Abstract
Diabetic nephropathy (DN) is a common microvascular complication of diabetic patients, along with hypertension, hyperlipemia, proteinuria, edema, and other clinical manifestations. Astragalus membranaceus (AM) is a traditional Chinese medicine and has shown significant clinical efficacy against DN. However, the overall molecular mechanism of this therapeutic effect has not been entirely elucidated. Using network pharmacology, we aimed to identify the key active ingredients and potential pharmacological mechanisms of AM in treating DN and provide scientific evidence of its clinical efficacy.
The active ingredients of AM were obtained from the traditional Chinese medicine systems pharmacology database, and the potential targets of AM were identified using the therapeutic target database. DN-related target genes were acquired from the Gene Expression Omnibus microarray dataset GSE1009 and 3 widely used databases-DisGeNET, GeneCards, and Comparative Toxicogenomics Database. The DN–AM common target protein interaction network was established by using the STRING database. Active ingredients candidate targets proteins networks were constructed using Cytoscape software for visualization. Additionally, gene ontology (GO) and Kyoto encyclopedia of genes and genomes pathway analyses were performed using the Database for Annotation, Visualization, and Integrated Discovery database. Target-regulating microRNAs (miRNAs) of these hub genes were obtained from the therapeutic target database, which could then be used for further identification of AM-regulated key miRNAs.
A total of 17 active ingredients and 214 target proteins were screened from AM. 61 candidate co-expressed genes with therapeutic effects against DN were obtained and considered as potential therapeutic targets. GO and Kyoto encyclopedia of genes and genomes enrichment analysis showed that these genes were mainly involved in inflammatory response, angiogenesis, oxidative stress reaction, HIF signaling pathway, tumor necrosis factor signaling pathway, and VEGF signaling pathway. In all, 636 differentially expressed genes were identified between the DN patients and control group by using microarray data, GSE1009. Lastly, VEGFA, epidermal growth factor receptor, STAT1, and GJA1 were screened as hub genes. The relationships between miRNAs and hub genes were constructed, which showed that miR-302-3p, miR-372-3p, miR-373-3p, and miR-520-3p were regulated by VEGFA and epidermal growth factor receptor. Meanwhile, VEGFA also influenced miR-15-5p, miR-16-5p, miR-17-5p, miR-20-5p, miR-93-5p, miR-106-5p, miR-195-5p, miR-424-5p, miR-497-5p, and miR-519-3p. In addition, miR-1-3p and miR-206 were regulated by VEGFA and GJA1, and miR-23-3p was regulated by STAT1 and GJA1.
To our knowledge, this study revealed for the first time the characteristic multiple components, multiple targets, and multiple pathways of AM that seem to be the underlying mechanisms of action of AM in the treatment of DN with respect to miRNAs.
Private information from individuals will not be published. This systematic review also does not involve endangering participant rights. Ethical approval will not be required. The results may be published in a peer-reviewed journal or disseminated at relevant conferences.
Keywords: Astragalus membranaceus, diabetic nephropathy, microRNAs, molecular, network pharmacology
1. Introduction
Diabetic nephropathy (DN) is a microvascular complication associated with type 2 diabetes mellitus (T2DM), and it is characterized by glomerulosclerosis due to accumulation of the extracellular matrix. Albuminuria, edema, and transiently increased glomerular filtration rate are the distinguishing features of DN.[1] Despite continuous advancements in medicine, DN remains a global public health issue with high morbidity and mortality. Specific treatment of patients with DN can be divided into the following categories: cardiovascular risk reduction, glycemic control, blood pressure control, and inhibition of the renin–angiotensin system.[2] However, treatments targeting these categories have numerous side effects such as hypoglycemia, gastrointestinal discomfort, and so on. Therefore, it is necessary to develop more efficient and safer therapeutic strategies to treat DN to supplement the above treatment limitations. At present, the molecular mechanism of DN is gradually being understood and has attracted considerable attention. Previous research studies have shown that mitogen-activated protein kinase, TGF-β, and AngII had different effects on mesangial matrices and cells, leading to glomerular hypertrophy in DN progression.[3] Other studies also revealed that ERK1/2, Akt, interleukin (IL)-6/JAK2/STAT3, and the mTOR signaling pathway activities had important roles in the development and progression of DN.[4] However, investigations regarding the specific molecular mechanism of DN are still ongoing. In recent years, research teams have focused on the regulatory role of microRNAs (miRNAs) in genes, which may be a new diagnostic marker and therapeutic target for DN.
miRNAs are a class of single-stranded, endogenous, noncoding small RNAs that negatively modulate gene expression at the post-transcriptional levels via mRNA cleavage or translational repression in plants and animals.[5] In recent years, the influence of miRNAs in the physiological mechanisms and therapeutic interventions for diseases have gained increasing attention, especially in diabetes and the related metabolic syndrome. Studies have shown that miRNA-337 expression increased in T2DM mice with DN, which lead to podocyte injury by up-regulating levels of IL-6 and IL-18.[6] miR-214 could regulate DN symptoms through ROS/Akt/mTOR signaling pathway by uncoupling proteins in the proximal tubular cells.[7] Although various DN-related miRNAs have been identified, more efforts are needed to discover new targets and to provide new ideas for the diagnosis and treatment of DN.
Astragalus membranaceus (AM) has been used for several centuries as a common Qi-tonifying and immunomodulating herb in traditional Chinese medicine. It is widely used to treat diabetes and metabolic syndrome, and especially for the treatment of DN.[8] Many clinical experiments have shown that AM can alleviate blood glucose, albuminuria, and serum creatinine levels in DN patients.[9,10] In addition, it has a wide range of pharmacological effects such as antirheumatic, antiangiogenic, antiallergic, anti-inflammatory, and antibacterial activities.[11] In particular, astragaloside IV – a crucial active component in AM – could inhibit oxidative stress and inflammatory reaction and reduce glucose metabolic disorders and hemorheology anomalies.[12]
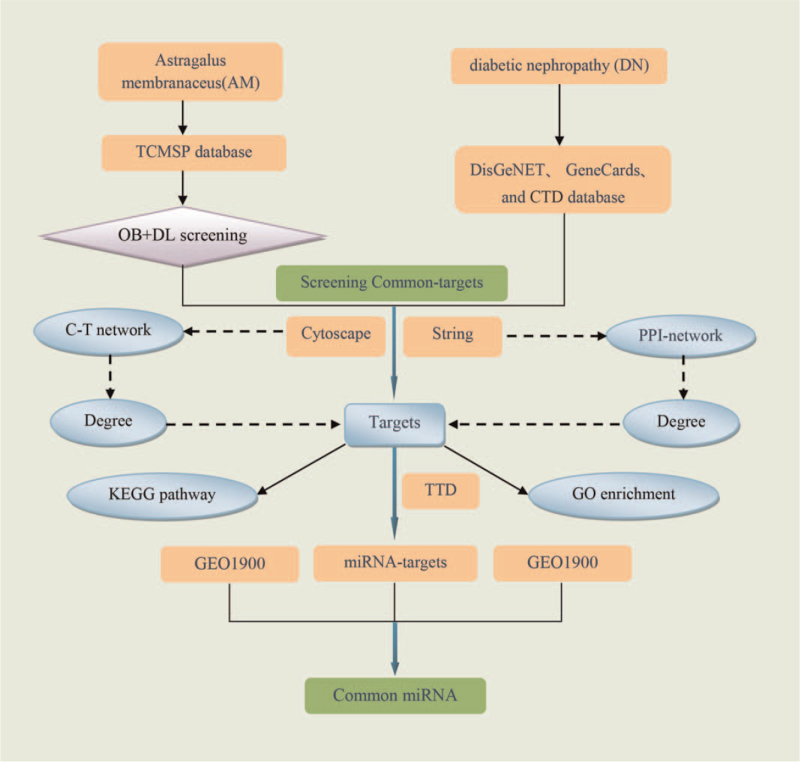
Network pharmacology is a network construction and network topology analysis strategy that combines pharmacology and pharmacodynamics. It is a novel research field which is implicated in the application of omics and systems biology-based technologies.[13,14] In recent years, the application of network pharmacology has become a comprehensive tool to systematically reveal the complex network relationships between bioactive components and potential mechanisms of traditional Chinese medicine formulas.[15] The aim of this study was to reveal the mechanism of AM in the treatment of DN through the regulation of miRNA expression and provide a new target to supports the clinical use of AM for DN. A detailed flow chart of the network pharmacology analysis is shown in Figure 1.
Figure 1.
Flowchart of the network pharmacology analysis. CTD = Comparative Toxicogenomics Database, DL = drug-likeness, OB = oral bioavailability, PPI = protein–protein interaction, GEO = gene expression, omnibus, GO = gene ontology, KEGG = Kyoto encyclopedia of genes and genomes, miRNAs = microRNAs, TCMSP = traditional Chinese medicine systems pharmacology database, TTD = therapeutic target database.
2. Materials and methods
2.1. Screening active components of AM
All components related to AM were screened by using the traditional Chinese medicine systems pharmacology (http://lsp.nwsuaf.edu.cn/tcmsp.php), which contains 12 pharmacokinetic parameters including oral bioavailability (OB), drug-likeness (DL), half-life, intestinal epithelial permeability (Caco-2), blood–brain barrier, and etc. OB is one of the most important pharmacokinetic parameters of the absorption, distribution, metabolism, and excretion characteristics of drugs. DL evaluation helps to screen out candidate compounds, which can act as an indicator of its proximity to a listed drug. Hence, the Tanimoto coefficient was applied to evaluate the DL of molecules in AM, using the following formula: T(a, b) = (a, b)/(|a|2 + |b|2 − a × b). High OB and excellent DL are usually key indicators of a molecule that is biologically active.[16] Therefore, the selected active components meeting the demands of both OB ≥30% and DL ≥0.18 were considered as the screening criteria for further analyses.
2.2. Screening of potential DN targets
The related DN target genes were screened from DisGeNET (https://www.disgenet.org/home/), GeneCard (https://www.genecards.org/), and Comparative Toxicogenomics Database (CTD, http://ctdbase.org/) and by using “diabetic nephropathy” as the keyword. DisGeNet is a widely used database dedicated to collecting information on genes and mutation sites related to human diseases. As a comprehensive database, GeneCards provides concise genome, proteome, transcription, genetic and functional information of all known and predicted human genes. CTD is a powerful and open research resource, which is used to describe the relationship between chemicals, genes and human diseases. The UniProt (https://www.uniprot.org/) database was used to officially correct names and collect corresponding ID of the related genes.
2.3. Extracting co-expressed genes
The co-expressed target genes of AM and DN were inputted into the ImageGP (http://www.ehbio.com/ImageGP/index.php) platform for analysis. Next, a visual Venn diagram was drawn and the co-expressed genes were extracted for further research.
2.4. Network construction
The active components and targets (C-T) network of AM was introduced into the visualization software Cytoscape (http://cytoscape.org/) to construct a visual network. This reflects the relationship between the candidate compounds and the corresponding targets. Protein–protein interaction (PPI) network was used to illustrate the relationship between the potential targets, which reflected the intensity of interaction with proteins.[17] The common genes were analyzed by STRING (http://string-db.org) database, and medium-confidence data (>0.4) were obtained for further exploration. The PPI network was established using the Cytoscape software, and the degree of relationships were calculated by CytoHubbpa plugin. In bilateral networks, the degree value represented the interaction of nodes (compounds, proteins, or targets), which indicated how many nodes one is related with. The larger the degree value, the more critical a node.
2.5. Gene Ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis
To comprehend the function of co-expressed genes and its role in signal transduction, the Database for Annotation, Visualization, and Integrated Discovery (https://david.ncifcrf.gov/) database was used.[18] The GO function and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment of the results were selected with a threshold value of P < .05, and the image was visualized using the R software (http://bioconductor.org/, New Zealand) and its cluster profiler package.
2.6. Screening of key differentially expressed genes (DEGs)
The Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) database is a high-throughput gene expression database, which stores about 1 billion individual genes from more than 100 organisms, involved in various biological problems.[19] Therefore, the related gene expression profiles of DN were obtained from microarray data GSE1900 by using “Diabetic nephropathy”, “Homo sapiens”, and “Expression profiling by array” as the search terms. The online analysis tool GEO2R was used to preprocess and analyze differentially expressed genes (DEGs), and a fold change |FC| > 2 and P < .05 were set as the criteria for differential expression.
2.7. Predicting the hub miRNAs
We intersected the returned co-expressed genes with the DEGs that were associated with DN and illustrated the intersection using a Venn diagram. Then, the key genes were screened and exported into the therapeutic target database to predict the corresponding miRNAs. The miRNA–protein interaction networks were constructed through Cytoscape software (US), and the node degree was calculated by the network analysis plugin.
3. Results
3.1. Screening active components
According to the absorption, distribution, metabolism, and excretion thresholds of OB ≥30% and DL ≥0.18, 17 active components were screened out in AM from the traditional Chinese medicine systems pharmacology database. Meanwhile, 289 related targets were obtained, corresponding to these active ingredients. The composition of the compounds are shown in Table 1.
Table 1.
The compound ingredients of AM.
| MOL ID | Molecule name | OB (%) | DL |
| MOL000211 | Mairin | 55.38 | 0.78 |
| MOL000239 | Jaranol | 50.83 | 0.29 |
| MOL000296 | Hederagenin | 36.91 | 0.75 |
| MOL000033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-Dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | 36.23 | 0.78 |
| MOL000354 | Isorhamnetin | 49.6 | 0.31 |
| MOL000371 | 3,9-di-O-Methylnissolin | 53.74 | 0.48 |
| MOL000378 | 7-O-Methylisomucronulatol | 74.69 | 0.3 |
| MOL000379 | 9,10-Dimethoxypterocarpan-3-O-β-D-glucoside | 36.74 | 0.92 |
| MOL000380 | (6aR,11aR)-9,10-Dimethoxy-6a,11a-dihydro-6H-benzofurano[3,2-c]chromen-3-ol | 64.26 | 0.42 |
| MOL000387 | Bifendate | 31.1 | 0.67 |
| MOL000392 | Formononetin | 69.67 | 0.21 |
| MOL000417 | Calycosin | 47.75 | 0.24 |
| MOL000422 | Kaempferol | 41.88 | 0.24 |
| MOL000433 | FA | 68.96 | 0.71 |
| MOL000439 | Isomucronulatol-7,2′-di-O-glucosiole | 49.28 | 0.62 |
| MOL000442 | 1,7-Dihydroxy-3,9-dimethoxy pterocarpene | 39.05 | 0.48 |
| MOL000098 | Quercetin | 46.43 | 0.28 |
3.2. Compound-target network
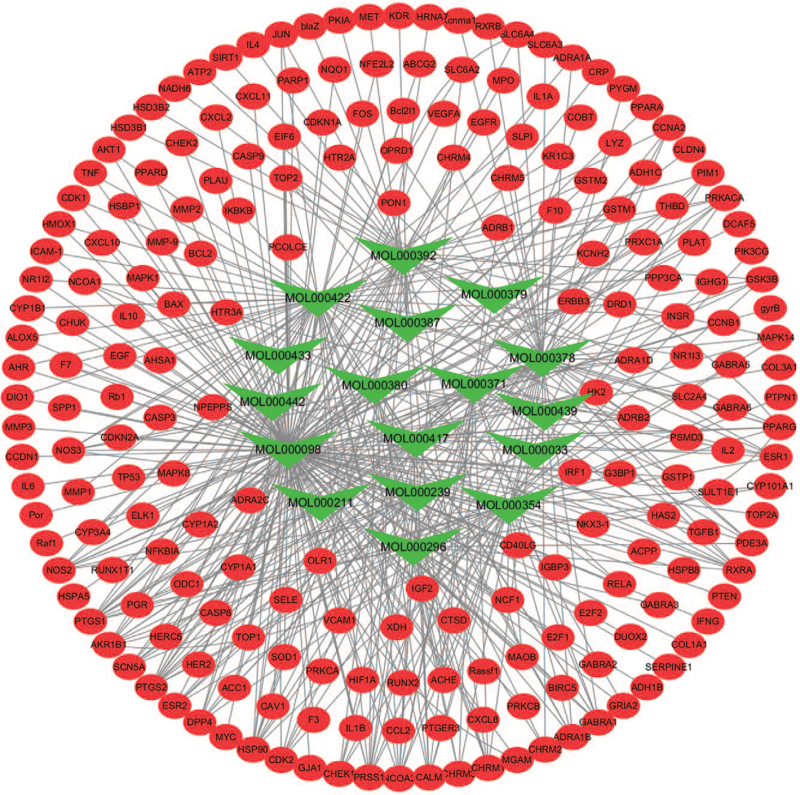
The compounds from AM corresponded with multiple targets, and each target was involved with a variety of compounds. The interactions between compounds and targets embodied 151 nodes and 296 edges (Fig. 2). Our analysis showed that quercetin, kaempferol, 7-O-methylisomucronulatol, formononetin, and isorhamnetin were the top 5 compounds linked to 146, 58, 44, 38, and 36 targets, separately. The results implied that these targets might be synergistically regulated by active compounds, and the therapeutic effect on DN is through multi-component, multi-target regulation of AM.
Figure 2.
Compound-target network of potential targets in AM. The green nodes are active compounds and the red nodes are targets of the compounds. AM = Astragalus membranaceus.
3.3. Co-expressed genes
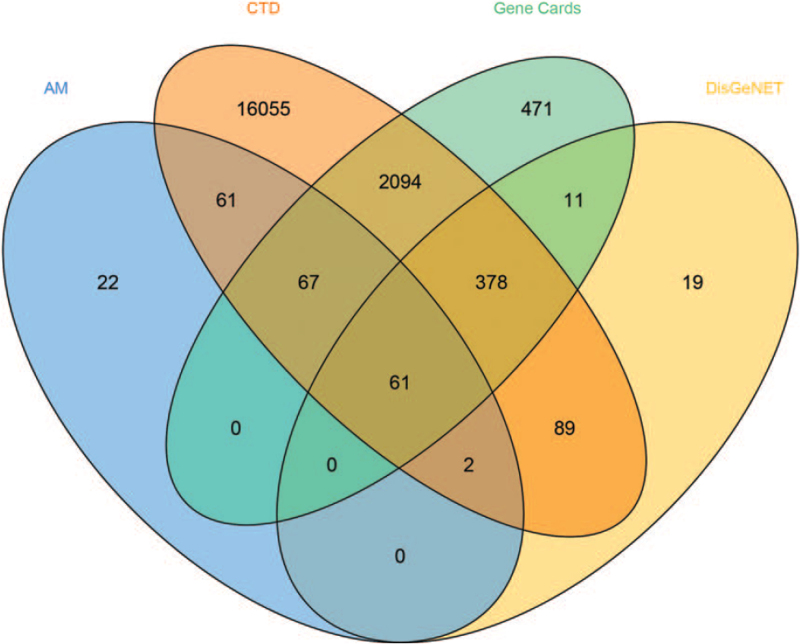
To identify the relationship between AM and DN via the method of integration of 3 databases, the target data of DN from the databases of DisGeNET, GeneCards, and CTD were integrated (Fig. 3). Subsequently, 61 co-expressed genes were selected for further investigation based on the intersection of protein targets acting on AM components and these were related to DN.
Figure 3.
The co-expressed genes of AM and DN. AM = Astragalus membranaceus, CTD = Comparative Toxicogenomics Database, DN = diabetic nephropathy.
3.4. PPI of co-expressed genes
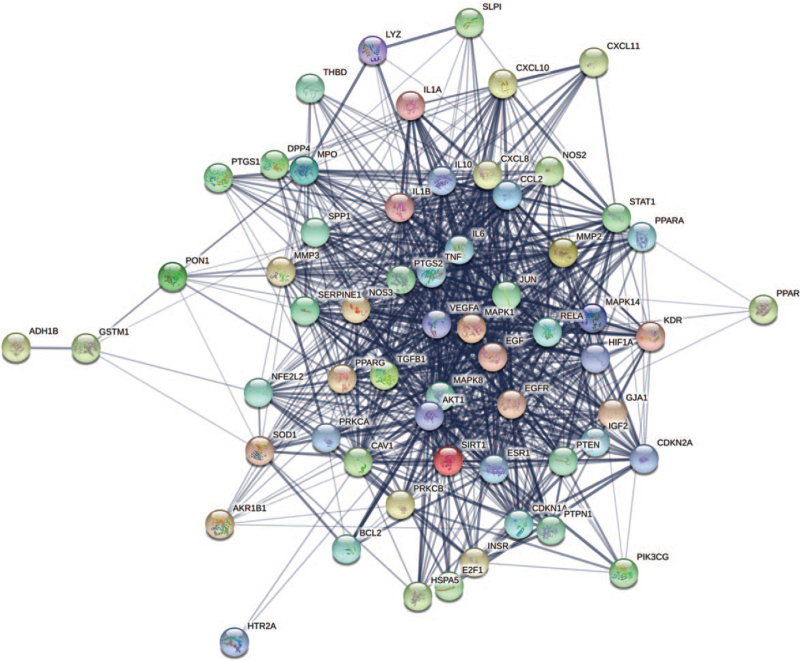
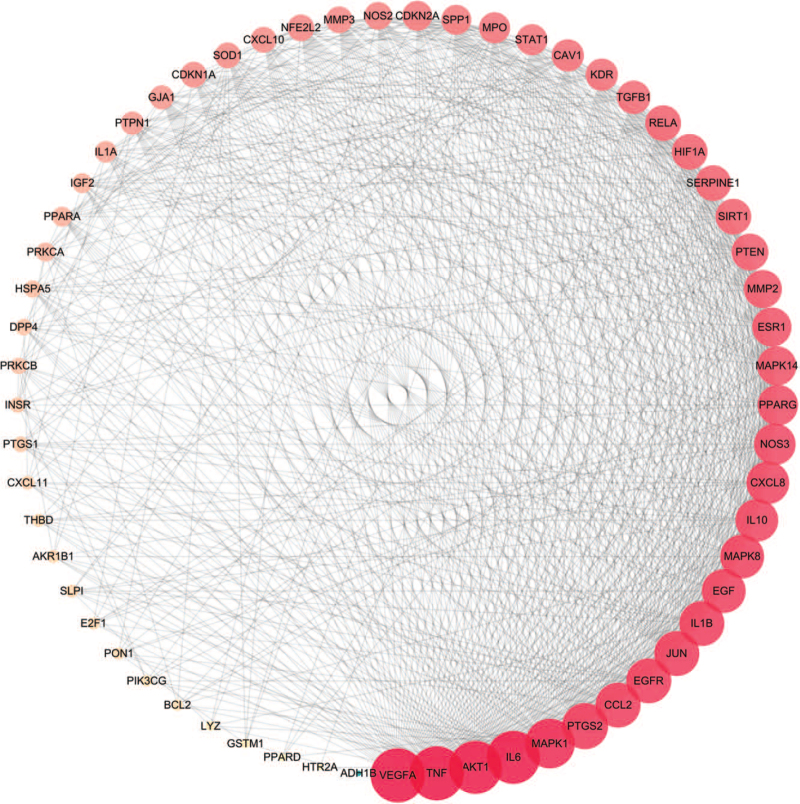
Sixty-one co-expressed genes were uploaded to the STRING platform to identify the interactions. Then, we constructed a PPI network (Fig. 4) consisting of 61 nodes and 811 edges; the average node degree was 26.6. According to the degree value, the node size was divided anticlockwise from large to small, as shown in Figure 5. In addition, the degree of the top 20 target genes exceeded the average in this process, which indicated the significance and more attention required in further analysis. Genes such as VEGFA, tumor necrosis factor (TNF), AKT1, IL6, mitogen-activated protein kinase 1, and prostaglandin G/H synthase 2 might be the key genes in the PPI (Table 2).
Figure 4.
Co-expressed genes PPI network. The thicker the edge, the closer the relationship among proteins. PPI = protein–protein interaction.
Figure 5.
The nodes represent gene targets, and the edges represent the protein interaction between them; the node size is proportional to the degree.
Table 2.
Information on potential targets and the topological attributes.
| No. | Gene name | Protein name | UniProt ID | Degree |
| 1 | VEGFA | Vascular endothelial growth factor A | P15692 | 50 |
| 2 | TNF | Tumor necrosis factor | P01375 | 50 |
| 3 | AKT1 | RAC-alpha serine/threonine-protein kinase | P31749 | 49 |
| 4 | IL6 | Interleukin-6 | P05231 | 49 |
| 5 | MAPK1 | Mitogen-activated protein kinase 1 | P28482 | 46 |
| 6 | PTGS2 | Prostaglandin G/H synthase 2 | P35354 | 43 |
| 7 | EGFR | Epidermal growth factor receptor | P00533 | 42 |
| 8 | JUN | Transcription factor AP-1 | P05412 | 42 |
| 9 | IL1B | Interleukin-1 beta | P01584 | 42 |
| 10 | CCL2 | C-C motif chemokine 2 | P13500 | 42 |
| 11 | EGF | Pro-epidermal growth factor | P00533 | 41 |
| 12 | MAPK8 | Mitogen-activated protein kinase 8 | P01133 | 41 |
| 13 | IL10 | Interleukin-10 | P22301 | 40 |
| 14 | CXCL8 | Interleukin-8 | P10145 | 40 |
| 15 | NOS3 | Nitric-oxide synthase, endothelial | P29474 | 39 |
| 16 | PPARG | Peroxisome proliferator activated receptor gamma | P37231 | 38 |
| 17 | MAPK14 | Mitogen-activated protein kinase 14 | Q16539 | 37 |
| 18 | ESR1 | Estrogen receptor | P03372 | 37 |
| 19 | MMP2 | 72 kDa type IV collagenase | P08253 | 36 |
| 20 | PTEN | Phosphatase and tensin homolog deleted on chromosome 10 | P60484 | 35 |
3.5. GO enrichment analysis
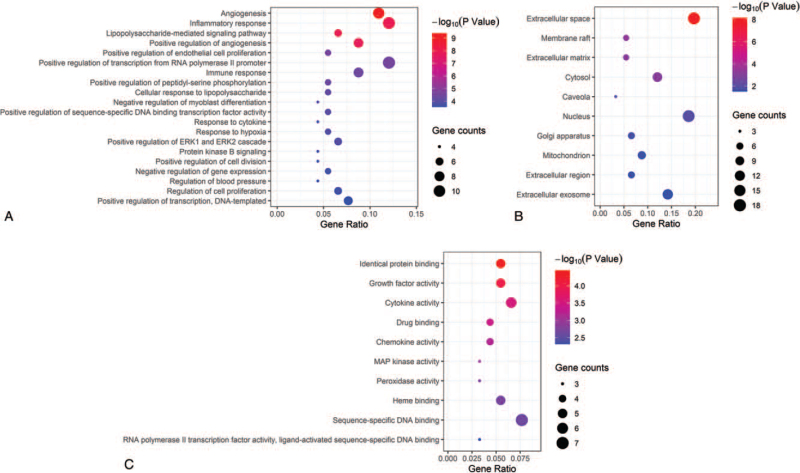
GO functional enrichment analysis was performed on the co-expression targets associated with DN from the aspects of biological process (BP), molecular function (MF), and cellular component (CC). The enrichment analysis was performed with the Database for Annotation, Visualization and Integrated Discovery system on 61 targets, and the screening threshold was P < .05. We retrieved 101 BP, 18 MF, and 11 CC GO items. The BP results demonstrated that the functions of these targets were mainly involved in angiogenesis, inflammatory response, and immune response (Fig. 6A). Furthermore, the processes of extracellular space, membrane raft, extracellular matrix, and nucleus were revealed by the CC analysis (Fig. 6B). In addition, MF enrichment analysis showed that most of these targets including growth factor activity, peroxidase activity, MAP kinase activity, prostaglandin–endoperoxide synthase activity, and etc (Fig. 6C). The top 20 BP results and top 10 MF, CC, results are shown in Fig. 6.
Figure 6.
Enrichment gene ontology (GO) terms for analysis of the co-expression targets. (A) biological process (BP), (B) cellular components (CC), (C) molecular function (MF). The larger the node, the more the count; the redder the color, the smaller the P value.
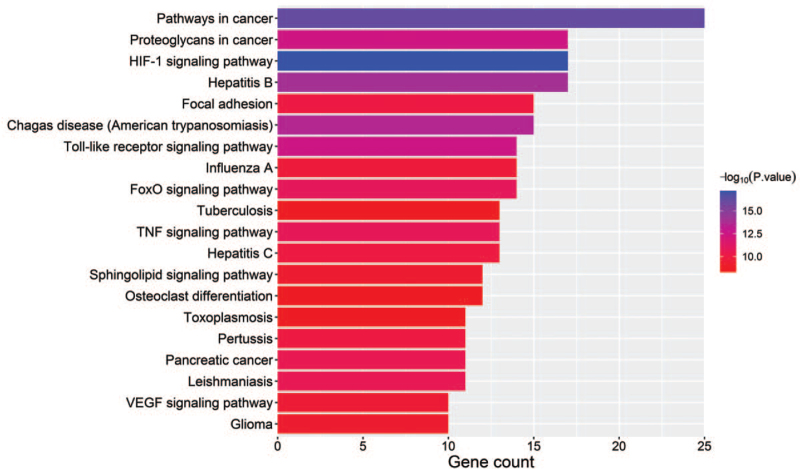
3.6. KEGG pathway enrichment analysis
The KEGG pathway enrichment analysis on 61 targets were performed using R software. Based on the threshold of P < .05, 100 signaling pathways based on the results of KEGG were screened and constructed. The results included Pathways in cancer, HIF-1 signaling pathway, TNF signaling pathway, Toll-like receptor signaling pathway, and VEGF signaling pathway, among others. The top 20 analysis results are shown in Figure 7.
Figure 7.
KEGG pathway analysis of co-expressed genes. KEGG = Kyoto encyclopedia of genes and genomes, TNF = tumor necrosis factor.
3.7. Genes related to DN
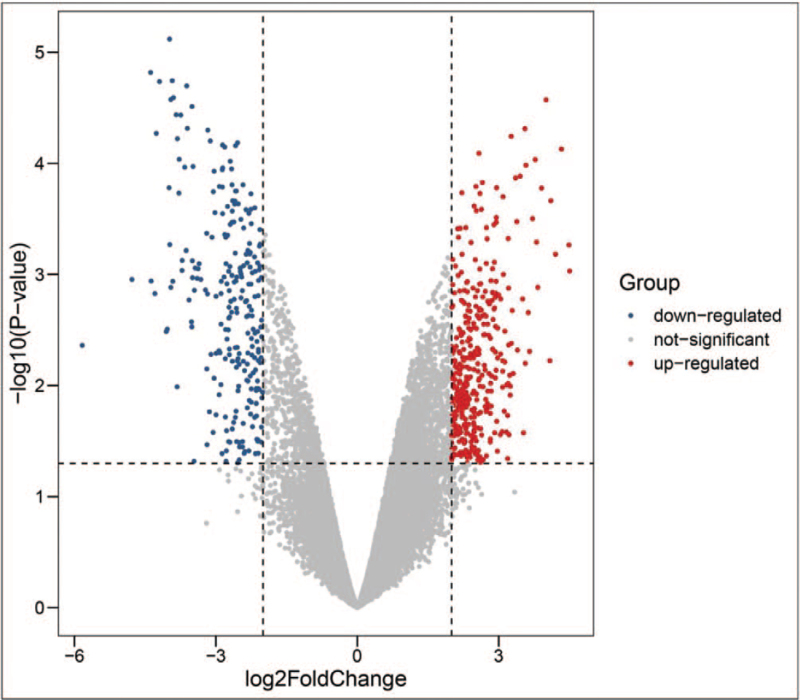
A total of 636 DEGs were extracted from microarray data GSE1009, including 390 up-regulated genes and 246 down-regulated genes (Fig. 8). After combination and intersection with the 61 screened targets, 4 hub DN-related genes were identified, including VEGFA, epidermal growth factor receptor (EGFR), STAT1, and GJA1.
Figure 8.
The volcano map of commonly expressed DEGs between DN patients and the control group in GSE1009. DEGs = differentially expressed genes, N = diabetic nephropathy.
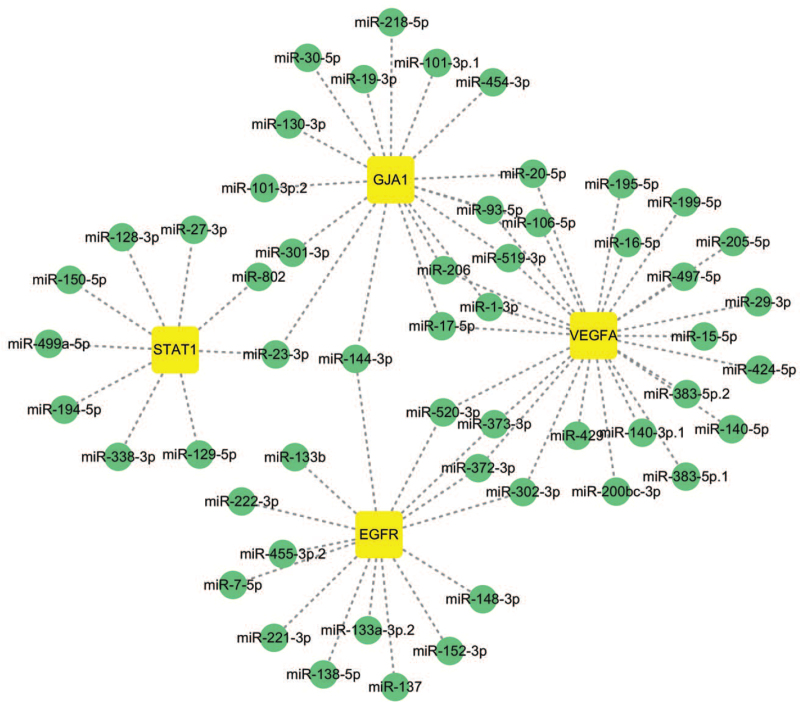
3.8. The key miRNAs
Related miRNA targets of the 4 hub genes were collected by the therapeutic target database; 64 miRNAs were screened in this process. As shown in Figure 9, a visualized network graph was established by Cytoscape software. The results showed that miR-372-3p, miR-373-3p, miR-520-3p, and miR-302-3p were all regulated by EGFR and VEGFA. Meanwhile, VEGFA also influenced 10 other miRNAs, namely miR-16-5p, miR-195-5p, miR-424-5p, miR-497-5p, miR-15-5p, miR-17-5p, miR-20-5p, miR-93-5p, miR-106-5p, and miR-519-3p. In addition, miR-1-3p and miR-206 were regulated by VEGFA and GJA1, miR-23-3p was also regulated by STAT1 and GJA1.
Figure 9.
The hub genes corresponding to key miRNAs. EGFR = epidermal growth factor receptor, miRNAs = microRNAs, VEGFA = vascular endothelial growth factor A.
4. Discussion
DN is a glomerular microvascular complication characterized by hyperglycemia, hypertension, proteinuria, and edema and is a common cause of end-stage renal disease.[20,21] It is generally believed that the pathogenesis of DN is related to disorders of glucose and lipid metabolism, oxidative stress, inflammatory reaction, and abnormal vasoactive substances.[22,23] However, the etiology of this disease is complex and the pathogenesis of DN requires further investigation.
Studies have reported that the extract component of AM can increase telomerase activity, and has antioxidant, anti-inflammatory, immunoregulatory, hypolipidemic, hepatoprotective, expectorant, and diuretic properties.[24,25] It was reported that AM exerts its therapeutic effects in DN by regulating the Nrf2/HO-1 signaling pathway, the PI3K/AKT/mTOR signaling pathway, and immunoregulation.[26,27] However, the pharmacological mechanism and material basis of AM are still unclear.
After analyzing the network, 17 active ingredients and 61 targets of AM were determined, and the pharmacological mechanisms of AM in treating DN were elucidated by enrichment analysis of the 61 targets. The “Compound-Target” network identified quercetin, kaempferol, 7-O-methylisomucronulatol, formononetin, and isorhamnetin as the key active components of AM that exhibit therapeutic effects against DN. Moreover, these key ingredients targeted 146, 58, 44, 38, and 36 targets, respectively. Interestingly, it was reported that quercetin liposomes ameliorate streptozotocin-induced DN symptoms by reduce the content of serum creatinine and urea nitrogen.[28] Meanwhile, kaempferol and 7-O-methylisomucronulatol ameliorate renal injury and fibrosis by enhancing the release of GLP-1 and insulin and inhibiting RhoA/Rho kinase in DN patients.[29] Formononetin could increase the expression of SIRT1 in kidney tissue and attenuated kidney damage in rats with T2DM.[30] In addition, isorhamnetin ameliorates inflammatory responses and articular cartilage damage in streptozotocin-induced diabetic rats via attenuation of oxidative stress, inflammation, and apoptosis.[31,32]
In recent years, significant progress has been made in the field of bioinformatics to better understand disease mechanisms, especially diabetes and its metabolic syndrome such as DN. Based on bioinformatics analysis, miRNAs have not only emerged as diagnostic and prognostic biomarkers but also provided new perspectives for researchers to study the potential molecular mechanisms and regulatory targets of DN. For instance, miR-29 and miR-200 simultaneously target VEGFA, which mediates ECM-receptor interaction and PI3K/Akt signaling pathways to initiate the pathogenesis of DN.[33,34] In DN patients with T2DM, miR-31 was related to the recruitment of leukocytes to vascular walls induced by pro-inflammatory and adhesion molecules, which was positively correlated with leukocyte rolling velocity and negatively associated with leukocyte adhesion, TNF-α, IL-6, and ICAM-1 levels.[35]
VEGFA is an important vascular endothelial growth factor and plays a vital role in angiogenesis, migration, permeability, and cell survival, with studies linking its abnormal expression in the kidney to a large array of renal diseases.[36] This study provides experimental evidence of the possible correlation between adipose tissues miR-20b and miR-296 with T2DM, which might regulate VEGFA CX3CL1, HIF1A, and STAT3 to alleviate diabetes and its complications.[37] Moreover, inhibition of miR-17 prevents high glucose-induced impairment of angiogenesis and improves cardiac function after myocardial infarction by targeting VEGFA in diabetic mice.[38] Other outcomes suggested that miR-15a/16 maintains the retinal endothelial cell barrier by reducing TGF-β3/VEGF signaling and increasing levels of key tight junction proteins.[39] The research revealed that miR-93 plays a role in the VEGF signaling pathway and offers a potentially novel target in preventing the progression of DN.[40] Our research has demonstrated that VEGFA was regulated by multiple miRNAs including miR-372-3p, miR-373-3p, miR-15/16/17, miR-195-5p, miR-424-5p, miR-497-5p, miR-20-5p, and miR-93-5p, which are consistent with former reports.
Previous studies have indicated that EGFR-mediated oxidative stress is activated in DN and inhibiting EGFR expression may hence serve as a potential therapeutic strategy in diabetic kidney diseases.[41,42] In addition, studies have shown that EGFR is highly or abnormally expressed in many solid tumors, especially in proliferation, angiogenesis, invasion, metastasis, and apoptosis of tumor cells. This study showed that EGFA was regulated by miR-302-3p and miR-372. In addition, miR-302a-3p may modulate renal epithelial-mesenchymal transition in DN by targeting ZEB1.[43] miR-372-3p and miR-373-3p not only inhibited the progression of renal fibrosis, but also prevented podocyte apoptosis in the pathogenesis of hepatic fibrosis in non-alcoholic steatohepatitis.[44]
STAT1, as a signal transduction protein between the cell membrane receptor and effector, can be activated by phosphorylation by stimulation of extracellular signal. STAT1 reportedly alleviates tubulointerstitial fibrosis in diabetic kidney disease via reduced tubule apoptosis and inhibition of the JAK-STAT signaling pathway.[45,46] Together, previous studies have reported that miR-30a targets the transcription factor STAT1 to limit the actions of proinflammatory cytokine interferon and alleviate insulin sensitivity.[47] Our research has found that miR-23-3p regulated by STAT1 and GJA1. However, STAT1 was regulated by miR-23-3p, which has not been previously reported in the field of DN and may be a starting point for future studies.
The knockout of miR-206 in mice may have a beneficial effect on glucose metabolism, owing to the regulation of glucokinase expression in pancreatic islets, which in turn affects glucose induction and insulin sensitivity. miR-206 has been shown to be an excellent target for studying the pathophysiology of T2DM and metabolic syndrome.[48] The study confirms that VEGFA and GJA1 were targeted by miR-206, which may mediate multiple signaling pathways promoting AM to inhibit or delay the development of DN.
5. Conclusion
Collectively, the active ingredients of AM and their corresponding targets were analyzed by network pharmacological methods, and the co-expressed genes, key genes, and key miRNAs were identified through the database and visualization software. These results further elaborate the molecular biological mechanism of AM treatment of DN and provide a theoretical basis for the clinical treatment of DN using AM.
Author contributions
Conceptualization: Mingfei Guo.
Data curation: Yaji Dai, Mingfei Guo.
Funding acquisition: Jiarong Gao.
Investigation: Yaji Dai, Lei Jiang.
Methodology: Yaji Dai.
Project administration: Jiarong Gao.
Resources: Mingfei Guo.
Software: Yaji Dai, Mingfei Guo.
Supervision: Lei Jiang.
Validation: Yaji Dai, Mingfei Guo.
Visualization: Mingfei Guo.
Writing – original draft: Yaji Dai.
Writing – review & editing: Mingfei Guo.
Footnotes
Abbreviations: AM = Astragalus membranaceus, BP = biological process, CC = cellular component, CTD = Comparative Toxicogenomics Database, DEGs = differentially expressed genes, DL = drug-likeness, DN = diabetic nephropathy, EGFR = epidermal growth factor receptor, GO = gene ontology, IL = interleukin, KEGG = Kyoto encyclopedia of genes and genomes, MF = molecular function, miRNAs = microRNAs, OB = oral bioavailability, PPI = protein–protein interaction, T2DM = type 2 diabetes mellitus, TNF = tumor necrosis factor, VEGFA = vascular endothelial growth factor A.
How to cite this article: Dai Y, Guo M, Jiang L, Gao J. Network pharmacology-based identification of miRNA expression of Astragalus membranaceus in the treatment of diabetic nephropathy. Medicine. 2022;101:5(e28747).
This study was financially supported by National Natural Science Foundation of China (grant no. 81973546) and the Research Fund of Anhui Medical University (grant no. 2021xkj056).
The authors have no conflicts of interest to disclose.
The data used to support the findings of this study are available from the corresponding author upon request.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 2018;71:884–95. [DOI] [PubMed] [Google Scholar]
- [2].Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab 2016;27:820–30. [DOI] [PubMed] [Google Scholar]
- [3].Wang Q, Shao X, Xu W, et al. Astragalosides IV inhibits high glucose-induced cell apoptosis through HGF activation in cultured human tubular epithelial cells. Ren Fail 2014;36:400–6. [DOI] [PubMed] [Google Scholar]
- [4].Dai H, Liu Q, Liu B. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res 2017;2017:2615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abla M, Sun H, Li Z, et al. Identification of miRNAs and their response to cold stress in Astragalus membranaceus. Biomolecules 2019;9:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao SM, Zhang T, Qiu Q, et al. MiRNA-337 leads to podocyte injury in mice with diabetic nephropathy. Eur Rev Med Pharmacol Sci 2019;23:8485–92. [DOI] [PubMed] [Google Scholar]
- [7].Yang S, Fei X, Lu Y, et al. miRNA-214 suppresses oxidative stress in diabetic nephropathy via the ROS/Akt/mTOR signaling pathway and uncoupling protein 2. Exp Ther Med 2019;17:3530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang R, Zhu X, Bai H, et al. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol 2019;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li J, Huang Y, Zhao S, et al. Based on network pharmacology to explore the molecular mechanisms of Astragalus membranaceus for treating T2 diabetes mellitus. Ann Transl Med 2019;7:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang R, Xing B, Zhao J, et al. Astragaloside IV relieves gestational diabetes mellitus in genetic mice through reducing hepatic gluconeogenesis. Can J Physiol Pharmacol 2020;98:466–72. [DOI] [PubMed] [Google Scholar]
- [11].Yue SJ, Liu J, Feng WW, et al. System pharmacology-based dissection of the synergistic mechanism of Huangqi and Huanglian for diabetes mellitus. Front Pharmacol 2017;8:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen X, Yang Y, Liu C, et al. Astragaloside IV ameliorates high glucose-induced renal tubular epithelial–mesenchymal transition by blocking mTORC1/p70S6K signaling in HK–2 cells. Int J Mol Med 2019;43:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luo TT, Lu Y, Yan SK, Xiao X, Rong XL, Guo J. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med 2020;26:72–80. [DOI] [PubMed] [Google Scholar]
- [14].Wang N, Zhu F, Shen M, et al. Network pharmacology-based analysis on bioactive anti-diabetic compounds in Potentilla discolor Bunge. J Ethnopharmacol 2019;241:111905. [DOI] [PubMed] [Google Scholar]
- [15].Huang J, Cheung F, Tan HY, et al. Identification of the active compounds and significant pathways of yinchenhao decoction based on network pharmacology. Mol Med Rep 2017;16:4583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu T, Wang Q, Liu M. A Network pharmacology approach to explore the potential mechanisms of Huangqin-Baishao herb pair in treatment of cancer. Med Sci Monit 2020;26:e923199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Athanasios A, Charalampos V, Vasileios T, et al. Protein–protein interaction (PPI) network: recent advances in drug discovery. Curr Drug Metab 2017;18:05–10. [DOI] [PubMed] [Google Scholar]
- [18].Qin T, Wu L, Hua Q, et al. Prediction of the mechanisms of action of Shenkang in chronic kidney disease: a network pharmacology study and experimental validation. J Ethnopharmacol 2020;246:112128. [DOI] [PubMed] [Google Scholar]
- [19].Guo A, Wang W, Shi H, et al. Identification of hub genes and pathways in a rat model of renal ischemia-reperfusion injury using bioinformatics analysis of the gene expression omnibus (GEO) dataset and integration of gene expression profiles. Med Sci Monit 2019;25:8403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen HW, Yang MY, Hung TW, et al. Nelumbo nucifera leaves extract attenuate the pathological progression of diabetic nephropathy in high-fat diet-fed and streptozotocin-induced diabetic rats. J Food Drug Anal 2019;27:736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang ZH, Tang YZ, Song HN, et al. miRNA-342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med 2020;45:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang C, Zhu X, Li L, et al. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes Metab Syndr Obes 2019;12:1297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rai U, Kosuru R, Prakash S, et al. Tetramethylpyrazine alleviates diabetic nephropathy through the activation of Akt signalling pathway in rats. Eur J Pharmacol 2019;865:172763. [DOI] [PubMed] [Google Scholar]
- [24].Guo MF, Dai YJ, Gao JR, et al. Uncovering the mechanism of Astragalus membranaceus in the treatment of diabetic nephropathy based on network pharmacology. J Diabetes Res 2020;2020:5947304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sun S, Yang S, An N, et al. Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J Ethnopharmacol 2019;238:111857. [DOI] [PubMed] [Google Scholar]
- [26].Abdel-Moneim A, Mahmoud B, Nabil A, et al. Correlation between oxidative stress and hematological profile abnormalities in diabetic nephropathy. Diabetes Metab Syndr 2019;13:2365–73. [DOI] [PubMed] [Google Scholar]
- [27].Song Y, Liu W, Tang K, et al. Mangiferin alleviates renal interstitial fibrosis in streptozotocin-induced diabetic mice through regulating the PTEN/PI3K/Akt signaling pathway. J Diabetes Res 2020;2020:9481720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tang L, Li K, Zhang Y, et al. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep 2020;10:2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sharma D, Kumar Tekade R, Kalia K. Kaempferol in ameliorating diabetes-induced fibrosis and renal damage: an in vitro and in vivo study in diabetic nephropathy mice model. Phytomedicine 2020;76:153235. [DOI] [PubMed] [Google Scholar]
- [30].Oza MJ, Kulkarni YA. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci 2019;219:109–21. [DOI] [PubMed] [Google Scholar]
- [31].Tsai SW, Lin CC, Lin SC, et al. Isorhamnetin ameliorates inflammatory responses and articular cartilage damage in the rats of monosodium iodoacetate-induced osteoarthritis. Immunopharmacol Immunotoxicol 2019;41:504–12. [DOI] [PubMed] [Google Scholar]
- [32].Jamali-Raeufy N, Baluchnejadmojarad T, Roghani M, et al. Isorhamnetin exerts neuroprotective effects in STZ-induced diabetic rats via attenuation of oxidative stress, inflammation and apoptosis. J Chem Neuroanat 2019;102:101709. [DOI] [PubMed] [Google Scholar]
- [33].Yang F, Cui Z, Deng H, et al. Identification of miRNAs-genes regulatory network in diabetic nephropathy based on bioinformatics analysis. Medicine (Baltimore) 2019;98:e16225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen L, Bai J, Li Y. miR-29 mediates exercise–induced skeletal muscle angiogenesis by targeting VEGFA, COL4A1 and COL4A2 via the PI3K/Akt signaling pathway. Mol Med Rep 2020;22:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rovira-Llopis S, Escribano-Lopez I, Diaz-Morales N, et al. Downregulation of miR-31 in diabetic nephropathy and its relationship with inflammation. Cell Physiol Biochem 2018;50:1005–14. [DOI] [PubMed] [Google Scholar]
- [36].Tziastoudi M, Stefanidis I, Zintzaras E. The genetic map of diabetic nephropathy: evidence from a systematic review and meta-analysis of genetic association studies. Clin Kidney J 2020;13:768–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gentile AM, Lhamyani S, Coín-Aragüez L, et al. miR-20b, miR-296, and Let-7f expression in human adipose tissue is related to obesity and type 2 diabetes. Obesity (Silver Spring) 2019;27:245–54. [DOI] [PubMed] [Google Scholar]
- [38].Yan M, Chen K, Sun R, et al. Glucose impairs angiogenesis and promotes ventricular remodelling following myocardial infarction via upregulation of microRNA-17. Exp Cell Res 2019;381:191–200. [DOI] [PubMed] [Google Scholar]
- [39].Ye EA, Liu L, Steinle JJ. miR-15a/16 inhibits TGF-beta3/VEGF signaling and increases retinal endothelial cell barrier proteins. Vision Res 2017;139:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Long J, Wang Y, Wang W, et al. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem 2010;285:23457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Z, Li Y, Overstreet JM, et al. Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes 2018;67:1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu Z, Zhao Y, Zhong P, et al. EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget 2017;8:32655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tang WB, Zheng L, Yan R, et al. miR302a-3p may modulate renal epithelial-mesenchymal transition in diabetic kidney disease by targeting ZEB1. Nephron 2018;138:231–42. [DOI] [PubMed] [Google Scholar]
- [44].Gerhard GS, Hanson A, Wilhelmsen D, et al. AEBP1 expression increases with severity of fibrosis in NASH and is regulated by glucose, palmitate, and miR-372-3p. PLoS One 2019;14:e0219764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huang F, Wang Q, Guo F, et al. FoxO1-mediated inhibition of STAT1 alleviates tubulointerstitial fibrosis and tubule apoptosis in diabetic kidney disease. EbioMedicine 2019;48:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sun M, Bu W, Li Y, et al. Danzhi Jiangtang capsule ameliorates kidney injury via inhibition of the JAK-STAT signaling pathway and increased antioxidant capacity in STZ-induced diabetic nephropathy rats. Biosci Trends 2019;12:595–604. [DOI] [PubMed] [Google Scholar]
- [47].Koh EH, Chernis N, Saha PK, et al. miR-30a remodels subcutaneous adipose tissue inflammation to improve insulin sensitivity in obesity. Diabetes 2018;67:2541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lazzara F, Trotta MC, Platania CBM, et al. Stabilization of HIF-1α in human retinal endothelial cells modulates expression of miRNAs and proangiogenic growth factors. Front Pharmacol 2020;11:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]