Abstract
Background:
It is still unclear if and to what extent antenatal or infant or childhood vitamin D supplementation would affect the development of allergy diseases later in life. This study aimed to review the efficacy of vitamin D supplementation in pregnant women, infants, or children for the prevention of allergies.
Methods:
MEDLINE (PubMed), EMBASE (OVID), and the Cochrane Central Register of Controlled Trials were searched up to March 1, 2020. We included only randomized controlled trials (RCTs). We performed a systematic review and meta-analysis for vitamin D supplementation in primary allergy prevention. These trials were assessed for risk of bias using the Cochrane Collaboration domains and the consensus was reached via discussion with the full study group. We descriptively summarized and quantitatively synthesized original data to evaluate vitamin D supplementation in primary allergy prevention by using Review Manager software for meta-analysis.
Results:
The search yielded 1251 studies. Seven RCTs were included in this analysis. A meta-analysis revealed that vitamin D supplementation for pregnant women or infants may not decrease the risk of developing allergic diseases, such as asthma or wheezing (supplementation for pregnant women, risk ratio [RR]: 1.01, 95% confidence interval [CI]: 0.81–1.26, P = 0.90, I2 = 47%; supplementation for infants, RR: 1.00, 95% CI: 0.70–1.43, P = 0.99, I2 = 0%; supplementation for pregnant women and infants, RR: 0.35, 95% CI: 0.10–1.25, P = 0.11), eczema (supplementation for pregnant women, RR: 0.95, 95% CI: 0.80–1.13, P = 0.77, I2 = 0%; supplementation for infants, RR: 0.84, 95% CI: 0.64–1.11, P = 0.19, I2 = 42%), allergic rhinitis (supplementation for pregnant women, RR: 0.93, 95% CI: 0.78–1.11, P = 0.15, I2 = 47%), lower respiratory tract infection (LRTI) (supplementation for pregnant women, RR: 0.97, 95% CI: 0.85–1.11, P = 0.59, I2 = 0%), or food allergy.
Conclusions:
Supplementation of vitamin D in pregnant women or infants does not have an effect on the primary prevention of allergic diseases.
Systematic Review Registration:
PROSPERO (CRD42020167747)
Keywords: Vitamin D, Prevention, Pregnancy, Infants, Allergy, Meta-analysis
Introduction
Allergic diseases represent a spectrum of health conditions with a large worldwide burden, which result from an interaction between individual genetic susceptibility and exposure to environmental factors. The prevalence of allergic diseases is approximately 10% in infants whose parents and siblings do not have allergic diseases and 20% to 30% in those with an allergic first-degree relative.[1] However, it has become increasingly evident that there is an important role for environmental factors in the onset of allergic diseases.[2] Most studies have shown correlations between vitamin D and the development of allergic diseases. Low vitamin D status during the first few years of life has also been associated with increased risk of asthma,[3–5] eczema,[6] food allergy,[7] wheezing,[8] allergic rhinitis,[9] and respiratory infections.[10]
Vitamin D is the generic term for two molecules: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). The former can be obtained from plant sources. The latter can be obtained from animal foods and sunlight exposure. The action of sunlight on the skin converts 7-dehydrocholesterol to pre-vitamin D, which is metabolized to vitamin D.[11] Vitamin D status is easily modified by sun exposure or diet and therefore makes it an attractive target biomarker for monitoring and prevention. It modulates the immune system in a complex manner with facilitating effects on the innate immune system and impeding effects on the adaptive immune system.[12] Vitamin D deficiency is highly prevalent worldwide, especially pregnant women are at high risk.[13]
Given the presence of vitamin D receptors on immune cells and the airways,[14] and its multiple effects on the developing lung and immune system, immunomodulation, and regulation of inflammation,[15–18] vitamin D has gained much attention in recent years as a key modifiable risk factor of asthma and allergy in childhood. Previous related systematic review and meta-analysis were mainly based on observational studies and mainly focused on the relationship between maternal 25-hydroxyvitamin D levels and risk of allergy diseases, which indicated that maternal vitamin D blood level was associated with risk of allergy diseases in the offspring.[19–21] One systematic review investigated the effects of vitamin D supplementation in pregnancy on the prevention of allergic outcomes in offspring, but it did not include any randomized controlled trials (RCTs) in which vitamin D supplementation was given to infants.[22] A recently published systematic review covered vitamin D supplementation in both pregnancy and early childhood, but it excluded studies that reported vitamin D supplementation combined with other vitamins or nutrients, and only one RCT was included.[23] It is still unclear if and to what extent antenatal or infant or childhood vitamin D supplementation would affect the development of allergy diseases later in life. In this study, the discussion of vitamin D will primarily aim to summarize the existing evidence from RCTs for whether vitamin D supplementation in pregnant women, infants, or children has any efficacy for the prevention of allergic diseases.
Methods
Data sources and searches
This systematic review and meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews (No. CRD42020167747). This review of the literature was performed by searching three computerized databases for peer-reviewed articles published in English from inception to March 1, 2020. The research articles were searched for and selected from electronic databases, including MEDLINE (PubMed), EMBASE (OVID), and the Cochrane Central Register of Controlled Trials. The search process was conducted using the following keywords (pregnancy OR lactation OR pregnan∗ OR antenatal mother OR maternal OR child OR adolescent OR infant OR baby OR newborn∗ OR toddler∗ OR preschool∗ OR schoolchild∗) AND (vitamin D OR Cholecalciferol OR Hydroxycholecalciferols OR Calcifediol OR Dihydroxycholecalciferols OR Calcitriol OR Ergocalciferols OR Dihydrotachysterol OR 25-Hydroxyvitamin D 2 OR “vitamin D deficiency”) AND (asthma OR “asthma prevention” OR wheez∗ OR “respiratory health” OR allergic rhinitis OR hay fever OR food allergy OR atopic dermatitis). We checked reference lists of reviewed articles and contacted clinical experts in the specialty for additional references.
Study selection and eligibility criteria
Two investigators independently reviewed titles, abstracts, and full-text articles using prespecified inclusion criteria, with disagreements about inclusion resolved by discussion. If additional information or clarification was needed about a study, we contacted the authors of the relevant article. Full-length articles of studies evaluating the associations between vitamin D supplementation in pregnancy or infants or children and allergic diseases were examined and subsequently selected if they fulfilled the following inclusion criteria: (1) the design was an RCT; (2) healthy pregnant or lactating females and/or healthy children from birth to 5 years of age were included; (3) vitamin D protocol was specified in the treatment group; (4) outcomes were asthma/wheeze, eczema (atopic dermatitis), allergic rhinitis, lower respiratory tract infection (LRTI), or food allergy; and (5) the study contained relevant data to calculate the effect size.
Articles were excluded if (1) conference abstract only; (2) cohort study, case-control study, cross-sectional study, review, case report, and other uninterested study design; and (3) animal studies. The reference lists of all papers of interest were scrutinized to obtain other relevant articles. Disagreements over inclusion were resolved through consensus, and where necessary, a third reviewer was involved.
Data extraction and risk of bias assessment
For each included study, one independent evaluator read all reports of eligible studies in detail and extracted the following data from each selected study: the total number of participants, population, geographical location, trial duration, exposure measurement, the interval of follow-up, and outcomes (asthma or wheeze, eczema, allergic rhinitis, LRTI, and food allergy). A second investigator reviewed for completeness and accuracy. We discussed any disagreements and aimed to reach an agreement by consensus with a third reviewer if necessary. These trials were assessed for risk of bias according to the PRISMA recommendations and consensus was reached via discussion with the full study group.
Data synthesis
We extracted data from the included studies and prepared the data in table format. The study outcomes were described in the Results section. We calculated pooled relative risk ratios (RRs) with a 95% confidence interval (CI) for categorical outcomes. Heterogeneity was assessed using the I2 test and Chi-square statistics. For all of the outcomes measured, a fixed-effects meta-analysis was used when low heterogeneity was present (P ≥ 0.10 and I2 < 50%), and a random-effects meta-analysis was performed to address the variation across the included studies when high heterogeneity was detected (P < 0.10 and I2 ≥ 50%). Subgroup analyses were conducted to explore the source of heterogeneity. Publication bias assessment was conducted through funnel plots if more than ten trials were included. Sensitivity analysis was used to explore the stability of the results. The statistical analysis was performed using the Review Manager (RevMan; Version 5.3.4. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Search results
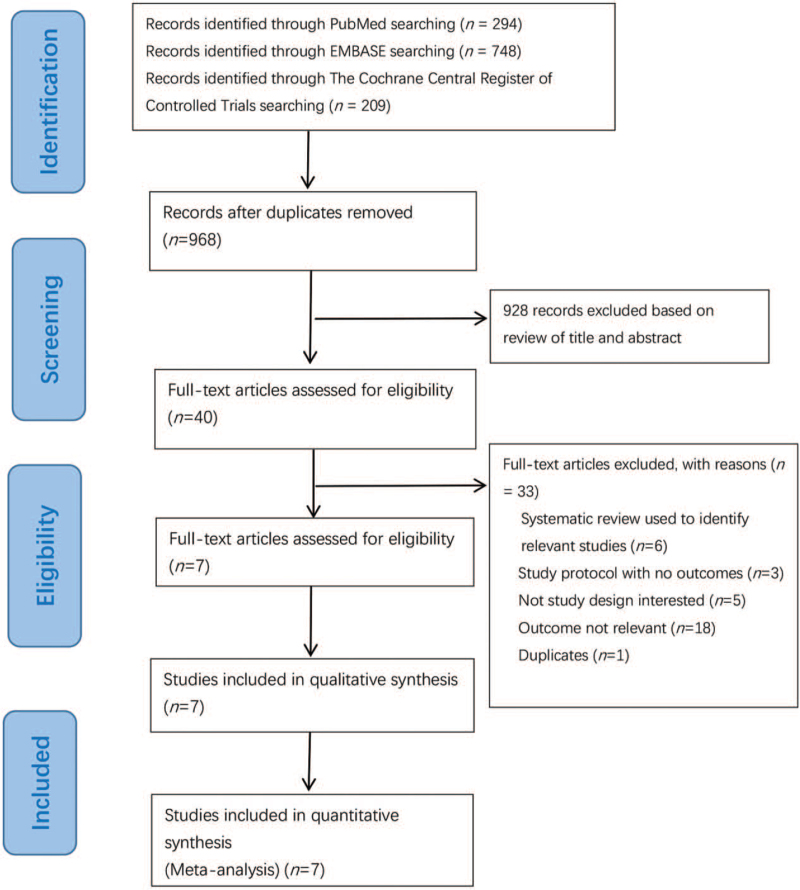
We have identified 294 potentially relevant publications from PubMed, 748 from EMBASE, and 209 from The Cochrane Central Register of Controlled Trials. After excluding duplicates and publications that did not meet the inclusion criteria, seven RCTs[24–30] were included [Figure 1].
Figure 1.
Flowchart for selection of studies of vitamin D supplementation in pregnant women or infants or children for preventing allergic diseases.
Study characteristics
Description of included studies
Five of the studies[24,25,27,29,30] administered vitamin D3 (cholecalciferol); one study[29] administered both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol); and one study administered vitamin D.[26] The numbers of studies according to the population exposed to vitamin D were as follows: four RCTs in pregnant women,[25,28–30] one RCT in pregnant women and infants,[26] two RCTs in infants,[24,27] and no RCTs in children or breastfeeding women. The follow-up time of the studies ranged from 6 months to 6 years. The supplementation dosage also varied from study to study, ranging from 400 U/day to 200,000 U/day. All the seven RCTs reported information about the primary prevention of allergic diseases, including asthma, wheezing, allergic rhinitis, food allergy, airborne allergens, and eczema. Table 1 outlines the characteristics of each study. Except for one study[29] that did not report the specific subjects’ baseline vitamin D serum levels, the other six studies all reported the mean concentration of serum 25-hydroxyvitamin D. All studies reported that enrolment mean serum 25-hydroxyvitamin D levels showed no clinically important differences between the vitamin D group and control group [Table 1].
Table 1.
Characteristics of studies on the effects of vitamin D supplementation on the risk of allergic diseases.
| Study | Design | Location | Participant characteristics | Intake of intervention from/until | Intervention | Comparator | Follow-up | Total N | Outcome assessment |
| Goldring et al[29] | Single-center double-blinded placebo RCT | UK | (1) Pregnant women (Asian, Middle, Eastern, Black, and White). (2) The history of allergic diseases was not mentioned. (3) 3–5% mothers were smoking. | From 27 weeks of gestation to delivery | 800 U/day vitamin D2 (n = 60); 200,000 U/day vitamin D3 (n = 60) | No treatment (n = 60) | 3 years | 180 | (1) Wheeze ever. (2) Recurrent wheezing. (3) Eczema ever. (4) Allergic rhinitis. (5) Food allergy. (6) Atopy. |
| Grant et al[26] | Single-center randomized, double-blind, placebo-controlled, parallel-group study | New Zealand | (1) Pregnant women and healthy infants. (2) 23% of the mothers and 11% of the fathers had a history of doctor-diagnosed asthma. (3) 16–21% of mothers were smoking. | Pregnant women (from 27 weeks of gestation to birth); infants (from birth to 6 months) | 1000 U/day (pregnant women) + 400 U/day vitamin D (infants) (n = 86); 2000 U/day (pregnant women) + 800 U/day vitamin D (infants) (n = 87) | Placebo (n = 87) | 18 months | 260 | (1) Positive skin prick test. (2) Acute primary care visits. |
| Chawes et al[25] | Single-center double-blind, RCT | Denmark | (1) Pregnant women. (2) 26% of mothers had a history of asthma. (3) 8% of mothers were smoking. | From 24 weeks of gestation to 1 week after delivery | 2800 U/day vitamin D3 (n = 315) | Placebo pill plus 400 IU/day of vitamin D3 (n = 308) | 3 years | 623 | (1) Persistent wheeze. (2) Episode of troublesome lung symptoms. (3) Asthma. (4) Upper respiratory tract infections. (5) LRTIs. (6) Eczema. (7) Allergic sensitization. |
| Rueter et al[27] | Single-center, double-blind, placebo-controlled RCT | Bahama | (1) Healthy singleton infants. (2) Infants had a first-degree relative (mother, father, or sibling) with a history of allergic disease (asthma, eczema, and allergic rhinitis). (3) Mothers were non-smokers. | From 28 days after birth to 6 months of age | 400 U/day vitamin D3 (n = 97) | An identical product of coconut and palm kernel oil but containing no vitamin D3 (n = 98) | 3/6 months | 195 | (1) 25(OH)D levels. (2) Eczema. (3) Wheeze. |
| Brustad et al[30] | Single-center double-blinded placebo RCT | Denmark | (1) Pregnant women. (2) 26% of mothers had a history of asthma. (3) 8% of mothers were smoking. | From 24 weeks of gestation to 1 week after delivery | 2800 U/day of vitamin D3 (n = 315) | Placebo plus 400 U/day of vitamin D3 (n = 308) | 6 years | 623 | (1) Asthma. (2) Allergic rhinitis. |
| Rosendahl et al[24] | Single-center double-blinded placebo RCT | Finland | (1) Healthy infants. (2) Some mothers and fathers had a history of allergic diseases. (3) Some mothers and fathers were smoking. | From 2 weeks to 24 months of age | 1200 U/day of vitamin D3 (n = 486) | 400 U/day vitamin D3 (n = 489) | 12 months | 975 | (1) Allergic sensitization to food or aeroallergens. (2) Allergic disease and allergy symptom. |
| Litonjua et al[28] | Multicenter (3) double-blinded placebo RCT | USA | (1) Pregnant women (age 18–39 years, 10–18 gestational weeks), English or Spanish speaking. (2) Pregnant women or their partner had a history of allergic disease. (3) Women were not smoking. | From 10 weeks to 18 weeks of gestation until birth | 4000 U/day vitamin D3 plus a multivitamin with 400 U/day vitamin D3 (n = 442) | Placebo pill plus a multivitamin with 400 U/day vitamin D3 (n = 439) | 6 years | 881 | (1) Active asthma. (2) Recurrent wheeze without asthma diagnosis. (3) Late-onset wheeze. (4) Eczema with typical rash. (5) Allergic rhinitis. (6) LRTI. (7) Total immunoglobulin E. (8) Any allergic sensitization. |
RCT: Randomized controlled trials; LRTI: Lower respiratory tract infection; 25(OH)D: 25-hydroxyvitamin D.
Risk of bias in included trials
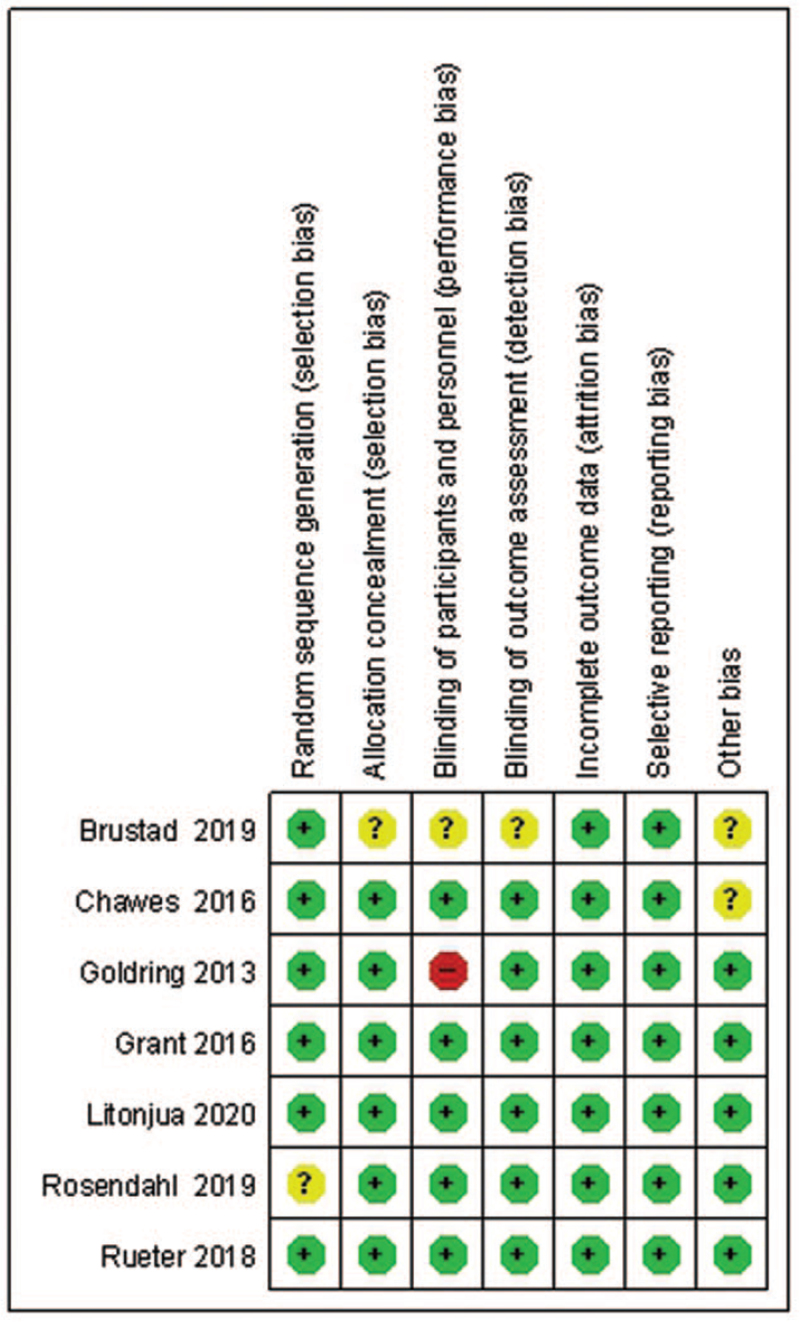
Of the seven trials, four trials were deemed to have a low risk of bias across all domains.[25–28] Only one trial was deemed to have a high risk of bias in blinding of participants and personnel.[29] And one trial was deemed to have an unclear risk of bias in allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other bias.[30] One trial was deemed to have an unclear risk of bias in random sequence generation due to insufficient information[24] [Supplementary Table 1]. The results of the risk of bias assessment are shown in Figure 2.
Figure 2.
Summary of risk of bias for studies on the effects of vitamin D supplementation on the risk of allergic diseases.
Effects of vitamin D supplementation on allergic diseases
Asthma or wheeze
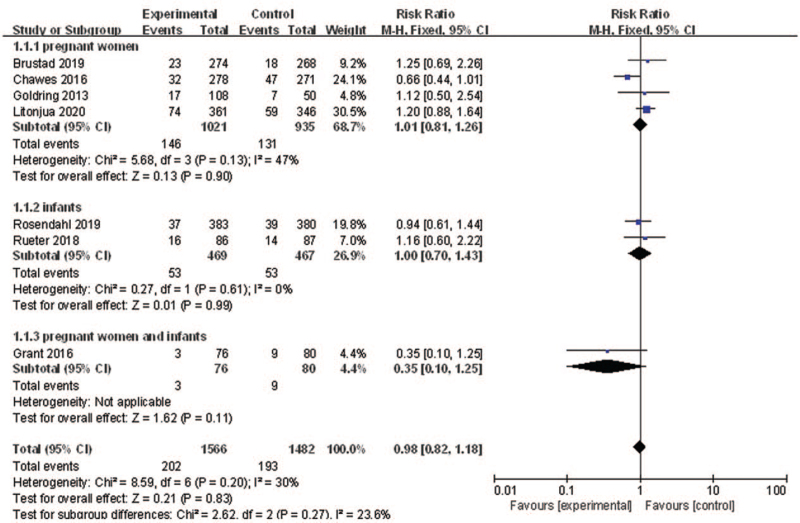
The association between vitamin D supplementation and asthma or wheeze prevention was investigated by seven trials.[24–29] The results combined with fixed-effects model showed that vitamin D supplementation in pregnant women or infants was not associated with the risk of asthma or wheeze (RR: 0.98, 95% CI: 0.82–1.18, P = 0.83, I2 = 30%).
As there was heterogeneity among the studies, subgroup analysis was conducted according to different types of populations (pregnant women/infants/pregnant women and infants). The results of subgroup analyses showed that vitamin D supplementation for pregnant women/infants/pregnant women and infants may not decrease the risk of developing asthma/wheezing (RR: 1.01, 95% CI: 0.81–1.26, P = 0.90, I2 = 47%; RR: 1.00, 95% CI: 0.70–1.43, P = 0.99, I2 = 0%; RR: 0.35, 95% CI: 0.10–1.25, P = 0.11, respectively) [Figure 3]. Sensitivity analysis indicated that the result was robust.
Figure 3.
Forest plot showing the effects of vitamin D supplementation on the risk of asthma or wheeze. CI: Confidence interval.
Eczema
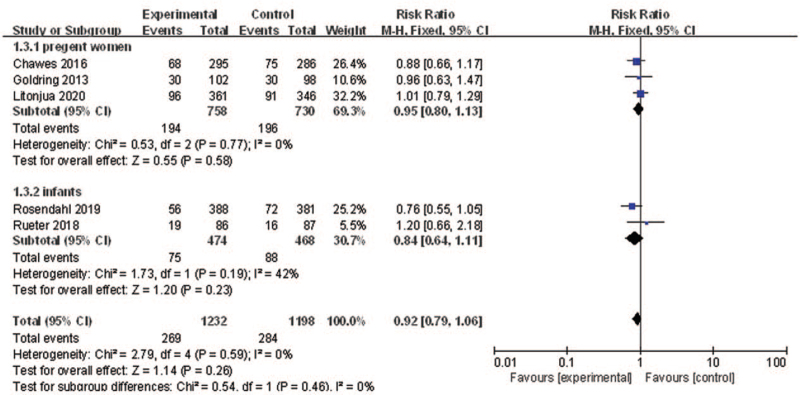
Five RCTs[24,25,27–29] reported the effects of vitamin D supplementation in pregnant women on preventing the development of eczema. Pooling of these estimates found non-significant effects of vitamin D supplementation in pregnant women or infants on preventing the development of eczema (RR: 0.92, 95% CI: 0.79–1.06, P = 0.26, I2 = 0%). The results of subgroup analysis according to different types of population (pregnant women/infants) showed that there were no significant differences in the outcome between supplementation of vitamin D to pregnant women and infants for eczema prevention (test for subgroup differences: P = 0.46) [Figure 4]. Sensitivity analysis indicated that the result was robust.
Figure 4.
Forest plot showing the effects of vitamin D supplementation on the risk of eczema. CI: Confidence interval.
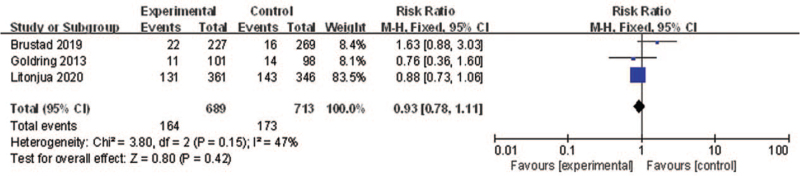
Allergic rhinitis
Three of the included RCTs reported the associations between vitamin D supplementation in pregnant women and allergic rhinitis. Pooling of the three estimates for vitamin D supplementation vs. placebo showed that there were non-significant effects of vitamin D supplementation in pregnant women on preventing the development of allergic rhinitis (RR: 0.93, 95% CI: 0.78–1.11, P = 0.42, I2 = 47%) [Figure 5]. Sensitivity analysis indicated that the result was robust.
Figure 5.
Forest plot showing the effects of vitamin D supplementation on the risk of allergic rhinitis. CI: Confidence interval.
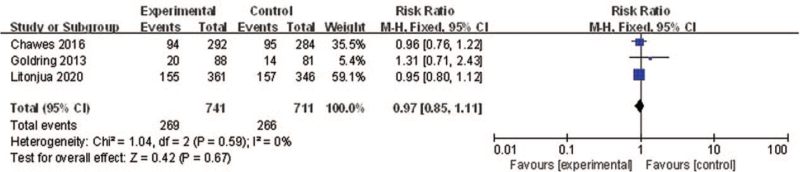
Lower respiratory tract infection
The association between vitamin D supplementation in pregnant women and LRTI was investigated by three RCTs. Pooling of the three estimates for vitamin D supplementation vs. placebo showed that vitamin D supplementation in pregnant women had non-significant effects on preventing the development of LRTI (RR: 0.97, 95% CI: 0.85–1.11; P = 0.67, I2 = 0) [Figure 6]. Sensitivity analysis indicated that the result was robust.
Figure 6.
Forest plot showing the effects of vitamin D supplementation on the risk of LRTI. CI: Confidence interval; LRTI: Lower respiratory tract infection.
Food allergy
Two RCTs investigated the association between vitamin D supplementation in pregnant women or infants and food allergy prevention. One of the RCTs was vitamin D supplementation for pregnant women, and the other RCT was vitamin D supplementation for infants. These two studies showed a non-significant protective effect of vitamin D supplementation for food allergy prevention.
Discussion
In the present systematic review, the available published randomized evidence on vitamin D supplementation in pregnant women or infants or children for preventing allergic diseases across seven RCTs was systematically assessed. Our systematic review showed non-significant effects of vitamin D supplementation in pregnant women or infants on the primary prevention of allergic diseases.
Animal, laboratory, and gene expression studies have suggested significant effects of vitamin D on lung structure and function during the early lung development period. Vitamin D has many immunomodulatory effects.[31–33] Present systematic review found supplementation of vitamin D in pregnant women or infants did not have an effect on the primary prevention of allergic diseases. There are several concerns that need to be addressed. First, does prenatal vitamin D supplementation affect only a few wheezing phenotypes in early childhood? Some scholars[34] hold that prenatal vitamin D supplementation had an early effect on only a minority of the wheezing phenotypes in early childhood. These different phenotypes of early wheezing probably have different causes, and combinations of different types of interventions may be needed to more fully prevent the development of asthma by school age and adolescence. Second, whether vitamin D should be supplemented from the beginning of pregnancy and continue throughout childhood? Some scholars indicated that initiating vitamin D3 supplementation at earlier pregnancy stages may be beneficial, as recent data in humans suggest vitamin D affects fetal lung development as early as the start of the second trimester.[33] Among the seven trials with outcomes of asthma and wheezing, only one study began vitamin D supplementation in the second trimester. Some scholars acknowledged lung development continues throughout childhood,[35] so vitamin D may continue to exert its influence on the respiratory and immune systems in the postnatal period. It was suggested that vitamin D supplementation should be from pregnancy to childhood. However, no RCTs began vitamin D supplementation from pregnancy to childhood in our review. Third, for pregnant women or infants or children from birth to 5 years of age, what dose of vitamin D should be added every day to achieve the desired level of 25-hydroxyvitamin D in them to have an influential effect on the fetal immune programming and lung function. In our review of included literature, the dose of vitamin D3 supplementation ranges from 400 U/day to 200,000 IU/day. It is possible to hypothesize that a lower dose of vitamins may have failed to reach the desirable level of 25-hydroxyvitamin D. For example, in one trial, 200,000 U/day vitamin D3 supplementation can result in at least 50% higher cord blood 25-hydroxyvitamin D concentrations compared with no treatment, but cord blood levels (the median cord-blood level of 25-hydroxyvitamin D, 42 nmol/L, range [27–68 nmol/L]) were still lower in the intervention groups than in some observational studies confirming the correlation between vitamin D and allergic diseases (the median cord-blood level of 25-hydroxyvitamin D, 44 nmol/L [interquartile range: 29–78 nmol/L]).[36] For the dose of vitamin D supplementation, the Endocrine Society Clinical Practice Guideline[37] suggests that infants and children aged 0 to 1 year require an intake of vitamin D of at least 400 U/day, and children aged 1 year and older, at least 600 U/day to maximize bone health. However, this report is based almost exclusively on skeletal considerations and has been criticized by experts in the vitamin D field.[38,39] It has been argued that in populations with limited sun exposure, the current vitamin D recommendations are inadequate for non-skeletal effects, and intakes of 2000 U/day may be required.[37] The American Academy of Pediatrics (AAP)[40] recommends, on an individual basis, pregnant women should receive adequate amounts of vitamin D3 to ensure that their 25-hydroxyvitamin D levels are sufficiently high (>80 nmol/L).
This systematic review and meta-analysis was based on RCTs to summarize whether vitamin D exposure in pregnant women, infants, or children has any efficacy for the prevention of allergic diseases. And it followed the standard guidelines with a comprehensive search strategy that included pertinent records. All steps were carried out independently and in duplicate. Any disagreements were discussed with a third author. And we included only RCTs, which avoided the influence of confounding factors as much as possible and were more likely to get real results. There are some limitations of this systematic review and meta-analysis. There are discrepancies in different intervention doses and different windows of exposure assessment. Moreover, three RCTs of the included studies were conducted in populations at high risk due to family history of allergic diseases; three RCTs were conducted in populations at an average risk, and one study did not mention it. These may have affected the results.
In conclusion, our meta-analysis shows that supplementation of vitamin D in pregnant women or children from birth to 5 years of age does not have an effect on the primary prevention of allergic diseases. Future clinical trials should identify different phenotypes of asthma and response to vitamin D supplementation, and consider the window of vitamin D supplementation (during pregnancy, infancy, or childhood). And long-term follow-up of such studies to evaluate long-term effects is also needed.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Luo C, Sun Y, Zeng Z, Liu Y, Peng S. Vitamin D supplementation in pregnant women or infants for preventing allergic diseases: a systematic review and meta-analysis of randomized controlled trials. Chin Med J 2022;135:276–284. doi: 10.1097/CM9.0000000000001951
Supplemental digital content is available for this article.
References
- 1.Yepes-Nuñez JJ, Fiocchi A, Pawankar R, Cuello-Garcia CA, Zhang Y, Morgano GP, et al. World allergy organization-McMaster university guidelines for allergic disease prevention (GLAD-P): vitamin D. World Allergy Organ J 2016; 9:17.doi: 10.1186/s40413-016-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health 2012; 11:42.doi: 10.1186/1476-069x-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss ST, Litonjua AA. Childhood asthma is a fat-soluble vitamin deficiency disease. Clin Exp Allergy 2008; 38:385–387. doi: 10.1111/j.1365-2222.2007.02920.x. [DOI] [PubMed] [Google Scholar]
- 4.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr 2010; 156:948–952. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm JM, Celedón JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009; 179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer DJ, Sullivan TR, Skeaff CM, Smithers LG, Makrides M. DOMInO Allergy Follow-up Team. Higher cord blood 25-hydroxyvitamin D concentrations reduce the risk of early childhood eczema: in children with a family history of allergic disease. World Allergy Organ J 2015; 8:28.doi: 10.1186/s40413-015-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol 2013; 131:1109–1116. doi: 10.1016/j.jaci.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Baïz N, Dargent-Molina P, Wark JD, Souberbielle JC, Annesi-Maesano I. EDEN Mother-Child Cohort Study Group. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J Allergy Clin Immunol 2013; 133:147–153. doi: 10.1016/j.jaci.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Dogru M, Suleyman A. Serum 25-hydroxyvitamin D3 levels in children with allergic or nonallergic rhinitis. Int J Pediatr Otorhinolaryngol 2015; 80:39–42. doi: 10.1016/j.ijporl.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy 2012; 67:10–17. doi: 10.1111/j.1398-9995.2011.02711.x. [DOI] [PubMed] [Google Scholar]
- 11.Charoenngam N, Shirvani A, Holick MF. Vitamin D for skeletal and non-skeletal health: what we should know. J Clin Orthop Trauma 2019; 10:1082–1093. doi: 10.1016/j.jcot.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giustina AD, Landi M, Bellini F, Bosoni M, Ferrante G, Onorari M, et al. Vitamin D, allergies and asthma: focus on pediatric patients. World Allergy Organ J 2014; 7:27.doi: 10.1186/1939-4551-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez A, Marina LS, Jimenez AM, Esplugues A, Ballester F, Espada M, et al. Vitamin D status in pregnancy and determinants in a Southern European cohort study. Paediatr Perinat Epidemiol 2016; 30:217–228. doi: 10.1111/ppe.12281. [DOI] [PubMed] [Google Scholar]
- 14.Bikle DD. Extraskeletal actions of vitamin D. Ann N Y Acad Sci 2016; 1376:29–52. doi: 10.1111/nyas.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saadoon A, Ambalavanan N, Zinn K, Ashraf AP, MacEwen M, Nicola T, et al. Effect of prenatal versus postnatal vitamin D deficiency on pulmonary structure and function in mice. Am J Respir Cell Mol Biol 2017; 56:383–392. doi: 10.1165/rcmb.2014-0482OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornsby E, Pfeffer PE, Laranjo N, Cruikshank W, Tuzova M, Litonjua AA, et al. Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. J Allergy Clin Immunol 2018; 141:269–278. doi: 10.1016/j.jaci.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 17.May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy 2004; 3:377–393. doi: 10.2174/1568010042634596. [DOI] [PubMed] [Google Scholar]
- 18.Tamblyn JA, Hewison M, Wagner CL, Bulmer JN, Kilby MD. Immunological role of vitamin D at the maternal-fetal interface. J Endocrinol 2015; 224:R107–R121. doi: 10.1530/joe-14-0642. [DOI] [PubMed] [Google Scholar]
- 19.Wei Z, Zhang J, Yu X. Maternal vitamin D status and childhood asthma, wheeze, and eczema: a systematic review and meta-analysis. Pediatr Allergy Immunol 2016; 27:612–619. doi: 10.1111/pai.12593. [DOI] [PubMed] [Google Scholar]
- 20.Song H, Yang L, Jia C. Maternal vitamin D status during pregnancy and risk of childhood asthma: a meta-analysis of prospective studies. Mol Nutr Food Res 2017. 61.doi: 10.1002/mnfr.201600657. [DOI] [PubMed] [Google Scholar]
- 21.Feng H, Xun P, Pike K, Wills AK, Chawes BL, Bisgaard H, et al. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: a meta-analysis of birth cohort studies. J Allergy Clin Immunol 2017; 139:1508–1517. doi: 10.1016/j.jaci.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 22.Vahdaninia M, Mackenzie H, Helps S, Dean T. Prenatal intake of vitamins and allergic outcomes in the offspring: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2016; 5:771–778. doi: 10.1016/j.jaip.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Yepes-Nuñez JJ, Brożek JL, Fiocchi A, Pawankar R, Cuello-García C, Zhang Y, et al. Vitamin D supplementation in primary allergy prevention: systematic review of randomized and non-randomized studies. Allergy 2017; 73:37–49. doi: 10.1111/all.13241. [DOI] [PubMed] [Google Scholar]
- 24.Rosendahl J, Pelkonen AS, Helve O, Hauta-Alus H, Holmlund-Suila E, Valkama S, et al. High-dose vitamin D supplementation does not prevent allergic sensitization of infants. J Pediatr 2019; 209:139–145. doi: 10.1016/j.jpeds.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Chawes BL, Bønnelykke K, Stokholm J, Vissing NH, Bjarnadóttir E, Schoos AM, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA 2016; 315:353–361. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 26.Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy 2016; 71:1325–1334. doi: 10.1111/all.12909. [DOI] [PubMed] [Google Scholar]
- 27.Rueter K, Jones AP, Siafarikas A, Lim EM, Bear N, Noakes PS, et al. Direct infant UV light exposure is associated with eczema and immune development. J Allergy Clin Immunol 2018; 143:1012–1020. doi: 10.1016/j.jaci.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, O’Connor GT, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med 2020; 382:525–533. doi: 10.1056/NEJMoa1906137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLoS One 2013; 8:e66627.doi: 10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brustad N, Eliasen AU, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. High-dose vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA 2019; 321:1003–1005. doi: 10.1001/jama.2019.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Wilson R, Bennett E, Zosky GR. Identification of vitamin D sensitive pathways during lung development. Respir Res 2016; 17:47.doi: 10.1186/s12931-016-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foong RE, Bosco A, Jones AC, Gout A, Gorman S, Hart PH, et al. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am J Respir Cell Mol Biol 2015; 53:664–675. doi: 10.1165/rcmb.2014-0356OC. [DOI] [PubMed] [Google Scholar]
- 33.Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, et al. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics 2013; 6:47.doi: 10.1186/1755-8794-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016; 315:362–370. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres A, Bauer TT. Fishman's Pulmonary Diseases and Disorders. New York: McGraw-Hill Book Company; 2008. [Google Scholar]
- 36.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011; 127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 38.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 2011; 26:455–457. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 39.Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/mL). Best Pract Res Clin Endocrinol Metab 2011; 25:681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008; 122:1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.