Significance
Aggression is dependent on the sex of the conspecific in almost all animal species. But the neuronal basis of how sex-specific chemosensory signals regulate aggression is poorly understood. Using the fruit fly model of Drosophila melanogaster, we demonstrate that activation of a group of GABAergic central brain neurons, known to respond to sex-specific pheromonal stimuli, enhances aggression in dyadic male encounters. Inactivation of this neuronal group decreases aggression and increases the reciprocal social behavior of courtship. Our results can help trace the neural circuit from pheromone processing in the sensory neurons to behavior integration in the central brain and ultimately help understand how neurons encode the behavior of aggression.
Keywords: aggression, GABA, mAL, Drosophila melanogaster, males
Abstract
Aggression is known to be regulated by pheromonal information in many species. But how central brain neurons processing this information modulate aggression is poorly understood. Using the fruit fly model of Drosophila melanogaster, we systematically characterize the role of a group of sexually dimorphic GABAergic central brain neurons, popularly known as mAL, in aggression regulation. The mAL neurons are known to be activated by male and female pheromones. In this report, we show that mAL activation robustly increases aggression, whereas its inactivation decreases aggression and increases intermale courtship, a behavior considered reciprocal to aggression. GABA neurotransmission from mAL is crucial for this behavior regulation. Exploiting the genetic toolkit of the fruit fly model, we also find a small group of approximately three to five GABA+ central brain neurons with anatomical similarities to mAL. Activation of the mAL resembling group of neurons is necessary for increasing intermale aggression. Overall, our findings demonstrate how changes in activity of GABA+ central brain neurons processing pheromonal information, such as mAL in Drosophila melanogaster, directly modulate the social behavior of aggression in male–male pairings.
Aggression is an evolutionary conserved behavior required for acquiring food, territory, and mating partners. It is highly dependent on the sex of the conspecific. In Drosophila, as in most species, males fight males and never females. Females are instead courted by males as potential mates for reproduction. A dyadic male encounter in Drosophila is characterized by high levels of aggression with little or no courtship (1–4). Therefore, proper recognition of the sex of the conspecific is essential for appropriate displays of aggression. In fruit flies, pheromones constitute one of the principal sensory modalities guiding gender identification and behavioral decisions. Indeed, feminization of cuticular hydrocarbons (CHCs), a form of nonvolatile pheromones, in a tester male fly elicits reduced aggression and vigorous courtship from a wild-type male fly (1, 5, 6). One of the male-enriched CHCs, the (z)-7-tricosene (7-T), is necessary for maintaining the proper balance of aggression and courtship in male–male encounters. A group of sensory neurons mediating this role of 7-T is the Gr32a gustatory receptor neurons (GRNs) (2, 6, 7). Volatile pheromones can also influence aggression. Acute exposure to the male-specific volatile pheromone, 11- cis-vaccenyl acetate (cVA), increases male aggression by activating a population of odorant receptor neurons (ORNs) expressing the cVA receptor Or67d (6, 8). Interestingly, chronic exposure to cVA reduces male aggression by activating a group of ORNs expressing another cVA receptor, Or65a (9). In another study, males lacking Or67d were found to display increased courtship toward wild-type males (10). While these findings have made valuable inroads into understanding how some of the pheromone receptors and sensory neurons expressing them regulate aggression, a vast majority of central brain neurons poised to process the sex-specific chemosensory information remain to be investigated.
One group of central brain neurons known to be activated by both male and female pheromones is the mAL cluster (11, 12). The mAL neurons were originally identified as a sexually dimorphic cluster of FruM+, GABA+ interneurons, with ∼30 cells per hemisphere in males and only ∼5 in females. The mAL cell bodies are medially located above antennal lobes (ALs), and mAL presynaptic and postsynaptic terminals arborize in the superior lateral protocerebrum (SLP) and suboesophageal ganglion (SOG), respectively (13). Activation of the mAL cluster decreases Drosophila male courtship towards a female (11, 12, 14). A pair of elegant studies by Clowney et al. (12) and Kallman et al. (11) have shown that, at the circuit level, this regulation is achieved by inhibitory GABA release from mAL cluster into the main courtship command center of FruM+ P1 neurons in the fly brain. Compared to mAL’s role in courtship behavior, its role in regulating the reciprocal behavior of aggression is less well studied (14, 15). In this study, we systematically characterize mAL’s role in aggression. We demonstrate that mAL activation robustly increases male aggression, while mAL inactivation decreases aggression and increases intermale courtship in a concomitant manner. We also show that this behavioral regulation involves GABA neurotransmission from the mAL cluster. We were prompted to investigate mAL’s role in aggression by the results of a small-scale screening performed at the beginning of our study. To find aggression-promoting neurons with potential roles in chemosensory processing, we screened a set of GAL4 drivers targeting at least one or more central brain neuropils implicated in pheromonal signaling. Our screen yielded a line, R72D07-GAL4, whose thermogenetic activation resulted in a robust and simultaneous increase in intermale aggression and intermale courtship. Among the neurons targeted by the R72D07-GAL4 was a small group of approximately three to five GABA+ neurons per brain hemisphere with striking anatomical similarities with mAL. We subsequently found that activation of this mAL-resembling group was required for the increased aggression phenotype but not the increased courtship phenotype of the thermogenetically activated R72D07-GAL4 fights. Overall, our findings uncover a previously unknown role of mAL neurons in fruit fly aggression and yield a more complete understanding of its regulation of the behaviors of aggression and courtship in dyadic male encounters. In addition, we identify a small group of mAL-resembling neurons, whose activation was required for increasing male aggression.
Results
dTrpA1-Mediated Activation of R72D07-GAL4 Neurons Increases Aggression and UWEs in Drosophila Intermale Fights.
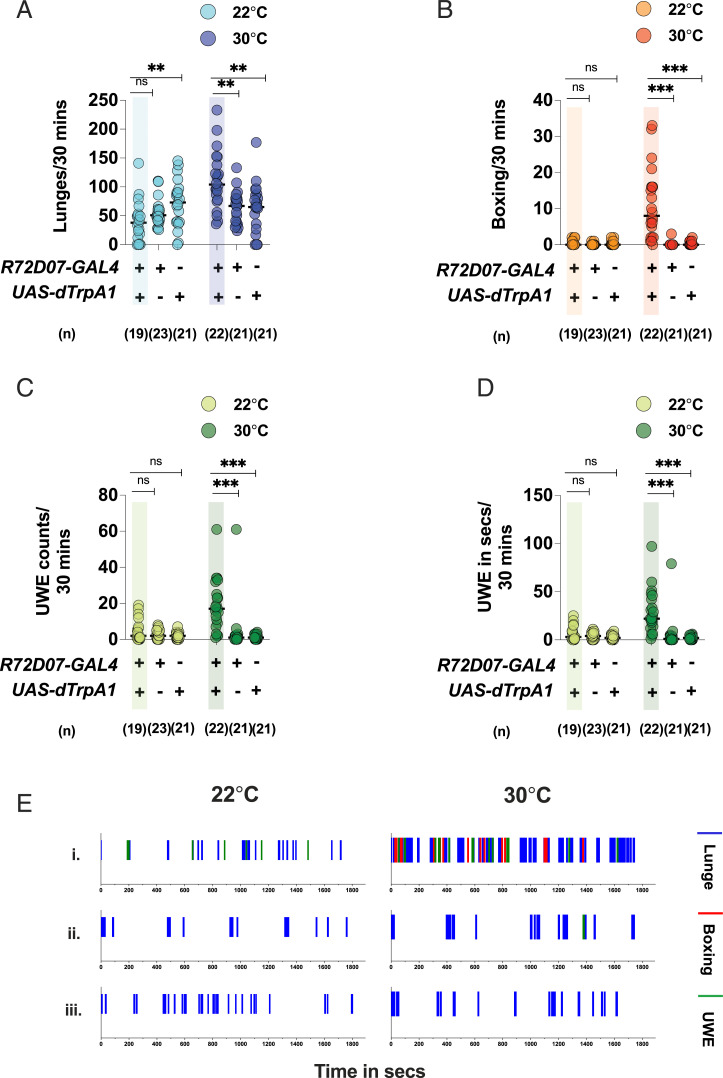
Some of the neuropils in fruit fly central brain implicated in processing pheromonal information include the AL (16), SOG (17), and ventrolateral protocerebrum (VLP) (18), among others. To find neurons that could regulate aggression by potentially participating in chemosensory processing, we visually screened the confocal stacks of the Janelia FlyLight collection (https://flweb.janelia.org/cgi-bin/flew.cgi) (19) and selected 12 GAL4 lines that included innervation in one or more of the above-mentioned neuropils. Using the GAL4/UAS binary system (20), we expressed the thermosensitive cation channel of dTrpA1 in the GAL4-labeled neurons, activated them at an ambient temperature of 30 °C (21), and screened for increased aggressive behavior in intermale fights. To quantify aggression, we scored the number of lunging and boxing events in each fight. The “lunge” is the predominant motor pattern used in a male fight, where a fly stands on its hind legs and snaps down on its opponent with its front legs. The motor pattern of “boxing” consists of two flies striking at one another with front legs and is characteristic of very high intensity aggression (22). Our preliminary screen yielded a line, R72D07-GAL4, which robustly increased the number of lunges and boxing when dTrpA1 activated at 30 °C (SI Appendix, Fig. S1) We retested this line for detailed analysis and found that, in addition to an increased aggression phenotype (Fig. 1 A and B), dTrpA1-activated R72D07-GAL4 males also exhibited a striking increase in unilateral wing extensions (UWEs), a marker for courtship behavior (23, 24). In dTrpA1-activated R72D07-GAL4 males, the increase in courtship was reflected in the number of UWEs executed as well as the total time spent in wing extension per fight (Fig. 1 C and D). Since the thermogenetic activation of R72D07-GAL4 significantly increased the reciprocal behaviors of aggression and courtship in male–male pairings, we decided to investigate this line further. Next, we studied the time course of lunging, boxing, and UWEs displayed in each fight. The time course study revealed that aggression and UWEs occurred in interspersed bouts throughout the observation period and were clearly increased in the temperature-activated R72D07-GAL4 males compared to the parental controls (Fig. 1E). Taken together, our behavioral analysis revealed that thermogenetic activation of R72D07-GAL4 neurons significantly promoted both aggression and courtship in intermale fights (Movies S1 and S2).
Fig. 1.
Thermogenetic activation of R72D07-GAL4 neurons increases aggression and UWEs in intermale fights: Thermogenetic activation of R72D07-GAL4 neurons at 30 °C increases (A) lunges, (B) boxing, (C) UWE counts, and (D) UWE time, compared to the parental controls. This phenotype is lost at the control temperature of 22 °C. For A–D, each circle represents lunges per 30 min, boxing per 30 min, UWE counts per 30 min, and UWE time in seconds per 30 min, respectively. For A–D, Kruskal–Wallis test followed by post hoc Dunn’s was performed for both the temperatures (**P < 0.01, ***P <0.001, ns = not significant at P > 0.05). Genotypes and number of pairs used are shown below each plot. (E) Thermogenetically activated R72D07-GAL4 males display increased aggression and UWEs in interspersed bouts: The x axis represents the observation period, which is 1,800 s (or 30 min). Representative fights of (i) R72D07-GAL4/UAS-dTrpA1 at 22 °C (Left) and 30 °C (Right), (ii) R72D07-GAL4-only control at 22 °C (Left) and 30 °C (Right), and (iii) UAS-dTrpA1-only control at 22 °C (Left) and 30 °C (Right).
Activation of Central Brain Neurons in R72D07-GAL4 Is Sufficient to Generate the Increased Aggression and Increased UWE Phenotype.
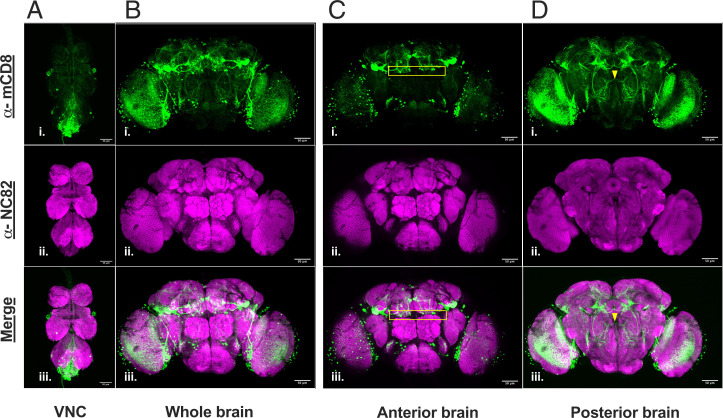
To investigate which neurons were targeted by the R72D07-GAL4, we combined it with the UAS-mCD8:GFP reporter and performed immunostaining with an α-mCD8 Ab. Our immunofluorescence results revealed that the R72D07-GAL4 targeted multiple neurons in the central brain and the ventral nerve cord (VNC) (Fig. 2 A and B). In the central brain itself, ∼125 neurons (125 mean SD, n = 6) were targeted. This included neurons above ALs, in the optic lobes, mushroom bodies, and anterior and posterior VLP, among others.
Fig. 2.
R72D07-GAL4 labels many neurons in the central brain, including a small group of mAL-resembling neurons: Expression of UAS-mCD8:GFP by R72D07-GAL4 reveals neurons in adult (A) VNC and (B) central brain. α-mCD8 (green) marks the R72D07-GAL4–targeted neurons. Neuropil marker α-NC82 (magenta) visualizes the brain and VNC. Among the neurons targeted by the R72D07-GAL4 is a small group of approximately three to five neurons per hemisphere with anatomical similarities to mAL. (C) Cell bodies of this group are located above ALs (yellow rectangle), like the mAL. (D) Processes of this group exhibit midline crossing (yellow arrow), and descend to the SOG, like the mAL. A and B are maximum projection through all the optical slices of the confocal stack. C and D are maximum projection of optical slices containing the cell bodies and processes of the mAL-resembling group, respectively. (Scale bars, 50 µm.)
As the first step in identifying the neuron(s) triggering the increased aggression and increased UWE phenotype, we determined whether the phenotype was derived from the central brain or the VNC. We restricted the GAL4 activity in the VNC by using the Tshirt-Gal80 transgene (25) and observed a reduction in the α-mCD8 signal in the VNC (SI Appendix, Fig. S2 E, Lower). Interestingly, dTrpA1 activation of the R72D07-GAL4 neurons in the presence of Tshirt-Gal80 significantly increased both aggression (SI Appendix, Fig. S2 A and B) and UWEs in intermale fights (SI Appendix, Fig. S2 C and D). In short, these observations suggested that activation of the R7D07-GAL4 central brain neurons was sufficient for generating the increased aggression and increased UWE phenotype.
To further narrow down neuronal population in the central brain behind the increased aggression and increased UWE phenotype, we adopted an intersectional genetic strategy. Briefly, a UAS<STOP>dTrpA1myc; R72D07-GAL4 line was screened against a library of 32 enhancer trap flippase lines (Et-FLP) developed in our laboratory (26). Each of these Et-FLP lines drove FLP recombinase expression in different neuronal populations in the central nervous system. Therefore, in a UAS<STOP>dTrpA1myc; R72D07-GAL4/Et-FLP fly (henceforth denoted as R72D07∩Et-FLP), dTrpA1-mediated activation is only possible in neurons targeted by both the R72D07-GAL4 and the Et-FLP (SI Appendix, Fig. S3A). Upon thermogenetic activation at 30 °C, the intersectional screen yielded the R72D07∩293-FLP combination which had the highest median for lunges and the highest fighting frequency (SI Appendix, Fig. S3 B and D). Seventy percent of the R72D07∩293-FLP fights had lunges above the 90th percentile of the R72D07∩No-FLP control fights. Interestingly, the R72D07∩293-FLP fights did not exhibit any notable increase in either boxing (SI Appendix, Fig. S3C) or UWEs (Movie S3). α-Myc staining revealed ∼62 cells (62.5 , mean SD, n = 6) in different parts of the R72D07∩293-FLP central brain (SI Appendix, Fig. S4B). Overall, our data suggested that R72D07∩293-FLP targeted a group of neurons whose activation increased lunges without increasing boxing or UWEs. Our intersectional strategy also raised the hypothesis that increased aggression and increased UWEs were triggered by separate sets of central brain neurons in the R72D07-GAL4 line. Right now, we do not know whether the increased boxing phenotype of the dTrpA1-activated R72D07-GAL4 males was triggered by a set of neurons not elicited in any of the intersectional lines or is a feedback response to the repeated events of lunging and UWEs or a combination of both.
Although the starting population of neurons in the central brain was reduced from ∼125 to ∼62 in the R72D07∩293-FLP combination, it was not small enough to attribute the increased aggression phenotype to any specific neuron or cluster of neurons. Upon further exploration, we found that the R72D07-GAL4 labeled a small group of approximately three to five central brain neurons per hemisphere with anatomical similarities to mAL. Cell bodies of these neurons were located above the ALs (Fig. 2C and SI Appendix, Fig. S5A), and their neuronal processes exhibited midline crossing and descended to the SOG (Fig. 2D and SI Appendix, Fig. S5B) in a manner reminiscent of the mAL neurons (11–13). The R72D07∩293-FLP combination whose activation increased intermale lunging also included the small group of mAL-resembling cells (SI Appendix, Fig. S4 C and D). Taken together, these findings prompted us to investigate mAL’s role in aggression. While courtship suppression upon mAL activation is well known (11, 12, 23), its effect on aggression is less well studied (14, 15).
Activation of mAL Neurons Increases Intermale Aggression in a GABA-Dependent Manner.
To investigate how mAL neurons influenced intermale aggression, we used the R43D01-GAL4 line which primarily drove expression into the mAL cluster (11, 12, 14). The R43D01-GAL4 targets ∼22 to 24 FruM+ mAL cells per hemisphere (left hemisphere: , right hemisphere: , mean SD, n = 4) (SI Appendix, Fig. S6).
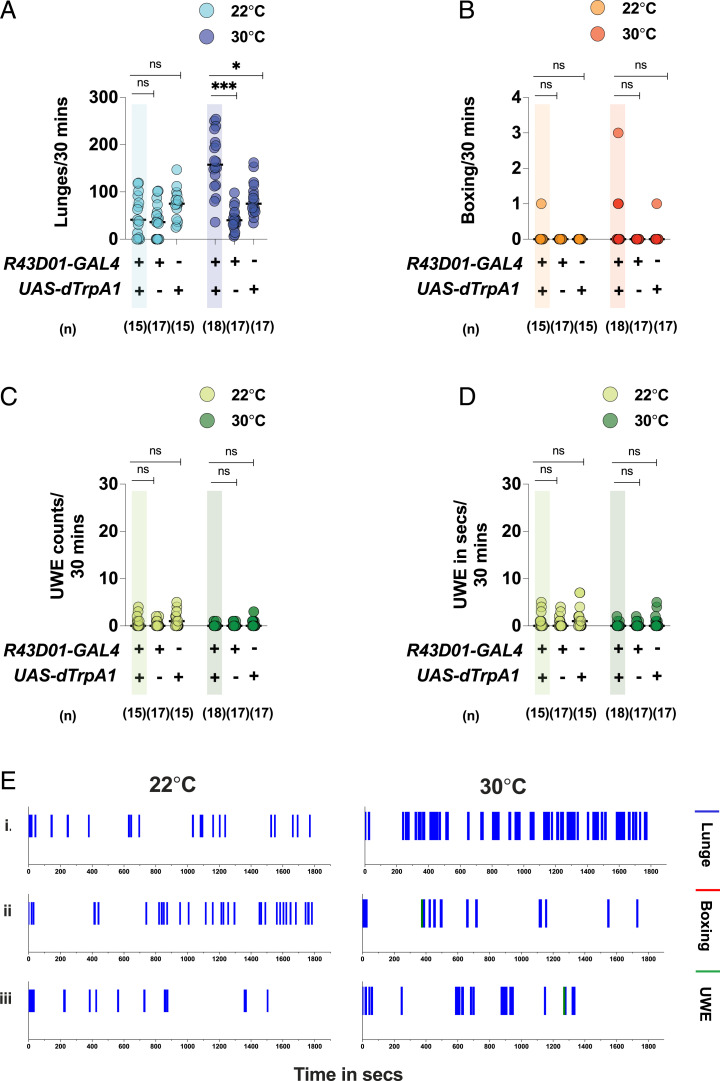
dTrpA1-mediated activation of the mAL neurons in males robustly increased lunges (Fig. 3 A and E), without any significant change in boxing (Fig. 3 B and E) or UWEs (Fig. 3 C–E). Thus, mAL activation in males increased aggression by increasing the number of lunges (Movie S4). The mAL neurons are also present in females and are sexually dimorphic in number and arborization patterns (13). Intriguingly, dTrpA1-mediated activation of mAL neurons in females also resulted in significantly increased interfemale aggression (SI Appendix, Fig. S7). Two motor patterns characteristic of female aggression, “headbutts” and “wings-up and charging,” were scored (27, 28) and found to be significantly elevated in females with mAL activation. Taken together, our data indicated that mAL activation increased aggression in both intermale and interfemale fights. However, in rest of this study, we will focus on mAL’s role in male aggression.
Fig. 3.
Thermogenetic activation of mAL neurons increases lunges in intermale fights: Thermogenetic activation of mAL neurons at 30 °C increases (A) lunges but does not change (B) boxing, (C) UWE counts, or (D) UWE time, compared to the parental controls. At the control temperature of 22 °C, the phenotype of increased lunges is lost. For A–D, each circle represents lunges per 30 min, boxing events per 30 min, UWE counts per 30 min, and UWE time in seconds per 30 min, respectively. For A–D, Kruskal–Wallis test followed by post hoc Dunn’s was performed for both the temperatures (*P < 0.05, ***P <0.001, ns = not significant at P > 0.05). Genotypes and number of pairs used are indicated below each plot. (E) The mAL activation increases lunging without any effect on boxing or UWEs: The x axis represents the observation period, which is 1,800 s (or 30 min). Representative fights of (i) R43D01-GAL4/UAS-dTrpA1 at 22 °C (Left) and 30 °C (Right), (ii) R43D01-GAL4-only control at 22 °C (Left) and 30 °C (Right), and (iii) UAS-dTrpA1-only control at 22 °C (Left) and 30 °C (Right).
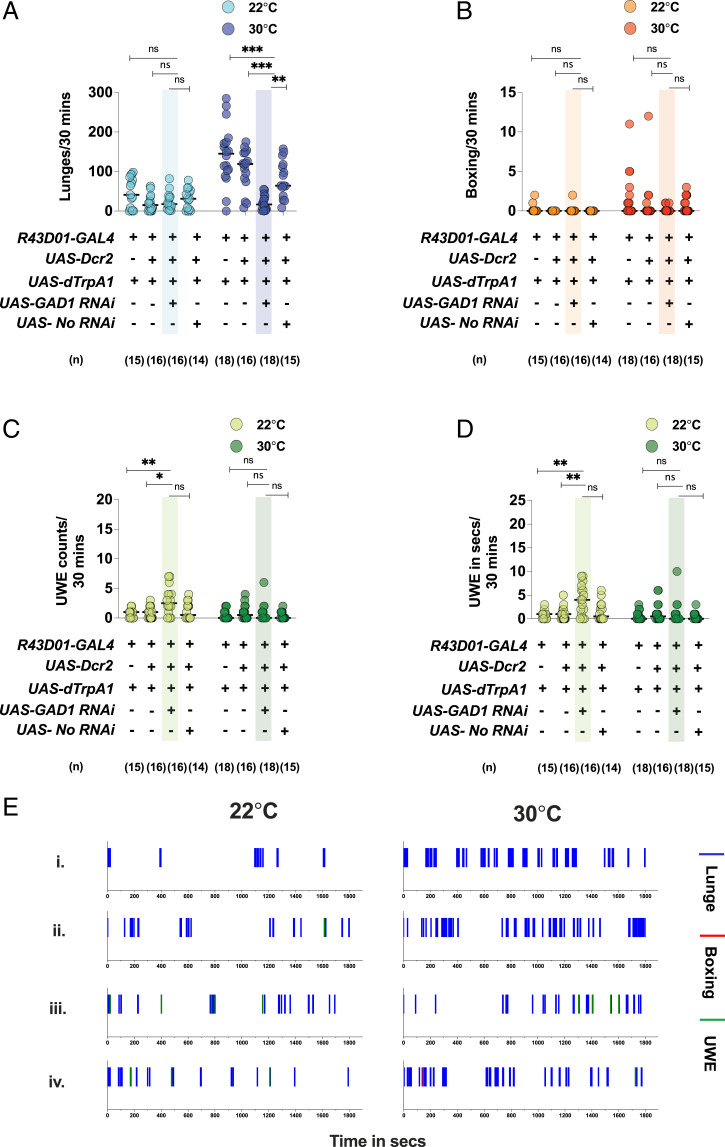
Ninety-eight percent of the cells in the male mAL cluster were GABA+ (left hemisphere: , right hemisphere: , mean SD, n = 4) (SI Appendix, Fig. S6C). In addition, we found that the mAL neurons were the only GABA+ cells in the R43D01-GAL4 brain. To investigate whether GABA transmission from the activated mAL neurons was responsible for the increased aggression phenotype, we performed RNA interference (RNAi) against Glutamic Acid Decarboxylase 1 (GAD1), a major gene in the GABA synthesis pathway (14). RNAi-mediated knockdown of GAD1 strongly attenuated the ability of mAL to increase lunges upon dTrpA1 activation, an effect not seen with the empty-RNAi control (Fig. 4 A and E). In summary, these observations strongly suggested that GABA transmission from the activated mAL cluster was crucial for promoting aggression in intermale fights.
Fig. 4.
GABA transmission from activated mAL neurons is crucial for increased aggression: GAD1 RNAi in thermogenetically activated mAL fights attenuates the (A) increased lunging phenotype. For A–D, each circle represents lunges per 30 min, boxing per 30 min, UWE counts per 30 min, and UWE time in seconds per 30 min, respectively. For A–D, Kruskal–Wallis test followed by post hoc Dunn’s was performed for the control and experimental temperatures of 22 °C and 30 °C (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant at P > 0.05), respectively. Genotypes and number of pairs used are shown below each plot. (E) GAD1 RNAi attenuates the increased lunging phenotype of mAL-activated fights: The x axis represents the observation period, which is 1,800 s (or 30 min). Representative fights of (i) R43D01-GAL4/UAS-dTrpA1 at 22 °C (Left) and 30 °C (Right), (ii) UAS-Dcr2; R43D01-GAL4/UAS-dTrpA1 at 22 °C (Left) and 30 °C (Right), (iii) UAS-Dcr2/UAS-GAD1 RNAi; R43D01-GAL4/UAS-dTrpA1 at 22 °C (Left) and 30 °C (Right), and (iv) UAS-Dcr2/UAS- no RNAi; R43D01-GAL4/UAS-dTrpA1 at 22 °C (Left) and 30 °C (Right).
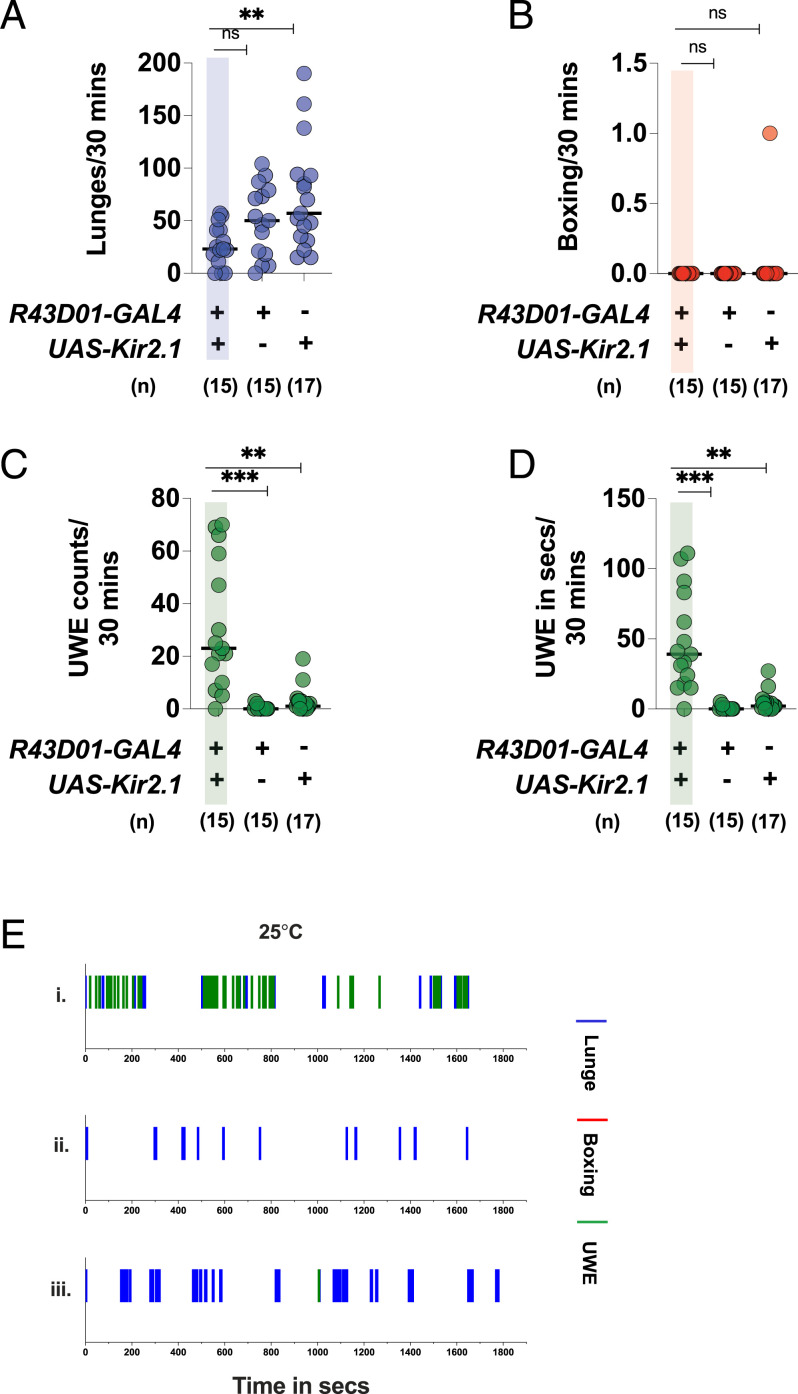
Kir2.1-Dependent Inactivation of mAL Neurons Decreases Aggression in Intermale Fights and Increases UWE.
Next, we asked whether mAL inactivation influenced baseline aggression. We inactivated the mAL neurons by expressing the hyperpolarizing inward rectifying potassium channel Kir2.1 (29). Kir2.1-dependent mAL inactivation in males significantly increased UWEs, as was reported before (Fig. 5 C and D) (11, 14). Interestingly, males with inactivated mAL exhibited a significant reduction in lunges (Fig. 5A) and a low fighting frequency (SI Appendix, Fig. S9B). Only 20% of fights with mAL inactivation had lunges above the 50th percentile of the R43D01-GAL4 only control fights. A time course study revealed that lunges and UWEs occurred in interspersed bouts upon mAL inactivation and that lunges were decreased and UWEs increased compared to the parental controls (Fig. 5E). Since mAL neurons are GABA+, we investigated whether RNAi-mediated GAD1 knockdown phenocopied the Kir2.1-dependent inactivation effects. GAD1 knockdown in the mAL cluster reduced the number of lunges, but the significance was lost after multiple comparisons (SI Appendix, Fig. S8 A and E). The fighting frequency was also reduced (SI Appendix, Fig. S9D). Only 18% of fights with mAL GAD1 RNAi had lunges above the 50th percentile of UAS-Dcr2; R43D01-GAL4 only control fights. The courtship marker UWE was significantly increased in mAL GAD1 RNAi fights, but the increase was not as robust as the Kir2.1-dependent inactivation (SI Appendix, Fig. S8 C–E). These observations indicated that Kir2.1-mediated mAL inactivation decreased aggression, with a concomitant increase in UWEs (Movie S5). RNAi-mediated GABA knockdown in mAL qualitatively recapitulated the Kir2.1 inactivation effects. In summary, these findings strongly suggested that optimum mAL activity was required for maintaining the appropriate balance of aggression and UWEs in male–male pairings.
Fig. 5.
The mAL inactivation by Kir2.1 decreases aggression and increases UWEs in intermale fights: mAL inactivation decreases (A) lunges and increases (C) UWE counts and (D) UWE time. (B) The mAL inactivation does not have any effect in boxing. For A–D, each circle represents lunges per 30 min, boxing per 30 min, UWE counts per 30 min, and UWE time in seconds per 30 min, respectively. For A–D, Kruskal–Wallis test followed by post hoc Dunn’s was performed (**P < 0.01, ***P <0.001, ns = not significant at P > 0.05). Genotypes and number of pairs used are shown below each plot. (E) The mAL inactivation decreases lunges and concomitantly increases UWEs: The x axis represents the observation period which is 1,800 s (or 30 min). Representative fights of (i) R43D01-GAL4/UAS-Kir2.1, (ii) R43D01-GAL4-only control, and (iii) UAS-Kir2.1-only control.
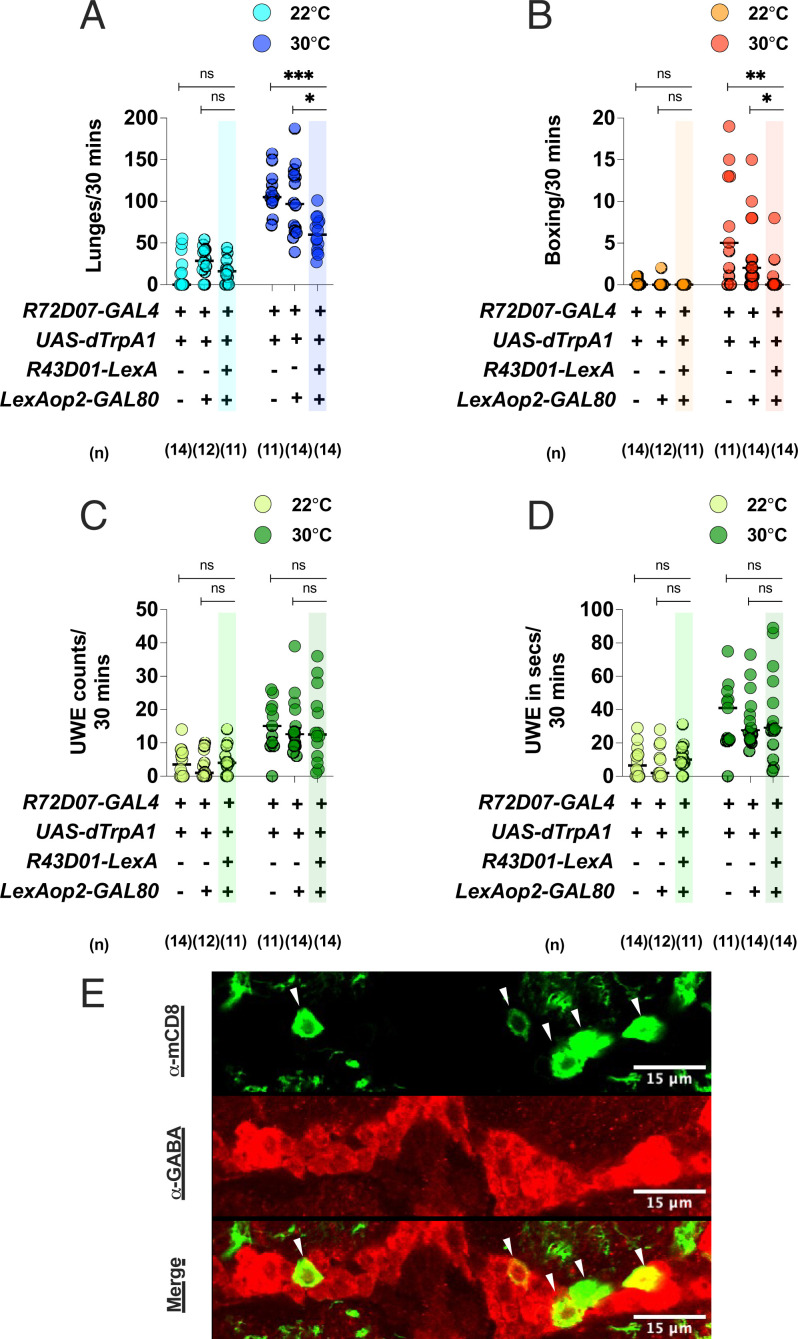
Activation of a Subpopulation of Approximately Three to Five mAL-Resembling Cells in the R72D07-GAL4 Line Contributes to Increased Male Aggression
Finally, we determined whether the small group of mAL-resembling cells in the R72D07-GAL4 line influenced the increased aggression and increased UWE phenotype of the dTrpA1-activated R72D07-GAL4 fights. Since mAL neurons are GABA+, we first determined whether the mAL-resembling cells were also GABA+. Staining with an α-GABA Ab revealed these cells to be GABA+ (Fig. 6E). Next, we investigated whether their activation was necessary for the increased aggression and increased UWE phenotype. Using the R43D01-LexA driver, known to primarily label the mAL neurons (11, 14), we expressed the GAL80 transgene to suppress GAL4 activity and effectively block dTrpA1 activation in these cells. Interestingly, the increased aggression phenotype of the dTrpA1-activated R72D07-GAL4 fights was strongly attenuated with GAL80 expression (Fig. 6 A and B), whereas the increased UWE phenotype was unaffected (Fig. 6 C and D). Therefore, these findings suggested that activation of the mAL-resembling group of cells in the R72D07-GAL4 line was necessary for the increased aggression phenotype but not the increased UWE phenotype. Along with our immunostaining results (Fig. 2 and SI Appendix, Figs. S4 and S5), these data strongly raised the possibility that these cells represent a subpopulation of the mAL cluster. However, to fully validate this possibility, we need a more detailed analysis of their projection patterns using experiments such as Multicolor FlpOut (30). Finally, these data also argued in favor of the hypothesis that the increased aggression and increased UWEs were triggered by activation of separate neurons in the R72D07-GAL4 line.
Fig. 6.
Activation of a small group of mAL-resembling cells in the R72D07-GAL4 line is required for increased male aggression but not increased UWEs: Driving GAL80 expression with the R43D01-LexA driver attenuates the (A) increased lunging and (B) increased boxing of the thermogenetically activated R72D07-GAL4 fights without any effect on the (C) increased UWE counts or (D) increased UWE time. For A–D, each circle represents lunges per 30 min, boxing events per 30 min, UWE counts per 30 min, and UWE time in seconds per 30 min, respectively. For A–D, Kruskal–Wallis test followed by post hoc Dunn’s was performed for the control and experimental temperatures of 22 °C and 30 °C, respectively (*P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant at P > 0.05). Genotypes and number of pairs used are shown below each plot. (E) R72D07-GAL4–labeled mAL-resembling neurons (green, α-mCD8) are GABA+ (red, α-GABA) and are denoted by white arrows. Overlap of signals in a single optical slice is shown for accurate representation. (Scale bars, 15 µm.)
Discussion
The neural circuit behind aggression is poorly understood. The initial goal of this study was to identify aggression-promoting neurons with potential roles in chemosensory processing. For that, we performed a thermogenetic activation screen of 12 GAL4s, each targeting at least one or more brain neuropils implicated in pheromonal signaling (SI Appendix, Fig. S1). Our screen yielded a line, R72D07-GAL4, whose thermogenetic activation robustly increased aggression and courtship in Drosophila male–male pairings (Fig. 1). Using intersectional genetics, we found a relatively reduced subset of neurons (R72D07∩293-FLP) in the R72D07-GAL4 brain whose activation increased intermale aggression but did not increase intermale courtship (SI Appendix, Fig. S3). Interestingly, the R72D07∩293-FLP labeled neuronal population contained a small group of approximately three to five GABA+ cells per brain hemisphere with a strong anatomical resemblance to mAL (SI Appendix, Fig. S4 C and D). Based on this, we next asked, do mAL neurons regulate aggression?
To answer this question, we used the R43D01-GAL4 driver which primarily labeled a group of ∼22 to 24 FruM+, GABA+ mAL neurons (SI Appendix, Fig. S6). Thermogenetic activation of the R43D01-GAL4–labeled mAL neurons robustly increased the number of lunges in intermale fights, a phenotype that was strongly attenuated upon GAD1 RNAi. This suggested GABA release from activated mAL neurons was crucial for increasing aggression (Figs. 3 and 4). Inactivating mAL neurons reduced baseline aggression and increased intermale courtship in a concomitant manner. RNAi-mediated GAD1 knockdown showed behavioral trends similar to Kir2.1-dependent silencing but lacked the robustness of the latter, possibly because RNAi-dependent silencing was not as strong as Kir2.1-dependent silencing in this case. Nevertheless, the Kir2.1 and GAD1 RNAi experiments suggested that mAL silencing resulted in low aggression and high UWEs (Fig. 5 and SI Appendix, Fig. S8). In wild-type encounters of Drosophila males, flies predominantly engage in aggression with little or no UWE (2, 6). Our data strongly suggest that optimal activation of the mAL cluster is necessary for maintaining a normal balance of aggression and courtship in male–male pairings. It is worth noting that, while we recorded a significant increase in UWEs upon mAL inactivation, we did not observe any decrease in UWEs (Fig. 3 C and D) upon mAL activation. Currently, we do not know whether the observable lack of decrease in UWEs required a stronger mAL activation than what was afforded by dTrpA1 or was simply a case of “floor effect.”
In contrast to our findings, a previous study by Koganezawa et al. (14) reported that mAL activation induced neither aggression nor courtship in male–male pairings. The discrepancy in findings could stem from the following sources: 1) The mAL subset in this study consisted of ∼22 to 24 mAL cells per hemisphere. A relatively smaller subset of ∼16 mAL cells was targeted in ref. 14. It is likely that the mAL subsets targeted in the two studies included at least a few nonoverlapping mAL neurons. 2) Genetically different dTrpA1 activators were used: UAS-dTrpA1 in this study v/s UAS<STOP>dTrpA1myc in ref. 14. UAS<STOP>dTrpA1myc is known to be a weaker activator than UAS-dTrpA1 (11, 31, 32). Due to points 1 and 2, one plausible explanation is that the two mAL subsets differing in at least few component mAL neurons were activated at different strengths, potentially resulting in different behavioral outputs. 3) The studies also differed in several technical aspects of the behavioral assays (such as thermoregulation for dTrpA1activation, behavior scoring, and chamber setups), making direct comparisons of results difficult. Clearly, these differences have a bearing on behavior outputs, since almost all the control fights in ref. 14 display little to no baseline aggression, whereas the control fights in our setups display clear baseline aggression.
The mAL neurons are known to be activated by pheromones (11, 12). Which sensory neurons could potentially communicate pheromonal stimuli to mAL? A strong candidate is the nonvolatile male CHC 7-T detecting Gr32a GRNs. Axons of Gr32a GRNs extensively arborize in the SOG, a region also innervated by mAL dendrites. Indeed, close apposition of Gr32a GRN axons and mAL dendrites has been detected in the SOG (7, 23). Previous studies have shown that males lacking Gr32a receptor exhibited reduced aggression and increased courtship in male–male pairings (2, 7, 18), a phenotype similar to the mAL inactivation found in this study. Therefore, it is possible that at least a subset of Gr32a GRNs physiologically communicates with central brain mAL neurons to transduce 7-T stimuli and help maintain an appropriate balance of aggression and courtship in male encounters. The mAL neurons can also potentially collect information from volatile pheromone-detecting sensory neurons. Males with mutant Or67d, a receptor for the male-specific volatile pheromone cVA, have been shown to display high courtship towards wild-type males (10). In another study, males without intact Or67d function have been shown to display reduced intermale aggression (8). Thus, loss-of-function of Or67d and mAL appear to have similar effects on aggression and courtship in male–male pairings. In the future, it would be interesting to explore whether a group of Or67d ORNs connects with a subset of mAL neurons to exert its role in Drosophila male encounters.
One group of neurons known to receive the GABAergic input from mAL activation is the male-specific, FruM+ P1 neurons. The mAL and P1 fibers intermingle in the lateral protocerebral complex of the central brain, and P1 is probably postsynaptic to mAL. Activation of the P1 group of neurons strongly promotes courtship in a Drosophila male toward a female, an effect robustly attenuated by simultaneous mAL activation (11, 12, 25, 33–35). A later study identified a subgroup of the P1 cluster (∼8 to 10 P1 neurons per hemisphere, also known as P1a), whose activation enhanced both aggression and courtship in male–male pairings (31). An important question here is, how does mAL activation influence P1a driven behaviors? Genetic epistatic experiments simultaneously activating both the mAL and P1a neurons could provide an insight into this question.
We find that activation of ∼22 to 24 mAL neurons en masse increases aggression in male–male pairings. Our experiments with the R72D07-GAL4 line identified a small group of ~3–5 GABAergic cells with morphological resemblance to mAL. Activation of this group was necessary for the increased aggression phenotype but not increased courtship phenotype of dTrpA1-activated R72D07-GAL4 fights (Fig. 6). This raises another question: Is the entire mAL cluster or just a small subgroup of it sufficient for increasing aggression? The mAL activation results in at least two behavioral outputs: 1) suppression of male courtship toward a female (11, 12, 14) and 2) promotion of intermale aggression (this study). It is plausible that mAL is a functionally heterogenous cluster, and courtship and aggression are regulated by different neuronal subgroups within the cluster. Consistent with this, Costa et al. (36) has classified the male mAL cluster into three main types and two subtypes based on positional differences in their dendritic and axonal arbors. Genetic mosaic approaches such as mosaic analysis with repressible cell marker (37) will be necessary to stochastically label subgroups of mAL neurons and systematically track their roles in aggression and courtship.
Our study systematically characterizes the role of mAL neurons in Drosophila intermale aggression. With an ever-expanding genetic and molecular toolkit of the fruit fly model, experiments delineating the circuit-driven mechanisms through which mAL neuronal subsets might regulate the reciprocal behaviors of aggression and courtship may not be far off. Along with previous research (11, 12, 14, 15), our findings provide an important framework in which to undertake these experiments.
Materials and Methods
Fly Stocks.
Dataset S1 contains a full list of the genotypes. The flies were reared on standard fly food containing cornmeal, sucrose, yeast, and agar with 50% humidity, on a 12 h light/12 h dark cycle. The following stocks were generously provided: Tshirt-Gal80 by Julie Simpson, University of California, Santa Barbara, and R43D01-LexA by Kristin Scott, University of California, Berkeley. The following stocks from Bloomington Stock Center were used: R72D07-GAL4 (# 39770), R72B09-GAL4 (# 46670), R13C09-GAL4 (#48555), R13F09-GAL4 (#48577), R31A02-GAL4 (#49654), R46D02-GAL4 (#50263), R72B03-GAL4 (#39610), R72B07-GAL4 (#39764), R72C01-GAL4 (#47729), R72C04-GAL4 (#49416), R72A10-GAL4 (#48306), R72C08-GAL4 (#49621), UAS-mCD8:GFP (#32185), UAS<STOP>dTrpA1myc (#66871), R43D01-GAL4 (#64345), UAS-dTrpA1 (#26264), UAS-Dcr2 (#24650), UAS-GAD1 RNAi (#51794), UAS- no RNAi (#36304), and LexAop2-GAL80 (#32214). An Et-FLP library was generated in the laboratory as described previously (26). The Et-FLP lines used for intersectional genetics are indicated in the figure (SI Appendix, Fig. S1)
Aggression Assays and Scoring.
Late-stage pupae were isolated and placed in individual glass vials (catalog #47729-576) containing 1.5 mL of standard fly food at 19 °C. Flies eclosed in these vials and were raised in social isolation for 12 d to 14 d. At least 4 d before behavioral experiments, each fly was briefly anesthetized with CO2, marked with a small dot of paint on the dorsal thorax for identification, and returned to the original vial for recovery.
All behavioral experiments were same-sex pairings of the same genotype and approximately the same age. Each chamber contained a food cup, filled with standard fly food and a dash of yeast paste in the center; see Movies S1, S3, S4, or S5. To mitigate temperature-mediated developmental differences (38), flies used for all the experiments were raised at 19 °C for 12 d to 14 d in social isolation after eclosion and subsequently assayed at either 30 °C for dTrpA1 activation or 25 °C for Kir2.1 or GAD1 RNAi-dependent silencing. Humidity of the behavior room was set to ∼50%. For the preliminary screening of 12 GAL4 lines (SI Appendix, Fig. S1), an observation period of 10 min (or 600 s) was used. Lunging and boxing events were counted to detect changes in male aggression. Once the R72D07-GAL4 was detected as a line with increased male aggression, it was retested for detailed analysis with additional genetic and temperature controls, along with a longer observation period of 30 min (or 1,800 s). Except for the preliminary screening in SI Appendix, Fig. S1, all fights were analyzed using an observation window of 30 min (or 1,800 s). Fights were videotaped and analyzed by QuickTime Player (10.4) for Macintosh. An intermale fight of 30 min was manually scored for the following: 1) number of lunges, 2) number of boxing events, and 3) UWEs. To reflect UWE abundance per fight, both the number of UWEs as well as the amount of time spent in performing UWEs were counted. An interfemale fight of 30 min was manually scored for 1) headbutts and 2) wings-up and charging (27, 28).
Immunohistochemistry.
Adult male brains and VNC were dissected, fixed, and stained as described previously (15). The following primary antibodies were used: rat α-mouse CD8 (1:100, Invitrogen, MCD0800), mouse α-nc82 (1:20, Developmental Studies Hybridoma Bank), rat α-DN-Cadherin (1:20, Developmental Studies Hybridoma Bank, DN-Ex #8), mouse α-myc (1:1,000, Abcam, ab32 9E10), rabbit α-GABA (1:500, Sigma-Aldrich, A2052), mouse- α-GFP (1:500, Invitrogen, A11120), and rabbit α-FruM (1:2,000). The following secondary antibodies from Invitrogen were used at 1:300 dilution: Alexa Fluor 488 goat α-rat, Alexa Fluor 594 goat α-mouse, Alexa Fluor 488 goat α-mouse, Alexa Fluor 568 goat α-rabbit, and Texas Red X goat α-rat. All images are confocal serial sections, which were acquired using Olympus FluoView FV1000 microscope with either 20×/NA 0.75(air) objective or 60×/NA1.42(oil) objective and processed in Fiji (https://fiji.sc/). Maximum projections were generated with the entire stack when GAL4 targeting of the entire brain or VNC is shown. Maximum projections were generated with a partial stack when GAL4 targeting of a specific group of neurons is shown. In other words, optical slices containing the cell bodies and/or processes of the specific group were used for generating the maximum projection image. To show colabeling of antibodies, staining in a single optical slice is shown for accurate representation.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism 9. For behavioral experiments in Figs. 1 A–D, 3 A–D, 4 A–D, 5 A–D, and 6 A–D and SI Appendix, Figs. S2 A–D, S7, and S8 A–D, data are presented as scatter dot plots with a line at the median. Since experiments in these figures included at least three groups, nonparametric Kruskal–Wallis followed by Dunn’s post hoc test was performed. Statistical significance was considered at P < 0.05. P values are represented as follows: *P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant at P > 0.05. No value was excluded from statistical analysis. Sample sizes used in this paper were based on previous literature in the aggression field and were not predetermined by power analysis. Experiments were not blinded. A list of exact P values is provided in Dataset S2. For SI Appendix, Figs. S1 A and B, S3 B and C, and S9 A and C, data are represented as boxplot with whiskers extending to the 10th and the 90th percentile with a line at the median. Individual data points for fights are overlaid on the respective boxplots. Descriptive statistics used for calculating fighting frequency was calculated in GraphPad Prism 9. Fighting frequency is represented by a bar graph in SI Appendix, Figs. S1C, S3D, and S9 B and D.
Supplementary Material
Acknowledgments
We thank members of the E.A.K. laboratory for helpful discussions on this project. We thank Drs. R. Wilson, K. Ressler, and D. Rogulja for helpful comments on the manuscript. We thank the imaging facility in the Department of Neurobiology at Harvard Medical School for helping with imaging experiments. This facility is supported by National Institute of Neurological Disorders and Stroke P30 Core Center Grant NS072030. We also thank the Nikon Imaging Center at Harvard Medical School for helpful discussions on confocal microscopy. These studies were supported by R35 GM118137 to E.A.K. and Louis Perry Jones Postdoctoral Fellowship to S.S. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Reviewers: S.G., University of Oxford; and J.L., University of Toronto.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117101119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Fernández M. P., et al. , Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 8, e1000541 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L., et al. , Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci. 14, 757–762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monyak R. E., et al. , Masculinized Drosophila females adapt their fighting strategies to their opponent. J. Exp. Biol. 224, jeb238006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vrontou E., Nilsen S. P., Demir E., Kravitz E. A., Dickson B. J., Fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 9, 1469–1471 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Ferveur J.-F., et al. , Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science 276, 1555–1558 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Fernández M. P., Kravitz E. A., Aggression and courtship in Drosophila: Pheromonal communication and sex recognition. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 1065–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews J. C., et al. , Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet. 10, e1004356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Anderson D. J., Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., et al. , Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosci. 14, 896–902 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Kurtovic A., Widmer A., Dickson B. J., A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Kallman B. R., Kim H., Scott K., Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 4, e11188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clowney E. J., Iguchi S., Bussell J. J., Scheer E., Ruta V., Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura K., Ote M., Tazawa T., Yamamoto D., Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229–233 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Koganezawa M., Kimura K., Yamamoto D., The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Curr. Biol. 26, 1395–1403 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Chan Y.-B., Kravitz E. A., Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 104, 19577–19582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott K., et al. , A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Dunipace L., Meister S., McNealy C., Amrein H., Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822–835 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto T., Amrein H., Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11, 874–876 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenett A., et al. , A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones W. D., The expanding reach of the GAL4/UAS system into the behavioral neurobiology of Drosophila. BMB Rep. 42, 705–712 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Hamada F. N., et al. , An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S., Lee A. Y., Bowens N. M., Huber R., Kravitz E. A., Fighting fruit flies: A model system for the study of aggression. Proc. Natl. Acad. Sci. U.S.A. 99, 5664–5668 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koganezawa M., Haba D., Matsuo T., Yamamoto D., The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol. 20, 1–8 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Pan Y., Robinett C. C., Baker B. S., Turning males on: Activation of male courtship behavior in Drosophila melanogaster. PLoS One 6, e21144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J. Y., Kanai M. I., Demir E., Jefferis G. S., Dickson B. J., Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20, 1602–1614 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Alekseyenko O. V., Chan Y.-B., Li R., Kravitz E. A., Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 110, 6151–6156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsen S. P., Chan Y.-B., Huber R., Kravitz E. A., Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 101, 12342–12347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palavicino-Maggio C. B., Chan Y.-B., McKellar C., Kravitz E. A., A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc. Natl. Acad. Sci. U.S.A. 116, 17029–17038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baines R. A., Uhler J. P., Thompson A., Sweeney S. T., Bate M., Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nern A., Pfeiffer B. D., Rubin G. M., Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc. Natl. Acad. Sci. U.S.A. 112, E2967–E2976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoopfer E. D., Jung Y., Inagaki H. K., Rubin G. M., Anderson D. J., P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, e11346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Philipsborn A. C., et al. , Neuronal control of Drosophila courtship song. Neuron 69, 509–522 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Sato K., Yamamoto D., Contact-chemosensory evolution underlying reproductive isolation in Drosophila species. Front. Behav. Neurosci. 14, 597428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohatsu S., Koganezawa M., Yamamoto D., Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Inagaki H. K., et al. , Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11, 325–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa M., Manton J. D., Ostrovsky A. D., Prohaska S., Jefferis G. S., NBLAST: Rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron 91, 293–311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J. S., Luo L., A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat. Protoc. 1, 2583–2589 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Al-Saffar Z., Grainger J., Aldrich J., Temperature and humidity affecting development, survival and weight loss of the pupal stage of Drosophila melanogaster, and the influence of alternating temperature on the larvae. J. Therm. Biol. 21, 389–396 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.