Summary
Fifteen percent of couples are globally estimated to be infertile, with up to half of these cases attributed to male infertility. Reactive oxidative species (ROS) are known to damage sperm leading to impaired quantity and quality. Although not routinely assessed, oxidative stress is a common underlying pathology in infertile men. Antioxidants have been shown to improve semen analysis parameters by reducing ROS and facilitating repair of damage caused by oxidative stress, but it remains unclear whether they improve fertility. Carnitines are naturally occurring antioxidants in mammals and are normally abundant in the epididymal luminal fluid of men. We conducted a systematic review and meta-analysis to evaluate the safety and efficacy of carnitine supplementation for idiopathic male infertility. We searched ClinicalKey, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, MEDLINE, PubMed and ScienceDirect for relevant studies published from 1 January 2000 to 30 April 2020. Of the articles retrieved, only eight randomised controlled trials were identified and included. Analysis showed that carnitines significantly improve total sperm motility, progressive sperm motility and sperm morphology, but without effect on sperm concentration. There was no demonstrable effect on clinical pregnancy rate in the five studies that included that outcome, although patient numbers were limited. Therefore, the use of carnitines in male infertility appears to improve some sperm parameters but without evidence of an increase in the chance of natural conception.
Lay summary
Although male infertility affects 1:15 men, there is no obvious reason in the vast majority of cases. Reactive oxidative species (ROS) are highly active molecules containing oxygen and are natural byproducts of normal metabolism. However, high concentrations of ROS have been shown to damage sperm, which negatively impacts a couple’s ability to conceive. Carnitines are natural antioxidants found in the body that counterbalance the damaging effects of ROS. We conducted a comprehensive review of published studies to assess whether carnitine supplements are safe and effective in improving sperm quality and pregnancy rates. Our analysis shows that carnitines improve sperm swimming and production of normal-shaped sperm cells but do not affect sperm count or pregnancy rates, although there are only a few studies and scientific evidence is limited. Whilst it is possible that carnitines may benefit male infertility, more evidence is required regarding chances of pregnancy after carnitine therapy.
Keywords: antioxidants, carnitine, male infertility, reactive oxidative species, sperm
Introduction
Infertility is the inability to conceive naturally within 1 year for a sexually active couple not using contraception (Rowe et al. 2000, World Health Organization 2020). Worldwide, 15% of couples are estimated to be infertile and approximately 50% of these cases are due to male factor, either as the sole underlying cause or a contributory factor (Agarwal et al. 2015). Diagnosis of male infertility usually follows semen analysis. The results may show abnormal semen parameters such as oligozoospermia, asthenozoospermia and teratozoospermia or a combination of these, or a complete absence of sperm in the ejaculate (azoospermia), which is identified in 10–15% of infertile men (Rowe et al. 2000, Gudeloglu & Parekattil 2013, Colpi et al. 2018). Notably, up to 75% of male infertility is thought to be idiopathic (i.e. with no cause identified) (Punab et al. 2017).
Oxidative stress (OS) occurs when there is an overproduction of oxidative free radicals and ROS, which damage spermatozoa and cause male infertility by impairing both the structure and function of sperm (Aitken et al. 2003, Aitken & Baker 2006, Valko et al. 2007, Venkatesh et al. 2011, Agarwal et al. 2014). Although the exact mechanism(s) of OS in reducing sperm quality is unknown, it is widely acknowledged that depleted intracellular ATP levels, insufficient axoneme phosphorylation and lipid peroxidation of the cell membrane manifests as poor motility and sperm dysfunction, including reduced ability of sperm to fertilise the oocyte (Storey 1997, Gomez et al. 1998, Valko et al. 2007). Sperm are vulnerable to OS as they have minimal cytoplasm and endogenous antioxidant protection (Martins da Silva 2019). This leads to the production of malondialdehyde (MDA) and 4-hydroxynonenal (4HNE), which oxidises the lipid membrane and causes fragmentation of both nuclear and mitochondrial DNA in sperm (de Lamirande & Gagnon 1992, Kodama et al. 1997, Gomez et al. 1998, Aitken et al. 2012, Iommiello et al. 2015).
There are currently no clinically established treatments available for unexplained male infertility (Martins da Silva et al. 2017). Empirical medical treatments such as human menopausal gonadotrophin (hMG)/human chorionic gonadotrophin (hCG), androgen, antioestrogens (clomiphene and tamoxifen), prolactin inhibitors (bromocriptine), and steroids have been used. However, beneficial effects on semen parameters are not proven (Isidori et al. 2006, Jungwirth et al. 2012). Lifestyle modification advice such as smoking, alcohol cessation and weight reduction programmes are therefore the mainstay of managing male infertility, before progressing to assisted reproduction. Vitamin and dietary supplements are widely marketed to improve male reproductive health and have gained considerable popularity in recent years. However, many formulations are not evidence based (Martins da Silva 2019).
Carnitines are naturally occurring compounds in mammals (Bremer 1983, Reuter & Evans 2012). Primary sources of carnitines are through dietary intake, de novo biosynthesis, and renal tubular reabsorption (Reuter & Evans 2012). Foods rich in carnitines include red meats, fish, poultry and dairy products (Steiber et al. 2004). Aside from dietary consumption, approximately 25% of total body carnitine is synthesised by the body from the essential amino acids lysine and methionine (Vaz & Wanders 2002, Shekhawat et al. 2013). Endogenous plasma and tissue concentrations of carnitines are preserved at relatively precise limits to facilitate mitochondrial and peroxisomal fatty acid oxidation (Bremer 1983, Reuter & Evans 2012). L-carnitine facilitates the β-oxidation of long-chain fatty acids, and in its active form of L-acetylcarnitine, is a vital antioxidant that protects the sperm mitochondria from oxidative stress (Kerner & Hoppel 1998, Russo et al. 2000, Abdelrazik et al. 2009). Carnitines participate in the metabolism of branch-chain amino acids and stabilise cellular membranes (Shalev et al. 1986, Adeva-Andany et al. 2017) and can also act as free radicle scavengers, thereby increasing antioxidative capabilities in spermatozoa resulting in reduction of OS (Balercia et al. 2005, Dokmeci 2005, Adewoyin et al. 2017). In vitro, addition of carnitine to culture media increases sperm motility and vitality (Tanphaichitr 1977, Banihani et al. 2014). Notably, men with abnormal semen parameters have been reported to have significantly lower carnitine serum levels (Zopfgen et al. 2000, Mongioi et al. 2016). In this review we have aggregated and analysed currently available data from clinical trials of L-carnitine and/or L-acetylcarnitine in idiopathic male infertility to determine whether carnitine supplements indeed improve sperm quality, and therefore male reproductive potential, in couples with male factor infertility (Haje & Naoom 2015, Moolenaar et al. 2015).
Materials and methods
Our study is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) and the systematic review is reported according to PRISMA guidelines (Moher et al. 2015, Shamseer et al. 2015). The study protocol is registered in PROSPERO (CRD42020181104).
Eligibility criteria
Analysis specifically included only Randomised Controlled Trials (RCTs). The RCTs had to be human studies with male patients between the ages of 18 and 65, with abnormal semen characteristics according to WHO normative ranges (2010) and treated with L-carnitine and/or L-acetyl-carnitine (World Health Organization 2010). Studies required at least one control group treated with placebo or without treatment.
Reviews, commentaries, observational studies, retrospective studies, quasi-randomised trials, case series and case reports were excluded. We also excluded literature with animal studies, laboratory and in vitro studies, female factor infertility, undiagnosed patients, infertility <1 year, couples with no regular sexual intercourse and other causes of male infertility not related to abnormal semen analysis.
Information sources
Literature search strategies were developed using medical subject heading (MeSH) terms and text relating to the impact of L-carnitine and L-acetylcarnitine on male reproductive potential. We searched ClinicalKey, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, MEDLINE, PubMed and ScienceDirect thoroughly according to PRISMA guidelines (Moher et al. 2015, Shamseer et al. 2015). The literature search was limited to the English language and published between 1 January 2000 and 30 April 2020. Articles were also sourced by screening through the references of included studies or relevant reviews during the selection process.
Search strategy
Our MeSH terms were ‘male infertility’ or ‘male reproductive potential’ or ‘male subfertility’ or ‘spermatozoa’ or ‘asthenozoospermia’ or ‘oligospermia’ or ‘oligoasthenozoospermia’ or ‘teratozoospermia’ or ‘DNA damage’ or ‘oxidative stress and ‘Carnitine’ or ‘Levocarnitine’ or ‘L-carnitine’ or ‘L-acetylcarnitine’ or ‘L-acetyl Carnitine’ or ‘L-acetyl-carnitine’ or ‘L-acetyl Carnitine’ or ‘Levoacetylcarnitine’ or ‘Levo-acetyl-carnitine’ or ‘Levoacetyl Carnitine’ or ‘Levo-acetyl Carnitine’ or ‘Acetyl-L-carnitine’ or ‘Acetyl L-carnitine’ or ‘Acetyl-L Carnitine’ or ‘Acetyl-Levocarnitine’ or ‘Acetyl Carnitine’.
Data management and collection
Covidence was used to filter duplicates and conduct the systematic review data collection process (Veritas Health Innovation). The selection was performed according to PRISMA guidelines (Moher et al. 2015, Shamseer et al. 2015). All available pieces of literature were thoroughly screened using the inclusion and exclusion criteria through Covidence (Veritas Health Innovation). Literature was first screened by title and abstract. Full-text articles that fulfilled the inclusion criteria were then reviewed. If two or more reports had repeated data, the study with the largest sample size, most extended follow-up, and most specific intervention and outcomes were selected. The screening and selection process was carried out by two independent review authors simultaneously (Khaw and Wong) using a standardised form to include study characteristics such as methodology, number of participants, demographics of participants, detailed test and control interventions, primary and secondary outcomes of the studies, the effect of treatment and risk of bias. Missing data were requested from study authors. Any discrepancies were resolved through consensus.
Risk of bias in individual studies
The Cochrane Collaboration’s tool for assessing the risk of bias in randomised trials was used to determine the six domains of bias (Higgins et al. 2011). Two independent review authors conducted this assessment (Khaw and Wong) and any discrepancies were resolved through consensus. Articles were assessed for bias based on the following aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias (Higgins et al. 2011). Each aspect was then classified as high, low, or unclear risk of material bias (Higgins et al. 2011). The risk of bias assessment chart was then generated using Review Manager (RevMan) 5.4 software (The Nordic Cochrane Centre 2014).
Data synthesis
We then carried out a descriptive analysis of included studies focusing on the methodology of the study, type and details of the intervention, target population demographics, primary outcomes, secondary outcomes, adverse outcomes and intervention effects. A meta-analysis was conducted for studies with the same intervention and comparator with equal outcome measures. For the meta-analysis, we conducted a random-effects meta-analysis using risk ratios for dichotomous outcomes and mean differences with s.d. The raw mean differences were used instead of standardised mean differences, as all studies used the same continuous outcomes and units of measure. We then used the statistical significance of 95% CIs and P-values for each outcome. Where there were results from multiple durations of therapy, the results after the most prolonged period of treatment was used. Higgins’s I2 test statistic (>50% indicative of substantial heterogeneity) was utilised to assess heterogeneity among the studies. Cochran’s Q test was not used to analyse heterogeneity as there were only small numbers of available studies. We then proceed with a stratified meta-analysis for study quality, trial size, concealment of allocation, blind adjudication of events, analysis according to the intention-to-treat principle, and intervention method. Assessment evidence of publication bias was carried out for the included studies and plots were generated to visually inspect the data through a funnel plot generated by RevMan 5.4 software (The Nordic Cochrane Centre 2014). In study outcomes that had substantial heterogeneity, the data were also synthesised through a narrative and qualitative approach.
The s.d. from Sigman et al. (2006) was calculated according to the Cochrane Handbook for Systematic Reviews of Interventions section 6.5.2.3(3) (Higgins et al. 2019).
Overall quality of evidence
The quality and consistency of each comparison was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines through GRADEpro (Balshem et al. 2011, Schünemann et al. 2013, Evidence Prime 2015). The strength of evidence for critical and essential outcomes was rated based on study design, risk of bias, consistency, limitations, directness, reporting precision and publication bias (Balshem et al. 2011, Schünemann et al. 2013, Evidence Prime 2015). Table 1 shows a summary of the included studies and their GRADE assessments. Effect (risk) of carnitine is expressed as mean difference (MD).
Table 1.
Summary of findings of carnitine compared to placebo or no treatment for idiopathic male infertility.
| Outcomes | Anticipated absolute effects (95% CI) | RR (95% CI) | Participants in studies | Certainty of evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Risk with placebo or no treatment, range | Risk with carnitine | |||||||
| Value | Range | n | Studies | Evidence | Grade | |||
| Sperm concentration | 0.8–33.73 million/mL | MD 2.7 million/mL higher | 2.04 lower to 7.44 higher | – | 438 | 6 RCTs | ⊕⊝⊝⊝ | VERY LOWa,b,c |
| Total sperm motility | 3.3–43.4% | MD 10.72% higher | 3.94 higher to 17.5 higher | – | 459 | 7 RCTs | ⊕⊕⊝⊝ | LOWa,c |
| Progressive sperm motility | 4–24.41% | MD 9.82% higher | 2.01 higher to 17.62 higher | – | 231 | 3 RCTs | ⊕⊕⊝⊝ | LOWa,c |
| Normal sperm morphology | 1.39–32.73% | MD 2.41% higher | 0.79 higher to 4.03 higher | – | 438 | 6 RCTs | ⊕⊕⊝⊝ | LOWa,c |
| Clinical pregnancy |
||||||||
| Study population | 113 per 1000 | 116 per 1000 | 61–221 | 1.03 (0.54–1.96) | 301 | 5 RCTs | ⊕⊕⊝⊝ | LOWa,b |
The population was men with abnormal semen characteristics. The intervention was l-carnitine and/or l-acetylcarnitine. The table compares placebo or no treatment. The outcomes measured were semen analysis parameters; clinical pregnancy; adverse events in a clinic or hospital.
aLack of blinding; bCrosses the line of no effect; cHiggins’s I2 test >50%.
Results
Study characteristics
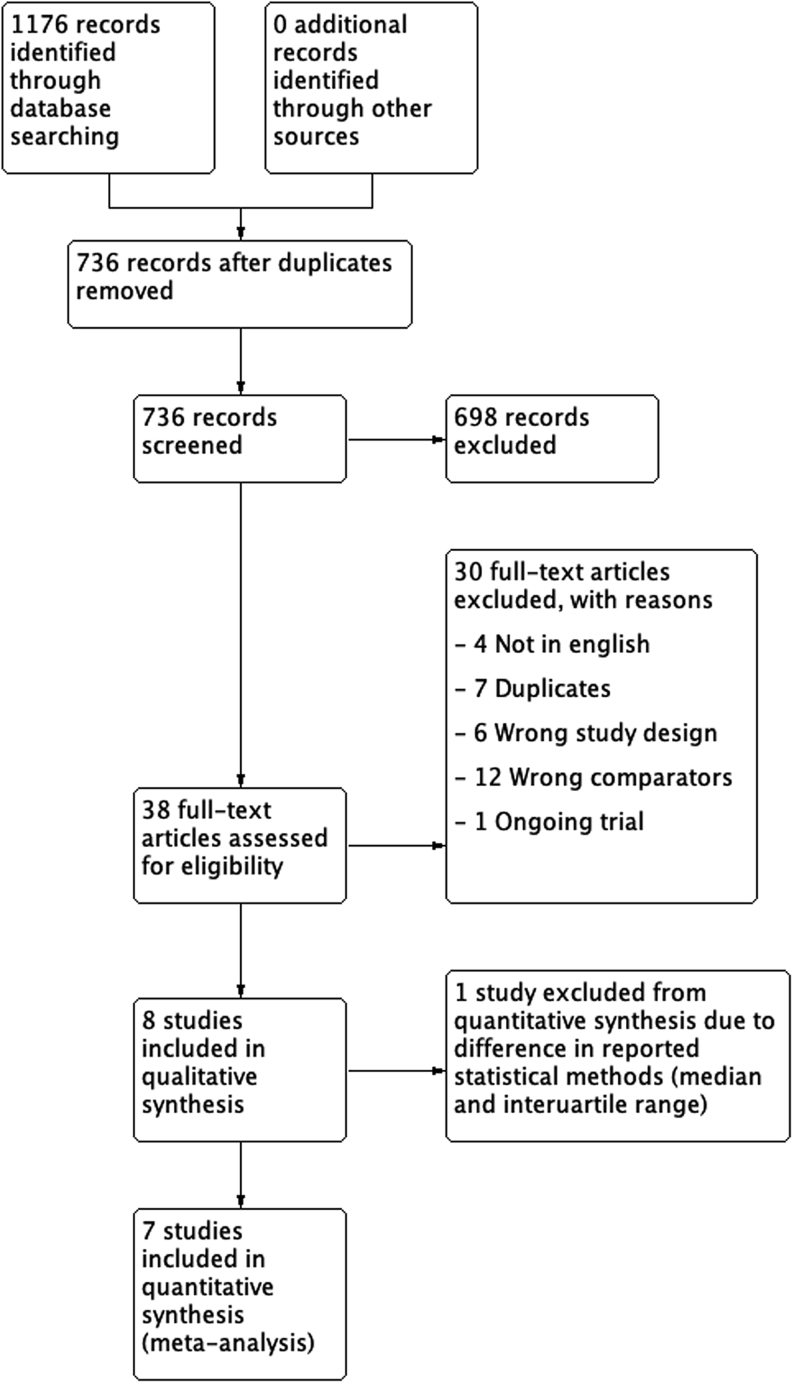
The search strategy identified 1176 citations dated 1 January 2000 to 30 April 2020. After 440 duplicates were removed, 736 abstracts were assessed. Six hundred and ninety-eight records were excluded as they did not meet inclusion criteria. Thirty-eight full-text articles were searched for eligibility according to inclusion and exclusion criterion. All included studies were of randomised controlled trials without cross over. We excluded Lenzi 2003 as it had a cross-over design (Lenzi et al. 2003). A total of eight studies were included in the review and the findings from seven studies were pooled into a meta-analysis (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). The data by Cavellini et al. was excluded from the meta-analysis as the authors reported their results as medians and interquartile ranges rather than means and s.d. (Cavallini et al. 2004). Our search process is summarised in the PRISMA flowchart (Fig. 1).
Figure 1.
PRISMA flowchart.
The included articles were assessed based on seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias (Figs 2 and 3). Where additional information was required, the study authors were contacted but this was unsuccessful. Overall, none of the included studies explicitly mentioned the method of randomisation (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Mehni et al. 2014, Dimitriadis et al. 2010, Haje & Naoom 2015, Tsounapi et al. 2018). Hence, all literature had an unclear risk of selection bias in this aspect. The majority of articles also had unclear risks of detection bias as the process of assessment was not reported in detail (Lenzi et al. 2004, Sigman et al. 2006, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018).
Figure 2.
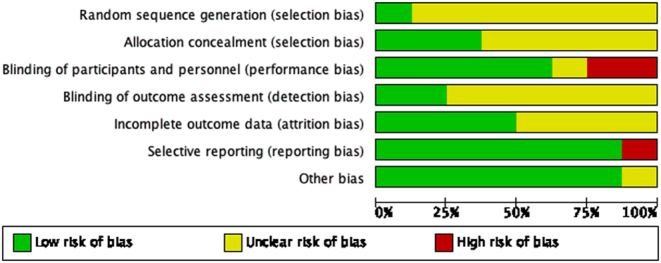
Risk of bias summary.
Figure 3.
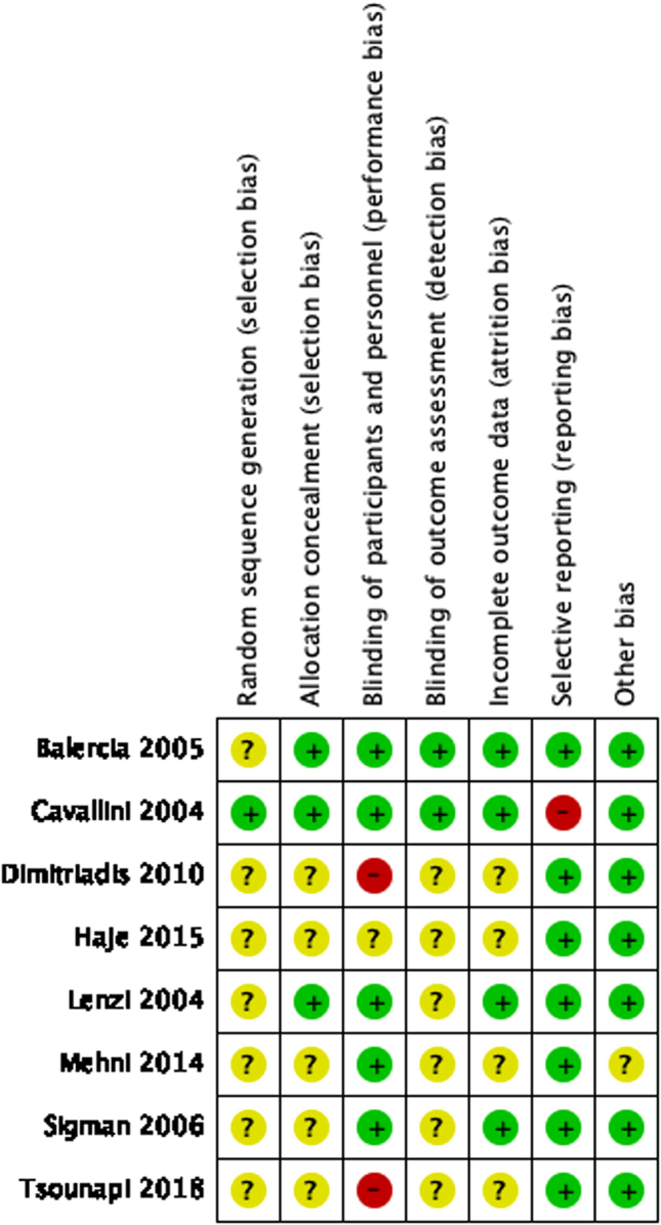
Risk of bias graph.
One study (Tsounapi et al. 2018) was five-armed, four studies (Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015) were four-armed, one study (Cavallini et al. 2004) was three-armed, and two studies (Lenzi et al. 2004, Sigman et al. 2006) were two-armed. The total duration of treatment ranged from 3 months (Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018) to 6 months (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Haje & Naoom 2015) while follow-up time varied between from 3 months (Mehni et al. 2014) to9 months (Cavallini et al. 2004, Balercia et al. 2005). Table 2 shows an overview of the included studies.
Table 2.
Study characteristics.
| Study | Study design | Age (years) | Treatment/day | Control | Arms | Duration of treatment (Weeks) | Sample size | Total follow-up time | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Therapy | Control | |||||||||
| 2004 | Cavallini et al. (2004) | RCT | 27–40 | 2 g LC + 1 g LAC | Placebo | 3 | 24 | 39 | 47 | 9 months |
| 2004 | Lenzi et al. (2004) | RCT | 20–40 | 2 g LC + 1 g LAC | Placebo | 2 | 24 | 30 | 26 | 8 months |
| 2005 | Balercia et al. (2005) | RCT | 24–38 | 2 g LC + 1 g LAC (n = 14) vs 3 g LC (n = 15) vs 3 g LAC (n = 15) | Placebo | 4 | 24 | 44 | 15 | 9 months |
| 2006 | Sigman et al. (2006) | RCT | 36.2 ± 1.7 | 2 g LC + 1 g LAC | Placebo | 2 | 16 | 12 | 9 | 6 months |
| 2010 | Dimitriadis et al. (2010) | RCT | NR | 1 g LC | No TT | 4 | 12 | 26 | 22 | 13 weeks (6 days after the experimental period) |
| 2014 | Mehni et al. (2014) | RCT | 25–40 | 1 g LC | Placebo | 4 | 12 | 51 | 59 | 3 months |
| 2015 | Haje & Naoom (2015) | RCT | 37.54 ± 2.46 | 1 g LC | Placebo | 4 | 12–24 | 20 | 29 | 4–7 months (as two samples were taken after treatment – 1 month apart) |
| 2018 | Tsounapi et al. (2018) | RCT | NR | 1 g LC | No TT | 5 | 12.8 | 44 | 42 | Experimental period of 90 days; up to 180 days for pregnancy rate |
NR, Not reported; TT, treatment.
Population
Our meta-analysis only included studies with idiopathic male infertility. The participants were treated with carnitine supplementation, placebo or did not receive any treatment. Six studies (438 men) recorded sperm concentration and morphology, seven studies (459 men) reported total sperm motility and three studies (231 men) evaluated the progressive sperm motility after carnitine therapy. Only four studies (252 men) reported pregnancy outcomes following carnitine supplementation. All participants were between the ages of 18 and 65 with infertility of more than 1 year. A study by Cavallini et al. (2004) was also included in our systematic review, but not in the meta-analysis, as their results were recorded in medians and interquartile ranges. Their study also enrolled men with varicocele but only the results from men with idiopathic male infertility were included in our review (Cavallini et al. 2004).
Interventions
Data included in our analysis compared l-carnitine (LC) and/or l-acetylcarnitine (LAC) to placebo or no treatment. Four studies (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006) compared LC and LAC to placebos, five studies compared LC to placebo (Balercia et al. 2005, Mehni et al. 2014, Haje & Naoom 2015) or no treatment (Dimitriadis et al. 2010, Tsounapi et al. 2018) while only one study (Balercia et al. 2005) compared LAC to placebo.
Outcomes
The primary outcomes for our review are sperm concentration, total sperm motility, progressive sperm motility, sperm morphology, pregnancy rate and live birth rate. We also included sperm DNA damage and adverse events such as side effects and miscarriage as secondary outcomes. Seven studies (Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018) recorded the total sperm motility while only four studies (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Tsounapi et al. 2018) reported progressive sperm motility. Sperm concentration and morphology were recorded by seven studies (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). None of the studies included DNA damage assessment. Although five studies (Cavallini et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Haje & Naoom 2015, Tsounapi et al. 2018) reported on pregnancy rate, none included live birth or miscarriage data. We contacted the authors where details were unclear or if a different statistical approach was used in their study; however, we received no response.
Sperm concentration
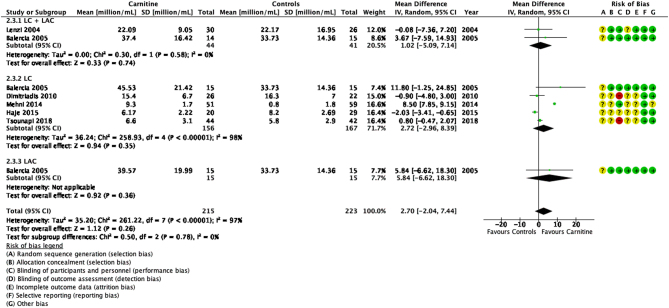
Seven studies reported the effects of carnitines on sperm concentration (Fig. 4) (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). Although the study by Cavallini et al. reported higher sperm concentrations after LC + LAC therapy (20.6%, IQR 24.9–15.1%) when compared to a placebo (10.9%, IQR 15.1–9.0%), findings were reported as median and interquartile range rather than mean and s.d. and no statistical analysis was reported (Cavallini et al. 2004). This study was therefore excluded from the meta-analysis.
Figure 4.
Forest plot of comparison for sperm concentration.
Overall, our findings showed that carnitines did not significantly improve sperm concentration (P > 0.05). However, the six studies showed a high heterogeneity (MD 2.70, 95% CI −2.04 to 7.44; n = 438, RCT = 6, P > 0.05, I2 = 97%) (Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018) primarily due to two studies with very small s.d. compared to the others (Mehni et al. 2014, Haje & Naoom 2015). A sensitivity analysis after removal of these two studies showed homogeneity between the remaining studies but the meta-analysis still showed no significant effects of carnitines on sperm concentration (MD 0.79, 95% CI −0.39 to 1.96; n = 279, RCT = 4, P > 0.05, I2 = 0%) (Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Tsounapi et al. 2018).
Total sperm motility
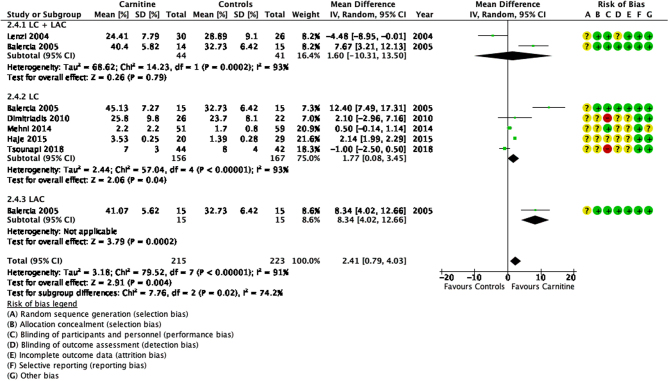
Seven studies compared the efficacy of carnitines to placebo or no treatment (Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). Analysis of the mean difference in total sperm motility showed that carnitines improved total sperm motility by 10.72% (95% CI 3.94–17.50; n = 459, RCT = 7, P < 0.05, I2 = 97%) (Fig. 5).
Figure 5.
Forest plot of comparison for total sperm motility.
The studies showed high heterogeneity. In studies comparing LC and LAC to placebo, Balercia et al. showed a significant increase in total sperm motility in all three study arms when compared to a placebo (MD 18.75, 95% CI 14.78–22.73; n = 30, P < 0.05) (Balercia et al. 2005). However two other studies did not show significant differences between the treatment and control groups (Lenzi et al. 2004, Sigman et al. 2006). Lenzi et al. showed a mean difference of 1.56 (95% CI −4.48 to 7.60; n = 56, P > 0.05), while Sigman et al. showed a mean difference of −7.70 (95% CI −20.68 to 5.28; n = 21, P > 0.05). In studies that compared LC to placebo or no treatment, four studies (Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015) showed significant improvements after receiving LC while one study (Tsounapi et al. 2018) reported no significant differences. Balercia et al. (2005) was the only study that assessed LAC treatment alone.
A detailed statistical analysis is shown in Fig. 5.
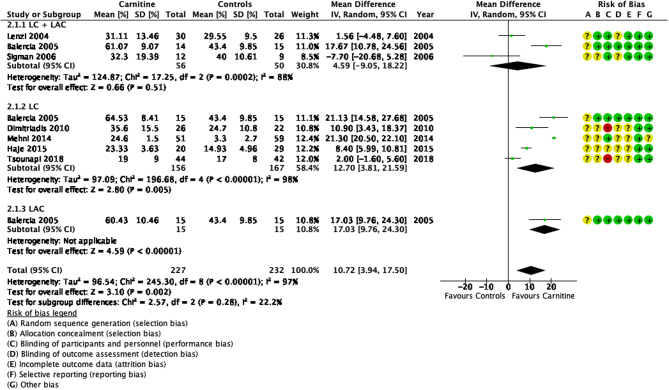
Progressive sperm motility
Four studies showed an increase in progressive sperm motility after carnitine when compared to control groups (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Tsounapi et al. 2018). Overall, carnitines significantly improved progressive sperm motility in idiopathic male infertility (MD 9.82, 95% CI 2.01, 17.62; n = 231, P < 0.05) (Fig. 6).
Figure 6.
Forest plot of comparison for progressive sperm motility.
This outcome also showed high heterogeneity (I2 = 94%). Balercia et al. (2005) showed significant improvement in progressive sperm motility in all LC, LAC and LC + LAC therapy groups (MD 16.02, 95% CI 11.98–20.06; n = 30, P < 0.05). Tousnapi et al. (2018) reported a significant increase in progressive sperm motility after LC therapy (MD 2.00, 95% CI 0.93–3.07; n = 86, P < 0.05). Lenzi et al. (2004) did not show a significant increase in progressive sperm motility after LC + LAC therapy (MD 0.59, 95% CI −5.30 to 6.48; n = 56, P > 0.05). Cavallini et al. (2004) reported an increase in progressive sperm motility after LC + LAC therapy (23.6%, IQR 28.9–16.0%) when compared to controls (13.2%, 18.6–9.0%) but no raw data or P-values were provided to draw a statistically significant conclusion.
Sperm morphology
The results of sperm morphology were recorded by seven studies (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). Overall, our results showed a significant improvement in sperm morphology (P < 0.05) as seen in Fig. 7.
Figure 7.
Forest plot of comparison for normal sperm morphology.
This outcome also had a high heterogeneity (I2 = 91%). Balercia et al. (2005) documented a significant improvement of 9.29% when compared to a placebo (MD 9.29, 95% CI 6.51–12.06; n = 30, P < 0.05). Lenzi et al. (2004) showed no significant differences between the treatment and control groups after LC + LAC therapy. Among the remaining studies of treatment after LC, only the study by Haje and Naoom (2015) showed a significant improvement (MD 2.14, 95% CI 1.99–2.29; n = 49, P < 0.05) in sperm morphology; while three other studies (Dimitriadis et al. 2010, Mehni et al. 2014, Tsounapi et al. 2018) recorded no significant changes. Cavallini et al. (2004) recorded an improvement of sperm morphology in their study after LC + LAC therapy (27.3%, IQR 32.0–22.6% vs 15.3%, IQR 22.0–12.1% in the placebo group) but the data provided is not sufficient for a test of statistical significance.
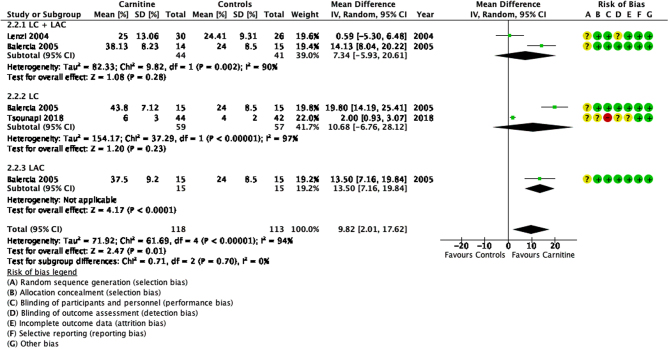
Clinical pregnancy rate
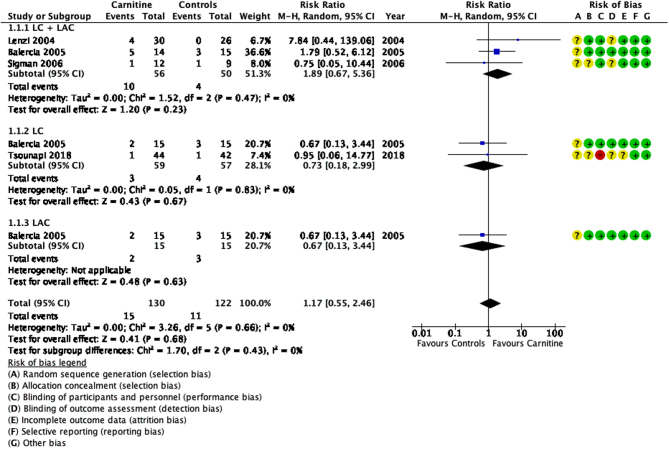
A total of five RCTs were analysed for their reported pregnancies and four studies were consolidated into a meta-analysis. There was low heterogeneity (I2 = 0%) and some concern with the risk of bias. The meta-analysis (Fig. 8) showed that there was no significant improvement in pregnancy rates when compared to control groups (RR 1.17, 95% CI 0.55–2.46; n = 252, RCT = 4, P > 0.05).
Figure 8.
Forest plot of comparison for clinical pregnancy.
In patients treated with a combination of LC and LAC, Cavallini et al. (2004) showed a significant improvement in pregnancy rate (X2 = 20.795, P < 0.01) when compared to controls. In contrast, the three other studies (Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006) did not show significant differences between the two groups (RR 1.89, 95% CI 0.67–5.36; n = 106, RCT = 3, P > 0.05, I2 = 0%). Patients treated with either LC (RR 0.73, 95% CI 0.28–1.87; n = 165, RCT = 3, P > 0.05, I2 = 0%) or LAC (RR 0.67, 95% CI 0.13–3.44; n = 30, RCT = 1, P > 0.05) did not show any significant changes in pregnancy rates in comparison to their control groups (Balercia et al. 2005, Haje & Naoom 2015, Tsounapi et al. 2018). Haje and Naoom (2015) studied the effect of carnitine supplementation in patients undergoing ICSI and reported no significant increase in pregnancy rates.
Overall, the results were imprecise as there were very few events and thus confidence intervals were wide. Moreover, the quality of evidence is also low.
Adverse events
Six studies did not report adverse events (Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). Sigman et al. (2006) confirmed that there were no adverse events in their study. Cavallini et al. (2004) reported four cases of mild euphoria (two in the LC + LAC group and two in control groups). Their study also recorded two cases of gastrointestinal side effects (mild epigastria and nausea) from both the treatment and control groups. However, these side effects were reported as negligible as they did not result in therapy suspension. None of the studies included data related to miscarriage.
Carnitine versus other arms in the included studies
Further studies were identified that compared carnitine to other compounds, rather than placebo or no treatment. They were therefore not included in the meta-analysis but are summarised in Table 3.
Table 3.
Carnitine versus other arms in the included studies.
| Published year | Study | Age (years) | Treatment/day | Improved Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Sperm concentration, (×106/mL) | Total sperm motility (%) | Progressive sperm motility (%) | Sperm morphology (%) | Clinical pregnancy rate (compared to carnitines or controls) | ||||
| 2004 | Cavallini et al. (2004) | 27–40 | LC + LAC and cinnoxicam | Improved* | Unchanged* | Improved* | Improved* | x2 = +5.743; P < 0.05 |
| 2010 | Dimitriadis et al. (2010) | NR | Vardenafil | +12.0, P < 0.05 | +19.9, P < 0.05 | NR | +16.3, P < 0.05 | NR |
| Sildenafil | +14.8, P < 0.05 | +21.4, P < 0.05 | NR | +17.7, P < 0.05 | ||||
| 2014 | Mehni et al. (2014) | 25–40 | LC and Pentoxifylline | P = 0.001$ | P = 0.045$ | NR | P = 0.052$ | NR |
| 2015 | Haje & Naoom (2015) | 37.54 ± 2.46 | Tamoxifen | +3.23, P = 0.016 | P > 0.05 | NR | +0.56, P = 0.25 | 48.9%, P > 0.05 |
| Tamoxifen and carnitine | +0.6, P = 0.01 | +5.75, P = 0.045 | NR | +1.11, P = 0.026 | 48.3%, P > 0.05 | |||
| 2018 | Tsounapi et al. (2018) | NR | Profertil | +2.1, P > 0.05 | +16, P < 0.05 | +9, P < 0.05 | +2, P > 0.05 | NR |
| Avanafil | +3.5, P > 0.05 | +30, P < 0.05 | +12, P < 0.05 | P > 0.05 | ||||
| Combination of Profertil and Avanafil | +2.9, P > 0.05 | +24, P < 0.05 | +7, P < 0.05 | +2, P > 0.05 | ||||
*Detailed statistical data was not reported by the authors, $The raw data was not provided by the authors.
NR, Not reported.
Discussion
Several published studies have reported that carnitines have beneficial effects on improving sperm quality in men with idiopathic male infertility (Steiber et al. 2004, Isidori et al. 2005, Isidori et al. 2006, Mongioi et al. 2016, Smits et al. 2019). Notably, concentrations of carnitine have also been documented to be higher in the sperm and seminal plasma of fertile men, compared to men with abnormal semen parameters (Zopfgen et al. 2000, Banihani et al. 2014, Mongioi et al. 2016, Smits et al. 2019). The scientific rationale behind this is a vital role played by carnitines during spermatogenesis (Jeulin & Lewin 1996, Agarwal & Sekhon 2011, Aliabadi et al. 2012). Carnitines are concentrated in the epididymal luminal fluid (Jeulin & Lewin 1996), and likely to be associated with sperm maturation. Carnitines also scavange free oxygen radicles and ROS, thus protecting against OS, as well as aiding cellular repair in mitochondria during β-oxidation of long-chain fatty acids (Fritz 1963, Steiber et al. 2004, Reuter & Evans 2012, Smits et al. 2019). However, whilst improved semen characterisitics have been reported, very few studies have recorded pregnancy outcomes after treatment of infertile men with carnitines, and none have considered live birth as a primary outcome (Zini et al. 1993, Shekarriz et al. 1995, Hakonsen et al. 2011, Poljsak 2011, Moolenaar et al. 2015).
This meta-analysis presents evidence supporting the improvement of sperm parameters with carnitine supplementation. Carnitines significantly improve total sperm motility (+10.72%), progressive sperm motility (+9.82%) and sperm morphology (+2.41%) (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). There does not appear to be a positive effect of carnitine supplementation on sperm concentration (Cavallini et al. 2004, Lenzi et al. 2004, Balercia et al. 2005, Dimitriadis et al. 2010, Mehni et al. 2014, Haje & Naoom 2015, Tsounapi et al. 2018). However, it is notable that the studies are characterised by high heterogeneity, and the quality of the evidence was low (total sperm motility, progressive sperm motility and sperm morphology) or very low (sperm concentration) when assessed through GRADEpro (Table 1).
The data indicate that carnitine does not significantly improve pregnancy rates in infertile couples with male infertility, despite improvements in sperm motility and morphology. However, natural conception was not a primary outcome in most studies, indeed most did not follow-up until pregnancy. Therefore, more evidence is required to study the effects of carnitines on pregnancy outcomes. Although multiple attempts have been made to encourage RCTs to report on fertility outcomes, very few RCTs achieve this in studies relating to male infertility (Tournaye, 2006). Nonetheless, two recently reported large RCTs showed that folic acid and zinc supplements (FAZST) or combination antioxidant treatment including Vitamin C, Vitamin E, folic acid, selenium, zinc, and l-carnitine (MOXI trial) did not improve clinical pregnancy or live birth rates when compared to placebo (Schisterman et al. 2020, Steiner et al. 2020).
Our findings are consistent with previously published systematic reviews researching the efficacy and safety of antioxidants in idiopathic male infertility. Two systematic reviews of empirical dietary and/or supplementary intervention recorded improved total sperm motility, progressive sperm motility and sperm morphology (Salas-Huetos et al. 2018, Omar et al. 2019). However, it is notable that our findings of effects on total sperm motility and morphology differ from a recent meta-analysis of carnitines in men with idiopathic oligoasthenoteratozoospermia conducted by Zhang et al. (2020). Their systematic review included studies of carnitine plus other antioxidants/compounds and one study that used active controls (Zhang et al. 2020). In contrast, we selected studies of carnitine-only treatment vs non-active controls so these studies were excluded during our full-text screening. We also included additional studies from other database searches (Zhang et al. 2020). However, the other authors similarly commented on inconsistent data and high heterogeneity amongst the published trials.
A major limitation of this systematic review, and others, is the inability to assess robustly the effect of carnitines on natural conception and pregnancy outcomes as this has not been comprehensively studied to date. Critically, our findings in regards to pregnancy rates did not support carnitine supplementation as an intervention for male infertility, which disagrees with Zhang et al. and is reflective of the different study data included (Zhang et al. 2020).
Conclusion
Overall, our systematic review shows that carnitine supplementation can improve sperm motility and morphology. However, there were only eight randomised controlled trials that specifically compared carnitine(s) to placebo or no treatment and study outcomes had high heterogeneity and were derived from low-quality evidence (Table 1). The majority of studies included found that carnitines were most effective in men with severe idiopathic infertility (Cavallini et al. 2004, Balercia et al. 2005, Sigman et al. 2006, Mehni et al. 2014), supporting their use as a potential treatment. However, whilst it is accepted that gains in male fertility are likely to be seen with improvement in total motile count, particularly when at the lower end of the range (Hamilton et al. 2015), studies included in this meta-analysis have not demonstrated increase in chance of conception, pregnancy and live birth with carnitine supplementation. It therefore remains unclear whether carnitines are a suitable intervention for idiopathic male infertility and randomised placebo‐controlled trials reporting on pregnancy and live births are required to clarify this.
Declaration of interest
S Martins da Silva has received research funding from AstraZeneca. The other authors declare no conflict of interests. S Martins da Silva is an Associate Editor of Reproduction and Fertility. S Martins da Silva was not involved in the review or editorial process for this paper, on which she is listed as an author.
Author contribution statement
S C K conceived and designed the study. S C K and Z Z W performed the acquision of data and quality assessment of included studies. S C K conducted the meta-analysis. S C K, R A A and S M d S analysed and interpreted the data. S C K wrote the manuscript with support and input from R A A and S M d S. R A A and S M d S revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript to be published.
Funding Statement
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Abdelrazik H, Sharma R, Mahfouz R, Agarwal A.2009. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertility and Sterility 91 589–5. ( 10.1016/j.fertnstert.2007.11.067) [DOI] [PubMed] [Google Scholar]
- Adeva-Andany MM, López-Maside L, Donapetry-García C, Fernández-Fernández C, Sixto-Leal C.2017. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 49 1005–1028. ( 10.1007/s00726-017-2412-7) [DOI] [PubMed] [Google Scholar]
- Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, Anuar MNN.2017. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases 5 9. ( 10.3390/diseases5010009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Sekhon LH.2011. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: is it justified? Indian Journal of Urology 27 74–85. ( 10.4103/0970-1591.78437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Virk G, Ong C, Du Plessis SS.2014. Effect of oxidative stress on male reproduction. World Journal of Men’s Health 32 1–17. ( 10.5534/wjmh.2014.32.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Mulgund A, Hamada A, Chyatte MR.2015. A unique view on male infertility around the globe. Reproductive Biology and Endocrinology 13 37. ( 10.1186/s12958-015-0032-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA.2006. Oxidative stress, sperm survival and fertility control. Molecular and Cellular Endocrinology 250 66–6. ( 10.1016/j.mce.2005.12.026) [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA, Sawyer D.2003. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reproductive Biomedicine Online 7 65–70. ( 10.1016/s1472-6483(1061730-0) [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Gibb Z, Mitchell LA, Lambourne SR, Connaughton HS, De Iuliis GN.2012. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biology of Reproduction 87 110. ( 10.1095/biolreprod.112.102020) [DOI] [PubMed] [Google Scholar]
- Aliabadi E, Soleimani Mehranjani M, Borzoei Z, Talaei-Khozani T, Mirkhani H, Tabesh H.2012. Effects of L-carnitine and L-acetyl-carnitine on testicular sperm motility and chromatin quality. Iranian Journal of Reproductive Medicine 10 77–82. [PMC free article] [PubMed] [Google Scholar]
- Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M.2005. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertility and Sterility 84 662–6. ( 10.1016/j.fertnstert.2005.03.064) [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris Set al. 2011. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 64 401–40. ( 10.1016/j.jclinepi.2010.07.015) [DOI] [PubMed] [Google Scholar]
- Banihani S, Agarwal A, Sharma R, Bayachou M.2014. Cryoprotective effect of L-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia 46 637–6. ( 10.1111/and.12130) [DOI] [PubMed] [Google Scholar]
- Bremer J.1983. Carnitine – metabolism and functions. Physiological Reviews 63 1420–14. ( 10.1152/physrev.1983.63.4.1420) [DOI] [PubMed] [Google Scholar]
- Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G.2004. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. Journal of Andrology 25 761–7; discussion 771–77. ( 10.1002/j.1939-4640.2004.tb02853.x) [DOI] [PubMed] [Google Scholar]
- Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, Krausz C, Giwercman A.2018. European Academy of Andrology Guideline Management of oligo-astheno-teratozoospermia. Andrology 6 513–524. ( 10.1111/andr.12502) [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C.1992. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. Journal of Andrology 13 368–3. [PubMed] [Google Scholar]
- Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, Gratsias S, Watanabe T, Saito M, Miyagawa Iet al. 2010. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: a randomized trial. BJU International 106 1181–118. ( 10.1111/j.1464-410X.2010.09243.x) [DOI] [PubMed] [Google Scholar]
- Dokmeci D.2005. Oxidative stress, male infertility and the role of carnitines. Folia Medica 47 26–30. [PubMed] [Google Scholar]
- Evidence Prime Inc. 2015. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University. [Google Scholar]
- Fritz IB.1963. Carnitine and its role in fatty acid metabolism. Advances in Lipid Research 1 285–334. ( 10.1016/B978-1-4831-9937-5.50014-4) [DOI] [PubMed] [Google Scholar]
- Gomez E, Irvine DS, Aitken RJ.1998. Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4-hydroxyalkenals in human spermatozoa: relationships with semen quality and sperm function. International Journal of Andrology 21 81–94. ( 10.1046/j.1365-2605.1998.00106.x) [DOI] [PubMed] [Google Scholar]
- Gudeloglu A, Parekattil SJ.2013. Update in the evaluation of the azoospermic male. Clinics 68 (Supplement 1) 27–34. ( 10.6061/clinics/2013(sup0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haje M, Naoom K.2015. Combined tamoxifen and L-carnitine therapies for the treatment of idiopathic male infertility attending intracytoplasmic sperm injection: a randomized controlled trial. International Journal of Infertility and Fetal Medicine 6 20–24. ( 10.5005/jp-journals-10016-1096) [DOI] [Google Scholar]
- Hakonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, Bungum M, Ernst EH, Hansen ML, Ernst EHet al. 2011. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reproductive Health 8 24. ( 10.1186/1742-4755-8-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA, Cissen M, Brandes M, Smeenk JM, De Bruin JP, Kremer JA, Nelen WL, Hamilton CJ.2015. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Human Reproduction 30 1110–11. ( 10.1093/humrep/dev058) [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAet al. 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343 d5928. ( 10.1136/bmj.d5928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch VE.2019. Cochrane Handbook for Systematic Reviews of Interventions , Version 6.0 (Updated July 2019). Cochrane. (available at: https://training.cochrane.org/handbook/archive/v6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iommiello VM, Albani E, Di Rosa A, Marras A, Menduni F, Morreale G, Levi SL, Pisano B, Levi-Setti PE.2015. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. International Journal of Endocrinology 2015 321901. ( 10.1155/2015/321901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A, Latini M, Romanelli F.2005. Treatment of male infertility. Contraception 72 314–31. ( 10.1016/j.contraception.2005.05.007) [DOI] [PubMed] [Google Scholar]
- Isidori AM, Pozza C, Gianfrilli D, Isidori A.2006. Medical treatment to improve sperm quality. Reproductive Biomedicine Online 12 704–7. ( 10.1016/s1472-6483(1061082-6) [DOI] [PubMed] [Google Scholar]
- Jeulin C, Lewin LM.1996. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Human Reproduction Update 2 87–102. ( 10.1093/humupd/2.2.87) [DOI] [PubMed] [Google Scholar]
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, Krausz C.European Association of Urology Working Group on Male Infertility 2012. European Association of Urology guidelines on male infertility: the 2012 update. European Urology 62 324–3. ( 10.1016/j.eururo.2012.04.048) [DOI] [PubMed] [Google Scholar]
- Kerner J, Hoppel C.1998. Genetic disorders of carnitine metabolism and their nutritional management. Annual Review of Nutrition 18 179–206. ( 10.1146/annurev.nutr.18.1.179) [DOI] [PubMed] [Google Scholar]
- Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T.1997. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertility and Sterility 68 519–5. ( 10.1016/s0015-0282(9700236-7) [DOI] [PubMed] [Google Scholar]
- Lenzi A, Lombardo F, Sgro P, Salacone P, Caponecchia L, Dondero F, Gandini L.2003. Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertility and Sterility 79 292–300. ( 10.1016/s0015-0282(0204679-4) [DOI] [PubMed] [Google Scholar]
- Lenzi A, Sgro P, Salacone P, Paoli D, Gilio B, Lombardo F, Santulli M, Agarwal A, Gandini L.2004. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertility and Sterility 81 1578–15. ( 10.1016/j.fertnstert.2003.10.034) [DOI] [PubMed] [Google Scholar]
- Martins da Silva SJ.2019. Male infertility and antioxidants: one small step for man, no giant leap for andrology? Reproductive Biomedicine Online 39 879–883. ( 10.1016/j.rbmo.2019.08.008) [DOI] [PubMed] [Google Scholar]
- Martins da Silva SJ, Brown SG, Sutton K, King LV, Ruso H, Gray DW, Wyatt PG, Kelly MC, Barratt CLR, Hope AG.2017. Drug discovery for male subfertility using high-throughput screening: a new approach to an unsolved problem. Human Reproduction 32 974–984. ( 10.1093/humrep/dex055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehni N, Ketabchi AA, Hosseini E.2014. Combination effect of pentoxifylline and L-carnitine on idiopathic oligoasthenoteratozoospermia. Iranian Journal of Reproductive Medicine 12 817–824. [PMC free article] [PubMed] [Google Scholar]
- Moher D Shamseer L Clarke M Ghersi D Liberati A Petticrew M Shekelle P & Stewart LA & PRISMA-P Group. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 4 [epub]. ( 10.1186/2046-4053-4-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongioi L, Calogero AE, Vicari E, Condorelli RA, Russo GI, Privitera S, Morgia G, La Vignera S.2016. The role of carnitine in male infertility. Andrology 4 800–80. ( 10.1111/andr.12191) [DOI] [PubMed] [Google Scholar]
- Moolenaar LM, Cissen M, De Bruin JP, Hompes PG, Repping S, Van Der Veen F, Mol BW.2015. Cost-effectiveness of assisted conception for male subfertility. Reproductive Biomedicine Online 30 659–66. ( 10.1016/j.rbmo.2015.02.006) [DOI] [PubMed] [Google Scholar]
- Omar MI, Pal RP, Kelly BD, Bruins HM, Yuan Y, Diemer T, Krausz C, Tournaye H, Kopa Z, Jungwirth Aet al. 2019. Benefits of empiric nutritional and medical therapy for semen parameters and pregnancy and live birth rates in couples with idiopathic infertility: a systematic review and meta-analysis. European Urology 75 615–625. ( 10.1016/j.eururo.2018.12.022) [DOI] [PubMed] [Google Scholar]
- Poljsak B.2011. Strategies for reducing or preventing the generation of oxidative stress. Oxidative Medicine and Cellular Longevity 2011 194586. ( 10.1155/2011/194586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, Korrovits P, Laan M.2017. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Human Reproduction 32 18–31. ( 10.1093/humrep/dew284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter SE, Evans AM.2012. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clinical Pharmacokinetics 51 553–5. ( 10.1007/BF03261931) [DOI] [PubMed] [Google Scholar]
- Rowe PJ, Comhaire FH, Hargreave TB, Mahmoud AMA.2000. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Male. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G, Barcellona ML, Vanella A.2000. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biology and Toxicology 16 91–9. ( 10.1023/a:1007685909018) [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A, Rosique-Esteban N, Becerra-Tomas N, Vizmanos B, Bullo M, Salas-Salvado J.2018. The effect of nutrients and dietary supplements on sperm quality parameters: a systematic review and meta-analysis of randomized clinical trials. Advances in Nutrition 9 833–848. ( 10.1093/advances/nmy057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, Lamb D, Chaney K, Van Voorhis BJ, Ryan Get al. 2020. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA 323 35–48. ( 10.1001/jama.2019.18714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, Oxman A.2013. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The Grade Working Group. [Google Scholar]
- Shalev DP, Soffer Y, Lewin LM.1986. Investigations on the motility of human spermatozoa in a defined medium in the presence of metabolic inhibitors and of carnitine. Andrologia 18 368–3. ( 10.1111/j.1439-0272.1986.tb01792.x) [DOI] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA.GROUP P-P 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350 g7647. ( 10.1136/bmj.g7647) [DOI] [PubMed] [Google Scholar]
- Shekarriz M, Thomas Jr AJ, Agarwal A.1995. Effects of time and sperm concentration on reactive oxygen species formation in human semen. Archives of Andrology 34 69–75. ( 10.3109/01485019508987833) [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Sonne S, Carter AL, Matern D, Ganapathy V.2013. Enzymes involved in L-carnitine biosynthesis are expressed by small intestinal enterocytes in mice: implications for gut health. Journal of Crohn’s and Colitis 7 e197–e. ( 10.1016/j.crohns.2012.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Glass S, Campagnone J, Pryor JL.2006. Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo-controlled trial. Fertility and Sterility 85 1409–14. ( 10.1016/j.fertnstert.2005.10.055) [DOI] [PubMed] [Google Scholar]
- Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG.2019. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 3 CD007411. ( 10.1002/14651858.CD007411.pub4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiber A, Kerner J, Hoppel CL.2004. Carnitine: a nutritional, biosynthetic, and functional perspective. Molecular Aspects of Medicine 25 455–4. ( 10.1016/j.mam.2004.06.006) [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, Krawetz SA, Usadi R, Baker VL, Coward RMet al. 2020. The effect of antioxidants on male factor infertility: the Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertility and Sterility 113 552, .e3–560.e3. ( 10.1016/j.fertnstert.2019.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey BT.1997. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Molecular Human Reproduction 3 203–2. ( 10.1093/molehr/3.3.203) [DOI] [PubMed] [Google Scholar]
- Tanphaichitr N.1977. In vitro stimulation of human sperm motility by acetylcarnitine. International Journal of Fertility 22 85–91. [PubMed] [Google Scholar]
- The Nordic Cochrane Centre 2014. Review Manager (RevMan). Version 5.3 ed. Copenhagen: The Cochrane Collaboration. [Google Scholar]
- Tournaye H.2006. Evidence-based management of male subfertility. Current Opinion in Obstetrics and Gynecology 18 253–25. ( 10.1097/01.gco.0000192994.37965.c6) [DOI] [PubMed] [Google Scholar]
- Tsounapi P, Honda M, Dimitriadis F, Koukos S, Hikita K, Zachariou A, Sofikitis N, Takenaka A.2018. Effects of a micronutrient supplementation combined with a phosphodiesterase type 5 inhibitor on sperm quantitative and qualitative parameters, percentage of mature spermatozoa and sperm capacity to undergo hyperactivation: a randomised controlled trial. Andrologia 50 e13071. ( 10.1111/and.13071) [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J.2007. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology 39 44–84. (. ( 10.1016/j.biocel.2006.07.001) [DOI] [PubMed] [Google Scholar]
- Vaz FM, Wanders RJ.2002. Carnitine biosynthesis in mammals. Biochemical Journal 361 417–4. ( 10.1042/0264-6021:3610417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S, Shamsi MB, Deka D, Saxena V, Kumar R, Dada R.2011. Clinical implications of oxidative stress & sperm DNA damage in normozoospermic infertile men. Indian Journal of Medical Research 134 396–39. [PMC free article] [PubMed] [Google Scholar]
- Veritas Health Innovation. Covidence Systematic Review Software [Online]. Melbourne, Australia. (available at: www.covidence.org). Accessed. [Google Scholar]
- World Health Organization 2010. Reference values and semen nomenclature. In WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization. [Google Scholar]
- World Health Organization 2020. Infertility definitions and terminology [Online]. World Health Organization. (available at: https://www.who.int/reproductivehealth/topics/infertility/definitions/en/). Accessed 16 July 2020. [Google Scholar]
- Zhang X, Cui Y, Dong L, Sun M, Zhang Y.2020. The efficacy of combined l-carnitine and l-acetyl carnitine in men with idiopathic oligoasthenoteratozoospermia: a systematic review and meta-analysis. Andrologia 52 e13470. ( 10.1111/and.13470) [DOI] [PubMed] [Google Scholar]
- Zini A, De Lamirande E, Gagnon C.1993. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. International Journal of Andrology 16 183–18. ( 10.1111/j.1365-2605.1993.tb01177.x) [DOI] [PubMed] [Google Scholar]
- Zopfgen A, Priem F, Sudhoff F, Jung K, Lenk S, Loening SA, Sinha P.2000. Relationship between semen quality and the seminal plasma components carnitine, alpha-glucosidase, fructose, citrate and granulocyte elastase in infertile men compared with a normal population. Human Reproduction 15 840–84. ( 10.1093/humrep/15.4.840) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a