Abstract
Mesenchymal stem cells (MSCs) are used extensively in developing tissue engineered constructs for bone and cartilage regeneration. An important factor in designing such constructs is that the MSCs are appropriately primed to differentiate along osteogenic or chondrogenic lineages. In contrast to the top-down method of tissue engineering where the differentiation of cells is guided by the scaffold and signals, a bottom-up method involves direct modulation of stem cell behavior without relying on the environmental cues. In this review, we discuss several bottom-up strategies that have emerged in engineering MSC behavior for bone and cartilage tissue engineering, including gene delivery, gene editing, and subpopulation isolation.
Keywords: Mesenchymal stem cell, tissue engineering, gene delivery, gene editing, subpopulation isolation
Graphical Abstract
Introduction
The development of traditional cell-laden tissue engineered constructs requires that the three fundamental components – cells, signals, and scaffolds – interact with each other in generating a functional tissue. In this paradigm, cells differentiate along the chosen pathway to become tissue-specific cells and secrete appropriate extracellular matrix components; signals (e.g., growth factors or bioactive factors) provide the biological cues to guide the differentiation and behavior of encapsulated/seeded cells; and scaffolds provide the framework to which the cells can adhere, differentiate, and deposit the matrix. This approach has been taken to develop constructs designed for the regeneration of a diverse array of tissues and organs.
Two of the most active areas of research in the development of tissue engineering strategies have been for the regeneration of bone and cartilage. Bone is characterized by its dynamic tissue environment, where it undergoes constant modeling and remodeling by bone-resorbing osteoclasts and bone-forming osteoblasts.[1,2] The activities of these two resident cell types are affected by the mechanical stimuli exerted to the bone and are tightly regulated to maintain bone homeostasis. Bone can also heal itself following an injury, although the self-regenerative capability decreases with age and is also defect size-dependent.[3] In general, segmental bone defects in humans longer than 2 cm are considered to be critical-sized defects and will not undergo spontaneous healing unless external interventions are applied.[3,4] Various clinical tools such as metallic fixtures and bone autografts/allografts have thus been developed, along with tissue engineered constructs that utilize synthetic materials as bone substitutes and growth factors. Although these methods are all currently being employed in the clinic as suitable surgical options, each has its own drawbacks such as donor site morbidity from autografts and ectopic bone formation from delivering bone morphogenetic protein-2 (BMP-2).[5,6]
Unlike bone, cartilage is an avascular tissue that obtains most of its nutrients from the surrounding synovial fluid.[7] Combined with its low cell density and dense extracellular matrix (ECM),[8,9] cartilage suffers from having a limited ability to heal itself following an injury. Various surgical techniques have been implemented thus far including MACI (Autologous Cultured Chondrocytes on a Porcine Collagen Membrane) which is the only FDA-approved cellular therapy product for the treatment of joint diseases.[10] Even with such improvements, however, the regeneration of cartilage remains elusive, as many surgical interventions have been shown to result in the formation of fibrous cartilage which is mechanically inferior compared to the hyaline cartilage from a healthy tissue.
The discovery and characterization of mesenchymal stem cells (MSCs) prompted an even greater interest from the community in developing tissue engineered constructs for bone and cartilage tissue engineering as MSCs can differentiate along both osteo- and chondro-genic lineages. Although MSCs can be derived from a number of tissue sources, those from the bone marrow and adipose tissue (also known as adipose-derived MSCs, or ADSCs) are most frequently utilized.[11,12] As MSCs have been shown to differentiate along osteogenic, chondrogenic, and adipogenic pathways readily,[13] many tissue engineering strategies have naturally started to investigate the potential of using MSCs within the tissue engineered constructs designed for the regeneration of bone and cartilage.
Most design strategies for fabricating stem cell-laden tissue engineered scaffolds can be classified into one of the two categories. The first approach, top-down, focuses on designing the extracellular environment to contain the appropriate signals for guiding the differentiation of stem cells towards the lineage of choice. Such signals can be presented by the material that constitutes the scaffold, as well as growth factors or other bioactive molecules delivered on the surface of the scaffold. This approach has been implemented for the past several decades as the guiding principle in designing countless tissue engineered constructs for bone and cartilage tissue regeneration.[14,15] For instance, combining bioceramics such as β-tricalcium phosphate and hydroxyapatite with growth factors such as BMP-2 has been proven to be a reliable strategy to guide the scaffold-seeded MSCs to undergo osteogenic differentiation.[16] Similarly, MSC chondrogenesis could be guided by encapsulating the cells in hydrogels synthesized from glycosaminoglycans found in cartilage (e.g., hyaluronic acid and chondroitin sulfate) and introducing transforming growth factor-β3 (TGF-β3).[7] In addition, natural materials that have tissue inductive properties such as decellularized bone and cartilage ECM have shown promising results in guiding the differentiation of MSCs toward osteogenic or chondrogenic lineages without the addition of external growth factors.[17]
The second approach, bottom-up, aims at fundamentally redirecting the stem cell behavior to enable the cells to naturally guide themselves to differentiate along the desired lineage. In this approach, the cells are reprogrammed via genetic modification, or they are sorted for a subpopulation of stem cells that has been shown to favor a specific differentiation pathway. In contrast to relying on external signals to induce changes in signaling pathways that lead to the desired phenotype of cells, these bottom-up strategies allow for more targeted control of MSC behavior. Recent advances in analytical methods in cell biology have allowed researchers to understand better how stem cells behave during proliferation and differentiation at the transcriptional and translational levels.[18–20] In turn, such new information has allowed tissue engineers to develop new tuned and refined approaches for reprogramming MSCs for bone and cartilage tissue engineering. This review will thus present an overview of the current advancements that have been made in the field of gene delivery, gene editing, and subpopulation isolation of MSCs within the scope of developing bone and cartilage tissue engineering solutions.
1. Guiding MSC Differentiation via Gene Delivery
Incorporating growth factors into tissue engineered scaffolds is one of the most reliable and historically demonstrated methods of priming the stem cell behavior towards the lineage of choice. Due to their short half-life, however, growth factors are usually required to be introduced at supra-physiological concentrations in order to produce therapeutic effects in vivo, which has been linked with off-target effects.[21] As such, gene delivery has been suggested as a viable alternative, since cellular behavior can be effectively modified by delivering certain genes that lead to either an overexpression or regulation of specific genes of interest. Although this strategy has been most widely implemented for gene therapy to treat disease conditions and cancers, numerous tissue engineering strategies have also been successfully developed that utilize genetically engineered MSCs as the main driving force for tissue regeneration. Specifically, efforts have focused on designing new delivery vectors to improve the transfection of primary MSCs, generally considered to be a cell type that is much more difficult to transfect than other cell lines.[22] In this section, we highlight some of the novel gene delivery vehicles that have recently been utilized for MSC transfection, as well as several DNA and RNA delivery applications developed for bone and cartilage tissue engineering specifically.
1.1. Gene Delivery Vehicles
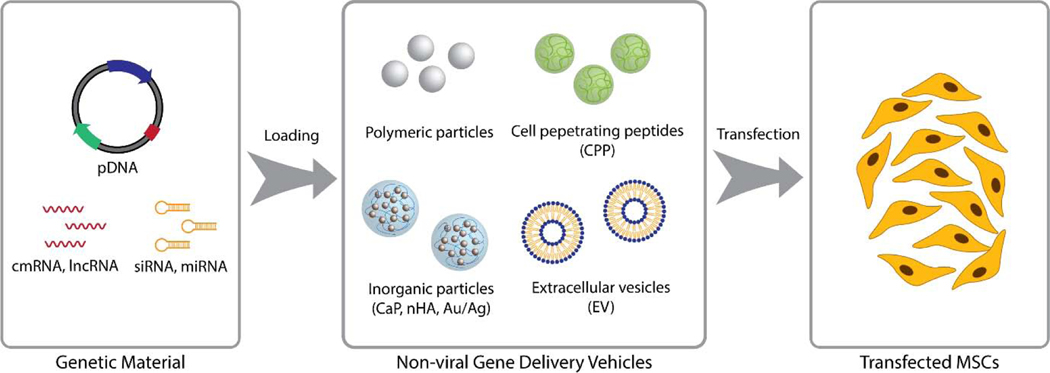
Although viral vectors such as lentiviruses, adenoviruses, and retroviruses have been widely used with promising results for gene delivery,[23] they possess several inherent drawbacks such as pathogenicity, immunogenicity, and potential insertion of the viral gene into the host genome.[24,25] Recombinant adeno-associated viruses (rAAV) have been suggested as an alternative viral delivery option, but rAAVs also suffer from low transduction efficiency and limited size of the gene that could be delivered.[26] To resolve these common issues with using viral vectors, efforts have been made to design alternative non-viral transfection methods (Figure 1).
Figure 1.
General principles of reprogramming MSC behavior via non-viral gene delivery. A gene vector or an RNA transcript is first loaded into a non-viral delivery vehicle of choice. Cells are then exposed to these vehicles either in suspension or in a scaffold for transfection.
By far the most widely utilized non-viral vectors for gene delivery are polymeric particles.[27] In particular, the works by Sung Wan Kim and his associates have led to the development of various novel polymeric gene delivery vehicles.[28] Some of their most notable delivery vehicles have been synthesized from poly(ethylene glycol)-based copolymers,[29–31] as well as from polycationic polymers such as poly(ethyleneimine) (PEI).[32,32,33] These fundamental works paved the way for the development of various polymeric gene delivery systems targeted specifically for MSC differentiation and tissue engineering applications.[34,35]
One of the active areas of research in polymeric gene delivery involves the enhancement of its targeting specificity for MSCs. In this approach, gene delivery vehicles are modified by either coating or chemical conjugation with molecules that are known to interact with cell-surface receptors. One such class of molecules is synthetic peptides, which are derived from ligand proteins known to interact with specific cell-surface receptors. Cell-specific gene delivery vehicles can thus be designed by conjugating these peptides to the surface of the particles.[34] In addition to synthetic peptides, hyaluronic acid has been successfully used to modify the surface of synthetic gene-delivery vehicles for enhanced specificity. In addition to being one of the components of tissue ECM, hyaluronic acid also has binding sites for several cell-surface receptors such as intercellular adhesion molecule-1 and CD44, which is one of the receptors that are present on the surface of MSCs.[36,37] Indeed, hyaluronic acid coating has been shown to enhance the interaction of polymeric particles with MSCs, resulting in improved gene delivery. For instance, MSCs in pellet cultures showed increased chondrogenic gene expression when they were transfected with hyaluronic acid-coated PEI nanogels loaded with SOX9 plasmid DNA (pDNA), compared to the uncoated nanogels.[35]
Inorganic materials such as nano-hydroxyapatite (nHA),[38,39] calcium phosphate (CaP),[40] and gold nanoparticles (AuNPs) [41] have also been employed as non-viral vectors. Nucleic acids can be loaded onto AuNPs via covalent or noncovalent interactions, and the resulting conjugates can be introduced into the cells via endocytosis.[42] In comparison, bioceramics such as CaP and nHA bind to nucleic acids via electrostatic interactions between the calcium ions (nHA and CaP) and phosphate groups (nucleic acids).[43] These bioceramic nanoparticles can then be introduced into the cells via endocytosis most likely via the membranolytic property of calcium ions.[44,45] nHA-mediated delivery of gene vectors has demonstrated higher transfection efficiency in vivo compared to a lipid-based delivery system.[46] In a rat cranial defect model, collagen-nHA scaffolds loaded with nHA vectors complexed with plasmids for BMP-2 and vascular endothelial growth factor (VEGF) resulted in higher percentage of new bone formation compared to the scaffolds loaded with PEI-plasmid complexes.[46] nHA has also been successfully used to locally deliver genes to MSCs encapsulated in an alginate hydrogel,[47] emphasizing the versatility of bioceramic gene delivery vectors in transfecting mammalian cells in a 3D environment. Considering their inherent osteoinductive properties,[48] bioceramics provides a promising approach to develop gene delivery vectors that not only deliver plasmids to the cells, but also possess specific tissue-inductive properties that could act synergistically with gene delivery for bone tissue regeneration.
Cell-penetrating peptides (CPP) are cell membrane-penetrating polypeptides (usually smaller than 30 base pairs) that have found their use as non-viral gene delivery vehicles.[49,50] When conjugated to payloads such as proteins and RNA/DNA, CPPs can be used as an effective intracellular delivery vehicle to influence cell behavior without eliciting significant immune responses from the host. Recently, a subclass of CPPs called glycosaminoglycan‐binding enhanced transduction (GET) peptides have been developed to incorporate a cell-binding domain along with CPP to further enhance the delivery efficiency of conjugated genes or proteins.[51] This system has been successfully used to deliver RUNX2 to human MSCs and promote their osteogenic differentiation in vitro.[52] GET has also been used in conjunction with tissue engineered scaffolds to enable controlled release of conjugated factors to cells in a 3D environment. [53,54] For instance, GET-RUNX2 loaded into poly(lactide-co-glycolide) (PLGA) microparticles induced osteogenic differentiation of human MSCs in vitro as well as resulted in higher bone volume in vivo when delivered in 3D printed PLGA/PEG scaffolds.[53]
Utilizing gene delivery vehicles of biological origin has also been explored. Extracellular vesicles (EVs) refer to a class of membrane-derived vesicles that are secreted by cells. Often less than 200 nm in diameter, EVs can hold various cargos ranging from short non-coding RNAs (ncRNAs) to large proteins and have been shown to participate in intercellular signaling as well as play various biological and pathological roles.[55,56] Prompted by their abundance and the ability to hold biological molecules, EVs – in particular, exosomes and microvesicles – have been actively studied as a drug delivery vehicle and, within the scope of gene delivery, as an alternative to viral or synthetic vectors. Indeed, EVs have been successfully used as delivery vehicles for various miRNAs and long non-coding RNAs (lncRNAs) to cells in vitro and in vivo and have shown to possess several advantages over other viral or synthetic vehicles. For instance, EVs may not induce an immunogenic response by the host if they have been isolated from cells of the host.
While most studies have introduced EVs to MSCs in monolayer simply by adding the gene-loaded exosomes to the culture media, successful fabrication of EV-activated matrices have been recently reported. One such study engineered the exosomes to express biotin on their surfaces, which were then conjugated to the streptavidin-activated electrospun chitosan/poly(L-lysine) scaffolds.[57] MSCs seeded on the surface of the scaffolds were shown to successfully internalize the exosomes, further supporting the potential of using EVs as carriers for localized gene delivery.
Along with the development of gene delivery vehicles, progress has been made in designing new methods to control their spatiotemporal release, and thus the behavior of seeded cells, in a 3D environment. Gene-activated matrix (GAM) is a term used to collectively refer to 3D scaffolds embedded with gene delivery vehicles, and has been used not only to enable a prolonged, local delivery of genes from the bulk construct,[58,59] but also to induce distinct cell behaviors in different layers within a scaffold with a homogeneous distribution of cells.[60] This approach has been successfully used for the fabrication of more spatially complex tissue constructs such as osteochondral tissue.[61,62] For instance, TGF-β3 and BMP-2 lentiviral vectors immobilized on the cartilage and bone layers of a cartilage-derived matrix scaffold, respectively, could successfully induce seeded MSCs to form distinct cartilage-like matrix formation and mineral deposition on the two separate layers of the scaffold in vitro.[62] Recently, 3D printing has also been proven to be an effective tool in enhancing the spatiotemporal control of gene delivery in vivo. Using alginate as the base bioink material and methylcellulose to control the porosity, a gene-activated bioink was used to fabricate dual-layered hydrogels with porous layer on the top and solid layer at the bottom, each with distinct sets of gene vectors.[63] When delivered subcutaneously to mice, the construct loaded with gene vectors resulted in an increased cartilage-like matrix formation in the cartilage region, and higher bone volume and blood vessel counts in the bone region.
1.2. pDNA Delivery
DNA-mediated cellular engineering is achieved by transfecting the cells with pDNA that encodes for a gene of interest, which subsequently is then inserted into the cellular genome and leads to the expression of the said gene. Unlike in RNA-based gene editing strategies where new targets for gene expression modification are constantly being revealed and tested, pDNA delivery has mainly focused on the genes that have been validated as being the main drivers of stem cell differentiation. Specific genes used to drive MSC osteogenesis and chondrogenesis have been thoroughly reviewed in multiple publications.[64–67] In general, delivering genes for transcription factors and growth factors that directly affect osteogenesis (e.g., RUNX2, BMP-2) and chondrogenesis (e.g., TGF-β3, SOX9) has shown promising results in promoting osteogenic or chondrogenic differentiation of MSCs.
Delivering combinations of genes have also been shown to improve the differentiation of MSCs.[68,69] One such example is SOX5, SOX6, and SOX9, also called the SOX trio. SOX9 is one of the main regulators of chondrogenesis, while SOX5 and SOX6 have been shown to play a supporting role. Compared to the delivery of SOX9 alone, delivering all three genes in a single polycistronic vector had higher impact on the chondrogenesis of MSCs in pellet cultures compared to delivering three separate vectors.[68] The SOX trio also outperformed the delivery of genes encoding growth factors related to chondrogenesis (e.g., TGF-β3, insulin-like growth factor, fibroblast growth factor).[69] The advantage of co-delivering TGF-β1 and SOX9 in enhancing MSC chondrogenesis was demonstrated in a separate study.[70] Using a pellet culture model, MSCs transfected simultaneously with pDNA encoding for the two genes via rAAV vectors showed greatly enhanced ability to undergo chondrogenesis, compared to other study groups that had the two genes delivered sequentially with a week between the transfections. Interestingly, the co-delivery group also underwent reduced hypertrophic differentiation compared to the other study groups, indicating that the co-delivery model could be used to reduce the prohypertrophic effects associated with TGF-β family.[71,72] The co-delivery model has also been successfully utilized to improve MSC osteogenesis over the single-gene delivery method. MSCs transfected with PEI-pDNA polyplexes encoding both BMP-2 and fibroblast growth factor-2 (FGF-2) secreted significantly higher amounts of BMP-2 compared to MSCs transfected with just BMP-2 or FGF-2.[73] In a calvarial bone defect study, ADSCs transfected with baculoviruses encoding genes for BMP-2 and miR-148b and delivered on porous PLGA scaffolds induced enhanced bone formation compared to other study groups where ADSCs were transfected with only one type of vector.[74]
1.3. mRNA Delivery
One of the inherent challenges with gene editing via pDNA delivery is that the method requires the pDNA vector to be delivered to the cell nucleus for the vector to be introduced into the host genome. Effective transfection is thus highly dependent on the rate of cell proliferation, as the nucleus is only accessible during mitosis. mRNA delivery can circumvent this challenge, as it can be delivered directly to the cytoplasm and be translated without relying on the cell nuclei to undergo transcription. Such features are especially significant for tissue engineering applications that incorporate cells in a 3D environment such as hydrogels, as cells in 3D tend to undergo slower proliferation compared to those in 2D.
Recently, it has been demonstrated that synthesizing mRNA using native as well as synthetic ribonucleotides allowed the mRNA to successfully penetrate the membrane and be translated without causing significant immune response by the host.[75] These chemically modified mRNA (cmRNA) also demonstrated increased stability within the cytoplasm, thus resulting in a prolonged expression of the encoded protein. Several studies have also demonstrated increased transfection efficiency of cmRNA compared to pDNAs.[76,77] For example, MSCs in spheroid cultures transfected with RUNX2-encoding cmRNA demonstrated enhanced osteogenic activity compared to the pDNA group, as demonstrated by higher ALP activity and expression of osteocalcin.[77]
The potential of cmRNA to be used in bone and cartilage tissue engineering has also been explored by applying the concept of GAM to cmRNA delivery and fabricating transcript-activated matrices to affect the behavior of encapsulated cells.[78–80] MSCs encapsulated in SOX9 cmRNA-activated fibrin hydrogels demonstrated higher levels of chondrogenic gene expression (ACAN and COL2A1) and secreted more cartilage-like matrix compared to MSCs in a non-activated hydrogel. In addition, using biphasic calcium phosphate ceramic granules as delivery vehicles for BMP2-encoding cmRNA had a synergistic effect on enhancing the degree of mineral deposition by MSCs encapsulated in fibrin hydrogels.[78]
1.4. ncRNA Delivery
Unlike mRNA which directly participates in affecting cellular behavior by being the target of translation, non-coding RNAs regulate gene expression by either enhancing or inhibiting the expression of endogenous mRNAs. This process is also known as RNA interference (RNAi) and plays an important role in post-transcriptional gene regulation. In particular, two types of short, non-coding RNAs – microRNAs (miRNAs) and small interfering RNAs (siRNAs) – have been thoroughly explored for their potential to be used in gene therapy as well as in regenerative medicine.
The role of RNAi during stem cell differentiation has been extensively researched and exploited in various therapeutic applications.[81,82] For bone and cartilage tissue engineering, the process starts by identifying the genes that are known to downregulate pathways involved in osteogenesis and chondrogenesis. Following their identification, the miRNAs or siRNAs are then delivered via viral or non-viral methods. The readers are encouraged to refer to the following reviews for a more comprehensive list of miRNAs and siRNAs used for bone and cartilage tissue engineering.[83,84]
Previously undiscovered miRNAs that affect bone and cartilage regeneration are continuously being identified,[85,86] along with novel delivery vehicles that allow for a finer temporal control of its release behavior.[41] Recently, a non-viral miRNA system that allows for temporal control of the release of multiple miRNAs has been developed. Using gold and silver nanoparticles that have absorbance maxima at different wavelengths, the release of two different miRNAs (miR-148b and miR-21 in gold and silver nanoparticles, respectively) could be tuned by controlling the light wavelength applied to the system.[41] The usage of antagomiRs, or anti-miRNAs, to inhibit endogenous miRNAs for tissue engineering is also an area of active research. For instance, human MSCs transfected with antagomiR-221 via lipotransfection not only demonstrated levels of cartilage-matrix formation and chondrogenic gene expression in vitro that were comparable to MSCs cultured with TGF-β3, but they also successfully stimulated chondrogenesis in vivo by silencing miR-221 which has been shown to be one of the antichondrogenic factors.[87]
Another class of non-coding RNA that have been shown to perform RNAi roles is long non-coding RNAs (lncRNAs). Although the identities of lncRNAs that are involved in MSC differentiation are continuously being elucidated,[88–90] their application in bone and cartilage tissue engineering settings have been rather limited. Most recently, the role played by ADAMTS9 antisense RNA 2 (ADAMTS9-AS2) during chondrogenic differentiation has been validated.[91] Using a micromass culture model, human MSCs transfected with ADAMTS9-AS2 lentivirus were shown to express higher levels of chondrogenic genes (SOX9, COL2A1, ACAN) and secrete denser cartilage-like matrix compared to non-transfected MSCs. Transfected MSCs in a 3D environment have been demonstrated to also enhance the regeneration of cartilage tissue in vivo when delivered in an alginate hydrogel.[91] In addition, single cell RNA sequencing (RNAseq) has been successfully used to reveal previously undiscovered lncRNAs that participate in chondrogenesis. For example, GRASLND is a lncRNA that was recently revealed via analyzing the RNAseq data to play an essential role in upregulating the proliferation and cartilage matrix production of MSCs.[92] Indeed, upregulating GRASLND enhanced not only the proliferation but also the secretion of cartilage-like matrix from MSCs in pellet culture.
2. Direct Reprogramming of MSC Behavior via CRISPR/Cas9 Mediated Gene Editing
Since its discovery in 2013, the clustered regularly interspaced short palindromic repeats/Cas9 (CRISPR/Cas9)-based gene editing system has made a significant impact in how genetic engineering is studied.[93] It has also proven itself to be a valuable tool in reprogramming stem cell behavior for tissue engineering. The most common method currently being used for CRISPR/Cas9 gene editing is by delivering a vector that encodes genes for the CRISPR/Cas9 components (e.g., Cas9 and single guide RNA) in vitro via viral transfection. The cell population is then sorted to isolate only the transfected cells using flow cytometry or antibiotics treatment, after which the isolated cells are expanded further before being utilized for tissue engineering applications. Several successful applications of CRISPR/Cas9 in tissue engineering have been reported. Deletion of IGFBP3 (insulin growth factor-β receptor protein 3) in human endometrium-derived MSCs led to a phenotypic change towards chondrocyte-like cells, with IGFBP3-knockout MSCs expressing higher levels of COL2A1 and lower levels of COL1A1, as well as secreting more cartilage-like matrix, compared to normal MSCs.[94]
Another application of the CRISPR-Cas9 system involves using a catalytically inactive Cas9 protein (dCas9) and combining it with either transcription repressors or activators to modulate the transcription of target genes, respectively.[95,96] These tools, known as CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa), have been recently used to control the expression of genes that either directly or indirectly affect the osteogenesis [97–99] and chondrogenesis [100] of MSCs. For instance, CRISPR interference/activation (CRISPRai) was used to evaluate whether simultaneously augmenting chondrogenesis and repressing adipogenesis in MSCs would ultimately lead to enhanced bone tissue regeneration.[97] Indeed, CRISPRai-engineered rat MSCs seeded on porous gelatin scaffolds could successfully enhance bone healing in rat calvarial defects compared to non-modified MSCs. In a similar study, CRISPRa was used to activate the expression of two genes (Wnt10b and Foxc2) in the Wnt signaling pathway,[98] which is one of the important pathways involved in stimulating osteogenesis.[101] The engineered MSCs not only demonstrated enhanced ability to undergo osteogenic differentiation in vitro, but also were able to improve bone healing in vivo when delivered to rat calvarial defects. CRISPRai has also been utilized to modify the expression of genes that participate in apoptotic and immunomodulatory signaling, with the objective of generating engineered stem cells that could be used in inflammatory environments. By using CRISPRi to inhibit the expression of inflammatory cytokine receptors TNFR1 and IL1R1, engineered human ADSCs in pellet culture could demonstrate an enhanced ability to secrete GAGs even in the presence of inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) compared to untreated cells.[102]
In some studies, a CRISPR/Cas9-mediated knockout was unable to replicate the results of other studies that used more transient gene editing methods such as treatment with inhibitors. For instance, although previous research has shown that inhibiting the expression of phosphatase and tensin homolog (PTEN) using a small molecule inhibitor could result in an increase in the expression of osteogenic markers from human MSCs,[103] complete knockout of PTEN using CRISPR/Cas9 had an opposite effect in rabbit MSCs.[104] Such results emphasize the complex cellular mechanisms involved, the multiple roles played by these genes, and that completely removing the genes will result in unforeseen consequences that may not readily be identified in a laboratory setting. For example, in addition to playing a role in regulating the Wnt signaling pathway, PTEN is also a known tumor suppressor where its reduction in expression has been linked with tumorigenesis.[105,106] The safety and long-term consequences of using CRISPR/Cas9-engineered stem cells will therefore need to be thoroughly assessed before they can be used in the clinic.
3. Enhancing the Therapeutic Potential of MSCs via Subpopulation Isolation
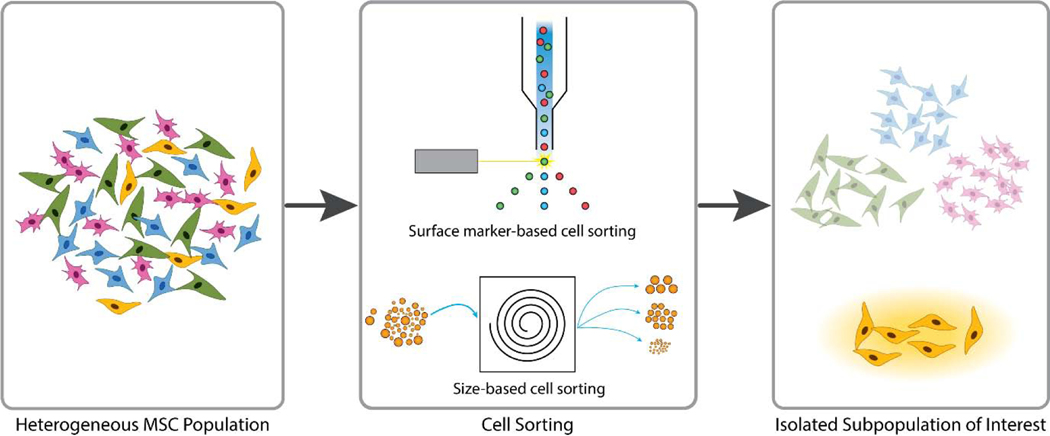
While MSCs have been widely used as a cell source for many successful tissue engineering applications, their inherent heterogeneity and donor-to-donor variability have also resulted in inconsistent results and raised significant questions to their therapeutic potential.[107] One of the solutions that has been suggested to resolve the issues resulting from heterogeneity is to isolate the MSCs that display the features that are known to be present in the cells of interest (Figure 2). Once the desired subpopulation is then separated, they can then undergo further expansion before being used for tissue engineering applications.
Figure 2.
General procedure for isolating MSC subpopulations. Using the features that are known to be overexpressed from the desired subpopulation as sorting criteria, MSCs are separated through either flow cytometry for surface marker-based cell sorting or other methods such as size-based cell sorting. The isolated subpopulation of interest is then expanded before being used for tissue engineering applications. Adopted from [108]; Published by BMC, and [109]; Published by the Royal Society of Chemistry.
The most widely utilized method for isolating the desired MSC subpopulation is by sorting for cells expressing specific surface markers.[110] MSCs are first stained with antibodies for the surface markers of choice, after which they are passed through a cell sorter to isolate the subpopulation of cells with the desired marker expression profile. Various markers for successful isolation of osteogenic and chondrogenic subpopulations have been identified.[111] One such surface marker explored widely as a potential subpopulation marker for enhanced chondrogenesis is CD146, or melanoma cell adhesion molecule.[112,113] CD146+ MSCs have shown to possess enhanced regenerative potential compared to the unsorted MSC population, mostly in their commitment toward the chondrogenic lineage.[114–116] The therapeutic benefit of using a CD146+ subpopulation was also demonstrated using a rabbit cartilage defect model, where the implantation of articular cartilage ECM-derived scaffolds seeded with CD146+ ADSCs induced enhanced cartilage regeneration compared to the non-sorted ADSC group.[115] In addition, in a preliminary study involving a direct injection of a cell suspension into rat articular cartilage, CD146+ ADSCs expressed IL-6 at a level comparable to that of the native tissue and significantly lower than that of non-sorted ADSCs. Similar results were also obtained in a collagen-induced arthritis model in mice, where it was demonstrated that CD146+ MSCs could suppress the activity of Th17, a pro-inflammatory T helper cell, and in turn promote chondrogenesis at the diseased site.[116]
In addition to surface markers, cell size has also been used as a criterion for selecting a specific subpopulation.[109,117] Using a microfluidics system, MSCs cultured in vitro were separated into five subgroups based on their size, with the average size of the cells ranging from 11–12 (smallest) to 23–25 μm (largest).[109] Among the subgroups, MSCs with the highest chondrogenic potential were the ones within the 17–21 μm size range, which constituted about 30–40% of the total cell population. In addition to achieving a fast sorting time (1.5 million cells/min), the cells did not need to be labeled for surface markers.
Recently, RNAseq has emerged as a powerful tool to provide additional insights about the characteristics of MSC subpopulations.[118,119] For instance, MSCs show an age-dependent therapeutic efficiency, where cells isolated from older donors tend to demonstrate impaired regenerative capacity compared to those from younger donors. Using single-cell transcriptomics analysis and machine learning algorithms, it has been revealed that MSCs from younger donors possess a unique quiescent subpopulation that does not exist in MSCs from older donors.[119] Continuous efforts to identify new subpopulations that show enhanced propensity to differentiate along a specific lineage will eventually lead to the isolation of stem cell populations with superior differentiation properties.
4. Concluding Remarks
One of the biggest challenges toward the development of cell-laden tissue engineered constructs is guiding the cells along a desired lineage and phenotype. Although countless bone and cartilage tissue engineering approaches have successfully harnessed the regenerative potential of MSCs, there is still need for improvement in guiding the cells to undergo osteogenesis and chondrogenesis more effectively and reliably. Part of the tissue engineering community has thus been focusing on modulating MSC behavior without necessarily relying on the environmental variables presented by the scaffold and other external factors such as growth factors or biomolecules. Reprogramming MSCs via gene delivery/editing and isolating the subpopulation with the desired therapeutic potential are some of the tools that have been leveraged with the goal of priming the MSCs for enhanced osteogenic or chondrogenic differentiation (Table 1).
Table 1.
List of cellular reprogramming method discussed, and their respective advantages and challenges.
| Methods | Advantages | Challenges | |
|---|---|---|---|
| Gene Delivery | Plasmid DNA (pDNA) | • Prolonged expression of the inserted gene | • Difficult to control the expression level of the inserted gene • Inefficient transfection of non-dividing cells |
| Messenger RNA (mRNA) | • Higher gene transcription efficiency compared to pDNA delivery | • Repeated delivery of mRNA required due to transient expression | |
| Non-coding RNA (ncRNA) | • Ability to silence gene expression without modifying the cell genome | • Off-target gene silencing (miRNA) • Rapid degradation by endogenous ribonuclease |
|
| CRISPR/Cas9 Mediated Gene Editing | • Simple yet highly specific and effective gene editing | • Off-target gene editing • Long-term safety not yet verified |
|
| Subpopulation Isolation | • Enhanced, uniform response to external stimuli following the isolation of homogeneous subpopulation | • Potential phenotypic change of cells during sorting and expansion | |
Gene delivery is an established field of research that has proven itself to be a useful tool in directing the differentiation of MSCs for therapeutic applications. However, a fundamental challenge with the gene editing approach is the complexity of the signaling pathways and genes involved during MSC differentiation. For instance, more than 40 miRNAs and lncRNAs have been identified that affect the translation of genes expressed during osteogenic and chondrogenic differentiation, and subsequent proliferation.[88,120] The diverse array of genes involved reduces the degree of control that can be achieved simply by delivering or editing a few genes from the cell. In addition, it has also been shown that MSCs from different donors may respond differently to the same gene delivery. For gene delivery and editing strategies to have a more reliable effect on guiding MSC differentiation, a more comprehensive understanding of the gene circuitry may be necessary. In-depth analyses of the transcriptomes of engineered MSCs will provide valuable insights into how the cells are behaving over time, as well as assist in discovering new pathways and genes that are triggered during the differentiation and maturation stages. Such genes can then be used as a new target for gene delivery.
The interaction between engineered MSCs and external stimuli must also be considered. Although gene editing is often discussed as an alternative approach for differentiating MSCs compared to the delivery of growth factors, utilizing both strategies simultaneously has been shown to further improve the effects seen from either. Indeed, few studies have demonstrated the synergistic effects of having both gene-edited MSCs and bioactive factors on enhancing the tissue-specific activity of differentiated MSCs.[69,121] Such results promise an exciting avenue for designing new tissue engineering strategies that combine the benefits of gene modification and growth factor delivery.
Although isolating specific subpopulations of MSCs presents an attractive approach to overcoming the inherent heterogeneity present in primary MSCs, it also presents a challenge of requiring additional expansion steps to acquire enough cells for in vivo delivery. Prolonged expansion in vitro is known to cause changes in MSC phenotype,[122] which can end up reverting the cellular characteristics of the isolated subpopulation. Advancements in microenvironment designs that provide optimal growth conditions for MSCs during in vitro expansion will thus be critical in maintaining the regenerative potential of the isolated MSCs until a therapeutically relevant number of cells is acquired.
Using alternative cell sources that are easier to source and to reprogram than MSCs should also be considered. One of the potential alternative cell sources is MSCs derived from induced pluripotent stem cells (iPSCs). With their ability to be derived from adult somatic cells and differentiate into multiple lineages,[123] iPSC-derived MSCs (iMSCs) have shown higher proliferation capacity compared to bone marrow-derived MSCs, demonstrating enhanced cell survival and regeneration of the tissue following transplantation in vivo.[124] The regenerative potential of iMSCs has also been shown to be independent of the age of the donor the cells were derived from, and demonstrated cellular phenotypes that are closer to MSCs from younger donors than to those from older donors.[125] Successful regeneration of bone and cartilage using iMSCs has also been reported.[126–130] Nevertheless, further work is needed to streamline the differentiation/induction protocol of iMSCs as well as better understand the safety of using iPSCs for in vivo tissue regeneration.
Careful considerations must also be put into overcoming the concerns associated with the clinical translation of modified stem cells. As mentioned previously, heterogeneity in engineered stem cell behavior not only reduces their potential clinical benefits but also increases the chance of health hazards due to unmodified or mis-modified cells. Further optimization and standardization of the methods involved during the harvest, expansion, and genetic modification of the isolated cells will be crucial in ensuring that the modified cells behave in a reliable manner once they are reintroduced into the patient.
In conclusion, enhancing the behavior of MSCs via gene delivery and isolating a more potent subpopulation hold promise for developing more effective cell-laden constructs for bone and cartilage tissue engineering. With the development of new gene delivery techniques that are safer and more effective than conventional methods, a more precise control of stem cell fate can be expected. Increased understanding of the regulatory network during stem cell differentiation and proliferation will reveal new genes and pathways that can be exploited not only to deliver such genes to direct stem cell behavior, but also to reveal more therapeutically relevant, previously undiscovered MSC subpopulations. Although there are still regulatory hurdles that need to be overcome before tissue engineered constructs with genetically modified MSCs could be used in the clinic, harnessing the regenerative potential of modified MSCs along with the environmental factors provided by the scaffold will offer tremendous benefits over just relying on the materials and signals alone.
HIGHLIGHTS.
Appropriate differentiation of MSCs is critical for developing functional tissue engineered constructs.
Gene delivery, gene editing, and subpopulation isolation are promising methods for enhancing MSC behavior in engineered constructs.
Recent advancements in gene editing and sequencing will provide powerful tools to further understand MSC differentiation.
5. Acknowledgments
This work was supported by the National Institutes of Health (R01 AR068073 and P41 EB023833). We thank Hannah A. Pearce for her help with the preparation of the manuscript.
Manuscript submitted for consideration and publication in the Journal of Controlled Release: Memorial Issue of Dr. Sung Wan Kim (Guest editors: Drs. David Grainger and You Han Bae)
Footnotes
CRediT Authorship Contribution Statement
Yu Seon Kim: Conceptualization, Literature Analysis, Visualization, Writing - Original Draft.
Antonios G. Mikos: Conceptualization, Funding Acquisition, Supervision, Validation, Writing - Review & Editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD, Effects of mechanical forces on maintenance and adaptation of form in trabecular bone, Nature. 405 (2000) 704–706. 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- [2].Raggatt LJ, Partridge NC, Cellular and Molecular Mechanisms of Bone Remodeling, J. Biol. Chem 285 (2010) 25103–25108. 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Keating JF, Simpson AHRW, Robinson CM, The Management of Fractures with Bone Loss, J. Bone Joint Surg. Br 87-B (2005) 142–150. 10.1302/0301-620X.87B2.15874. [DOI] [PubMed] [Google Scholar]
- [4].Nauth A, McKee MD, Einhorn TA, Watson JT, Li R, Schemitsch EH, Managing Bone Defects, J. Orthop. Trauma 25 (2011) 462–466. 10.1097/BOT.0b013e318224caf0. [DOI] [PubMed] [Google Scholar]
- [5].Rose FRAJ, Oreffo ROC, Bone Tissue Engineering: Hope vs Hype, Biochem. Biophys. Res. Commun 292 (2002) 1–7. 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- [6].James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C, A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2, Tissue Eng. Part B Rev 22 (2016) 284–297. 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Temenoff JS, Mikos AG, Review: Tissue Engineering for Regeneration of Articular Cartilage, Biomaterials. 21 (2000) 431–440. 10.1016/S0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- [8].Stockwell RA, The Cell Density of Human Articular and Costal Cartilage., J. Anat 101 (1967) 753–763. [PMC free article] [PubMed] [Google Scholar]
- [9].Gilmore RS, Palfrey AJ, Chondrocyte Distribution in the Articular Cartilage of Human Femoral Condyles, J. Anat 157 (1988) 23–31. [PMC free article] [PubMed] [Google Scholar]
- [10].Vericel Corporation | Advanced Cell Therapies, (n.d.). https://www.vcel.com/advanced-celltherapies/#maci (accessed August 28, 2020).
- [11].Gimble JM, Guilak F, Adipose-Derived Adult Stem Cells: Isolation, Characterization, and Differentiation Potential, Cytotherapy. 5 (2003) 362–369. 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- [12].Caplan AI, Mesenchymal Stem Cells, J. Orthop. Res 9 (1991) 641–650. 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- [13].Baksh D, Song L, Tuan RS, Adult Mesenchymal Stem Cells: Characterization, Differentiation, and Application in Cell and Gene Therapy, J. Cell. Mol. Med 8 (2004) 301–316. 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koons GL, Diba M, Mikos AG, Materials Design for Bone-Tissue Engineering, Nat. Rev. Mater 5 (2020) 584–603. 10.1038/s41578-020-0204-2. [DOI] [Google Scholar]
- [15].Yang J, Zhang YS, Yue K, Khademhosseini A, Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering, Acta Biomater. 57 (2017) 1–25. 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dimitriou R, Jones E, McGonagle D, Giannoudis PV, Bone Regeneration: Current Concepts and Future Directions, BMC Med. 9 (2011) 66. 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim YS, Majid M, Melchiorri AJ, Mikos AG, Applications of Decellularized Extracellular Matrix in Bone and Cartilage Tissue Engineering, Bioeng. Transl. Med 4 (2019) 83–95. 10.1002/btm2.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Del Sol A, Thiesen HJ, Imitola J, Carazo Salas RE, Big-Data-Driven Stem Cell Science and Tissue Engineering: Vision and Unique Opportunities, Cell Stem Cell. 20 (2017) 157–160. 10.1016/j.stem.2017.01.006. [DOI] [PubMed] [Google Scholar]
- [19].Camp JG, Wollny D, Treutlein B, Single-cell genomics to guide human stem cell and tissue engineering, Nat. Methods 15 (2018) 661–667. 10.1038/s41592-018-0113-0. [DOI] [PubMed] [Google Scholar]
- [20].Armstrong JPK, Stevens MM, Emerging Technologies for Tissue Engineering: From Gene Editing to Personalized Medicine, Tissue Eng. Part A 25 (2019) 688–692. 10.1089/ten.tea.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gautschi OP, Frey SP, Zellweger R, Bone Morphogenetic Proteins in Clinical Applications, ANZ J. Surg 77 (2007) 626–631. 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- [22].Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK, Efficient Transfection Method for Primary Cells, Tissue Eng. 8 (2002) 235–245. 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- [23].Zhang X, Godbey WT, Viral Vectors for Gene Delivery in Tissue Engineering, Adv. Drug Deliv. Rev 58 (2006) 515–534. 10.1016/j.addr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [24].Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P, Vectors and Delivery Systems in Gene Therapy, Med. Sci. Monit. Int. Med. J. Exp. Clin. Res 11 (2005) RA110–121. [PubMed] [Google Scholar]
- [25].Robbins PD, Ghivizzani SC, Viral Vectors for Gene Therapy, Pharmacol. Ther 80 (1998) 35–47. 10.1016/S0163-7258(98)00020-5. [DOI] [PubMed] [Google Scholar]
- [26].Calcedo RPD, Wilson JMMD, Humoral Immune Response to AAV, Front. Immunol 4 (2013). 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang R, Chen F, Guo J, Zhou D, Luan S, Recent Advances in Polymeric Biomaterials-Based Gene Delivery for Cartilage Repair, Bioact. Mater 5 (2020) 990–1003. 10.1016/j.bioactmat.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sung Y, Kim S, Recent Advances in the Development of Gene Delivery Systems, Biomater. Res 23 (2019) 8. 10.1186/s40824-019-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee M, Kim SW, Polyethylene Glycol-Conjugated Copolymers for Plasmid DNA Delivery, Pharm. Res 22 (2005) 1–10. 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- [30].Chang C-W, Choi D, Kim WJ, Yockman JW, Christensen LV, Kim Y-H, Kim SW, Non-Ionic Amphiphilic Biodegradable PEG–PLGA–PEG Copolymer Enhances Gene Delivery Efficiency in Rat Skeletal Muscle, J. Controlled Release. 118 (2007) 245–253. 10.1016/j.jconrel.2006.11.025. [DOI] [PubMed] [Google Scholar]
- [31].Jeong JH, Kim SW, Park TG, Biodegradable Triblock Copolymer of PLGA-PEG-PLGA Enhances Gene Transfection Efficiency, Pharm. Res 21 (2004) 50–54. 10.1023/B:PHAM.0000012151.05441.bf. [DOI] [PubMed] [Google Scholar]
- [32].Nam J-P, Kim S, Kim SW, Design of PEI-Conjugated Bio-Reducible Polymer for Efficient Gene Delivery, Int. J. Pharm 545 (2018) 295–305. 10.1016/j.ijpharm.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nam K, Jung S, Nam J-P, Kim SW, Poly(ethylenimine) Conjugated Bioreducible Dendrimer for Efficient Gene Delivery, J. Controlled Release 220 (2015) 447–455. 10.1016/j.jconrel.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Beloor J, Ramakrishna S, Nam K, Choi CS, Kim J, Kim SH, Cho HJ, Shin H, Kim H, Kim SW, Lee S-K, Kumar P, Effective Gene Delivery into Human Stem Cells with a Cell-Targeting Peptide-Modified Bioreducible Polymer, Small. 11 (2015) 2069–2079. 10.1002/smll.201402933. [DOI] [PubMed] [Google Scholar]
- [35].Park JS, Yi SW, Kim HJ, Park K-H, Receptor-Mediated Gene Delivery into Human Mesenchymal Stem Cells Using Hyaluronic Acid-Shielded Polyethylenimine/PDNA Nanogels, Carbohydr. Polym 136 (2016) 791–802. 10.1016/j.carbpol.2015.09.053. [DOI] [PubMed] [Google Scholar]
- [36].Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD, The Role of the Hyaluronan Receptor CD44 in Mesenchymal Stem Cell Migration in the Extracellular Matrix, STEM CELLS. 24 (2006) 928–935. 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- [37].Ramos TL, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N, López-Ruano G, Hernández-Hernández Á, Redondo A, Ortega R, Rodríguez C, Sánchez-Guijo F, del Cañizo C, MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry, Cell Commun. Signal. CCS 14 (2016). 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, O’Brien FJ, Innovative Collagen Nano-Hydroxyapatite Scaffolds Offer a Highly Efficient Non-Viral Gene Delivery Platform for Stem Cell-Mediated Bone Formation, Adv. Mater 24 (2012) 749–754. 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- [39].Castaño IM, Curtin CM, Shaw G, Mary Murphy J, Duffy GP, O’Brien FJ, A Novel Collagen-Nanohydroxyapatite microRNA-Activated Scaffold for Tissue Engineering Applications Capable of Efficient Delivery of Both Mir-Mimics and Antagomirs to Human Mesenchymal Stem Cells, J. Controlled Release 200 (2015) 42–51. 10.1016/j.jconrel.2014.12.034. [DOI] [PubMed] [Google Scholar]
- [40].Lee JE, Yin Y, Lim SY, Kim ES, Jung J, Kim D, Park JW, Lee MS, Jeong JH, Enhanced Transfection of Human Mesenchymal Stem Cells Using a Hyaluronic Acid/Calcium Phosphate Hybrid Gene Delivery System, Polymers. 11 (2019) 798. 10.3390/polym11050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Abu‐Laban M, Hamal P, Arrizabalaga JH, Forghani A, Dikkumbura AS, Kumal RR, Haber LH, Hayes DJ, Combinatorial Delivery of miRNA-Nanoparticle Conjugates in Human Adipose Stem Cells for Amplified Osteogenesis, Small. 15 (2019) 1902864. 10.1002/smll.201902864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ding Y, Jiang Z, Saha K, Kim CS, Kim ST, Landis RF, Rotello VM, Gold Nanoparticles for Nucleic Acid Delivery, Mol. Ther 22 (2014) 1075–1083. 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Okazaki M, Yoshida Y, Yamaguchi S, Kaneno M, Elliott JC, Affinity binding phenomena of DNA onto apatite crystals, Biomaterials. 22 (2001) 2459–2464. 10.1016/S0142-9612(00)00433-6. [DOI] [PubMed] [Google Scholar]
- [44].Giger EV, Puigmartí-Luis J, Schlatter R, Castagner B, Dittrich PS, Leroux J-C, Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles, J. Controlled Release 150 (2011) 87–93. 10.1016/j.jconrel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- [45].Uskokovid V, Uskokovid DP, Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents, J. Biomed. Mater. Res. B Appl. Biomater 96B (2011) 152–191. 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- [46].Curtin CM, Tierney EG, McSorley K, Cryan S-A, Duffy GP, O’Brien FJ, Combinatorial Gene Therapy Accelerates Bone Regeneration: Non-Viral Dual Delivery of VEGF and BMP2 in a Collagen-Nanohydroxyapatite Scaffold, Adv. Healthc. Mater 4 (2015) 223–227. 10.1002/adhm.201400397. [DOI] [PubMed] [Google Scholar]
- [47].Gonzalez-Fernandez T, Tierney EG, Cunniffe GM, O’Brien FJ, Kelly DJ, Gene Delivery of TGF-β3 and BMP2 in an MSC-Laden Alginate Hydrogel for Articular Cartilage and Endochondral Bone Tissue Engineering, Tissue Eng. Part A 22 (2016) 776–787. 10.1089/ten.tea.2015.0576. [DOI] [PubMed] [Google Scholar]
- [48].Lin L, Chow KL, Leng Y, Study of Hydroxyapatite Osteoinductivity with an Osteogenic Differentiation of Mesenchymal Stem Cells, J. Biomed. Mater. Res. A 89A (2009) 326–335. 10.1002/jbm.a.31994. [DOI] [PubMed] [Google Scholar]
- [49].Zorko M, Langel Ü, Cell-Penetrating Peptides: Mechanism and Kinetics of Cargo Delivery, Adv. Drug Deliv. Rev 57 (2005) 529–545. 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [50].Frankel AD, Pabo CO, Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus, Cell. 55 (1988) 1189–1193. 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- [51].Dixon JE, Osman G, Morris GE, Markides H, Rotherham M, Bayoussef Z, Haj AJE, Denning C, Shakesheff KM, Highly Efficient Delivery of Functional Cargoes by the Synergistic Effect of GAG Binding Motifs and Cell-Penetrating Peptides, Proc. Natl. Acad. Sci 113 (2016) E291–E299. 10.1073/pnas.1518634113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thiagarajan L, Abu‐Awwad HA-DM, Dixon JE, Osteogenic Programming of Human Mesenchymal Stem Cells with Highly Efficient Intracellular Delivery of RUNX2, STEM CELLS Transl. Med 6 (2017) 2146–2159. 10.1002/sctm.17-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Abu Awwad HA-DM, Thiagarajan L, Kanczler JM, Amer MH, Bruce G, Lanham S, Rumney RMH, Oreffo ROC, Dixon JE, Genetically-Programmed, Mesenchymal Stromal Cell-Laden & Mechanically Strong 3D Bioprinted Scaffolds for Bone Repair, J. Controlled Release 325 (2020) 335–346. 10.1016/j.jconrel.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Raftery RM, Walsh DP, Blokpoel Ferreras L, Mencía Castaño I, Chen G, LeMoine M, Osman G, Shakesheff KM, Dixon JE, O’Brien FJ, Highly Versatile Cell-Penetrating Peptide Loaded Scaffold for Efficient and Localised Gene Delivery to Multiple Cell Types: From Development to Application in Tissue Engineering, Biomaterials. 216 (2019) 119277. 10.1016/j.biomaterials.2019.119277. [DOI] [PubMed] [Google Scholar]
- [55].EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA, Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities, Nat. Rev. Drug Discov 12 (2013) 347–357. 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- [56].György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger É, Pap E, Kittel Á, Nagy G, Falus A, Buzás EI, Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles, Cell. Mol. Life Sci 68 (2011) 2667–2688. 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zha Y, Lin T, Li Y, Zhang X, Wang Z, Li Z, Ye Y, Wang B, Zhang S, Wang J, ExosomeMimetics as an Engineered Gene-Activated Matrix Induces In-Situ Vascularized Osteogenesis, Biomaterials. 247 (2020) 119985. 10.1016/j.biomaterials.2020.119985. [DOI] [PubMed] [Google Scholar]
- [58].Raisin S, Belamie E, Morille M, Non-Viral Gene Activated Matrices for Mesenchymal Stem Cells Based Tissue Engineering of Bone and Cartilage, Biomaterials. 104 (2016) 223–237. 10.1016/j.biomaterials.2016.07.017. [DOI] [PubMed] [Google Scholar]
- [59].Zhang K, Fang H, Qin Y, Zhang L, Yin J, Functionalized Scaffold for in Situ Efficient Gene Transfection of Mesenchymal Stem Cells Spheroids toward Chondrogenesis, ACS Appl. Mater. Interfaces 10 (2018) 33993–34004. 10.1021/acsami.8b12268. [DOI] [PubMed] [Google Scholar]
- [60].Bonadio J, Smiley E, Patil P, Goldstein S, Localized, Direct Plasmid Gene Delivery In Vivo: Prolonged Therapy Results in Reproducible Tissue Regeneration, Nat. Med 5 (1999) 753–759. 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- [61].Lee Y-H, Wu H-C, Yeh C-W, Kuan C-H, Liao H-T, Hsu H-C, Tsai J-C, Sun J-S, Wang T-W, Enzyme-Crosslinked Gene-Activated Matrix for the Induction of Mesenchymal Stem Cells in Osteochondral Tissue Regeneration, Acta Biomater. 63 (2017) 210–226. 10.1016/j.actbio.2017.09.008. [DOI] [PubMed] [Google Scholar]
- [62].Rowland CR, Glass KA, Ettyreddy AR, Gloss CC, Matthews JRL, Huynh NPT, Guilak F, Regulation of Decellularized Tissue Remodeling Via Scaffold-Mediated Lentiviral Delivery in Anatomically-Shaped Osteochondral Constructs, Biomaterials. 177 (2018) 161–175. 10.1016/j.biomaterials.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gonzalez-Fernandez T, Rathan S, Hobbs C, Pitacco P, Freeman FE, Cunniffe GM, Dunne NJ, McCarthy HO, Nicolosi V, O’Brien FJ, Kelly DJ, Pore-Forming Bioinks to Enable Spatio-Temporally Defined Gene Delivery in Bioprinted Tissues, J. Controlled Release 301 (2019) 13–27. 10.1016/j.jconrel.2019.03.006. [DOI] [PubMed] [Google Scholar]
- [64].Kim Y-D, Pofali P, Park T-E, Singh B, Cho K, Maharjan S, Dandekar P, Jain R, Choi Y-J, Arote R, Cho C-S, Gene Therapy for Bone Tissue Engineering, Tissue Eng. Regen. Med 13 (2016) 111–125. 10.1007/s13770-016-9063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fischer J, Kolk A, Wolfart St, Pautke C, Warnke PH, Plank C, Smeets R, Future of Local Bone Regeneration – Protein Versus Gene Therapy, J. Cranio-Maxillofac. Surg 39 (2011) 54–64. 10.1016/j.jcms.2010.03.016. [DOI] [PubMed] [Google Scholar]
- [66].Frisch J, Venkatesan JK, Rey-Rico A, Madry H, Cucchiarini M, Current Progress in Stem CellBased Gene Therapy for Articular Cartilage Repair, Curr. Stem Cell Res. Ther 10 (2015) 121–131. . [DOI] [PubMed] [Google Scholar]
- [67].Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, Stoddart MJ, Tissue Engineering for Articular Cartilage Repair--the State of the Art, Eur. Cell. Mater 25 (2013) 248–267. 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- [68].Park JS, Yi SW, Kim HJ, Kim SM, Kim J-H, Park K-H, Construction of PLGA Nanoparticles Coated with Polycistronic SOX5, SOX6, and SOX9 Genes for Chondrogenesis of Human Mesenchymal Stem Cells, ACS Appl. Mater. Interfaces 9 (2017) 1361–1372. 10.1021/acsami.6b15354. [DOI] [PubMed] [Google Scholar]
- [69].Raftery RM, Vazquez AGG, Chen G, O’Brien FJ, Activation of the SOX-5, SOX-6, and SOX-9 Trio of Transcription Factors Using a Gene-Activated Scaffold Stimulates Mesenchymal Stromal Cell Chondrogenesis and Inhibits Endochondral Ossification, Adv. Healthc. Mater 9 (2020) 1901827. 10.1002/adhm.201901827. [DOI] [PubMed] [Google Scholar]
- [70].Tao K, Frisch J, Rey-Rico A, Venkatesan JK, Schmitt G, Madry H, Lin J, Cucchiarini M, CoOverexpression of TGF-β and SOX9 via rAAV Gene Transfer Modulates the Metabolic and Chondrogenic Activities of Human Bone Marrow-Derived Mesenchymal Stem Cells, Stem Cell Res. Ther 7 (2016) 20. 10.1186/s13287-016-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Barry F, Boynton RE, Liu B, Murphy JM, Chondrogenic Differentiation of Mesenchymal Stem Cells from Bone Marrow: Differentiation-Dependent Gene Expression of Matrix Components, Exp. Cell Res 268 (2001) 189–200. 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- [72].Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B, The Chondrogenic Potential of Human Bone-Marrow-Derived Mesenchymal Progenitor Cells*, JBJS. 80 (1998) 1745–57. [DOI] [PubMed] [Google Scholar]
- [73].Khorsand B, Nicholson N, Do A-V, Femino JE, Martin JA, Petersen E, Guetschow B, Fredericks DC, Salem AK, Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model, J. Controlled Release 248 (2017) 53–59. 10.1016/j.jconrel.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li K-C, Lo S-C, Sung L-Y, Liao Y-H, Chang Y-H, Hu Y-C, Improved calvarial bone repair by hASCs engineered with Cre/loxP-based baculovirus conferring prolonged BMP-2 and MiR148b co-expression, J. Tissue Eng. Regen. Med 11 (2017) 3068–3077. 10.1002/term.2208. [DOI] [PubMed] [Google Scholar]
- [75].Kormann MSD, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C, Expression of Therapeutic Proteins After Delivery of Chemically Modified mRNA in Mice, Nat. Biotechnol 29 (2011) 154–157. 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- [76].Elangovan S, Khorsand B, Do A-V, Hong L, Dewerth A, Kormann M, Ross RD, Rick Sumner D, Allamargot C, Salem AK, Chemically Modified RNA Activated Matrices Enhance Bone Regeneration, J. Controlled Release 218 (2015) 22–28. 10.1016/j.jconrel.2015.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Uchida S, Yanagihara K, Matsui A, Kataoka K, Itaka K, mRNA as a Tool for Gene Transfection in 3D Cell Culture for Future Regenerative Therapy, Micromachines. 11 (2020) 426. 10.3390/mi11040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Balmayor ER, Geiger JP, Koch C, Aneja MK, van Griensven M, Rudolph C, Plank C, Modified mRNA for BMP-2 in Combination with Biomaterials Serves as a Transcript-Activated Matrix for Effectively Inducing Osteogenic Pathways in Stem Cells, Stem Cells Dev. 26 (2016) 25–34. 10.1089/scd.2016.0171. [DOI] [PubMed] [Google Scholar]
- [79].Ledo AM, Senra A, Rilo-Alvarez H, Borrajo E, Vidal A, Alonso MJ, Garcia-Fuentes M, mRNA-Activated Matrices Encoding Transcription Factors as Primers of Cell Differentiation in Tissue Engineering, Biomaterials. 247 (2020) 120016. 10.1016/j.biomaterials.2020.120016. [DOI] [PubMed] [Google Scholar]
- [80].Badieyan ZS, Berezhanskyy T, Utzinger M, Aneja MK, Emrich D, Erben R, Schüler C, Altpeter P, Ferizi M, Hasenpusch G, Rudolph C, Plank C, Transcript-Activated Collagen Matrix as Sustained mRNA Delivery System for Bone Regeneration, J. Controlled Release 239 (2016) 137–148. 10.1016/j.jconrel.2016.08.037. [DOI] [PubMed] [Google Scholar]
- [81].Beavers KR, Nelson CE, Duvall CL, miRNA Inhibition in Tissue Engineering and Regenerative Medicine, Adv. Drug Deliv. Rev 88 (2015) 123–137. 10.1016/j.addr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Curtin CM, Castaño IM, O’Brien FJ, Scaffold-Based microRNA Therapies in Regenerative Medicine and Cancer, Adv. Healthc. Mater 7 (2018) 1700695. 10.1002/adhm.201700695. [DOI] [PubMed] [Google Scholar]
- [83].Arriaga MA, Ding M-H, Gutierrez AS, Chew SA, The Application of microRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering, Biotechnol. J 14 (2019) 1900084. 10.1002/biot.201900084. [DOI] [PubMed] [Google Scholar]
- [84].Sriram M, Sainitya R, Kalyanaraman V, Dhivya S, Selvamurugan N, Biomaterials Mediated microRNA Delivery for Bone Tissue Engineering, Int. J. Biol. Macromol 74 (2015) 404–412. 10.1016/j.ijbiomac.2014.12.034. [DOI] [PubMed] [Google Scholar]
- [85].Cui Q, Xing J, Yu M, Wang Y, Xu J, Gu Y, Nan X, Ma W, Liu H, Zhao H, Mmu-miR-185 Depletion Promotes Osteogenic Differentiation and Suppresses Bone Loss in Osteoporosis Through the Bgn-Mediated BMP/Smad Pathway, Cell Death Dis. 10 (2019) 1–14. 10.1038/s41419-019-1428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Xia T, Dong S, Tian J, miR‑29b Promotes the Osteogenic Differentiation of Mesenchymal Stem Cells Derived from Human Adipose Tissue Via the PTEN/AKT/β‑Catenin Signaling Pathway, Int. J. Mol. Med 46 (2020) 709–717. 10.3892/ijmm.2020.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lolli A, Narcisi R, Lambertini E, Penolazzi L, Angelozzi M, Kops N, Gasparini S, van Osch GJVM, Piva R, Silencing of Antichondrogenic MicroRNA-221 in Human Mesenchymal Stem Cells Promotes Cartilage Repair In Vivo, STEM CELLS. 34 (2016) 1801–1811. 10.1002/stem.2350. [DOI] [PubMed] [Google Scholar]
- [88].Ju C, Liu R, Zhang Y-W, Zhang Y, Zhou R, Sun J, Lv X-B, Zhang Z, Mesenchymal Stem CellAssociated lncRNA in Osteogenic Differentiation, Biomed. Pharmacother 115 (2019) 108912. 10.1016/j.biopha.2019.108912. [DOI] [PubMed] [Google Scholar]
- [89].Zhu J, Yu W, Wang Y, Xia K, Huang Y, Xu A, Chen Q, Liu B, Tao H, Li F, Liang C, lncRNAs: Function and Mechanism in Cartilage Development, Degeneration, and Regeneration, Stem Cell Res. Ther 10 (2019) 344. 10.1186/s13287-019-1458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sun H, Peng G, Ning X, Wang J, Yang H, Deng J, Emerging Roles of Long Noncoding RNA in Chondrogenesis, Osteogenesis, and Osteoarthritis, Am. J. Transl. Res 11 (2019) 16–30. [PMC free article] [PubMed] [Google Scholar]
- [91].Huang M-J, Zhao J-Y, Xu J-J, Li J, Zhuang Y-F, Zhang X-L, lncRNA ADAMTS9-AS2 Controls Human Mesenchymal Stem Cell Chondrogenic Differentiation and Functions as a ceRNA, Mol. Ther. - Nucleic Acids 18 (2019) 533–545. 10.1016/j.omtn.2019.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Huynh NP, Gloss CC, Lorentz J, Tang R, Brunger JM, McAlinden A, Zhang B, Guilak F, Long Non-Coding RNA GRASLND Enhances Chondrogenesis Via Suppression of the Interferon Type ii Signaling Pathway, ELife. 9 (2020) e49558. 10.7554/eLife.49558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity, Science. 337 (2012) 816–821. 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ushakov RE, Skvortsova EV, Vitte MA, Vassilieva IO, Shatrova AN, Kotova AV, Kenis VM, Burova EB, Chondrogenic Differentiation Followed IGFBP3 Loss in Human Endometrial Mesenchymal Stem Cells, Biochem. Biophys. Res. Commun (2020). 10.1016/j.bbrc.2020.07.064. [DOI] [PubMed] [Google Scholar]
- [95].Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS, CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes, Cell. 154 (2013) 442–451. 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA, Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression, Cell. 152 (2013) 1173–1183. 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Truong VA, Hsu M-N, Kieu Nguyen NT, Lin M-W, Shen C-C, Lin C-Y, Hu Y-C, CRISPRai for Simultaneous Gene Activation and Inhibition to Promote Stem Cell Chondrogenesis and Calvarial Bone Regeneration, Nucleic Acids Res. 47 (2019) e74–e74. 10.1093/nar/gkz267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hsu M-N, Huang K-L, Yu F-J, Lai P-L, Truong AV, Lin M-W, Nguyen NTK, Shen C-C, Hwang SM, Chang Y-H, Hu Y-C, Coactivation of Endogenous Wnt10b and Foxc2 by CRISPR Activation Enhances BMSC Osteogenesis and Promotes Calvarial Bone Regeneration, Mol. Ther 28 (2020) 441–451. 10.1016/j.ymthe.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hsu M-N, Yu F-J, Chang Y-H, Huang K-L, Pham NN, Truong VA, Lin M-W, Kieu Nguyen NT, Hwang S-M, Hu Y-C, CRISPR Interference-Mediated Noggin Knockdown Promotes BMP2-Induced Osteogenesis and Calvarial Bone Healing, Biomaterials. 252 (2020) 120094. 10.1016/j.biomaterials.2020.120094. [DOI] [PubMed] [Google Scholar]
- [100].Farhang N, Davis B, Weston J, Ginley-Hidinger M, Gertz J, Bowles RD, Synergistic CRISPRaRegulated Chondrogenic Extracellular Matrix Deposition Without Exogenous Growth Factors, Tissue Eng. Part A (2020). 10.1089/ten.tea.2020.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Leucht P, Lee S, Yim N, Wnt Signaling and Bone Regeneration: Can’t Have One Without the Other, Biomaterials. 196 (2019) 46–50. 10.1016/j.biomaterials.2018.03.029. [DOI] [PubMed] [Google Scholar]
- [102].Farhang N, Brunger JM, Stover JD, Thakore PI, Lawrence B, Guilak F, Gersbach CA, Setton LA, Bowles RD, CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments, Tissue Eng. Part A 23 (2017) 738–749. 10.1089/ten.tea.2016.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liu X, Chen T, Wu Y, Tang Z, Role and Mechanism of PTEN in Adiponectin-Induced Osteogenesis in Human Bone Marrow Mesenchymal Stem Cells, Biochem. Biophys. Res. Commun 483 (2017) 712–717. 10.1016/j.bbrc.2016.12.076. [DOI] [PubMed] [Google Scholar]
- [104].Shen Y, Zhang J, Yu T, Qi C, Generation of PTEN Knockout Bone Marrow Mesenchymal Stem Cell Lines by CRISPR/Cas9-Mediated Genome Editing, Cytotechnology. 70 (2018) 783–791. 10.1007/s10616-017-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Carracedo A, Alimonti A, Pandolfi PP, PTEN Level in Tumor Suppression: How Much Is Too Little?, Cancer Res. 71 (2011) 629–633. 10.1158/0008-5472.CAN-10-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP, Subtle Variations in Pten Dose Determine Cancer Susceptibility, Nat. Genet 42 (2010) 454–458. 10.1038/ng.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].McLeod CM, Mauck RL, On the Origin and Impact of Mesenchymal Stem Cell Heterogeneity: New Insights and Emerging Tools for Single Cell Analysis, Eur. Cell. Mater 34 (2017) 217–231. 10.22203/eCM.v034a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hagmann S, Frank S, Gotterbarm T, Dreher T, Eckstein V, Moradi B, Fluorescence Activated Enrichment of CD146+ Cells During Expansion of Human Bone-Marrow Derived Mesenchymal Stromal Cells Augments Proliferation and GAG/DNA Content in Chondrogenic Media, BMC Musculoskelet. Disord 15 (2014) 322. 10.1186/1471-2474-15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yin L, Wu Y, Yang Z, Ann Tee C, Denslin V, Lai Z, Teck Lim C, Hin Lee E, Han J, Microfluidic Label-Free Selection of Mesenchymal Stem Cell Subpopulation During Culture Expansion Extends the Chondrogenic Potential In Vitro, Lab. Chip 18 (2018) 878–889. 10.1039/C7LC01005B. [DOI] [PubMed] [Google Scholar]
- [110].Mo M, Wang S, Zhou Y, Li H, Wu Y, Mesenchymal Stem Cell Subpopulations: Phenotype, Property and Therapeutic Potential, Cell. Mol. Life Sci 73 (2016) 3311–3321. 10.1007/s00018-016-2229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pérez-Silos V, Camacho-Morales A, Fuentes-Mera L, Mesenchymal Stem Cells Subpopulations: Application for Orthopedic Regenerative Medicine, Stem Cells Int. 2016 (2016) e3187491. 10.1155/2016/3187491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M, Isolation and Characterization of CD146+ Multipotent Mesenchymal Stromal Cells, Exp. Hematol 36 (2008) 1035–1046. 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- [113].Bowles AC, Kouroupis D, Willman MA, Orfei CP, Agarwal A, Correa D, Signature Quality Attributes of CD146+ Mesenchymal Stem/Stromal Cells Correlate with High Therapeutic and Secretory Potency, STEM CELLS. 38 (2020) 1034–1049. 10.1002/stem.3196. [DOI] [PubMed] [Google Scholar]
- [114].Wangler S, Menzel U, Li Z, Ma J, Hoppe S, Benneker LM, Alini M, Grad S, Peroglio M, CD146/MCAM Distinguishes Stem Cell Subpopulations with Distinct Migration and Regenerative Potential in Degenerative Intervertebral Discs, Osteoarthritis Cartilage. 27 (2019) 1094–1105. 10.1016/j.joca.2019.04.002. [DOI] [PubMed] [Google Scholar]
- [115].Li X, Guo W, Zha K, Jing X, Wang M, Zhang Y, Hao C, Gao S, Chen M, Yuan Z, Wang Z, Zhang X, Shen S, Li H, Zhang B, Xian H, Zhang Y, Sui X, Qin L, Peng J, Liu S, Lu S, Guo Q, Enrichment of CD146+ Adipose-Derived Stem Cells in Combination with Articular Cartilage Extracellular Matrix Scaffold Promotes Cartilage Regeneration, Theranostics. 9 (2019) 5105–5121. 10.7150/thno.33904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wu C-C, Liu F-L, Sytwu H-K, Tsai C-Y, Chang D-M, CD146+ Mesenchymal Stem Cells Display Greater Therapeutic Potential Than CD146– Cells for Treating Collagen-Induced Arthritis in Mice, Stem Cell Res. Ther 7 (2016). 10.1186/s13287-016-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yin L, Yang Z, Wu Y, Denslin V, Yu CC, Tee CA, Lim CT, Han J, Lee EH, Label-Free Separation of Mesenchymal Stem Cell Subpopulations with Distinct Differentiation Potencies and Paracrine Effects, Biomaterials. 240 (2020) 119881. 10.1016/j.biomaterials.2020.119881. [DOI] [PubMed] [Google Scholar]
- [118].Peffers MJ, Collins J, Fang Y, Goljanek-Whysall K, Rushton M, Loughlin J, Proctor C, Clegg PD, Age-Related Changes in Mesenchymal Stem Cells Identified Using a Multi-Omics Approach, Eur. Cell. Mater 31 (2016) 136–159. 10.22203/ecm.v031a10. [DOI] [PubMed] [Google Scholar]
- [119].Khong SML, Lee M, Kosaric N, Khong DM, Dong Y, Hopfner U, Aitzetmüller MM, Duscher D, Schäfer R, Gurtner GC, Single-Cell Transcriptomics of Human Mesenchymal Stem Cells Reveal Age-Related Cellular Subpopulation Depletion and Impaired Regenerative Function, STEM CELLS. 37 (2019) 240–246. 10.1002/stem.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Papaioannou G, Kozlova A, Kobayashi T, miRNA Regulation of Chondrogenesis, Curr. Mol. Biol. Rep 4 (2018) 208–217. 10.1007/s40610-018-0104-z. [DOI] [Google Scholar]
- [121].Cui Z-K, Sun JA, Baljon JJ, Fan J, Kim S, Wu BM, Aghaloo T, Lee M, Simultaneous Delivery of Hydrophobic Small Molecules and siRNA Using Sterosomes to Direct Mesenchymal Stem Cell Differentiation for Bone Repair, Acta Biomater. 58 (2017) 214–224. 10.1016/j.actbio.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Książek K, A Comprehensive Review on Mesenchymal Stem Cell Growth and Senescence, Rejuvenation Res. 12 (2009) 105–116. 10.1089/rej.2009.0830. [DOI] [PubMed] [Google Scholar]
- [123].Takahashi K, Yamanaka S, Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors, Cell. 126 (2006) 663–676. 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [124].Qizhou Lian, Yuelin Zhang, Jinqiu Zhang, Zhang Hua Kun Wu Xingang, Yang Zhang, Lam Francis Fu-Yuen Kang Sarang, Xia Jian Chuan Lai Wing-Hong, Au Ka-Wing Chow Yen Yen, Siu Chung-Wah Lee Chuen-Neng, Tse Hung-Fat, Functional Mesenchymal Stem Cells Derived From Human Induced Pluripotent Stem Cells Attenuate Limb Ischemia in Mice, Circulation. 121 (2010) 1113–1123. 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- [125].Spitzhorn L-S, Megges M, Wruck W, Rahman MS, Otte J, Degistirici Ö, Meisel R, Sorg RV, Oreffo ROC, Adjaye J, Human iPSC-Derived MSCs (iMSCs) from Aged Individuals Acquire a Rejuvenation Signature, Stem Cell Res. Ther 10 (2019) 100. 10.1186/s13287019-1209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Xie J, Peng C, Zhao Q, Wang X, Yuan H, Yang L, Li K, Lou X, Zhang Y, Osteogenic Differentiation and Bone Regeneration of iPSC-MSCs Supported by a Biomimetic Nanofibrous Scaffold, Acta Biomater. 29 (2016) 365–379. 10.1016/j.actbio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- [127].Liu J, Chen W, Zhao Z, Xu HHK, Reprogramming of Mesenchymal Stem Cells Derived from iPSCs Seeded on Biofunctionalized Calcium Phosphate Scaffold for Bone Engineering, Biomaterials. 34 (2013) 7862–7872. 10.1016/j.biomaterials.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sheyn D, Ben-David S, Shapiro G, Mel SD, Bez M, Ornelas L, Sahabian A, Sareen D, Da X, Pelled G, Tawackoli W, Liu Z, Gazit D, Gazit Z, Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects, STEM CELLS Transl. Med 5 (2016) 1447–1460. 10.5966/sctm.2015-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kang R, Zhou Y, Tan S, Zhou G, Aagaard L, Xie L, Bünger C, Bolund L, Luo Y, Mesenchymal Stem Cells Derived from Human Induced Pluripotent Stem Cells Retain Adequate Osteogenicity and Chondrogenicity but Less Adipogenicity, Stem Cell Res. Ther 6 (2015) 144. 10.1186/s13287-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang P, Ma T, Guo D, Hu K, Shu Y, Xu HHK, Schneider A, Metformin Induces Osteoblastic Differentiation of Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells, J. Tissue Eng. Regen. Med 12 (2018) 437–446. 10.1002/term.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]