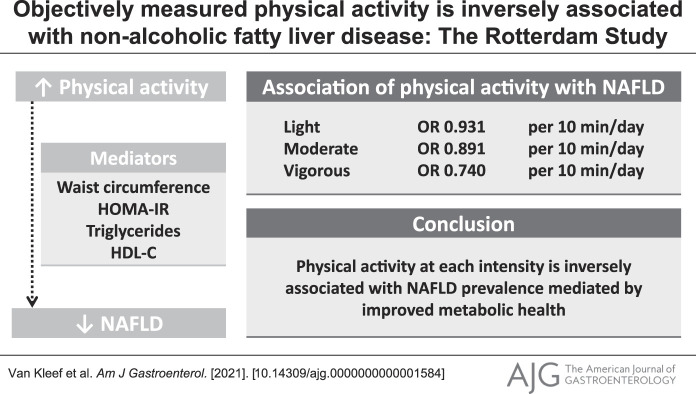
We included 667 participants (aged 63.3 ± 6.3 years, 53% female individuals), and 34.3% had NAFLD. Total physical activity was associated with lower NAFLD prevalence adjusted for demographic, lifestyle, and socioeconomic factors (odds ratio: 0.958 per 10 min/d, 95% confidence interval [CI]: 0.929–0.986). More intensive physical activity was more strongly associated with lower NAFLD prevalence: odds ratios for light, moderate, and vigorous physical activities were 0.931 (95% CI: 0.882–0.982), 0.891 (95% CI: 0.820–0.967), and 0.740 (95% CI: 0.600–0.906) per 10 min/d, respectively. These associations were explained by metabolic health, particularly homeostatic model assessment of insulin resistance (proportion mediated: 0.59, P < 0.001) and waist circumference (proportion mediated: 1.08, P < 0.001). Beyond this indirect effect, no direct effect could be demonstrated (P = 0.282–0.827).
DISCUSSION:
Physical activity at each intensity is inversely associated with NAFLD prevalence, with larger effects for higher intensities of physical activity. This association is mediated by better metabolic health, mainly lower insulin resistance and waist circumference. Physical activity should therefore be incorporated into NAFLD disease management and prevention programs.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has become the most prevalent chronic liver disease in the western world and is associated with severe hepatic and extrahepatic comorbidities and mortality (1,2). Moreover, the disease burden of NAFLD is expected to further increase in the following decades because of the rapid increase in adiposity and metabolic syndrome (3,4). Weight loss and improvements in metabolic health are important targets in prevention and disease management; in fact, NAFLD and even fibrosis can regress if 5% weight reduction is accomplished (5,6). These beneficial effects are driven by improved insulin resistance, stimulation of fat metabolism, and increased mitochondrial function (5,7). Many studies have therefore investigated the potential of dietary intake and composition in steatosis regression (5,8). In addition, previous studies demonstrated a beneficial association between physical activity and NAFLD to some extent (9–14). However, specific advice on physical activity duration and intensity is lacking.
Most studies to date that have been investigating the association between physical activity and NAFLD were hampered by several challenges. First, physical activity measurement was often self-reported. This limitation might be resolved by the recent progression in technology that has resulted in compact devices, which can accurately measure physical activity time and intensity over longer periods on a large scale (15). This type of continuous activity tracking is an objective approach and not subject to recall bias and differs significantly from conventional, self-reported information (16). Second, using serological algorithms instead of imaging to define steatosis is of concern because it lacks sensitivity and specificity. Third, these algorithms often include parameters (i.e., body mass index, waist circumference, fasting glucose, and high-density lipoprotein cholesterol [HDL-C]), which are directly linked with physical activity, which may therefore be mediators or confounders (17,18). Because such parameters are part of the algorithm, adjusting for and exploring the role of those variables in the association between physical activity and NAFLD is impossible, even in case of sufficient sample size and extensive data available. Therefore, it remains unclear whether physical activity is directly associated with NAFLD (11) or is effectuated by improvements in body composition and metabolic health (12).
In this study, we investigated the association between objectively measured physical activity and ultrasound-based NAFLD with emphasis on different intensities of physical activity, sedentary behavior, and the impact of metabolic health in these associations.
PATIENTS AND METHODS
Study design and population
This is a cross-sectional study within the Rotterdam Study, an ongoing prospective population-based cohort study. All citizens aged 45 years and older living in Ommoord, a suburb in Rotterdam, were eligible to participate and repeatedly invited for study visits. Further details about the Rotterdam Study have been described in detail elsewhere (19). The present study comprised all participants who visited the study site between March 2009 and June 2014 and participated in both the abdominal ultrasound and physical activity monitoring program. Exclusion criteria were secondary causes of liver steatosis, comprising excessive alcohol consumption, steatogenic drug use, and hepatitis B or C (20). In addition, participants were excluded if excessive alcohol intake could not be ruled out based on interview data and if food frequency questionnaire (FFQ) data were unavailable.
NAFLD diagnosis
Abdominal sonography was performed by a single experienced sonographer (Paulien van Wijngaarden) using a Hitachi Hi Vision 900. Hepatic steatosis was based on hyperechogenic liver parenchyma compared with the kidney cortex or spleen, according to Hamaguchi et al. (21), and was reassessed by an experienced hepatologist in abdominal ultrasound on request. Because secondary causes for steatosis were already excluded, NAFLD was diagnosed when liver steatosis was present.
Physical activity
A triaxial accelerometer (GeneActiv; Activinsights Ltd., Kimbolton UK) was used to assess physical activity duration, physical activity intensity, and sedentary time. The mean time between abdominal ultrasound and physical activity assessment was 40 days, and >90% underwent physical activity assessment within 3 months after the abdominal ultrasound. The participants were requested to wear the device continuously on the nondominant wrist for 1 week. Physical activity was measured relative to gravity (1 mg = 9.81 mm/sec2) with an interval of 20 ms and categorized based on intensity, according to White et al.(22), into time spent in sedentary behavior (<48 mg) and in light (48–154 mg, e.g., walking), moderate (154–389 mg, e.g., cycling), and vigorous activities (>389 mg e.g., running). Technical and statistical procedures have been described in detail earlier (16).
Covariates
At the study location, research assistants measured anthropometrics, which included waist circumference. Trained interviewers administered questionnaires at the participants' home to ensure completion and correct interpretation. Alcohol intake frequency and quantity were assessed during a home interview and with a validated self-administered FFQ. Excessive alcohol use was defined as >30 g/d for male individuals and >20 g/d for female individuals based on FFQ or interview data (20). Based on the same FFQ, coffee consumption, total caloric intake, and an overall diet quality score were calculated (23). Medication use was based on a digital linkage with the pharmacy of the participant. Systemic corticosteroids, amiodarone, methotrexate, and tamoxifen were defined as steatogenic drugs.
During fasting state, blood samples were collected from the participants. Glucose, HDL-C, triglycerides (TG), aspartate aminotransaminase, alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase were analyzed by automatic enzyme procedures, and insulin was analyzed with automatic immunoassay (Roche, Diagnostic GmbH, Mannheim, Germany). Homeostatic model assessment of insulin resistance (HOMA-IR) was based on glucose and insulin levels. Viral hepatitis was determined on hepatitis B surface antigen and anti-hepatitis C, analyzed by automatic immunoassay (Roche Diagnostic GmbH, Mannheim, Germany).
Metabolic factors were included both continuously and dichotomized according to the definition of the metabolic syndrome, following the Adult Treatment Panel III criteria (24): (i) fasting glucose > 5.6 mmol/L and/or antidiabetic drug use; (ii) waist circumference > 102 cm (male individuals) or >88 cm (female individuals); (iii) TG ≥ 1.7 mmol/L and/or lipid-lowering drug use; (iv) HDL-C < 1.04 mmol/L in female individuals or < 1.30 mmol/L in male individuals and/or lipid-lowering drug use; and (5) hypertension based on a systolic blood pressure(SBP) ≥ 130 mm Hg, diastolic blood pressure ≥ 85 mm Hg, and/or antihypertensive drug use.
Statistical analysis
The study sample was characterized using descriptive statistics. Associations between physical activity (per 10 min/d) and NAFLD (yes/no) were studied with logistic regression analyses. As independent variables, we separately studied the following: total physical activity; light, moderate and vigorous physical activities; and sedentary time. Potential confounders and mediators were visualized in a directed acyclic graph (see Figure, Supplementary Digital Content 1, http://links.lww.com/AJG/C332). We adjusted for demographics (age and sex; model 1) and lifestyle and socioeconomic status (smoking, education and daily alcohol consumption; model 2). In a third model, we assessed whether the association of physical activity and NAFLD was explained by metabolic health by additionally including all the components of the metabolic syndrome ([pre]diabetes, high waist circumference, abnormal TG, abnormal HDL-C, and hypertension; model 3). In additional analysis, model 2 was also adjusted for caloric intake, diet quality score, and coffee consumption.
To assess which of the metabolic health components contributed most, the subcriteria of the metabolic syndrome were added individually to model 2, 1 at a time, instead of all combined. For this additional analysis, we used both the categorical metabolic syndrome components (yes/no) and continuous measures of metabolic health, comprising SBP, TG, HDL-C, HOMA-IR, and waist circumference standardized for the upper limit of normal (ULN; 88 cm for female individuals and 102 cm for male individuals).
For one can debate whether the metabolic factors are only confounders or actually mediators, we performed a mediation analysis using the Lavaan package 0.6–9 to assess further the role of metabolic health in the pathway between physical activity and NAFLD. Investigated mediators were the individual continuous parameters of metabolic health: SBP, TG, HDL-C, HOMA-IR, and standardized waist circumference. These factors were included 1 at a time, and outcome of interest was the proportion of the total effect mediated by the particular metabolic health parameter.
To further investigate the associations of different intensities of physical activity with NAFLD, we analyzed them using a stepwise approach. First, we analyzed vigorous physical activity as an independent variable, and then, we added moderate physical activity (moderate-to-vigorous physical activity) and, finally, light physical activity (total physical activity).
Natural cubic splines were used to study nonlinear effects of physical activity variables with the outcome with the Splines package 4.0.2. All analyses were performed in R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). P values of <0.05 were considered statistically significant.
Ethics: The Rotterdam Study has been approved by the Medical Ethics Committee of Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare, and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. The Rotterdam Study has been entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalog number NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Patient characteristics
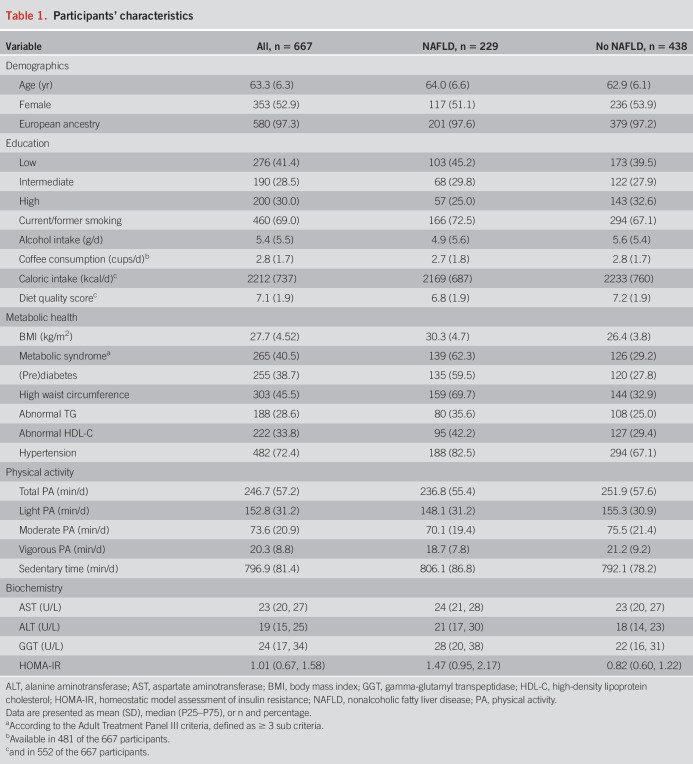
Of the 1,033 participants who participated in the GeneActiv program, 888 had valid accelerometry data and attended the abdominal ultrasound. Of this group, in total, 221 participants were excluded, 162 for the presence of secondary causes of steatosis and 59 for insufficient data on alcohol consumption, resulting in 667 participants for final analysis (Figure 1). Overall, the mean age of participants during the study visit was 63.3 years (SD 6.3), 53% was female individuals, and most of them were of European ancestry (97.3%). Total physical activity on average comprised 61.9% light, 29.8% moderate, and 8.2% vigorous activities. Metabolic comorbidity was common, resulting in a 40.5% prevalence of metabolic syndrome. Detailed information about the variables involved in the composition of the metabolic syndrome is provided in Supplementary Table 1, http://links.lww.com/AJG/C333. NAFLD was present in 229 participants (34.3%). These participants were on average older compared with the participants without NAFLD and had a higher prevalence of overweight, diabetes, hypertension, and lipid disorders (Table 1).
Figure 1.
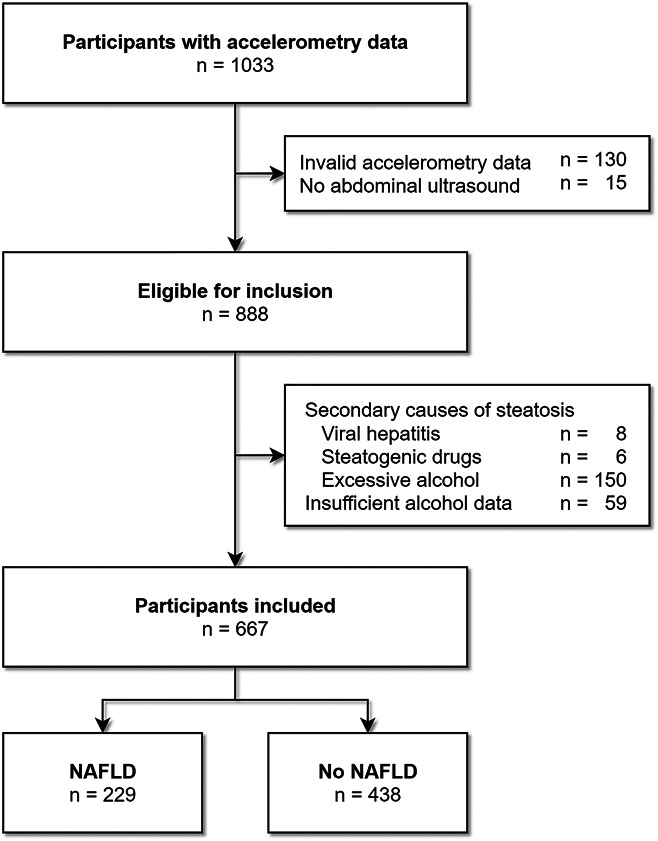
Flowchart of participant selection. Participants can have multiple exclusion criteria at once.
Table 1.
Participants' characteristics
Physical activity was associated with NAFLD
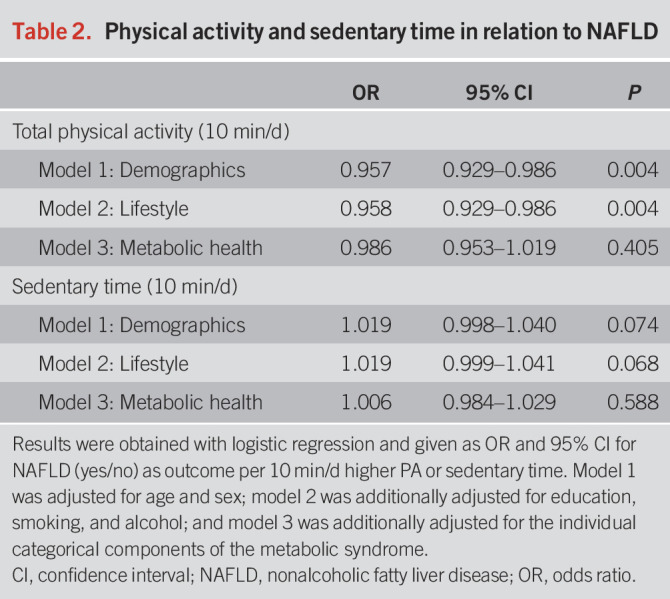
After adjustment for demographics (model 1), more physical activity was associated with a lower prevalence of NAFLD (odds ratio [OR]: 0.957, per 10 min/d, 95% confidence interval [CI]: 0.929–0.986, Table 2). No nonlinear effects for physical activity time with NAFLD were observed using natural cubic splines (df = 2, P = 0.38). Findings were consistent after additional adjustment for lifestyle and socioeconomic factors (model 2, OR 0.958, per 10 min/d, 95% CI: 0.929–0.986). Similar results were obtained when adjusting for caloric intake, diet quality score, and coffee consumption in addition to model 2 (OR 0.962, per 10 min/d 95% CI: 0.927–0.997), among a subset of participants with available dietary data (n = 480). For sedentary time, no statistically significant association was found after adjustment for covariates in model 2 (OR 1.019, per 10 min/d, 95% CI: 0.999–1.041).
Table 2.
Physical activity and sedentary time in relation to NAFLD
All intensities of physical activity were inversely associated with NAFLD
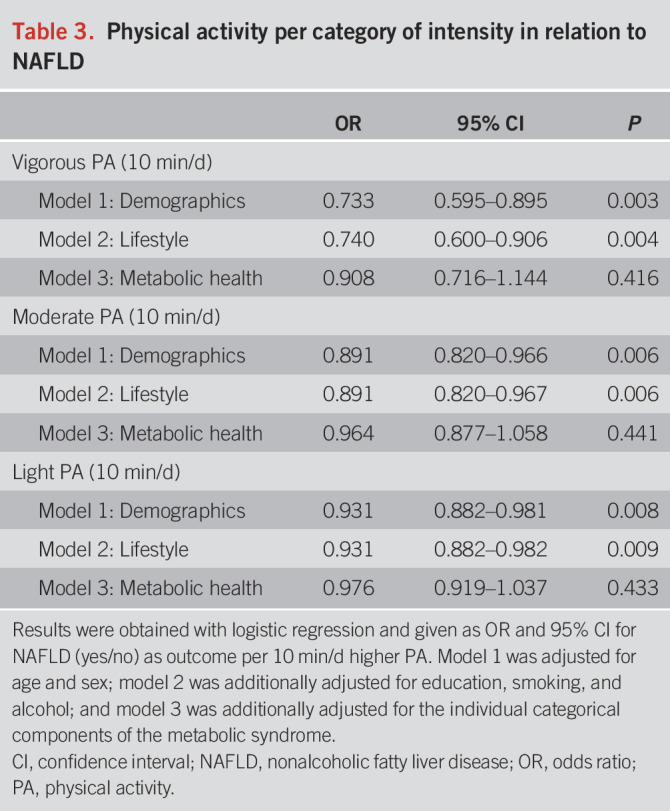
When we examined physical activity intensities separately, we observed that all levels of intensity studied were related to lower NAFLD risk (model 2). Generally, more intensive physical activity was more strongly associated with a lower prevalence of NAFLD (Table 3). Adjusted for demographics, lifestyle, and socioeconomic factors (model 2), we found an OR of 0.931 (95% CI: 0.822–0.982), 0.891 (95% CI: 0.820–0.966), and 0.740 (95% CI: 0.600–0.906) per 10 min/d for light, moderate, and vigorous activities, respectively.
Table 3.
Physical activity per category of intensity in relation to NAFLD
Similar trends for larger effects with higher levels of intensity were observed when different summed combinations of levels of intensity were analyzed; i.e., only vigorous activity (OR 0.740, 95% CI: 0.600–0.906), vigorous and moderate activities combined (OR 0.916, 95% CI: 0.861–0.972), light, moderate, and vigorous activities combined (total) (OR 0.958, 95% CI: 0.929–0.986) per 10 min/d (see Table, Supplementary Digital Content 2, http://links.lww.com/AJG/C334).
Metabolic health mediated the association between NAFLD and physical activity
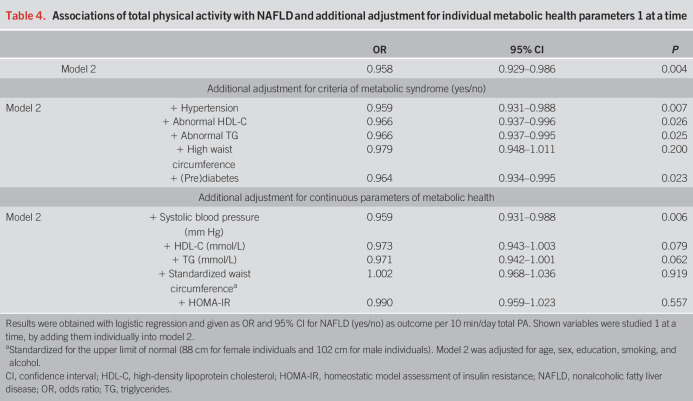
The association between total physical activity and NAFLD attenuated after adjusting for all the components of the metabolic syndrome (model 3, OR: 0.986, 95% CI 0.953–1.019, Table 2), per 10 min/d. Similar results were obtained for the individual intensities (Table 3). The metabolic syndrome components were also studied 1 at a time by adding them individually into model 2 (Table 4). The association between total physical activity and NAFLD attenuated most when adjusting for large waist circumference (OR: 0.979, 95% CI: 0.948–1.011) and prediabetes (OR 0.964, 95% CI: 0.934–0.995), per 10 min/d. When we adjusted the models for continuous measures of metabolic health, the attenuation became even more evident: HOMA-IR (OR 0.990, 95% CI: 0.968–1.036) and standardized waist circumference (OR: 1.002, 95% CI: 0.968–1.036), per 10 min/d.
Table 4.
Associations of total physical activity with NAFLD and additional adjustment for individual metabolic health parameters 1 at a time
Last, we assessed the impact of those continuous measures of metabolic health (SBP, TG, HDL-C, HOMA-IR, and standardized waist circumference) 1 at a time in mediation analysis. This revealed that the association of physical activity with NAFLD was mediated by metabolic health (Figure 2, see Table, Supplementary Digital Content 3, http://links.lww.com/AJG/C334). Likewise, our conventional logistic regression models, HOMA-IR (proportion mediated: 0.59, P < 0.001), and standardized waist circumference (proportion mediated: 1.08, P < 0.001) explained most of the observed effect. In this model, even a slight positive effect estimate was observed for the direct effect of physical activity in relation to NAFLD with waist circumference as mediator, resulting in a proportion-mediated effect exceeding 1.0. Beyond these indirect effects of HOMA-IR and waist circumference, no direct effect could be demonstrated (P = 0.282-0.827). SBP was the only investigated parameter not being a mediator in this association (proportion mediated: 0.03, P = 0.488).
Figure 2.
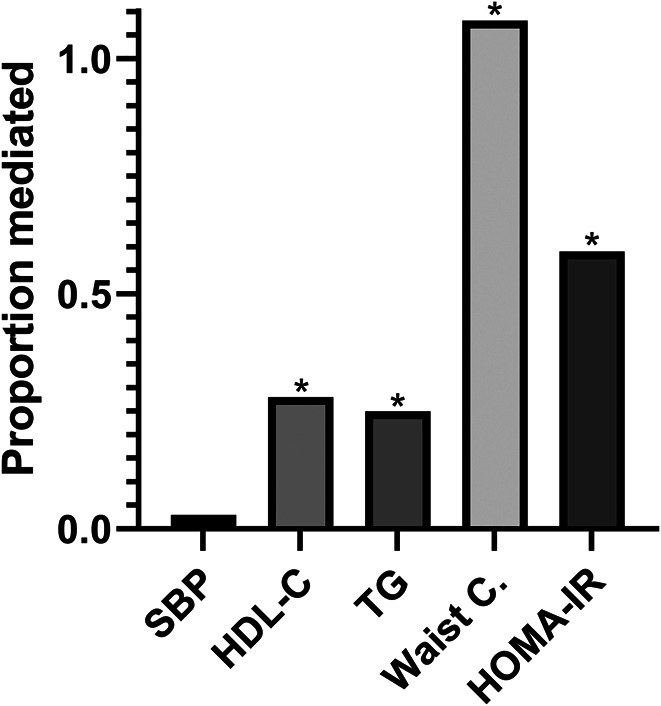
Proportion mediated of the total effect (OR 0.958 per 10 min total physical activity per day) for physical activity on nonalcoholic fatty liver disease (NAFLD). The shown variables were included 1 at a time in mediation analysis and were adjusted for age, sex, education, smoking, and alcohol consumption (model 2). In this analysis, even a slight positive effect estimate was observed for physical activity in relation to NAFLD with waist circumference as mediator, resulting in a proportion mediated of 1.08. *Indicates the particular parameter mediated a significant proportion of the total effect. Abbreviations: HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; SBP, systolic blood pressure; TG, triglycerides; waist C, waist circumference.
DISCUSSION
In this large population-based cohort study, physical activity measured with accelerometry was inversely associated with the presence of NAFLD, with larger effect sizes seen for more intensive activity. This association was mediated by improved metabolic traits, especially lower insulin resistance and waist circumference. More sedentary time was suggestively associated with higher NAFLD but did not reach statistical significance.
Previous studies have observed an association between total physical activity and lower NAFLD but were hampered by self-reported physical activity data, had no access to abdominal ultrasound, and/or lacked accurate confounder adjustment (9–14). We confirm this beneficial association of more physical activity with lower NAFLD in a large cohort with objective physical activity assessment, ultrasound-based NAFLD diagnosis, and adjustment for potential confounders. Furthermore, we add to the evidence that more intensive physical activity is more strongly associated with lower NAFLD prevalence. However, the beneficial effect was not only exclusively observed for vigorous activity but also in a lesser extent for moderate and light physical activities. We observed no significant association between sedentary time and NAFLD. To the best of our knowledge, this is the first study assessing different intensities of physical activity obtained with accelerometry in the association with NAFLD.
Studying the effect of intensity for this association is difficult because time spent in vigorous activity is undisputedly related to time spent in other intensities of physical activity. Therefore, the effect of vigorous activity could be reflected in the analysis investigating moderate and/or light activity alone. Hence, we did not only analyze the impact of different intensities per single category, but we additionally performed analyses with a composite physical activity duration, starting with vigorous activity alone and adding lower intensities resulting in moderate-to-vigorous activity and total physical activity. This analysis confirmed that compositions with overall more intensive activity resulted in a larger effect size.
Despite that vigorous activity was associated most strongly with NAFLD, additional benefits were shown for light and moderate physical activities, which confirm previous studies assessing nonvigorous activity and NAFLD. For example, George et al. (11) showed that a lifestyle intervention focusing on low-to-moderate intensity physical activity improved metabolic health in patients with chronic liver disease. Moreover, benefits were found in other studies for nonvigorous physical activity regarding ALT, intrahepatic TG, glucose management, weight, cardiovascular risk, and/or mortality, which are (or share) important risk factors of NAFLD (18,25–28). Vigorous activity is not only beneficial but also less intensive forms of exercise are especially relevant for individuals currently not physically active or those who might not be able to exercise vigorously.
Physical activity is known to improve metabolic health and reduce mortality, with stronger associations found for more intensive physical activity (26–28). For example, improved insulin resistance is a major benefit and effectuated by energy consumption during the activity. This, in turn, aids in achieving or maintaining a healthy body composition, which is a key factor in insulin sensitivity (29). Another consequence of physical activity is the enhancement of fatty acid metabolism in muscle tissue and increased muscle mass, resulting in an enhanced basal metabolic rate, which could not be achieved by dietary restrictions alone (17,29). For the metabolic syndrome, those effects are mainly reflected in (pre)diabetes and waist circumference. In our study, these 2 factors contributed most to the association between physical activity and NAFLD in both conventional and mediation analyses. In several other studies, the associations of physical activity with NAFLD attenuated as well after adjusting for (changes in) anthropometrics and glucose management (9,30,31). Improvements of liver enzymes have been demonstrated by physical activity also in the absence of weight loss, but this was not adjusted for glucose management (11,32). Thus, these results support that the association of physical activity with liver health is not only a matter of weight or waist circumference but is also affected and mediated by other metabolic health parameters, particularly glucose management.
Evidence for the effects of physical activity at different intensities on NAFLD is relevant to improve current guidelines for disease management and prevention programs. For example, in a recent intervention study among patients with NAFLD, 3 individually tailored counseling sessions to increase low-to-moderate physical activity resulted in a 1 hr/wk increase of physical activity in more than 60% of the participants and improved glucose management and ALT levels (11). Additional studies are needed to investigate lasting effects, NAFLD regression rates, impact on fibrosis or liver stiffness, feasibility of implementation on a large scale in NAFLD patients, and how more vigorous activity could be incorporated best. However, supported by our results, it seems feasible with a small intervention to achieve health benefits in patients with NAFLD focusing on physical activity.
Although this is one of the largest studies with accelerometric-based physical activity data and NAFLD diagnosis based on imaging, there are some limitations. First, this study had a cross-sectional design and the causality of relationships could therefore not be studied. For example, changes in physical activity habits to achieve weight loss in obese participants could have mitigated the associations. Second, although we adjusted for an extensive set of confounders, covering demographics, lifestyle, and metabolic health, residual confounding cannot be ruled out. Further studies should investigate potential interaction between diet, physical activity, and NAFLD. Third, despite being an objective measurement and not prone to recall bias, accelerometry is yet not able to accurately recognize different types of exercise. Therefore, we were unable to investigate the role of resistance compared with aerobic physical activity. Fourth, despite that abdominal ultrasound is the most used diagnostic tool to assess steatosis, it is not the gold standard and lacks sensitivity, particularly for the detection of mild steatosis, compared with liver biopsy (33). However, it is unethical to perform liver biopsy in healthy participants because it is an invasive intervention and prone to severe complications.
In conclusion, we have demonstrated that physical activity is associated with a lower prevalence of NAFLD, which was mediated by better metabolic health, particularly lower waist circumference and better glucose management. Although we observed strongest associations for vigorous physical activity, additional benefits were objectified for both moderate and light physical activities. This is especially relevant for those unable to reach vigorous physical activity and indicates that increasing time spent in lower intensities of physical activity may already be beneficial in achieving or maintaining good liver health. We recommend incorporating physical activity to its full extent in NAFLD disease management and prevention.
CONFLICTS OF INTEREST
Guarantor of the article: Robert J. de Knegt, MD, PhD.
Author contributions: L.v.K., A.H., T.V. and R.d.K. conceived the study. L.v.K. and A.H. performed statistical analysis, which was approved and interpreted by L.v.K., A.H., T.V. and R.d.K., L.v.K. and A.H. wrote the manuscript. L.v.K., A.H., T.V. and R.d.K. reviewed and approved the final manuscript.
Financial support: The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, the Netherlands Organization for the Health Research and Development (ZonMw), the Research institute for Diseases in the Elderly (Ride), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. Financial support was also provided by the Foundation for Liver and Gastrointestinal Research, Rotterdam, the Netherlands. The funding sources were not involved in study design, data collection, analysis and interpretation of the data, or the writing of the report or decision to submit for publication.
Potential competing interests: R.d.K. is a speaker for Echosens, consultant for AbbVie and received grants from AbbVie, Gilead, and Janssen. The remaining authors reported no relevant conflicts.
Data transparency statement: Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contribution of the participants of the Rotterdam Study, research assistants (particularly Paulien van Wijngaarden for performing the liver ultrasounds), the general practitioners, hospitals, and pharmacies in Rotterdam and Nicole Erler for providing statistical assistance.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C332, http://links.lww.com/AJG/C333, http://links.lww.com/AJG/C334, http://links.lww.com/AJG/C335
Contributor Information
Laurens A. van Kleef, Email: l.vankleef@erasmusmc.nl.
Amy Hofman, Email: amy.hofman@erasmusmc.nl.
Trudy Voortman, Email: trudy.voortman@erasmusmc.nl.
Robert J. de Knegt, Email: r.deknegt@erasmusmc.nl.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Scorletti E, Mosca A, et al. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020;111s:154170. [DOI] [PubMed] [Google Scholar]
- 3.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol 2017;960:1–17. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15(1):11–20. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67(4):829–46. [DOI] [PubMed] [Google Scholar]
- 6.Koutoukidis DA, Koshiaris C, Henry JA, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2021;115:154455. [DOI] [PubMed] [Google Scholar]
- 7.Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: Novel mechanisms and treatment strategies. Trends Endocrinol Metab 2017;28(4):250–60. [DOI] [PubMed] [Google Scholar]
- 8.Alferink LJ, Kiefte-de Jong JC, Erler NS, et al. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: The Rotterdam study. Gut 2019;68(6):1088–98. [DOI] [PubMed] [Google Scholar]
- 9.Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: A population-based study. Aliment Pharmacol Ther 2012;36(8):772–81. [DOI] [PubMed] [Google Scholar]
- 10.Joo JH, Kim HJ, Park EC, et al. Association between sitting time and non-alcoholic fatty live disease in South Korean population: A cross-sectional study. Lipids Health Dis 2020;19(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St George A, Bauman A, Johnston A, et al. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2009;50(1):68–76. [DOI] [PubMed] [Google Scholar]
- 12.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: A population-based study. Hepatology 2008;48(6):1791–8. [DOI] [PubMed] [Google Scholar]
- 13.Mansour-Ghanaei R, Mansour-Ghanaei F, Naghipour M, et al. The lifestyle characteristics in non-alcoholic fatty liver disease in the PERSIAN guilan cohort study. Open access Macedonian J Med Sci 2019;7(19):3313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallsworth K, Thoma C, Moore S, et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol 2015;6(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troiano RP, McClain JJ, Brychta RJ, et al. Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014;48(13):1019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koolhaas CM, van Rooij FJ, Cepeda M, et al. Physical activity derived from questionnaires and wrist-worn accelerometers: Comparability and the role of demographic, lifestyle, and health factors among a population-based sample of older adults. Clin Epidemiol 2017;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 2003;52(9):2191–7. [DOI] [PubMed] [Google Scholar]
- 18.Jeon CY, Lokken RP, Hu FB, et al. Physical activity of moderate intensity and risk of type 2 diabetes. Diabetes Care Mar 2007;30(3):744. [DOI] [PubMed] [Google Scholar]
- 19.Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol 2020;35(5):483–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatology 2016;64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102(12):2708–15. [DOI] [PubMed] [Google Scholar]
- 22.White T, Westgate K, Wareham NJ, et al. Estimation of physical activity energy expenditure during free-living from wrist accelerometry in UK adults. PLoS One 2016;11(12):e0167472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voortman T, Kiefte-de Jong JC, Ikram MA, et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol 2017;32(11):993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy Scott M, Cleeman James I, Daniels Stephen R, et al. Diagnosis and management of the metabolic syndrome. Circulation 2005;112(17):2735–52. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan S, Kirk EP, Mittendorfer B, et al. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012;55(6):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMonte MJ, Lewis CE, Buchner DM, et al. Both light intensity and moderate-to-vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: The objective physical activity and cardiovascular health (OPACH) study. J Am Heart Assoc 2017;6(10):e007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Murag S, Cholankeril G, et al. Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2020;19(6):1240–1247.e5. doi: 10.1016/j.cgh.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Saint‐Maurice Pedro F, Troiano Richard P, Berrigan D, et al. Volume of light versus moderate‐to‐vigorous physical activity: Similar benefits for all‐cause mortality?. J Am Heart Assoc 2018;7(7):e008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med 2017;2(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwak MS, Kim D, Chung GE, et al. The preventive effect of sustained physical activity on incident nonalcoholic fatty liver disease. Liver Int 2017;37(6):919–26. [DOI] [PubMed] [Google Scholar]
- 31.Kistler KD, Brunt EM, Clark JM, et al. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 2011;106(3):460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asada F, Nomura T, Hosui A, et al. Influence of increased physical activity without body weight loss on hepatic inflammation in patients with nonalcoholic fatty liver disease. Environ Health Prev Med 2020;25(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology (Baltimore, Md) 2011;54(3):1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.