Abstract
PURPOSE
Collection of family cancer histories (FCHs) can identify individuals at risk for familial cancer syndromes. The aim of this study is to evaluate the literature on existing strategies whereby providers use information technology to assemble FCH.
METHODS
A systematic search of online databases (Ovid MEDLINE, Cochrane, and Embase) between 1980 and 2020 was performed. Statistical heterogeneity was assessed through the chi-square test (ie, Cochrane Q test) and the inconsistency statistic (I2). A random-effects analysis was used to calculate the pooled proportions and means.
RESULTS
The comprehensive search produced 4,005 publications. Twenty-eight studies met inclusion criteria. Twenty-seven information technology tools were evaluated. Eighteen out of 28 studies were electronic surveys administered before visits (18, 64.3%). Five studies administered tablet surveys in offices (5, 17.8%). Four studies collected electronic survey via kiosk before visits (4, 14.3%), and one study used animated virtual counselor during visits (1, 3.6%). Among the studies that use an FCH tool, the pooled estimate of the overall completion rate was 86% (CI, 72% to 96%), 84% (CI, 65% to 97%) for electronic surveys before visits, 89% (CI, 0.74 to 0.98) for tablet surveys, and 85% (CI, 0.66 to 0.98) for surveys via kiosk. Mean time required for completion was 31.0 minutes (CI, 26.1 to 35.9), and the pooled estimate of proportions of participants referred to genetic testing was 12% (CI, 4% to 23%).
CONCLUSION
Our review found that electronic FCH collection can be completed successfully by patients in a time-efficient manner with high rates of satisfaction.
INTRODUCTION
In the United States, approximately four million individuals carry a pathogenic mutation in a cancer-associated gene.1,2 Guided personalized medicine, with a focus on genetics, leverages information about one's unique genetics to tailor cancer-preventative strategies.3,4 However, fewer than 20% of affected individuals are aware of their underlying genetic condition.2 Specifically, significant barriers to genetic testing and educational opportunities exist for patients with hereditary breast and ovarian cancer syndrome and Lynch syndromes, despite their lifetime elevated risk for gynecologic and nongynecologic cancers.5,6 The significance of a missed diagnosis is measured in lives lost, as medical and surgical interventions can often decrease mortality.7 Collection of an accurate family cancer history (FCH) can help to identify individuals with cancer-associated pathogenic mutations.
CONTEXT
Key Objective
The purpose of this study was to determine whether the utilization of health information technology to collect personal and family history improves the identification and care of individuals at risk for hereditary cancer syndromes.
Knowledge Generated
As the first large-scale, systematic review that focuses on the collection of family health histories via electronic or online tools, this study demonstrates that electronic family cancer history tools have high patient completion rates, acceptable time requirements, and high levels of user satisfaction and collection of complete health information.
Relevance
While health information technology for family cancer history collection holds the potential to improve detection rates of inherited cancer syndromes, additional studies are needed to measure the clinical efficacy through acceptance by patients and health care providers to increase genetic testing uptake, adaptation of the same tools in different clinical settings, and incorporation of the tools in electronic medical records.
Despite the known benefits of screening and testing for hereditary cancer syndromes, it has historically been difficult to execute and there is wide variability in the collection and accuracy of family health history across medical systems. Patients often cannot recollect the pertinent details about their relatives' cancer history in real time during a physician visit without assistance from other family members. Additionally, collecting a family health history is time-consuming, sometimes requiring more than 30 minutes. Limited appointment time and the lack of provider training with consistent documentation in the electronic medical record (EMR), therefore, can result in an incomplete or imprecise history.8,9 Ideally, an effective tool to collect FCH should be self-administered, easily updated over time, and allow for automatic cancer risk assessment, referral to genetic counseling and cascade testing.
A potential solution is health information technology (IT). Health IT has been shown to successfully improve clinical documentation, clinical workflows, quality of care, patient safety, communication, and clinical decision support compared with handwritten paper documentation and in-person collection during a medical appointment.10 For cancer-associated syndromes, health IT can be used to collect personal and family history, identify high-risk patients, and calculate disease risk. Additionally, following genetic assessment, IT can coordinate care with other providers, communicate results with patients, and assist patients in sharing their test results with relatives at risk for carrying the same familial mutation, thus allowing for cascade testing.10-13 With the growing recognition that it is critically important to identify individuals with cancer-associated pathogenic mutations and use health IT, we sought to systematically review the literature on health IT for family history collection as it is easily accessible and highly comprehensive.
METHODS
Inclusion Criteria
The current study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was preregistered with PROSPERO (registration no.: CRD42020193024).14 The literature search strategy was designed around the PICO format: Is there a difference in FCH completion rate and time, genetic counseling referral rate, and testing (outcome) in all patients presenting to outpatient clinics and hospitals (population of interest) following collection of FCH using IT tools (intervention) versus traditional paper and in-person FCH collection methods (comparison)? This study protocol received exemption status from institutional review board committee approval. The final literature search was conducted on July 1, 2020, and assessed online publications between 1980 and 2020. We searched the following bibliographic databases: Ovid MEDLINE, Ovid Embase, and the Cochrane Library (Wiley). There were no language, publication date, or article-type restrictions included in the search, with the exception that all articles had to be original. Inclusion criteria for the review included original research papers; human focus; manuscript in peer-reviewed journal; and primary focus on the use of health IT to capture, collect, and/or collate information on FCH. For all identified references, two independent investigators reviewed titles, index terms, and available abstracts to determine whether the articles appeared to meet inclusion criteria. If insufficient information was available to make a decision at this stage, the article was included for full-text retrieval. Each full-text article was then reviewed to determine final inclusion status, which was included in a data collection form. Any discrepancies were resolved by discussion between the investigators to reach consensus.
For each of the articles meeting inclusion criteria, primary outcomes of interest consisted of patient population and setting, level of kinship collected, completion rate of all questions in the tool, completion time, incorporation into the EMR, resulting referral to genetic assessment, and patient satisfaction. We classified kinship as follows: first-degree relatives (FDR)—parents, siblings, and children; second-degree relatives (SDR)—grandparents, grandchildren, uncles, aunts, nephews, nieces, and half-siblings; and third-degree relatives (TDR)—great grandparents, great grandchildren, great uncles and aunts, and first cousins. Risk of bias in each study was evaluated using the Newcastle-Ottawa Scale.
Statistics
Meta-analysis models were used to estimate the proportion of individuals completing the FCH survey, the mean time required for survey completion, and the proportion of individuals referred for genetic testing as a result of information obtained via the survey. Statistical analyses were conducted with R software (Version 3.6.1 [July 5, 2019]; R Foundation for Statistical Computing, Vienna, Austria). Meta-analyses were conducted by tool type (electronic survey before medical visit, electronic survey via a tablet in the medical office, and electronic survey via a kiosk before visit). Statistical heterogeneity was tested through the chi-square test (ie, Cochrane Q test) and a P value ≤ .20 was used to indicate the presence of heterogeneity. Statistical heterogeneity was also assessed by the inconsistency statistic (I2), with values > 50% considered as substantial heterogeneity. A random-effects analysis was used to calculate the pooled proportions and means. The random-effects analysis allows for more variability in the individual study proportion estimates when generating the pooled proportion and is more conservative. The pooled proportion was calculated using the Freeman-Tukey double arcsine transformation, and the 95% CI was calculated using the Clopper-Pearson interval, also called the exact binomial interval. We used the Freeman-Tukey double arcsine transformation because of its ability in stabilizing the variances. The studies included in this meta-analysis have a reported proportion of > 0.80, and when using other transformations, they grossly overstate the effects of proportions close to 0 and close to 1. The pooled mean time to completion was calculated by using the inverse variance method with untransformed means. To estimate the between-study variance, the DerSimonian-Laird estimator was used. For the outcome proportions of interest, the results of each study were expressed as binary proportions with exact 95% CIs.
Some of the included studies used both standard patient interview for collection of FCH and a health IT tool for FCH collection. For these studies, agreement between the two methods of FCH collection was assessed with Cohen's κ coefficient, reported in selected studies. Based on the Landis-Koch guidelines,15 a κ value < 0.20 was regarded as poor agreement; 0.21-0.40 as fair agreement; 0.41-0.60 as moderate agreement; 0.61-0.80 as good agreement; and > 0.81 as very good concordance between the two tool methods of FCH collection.
RESULTS
Electronic Family Health History Collection Tools
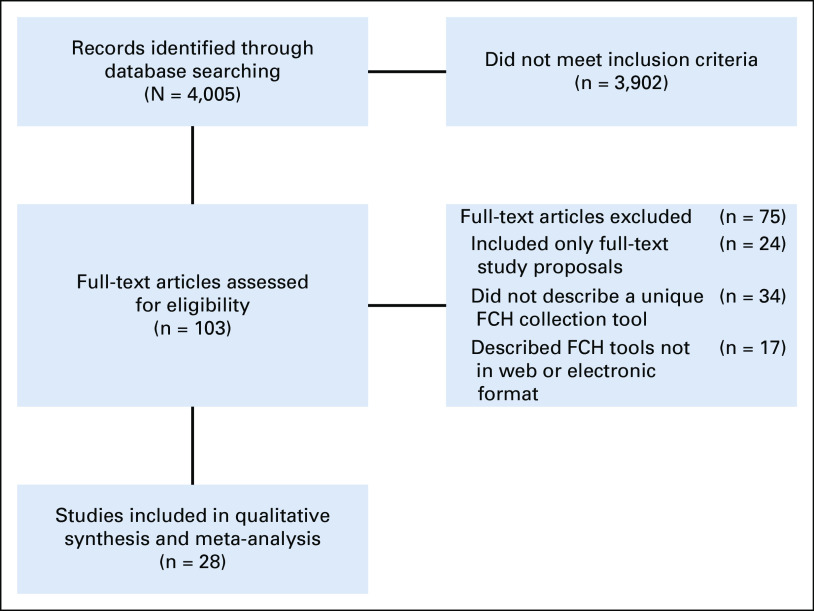
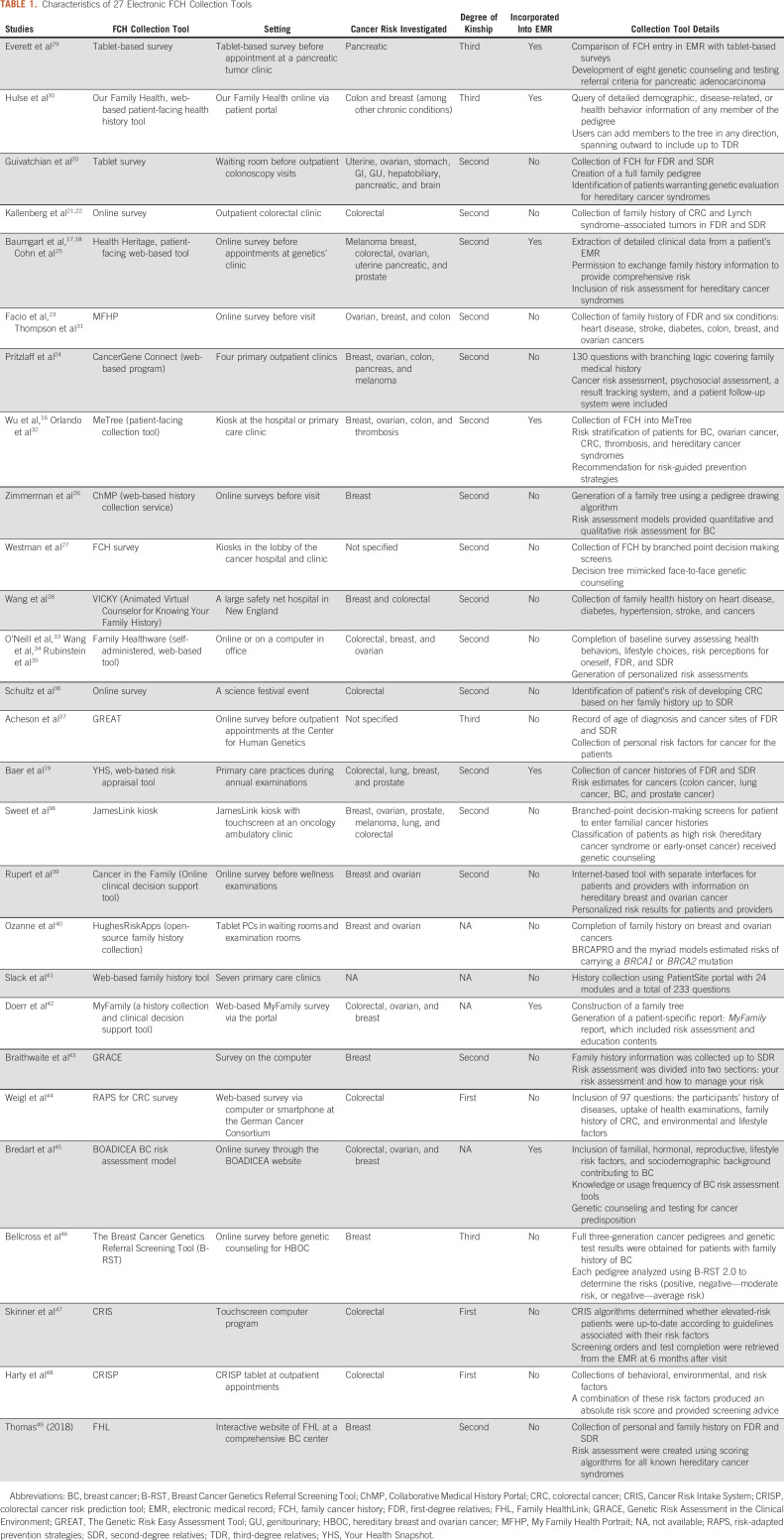
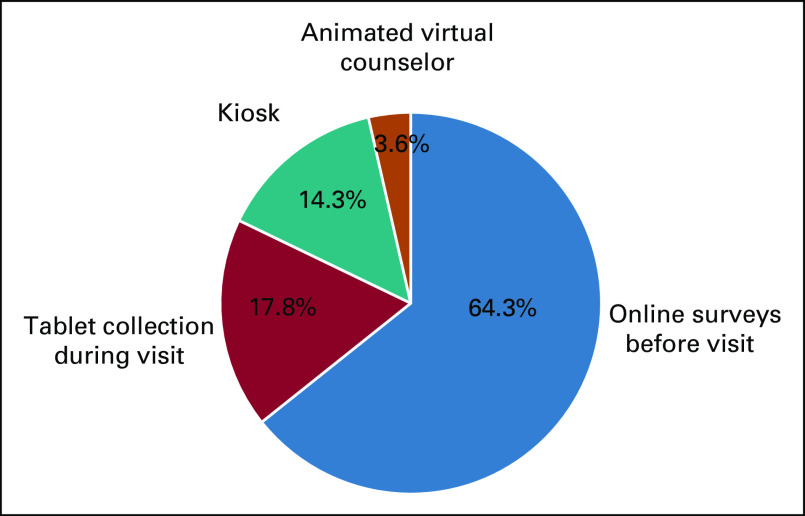
The initial literature search identified 4,005 potentially relevant articles. Following review of titles and abstracts and based on inclusion and exclusion criteria, 103 articles were selected for full-text review and 28 articles were selected for inclusion (Fig 1). The included articles were published from 2000 to 2019, with the majority published after 2010 (25 studies). The studies included 27 unique tools for collection of FCH (Table 1). Seven of the FCH collection tools were developed outside of the United States. From the 28 studies included, the following health IT methods were used for FCH collection (Fig 2): electronic survey administered before visit (18, 64.3%), electronic survey via tablet administered in the medical office (5, 17.8%), electronic survey via kiosk in the hospital or medical office (4, 14.3%), and animated virtual counselor in the medical office (1, 3.6%). The virtual counselor VICKY is an animated computer character that helps to collect FCH using a touchscreen in the office. From 74 patients who were invited to the study, 70 patients completed FCH collection with VICKY (94.6%).
FIG 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of search strategy and study selection. FCH, family cancer history.
TABLE 1.
Characteristics of 27 Electronic FCH Collection Tools
FIG 2.
Four strategies for family cancer history collection.
Patient Population
The 28 studies included 188,994 patients. The median patient age was 51.2 years (range 18-75 years). The percentage of female patients varied among publications (range 33%-100%). Studies were conducted in the inpatient or outpatient settings depending on the IT methods used. Targeted familial cancers differed based on the study design, patient population, and setting. Studies included a focus on cancer of the uterus, ovaries, stomach, GI tract, genitourinary system, hepatobiliary system, pancreas, and brain. Studies included variable levels of kinship, ranging from first degree to third degree.
Completion Rate
Among the 28 studies that used an FCH tool, the pooled estimate of the overall completion rate for health IT FCH tools was 86.0% (CI, 0.72 to 0.96; Fig 3A). The pooled estimated completion rate for health IT FCH collection via electronic survey using a tablet in the medical office was 89% (0.74 to 0.98), for health IT FCH collection completed via electronic survey before visit was 84.0% (CI, 0.65 to 0.97), and for health IT FCH collection completed via electronic survey using a kiosk was 85% (CI, 0.66 to 0.98); Figs 3B-3D). I2 values were 99%-100% across all pooled analyses. Animated virtual counselor in the medical office was not pooled via meta-analysis because of the small sample size of the studies that used the tool.
FIG 3.
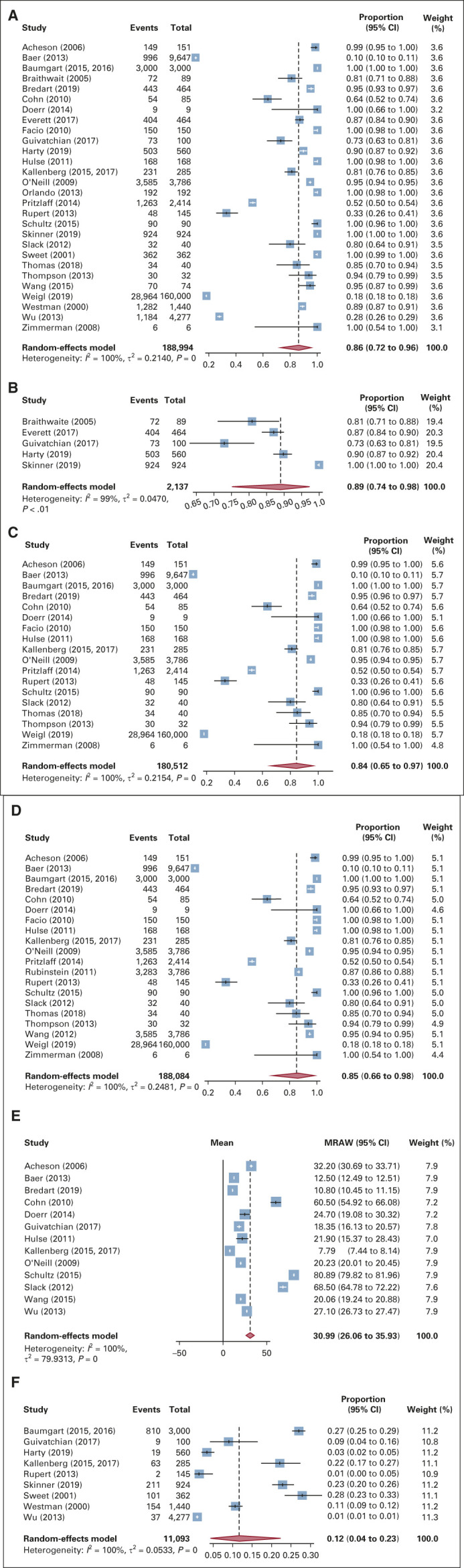
(A) Pooled proportion of patients completing any collection tool. (B) Pooled proportion of participants completing tablet collection tool. (C) Pooled proportion of participants completing online survey collection tool. (D) Pooled proportion of participants completing kiosk survey collection tool. (E) Pooled mean time to completion of family cancer histories collection tool. (F) Pooled proportion of participants referred to genetic testing. MRAW, pooled raw means.
Completion Time
Thirteen of the 28 studies (46.4%) reported a time needed for patients to complete the FCH tool, estimating a mean time pooled via meta-analysis of 31.0 minutes (CI, 26.1 to 35.9; Fig 3E). The quantile estimation method was used to estimate the sample mean and sample deviation for studies where median and range were reported. No material differences in the pooled mean completion time were observed among strata of kinship collected (FDR v SDR v TDR) as there were not enough data collected to quantitatively test for associations.
Interface With EMR
Seven (25.9%) of the 27 unique FCH collection tools had the capacity to automatically incorporate the health IT FCH tool results into the patient's individual EMR. In MeTree, provider reports were integrated into the medical record for use at the patient visit.16 Risk factors from family history addressed by Health Heritage (ie, adenomatous polyps, BRCA1 mutation, and elevated body mass index) were translated to the patient's record to enhance the geneticist's risk assessment through the EMR.17,18 The Your Health Snapshot tool as mentioned in Baer et al19 is a web-based risk appraisal tool that incorporates both family history and lifestyle factors in a patient's EMR. OurFamily Health stores family history simultaneously in a patient's EMR as the application is launched. Similarly, MyFamily delivers the patient's family history via a patient-specific report through the EMR. However, with all seven studies, the incorporation was limited because of the different structures of EMRs available, making compatibility a main challenge.
Referral to Genetic Counseling and Testing
Nine studies (32.1%) captured information on referral to genetic counseling following the use of an IT FCH tool. After undergoing collection of FCH, pooled estimate of the proportion of participants referred to genetic testing was 12% (CI, 0.04 to 0.23; Fig 3F). Seven FCH collection tools (The GREAT survey, Cancer in the Family, ChMP, Colorectal Cancer Risk Prediction Tool, Genetic Risk Assessment in the Clinical Environment, MyFamily, and Ontario-FHAT) generated a report following data input that could be shared with patients and their relatives detailing their cancer risk assessment.
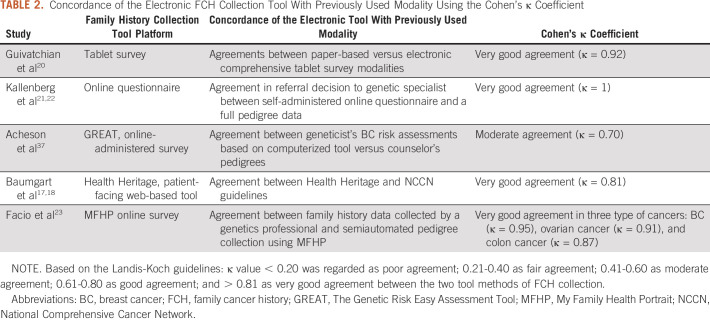
Correlation Coefficient
Five studies (17.8%) used both standard patient interview for collection of FCH and a health IT tool for FCH collection. These studies compared results from each method of information collection to assess for concordance using Cohen's κ coefficient. Overall, there was almost perfect agreement between the health IT FCH tools and previously used modalities in FCH collection. Four studies (80%; Guivatchian et al,20 Kallenberg et al,21,22 Baumgart et al,17,18 and Facio et al23) demonstrated very good agreement (κ > 0.81; Table 2). When comparing agreement between the IT FCH and standard patient interview via simple pooled κ values, the electronic tools administered before the medical visit were equally concordant compared with electronic tool administered at the visit via tablet (0.92, 0.70-1.00 respectively).
TABLE 2.
Concordance of the Electronic FCH Collection Tool With Previously Used Modality Using the Cohen's κ Coefficient
Patient Satisfaction
Eight studies (28.6%) included a qualitative assessment of patient satisfaction with the health IT FCH tool. For each of these studies, the majority of patients completing the IT tools (51%-100%) found the tool easy to use and expressed satisfaction with the experience. Patients described the electronic survey via tablet in the medical office to be user friendly, the time for completion to be appropriate,20 and the questions easy to understand.24 Patients stated that the electronic survey before the medical visit was easy to use and stated that they were satisfied with being able to answer the questions at one's own pace.16,21,22,25,26 For patients who used a kiosk system in the medical office, participants stated that it was conveniently available before the appointment with medical personnel present to help with troubleshooting.27 The animated virtual counselor was described as interactive and participants rated it as stress-free and comprehensive.28
Bias Assessment
The risk of bias assessed using the Newcastle-Ottawa Scale was found to be high in 19 studies and medium in 9 studies studies. Funnel plots were generated to test for the presence of publication bias, demonstrating a slight asymmetry on visual inspection (Figs 4A-4F).
FIG 4.
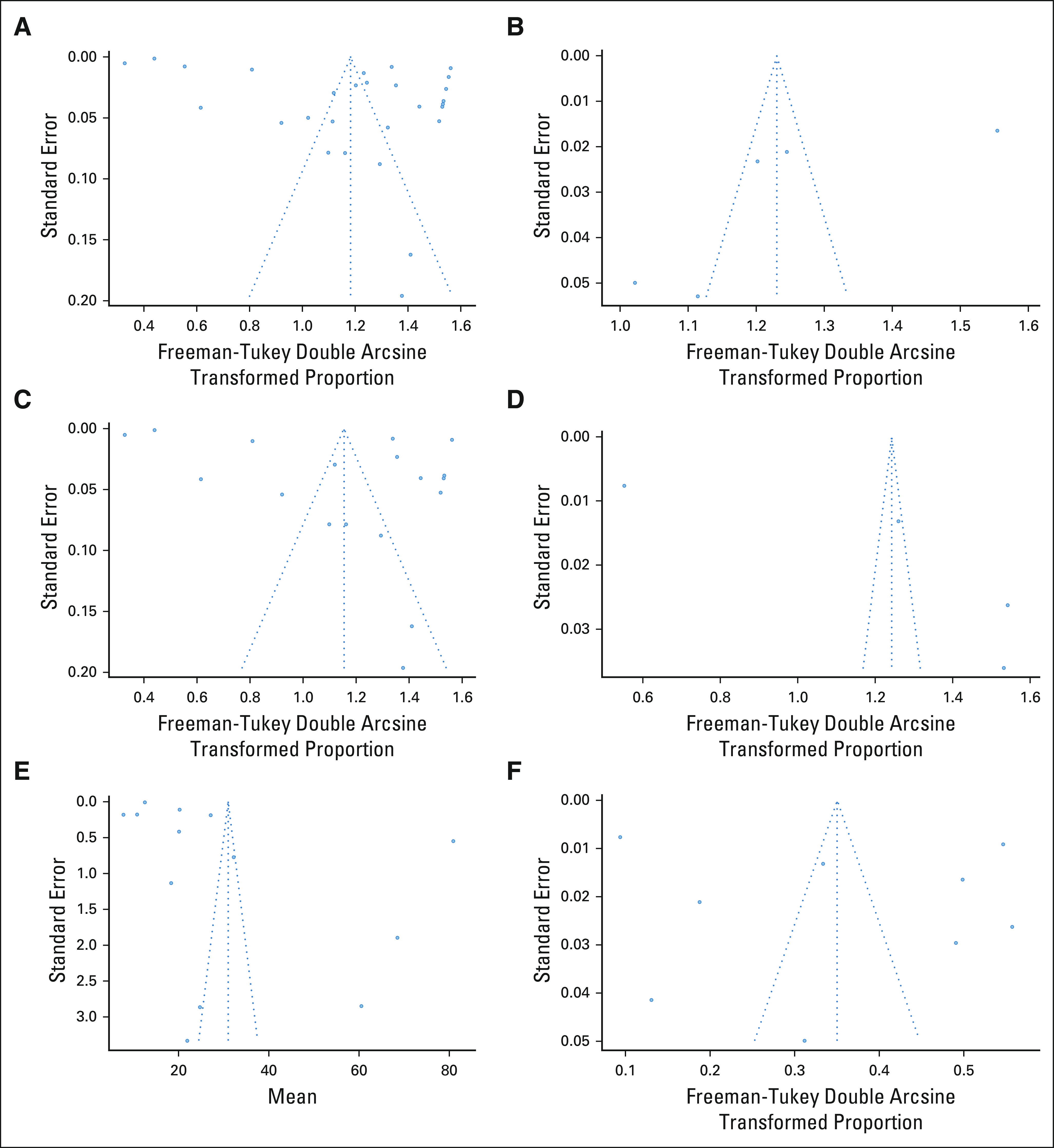
(A) Pooled proportion of patients completing any collection tool funnel plot. (B) Pooled proportion of participants completing tablet collection tool funnel plot. (C) Pooled proportion of participants completing online survey collection tool funnel plot. (D) Pooled proportion of participants completing kiosk survey collection tool funnel plot. (E) Pooled mean time to completion of family cancer histories collection tool funnel plot. (F) Pooled proportion of participants referred to genetic testing funnel plot.
DISCUSSION
In this systematic review and meta-analysis, we have evaluated the literature on health IT strategies used to capture FCH. We identified 28 peer-reviewed studies using 27 unique FCH collection tools. The most commonly reported strategy of health IT FCH tool was an electronic survey administered either before the office visit or via a tablet in the office. Other reported strategies included kiosk-based interface in an outpatient clinic and an animated virtual counselor.
Meta-analyses were conducted for all patients as well as for the most commonly reported IT tools (electronic survey before visit, and electronic survey via tablet in the medical office and via kiosk before visit). Across all IT tools, 86% (CI, 0.72 to 0.96) of patients completed FCH collection. Self-administered electronic surveys demonstrated a completion rate of 84% (CI, 0.65 to 0.97), tablet-based strategies had a completion rate of 89.0% (CI, 0.74 to 0.98), and kiosk-based strategies had a completion rate of 85% (CI, 0.66 to 0.98). Patients required approximately 31 minutes to complete the tool (CI, 26.1 to 35.9). Tools that collected FCH of higher degree of kinship did not always require more time for completion. Time potentially varied based on the branched-point decision-making tree: as higher degree of kinship is involved, the number of questions being asked would be affected. Completion of the FCH collection resulted in referral of 12% (CI, 0.04 to 0.23) of patients to genetic assessment. This result is similar to the national estimates of genetic testing in women with a history of breast or ovarian cancer: 15.3% underwent genetic testing with a history of breast cancer, and 10.5% underwent genetic testing with history of ovarian cancer.2 All studies including a κ coefficient demonstrated at least good agreement and 80% demonstrated very good agreement between the electronic FCH collection tool with previously used FCH collection modality. Seven (25.9%) family history tools were designed to electronically incorporate family history information to the patient's EMR.
While previous systematic reviews have assessed different forms of family history collection tools,50-52 this is the first large-scale, systematic review that focuses on the collection of family health histories via electronic or online tools. Paper-based questionnaires, telephone interviews, and face-to-face interviews during medical appointments can collect FCH and identify at-risk patients. However, there is a concern of standardization, exhausting of additional human resources, and whether these data can easily be retrieved and updated by all health care teams.52 Compared with prior archaic FCH collection methods, our findings demonstrate that electronic and online tools are feasible methods for collecting family histories effectively with high completion rate, short completion time, greater patient satisfaction, capacity of incorporation into EMR, referral to genetic counseling, and almost perfect agreement between the health IT FCH tools and prior FCH collection methods.
When used either before the office visit or in the office via tablet, kiosk, or virtual-based system, FCH IT tools collected valuable family history that is often incomplete because of time constraints. By designating time before the physician evaluation for thorough collection, FCH IT tools allow for more time for face-to-face discussion once patients are with the physician.16,27
Many of the studies included in this analysis suggest similar characteristics that improve completion of FCH collection tools for detection of hereditary cancer syndromes. These tool characteristics include wide accessibility, self-administered by the patients, easy to use, presenting medical terms in an easy-to-understand format, displaying a pedigree, and including management plans to help patients understand hereditary cancer syndrome risk. As the COVID-19 pandemic continues to drive improved utilization of telemedicine and limited in-person face-to-face patient experiences, medical systems and physicians should keep these characteristics in mind to design electronic tools that will be well received and achieve the intended goal in their patient population.
This study has several limitations. Although this is a systematic review including studies of different patient populations and study designs, we were unable to control for selection bias and were not able to match patient characteristics. Because of the heterogeneity in findings across studies, the random-effects model was used. However, because the I2 values are close to 100%, it is difficult to determine the generalizability of our results. Second, accessibility to an electronic device and internet connection from home can be a limitation in remote areas. While FCHs were promptly collected for FDR, SDR, and TDR, there was not a validation tool available among the different IT tools to confirm the histories collected besides the five studies that listed a Cohen's κ coefficient to demonstrate concordance (Table 2). Third, while missing data might be a significant issue, we do not have information regarding missing data available from the studies presented. Fourth, as the proportion estimates in this meta-analysis are heterogeneous across studies, the random‐effects model is used with the assumption that each study's true transformed proportions follow the normal distribution. However, one limitation is that the Freeman-Tukey double arcsine transformation might violate this assumption because of bounded domains.53 In terms of publication bias, while there does not appear to be strong publication bias present in the meta-analyses conducted in this study, there is a slight asymmetric nature to the funnel plots. Additionally, we were unable to obtain long-term data or follow-up results regarding patient compliance with genetic referrals or testing and therefore are unable to measure the clinical efficacy. However, a strength of this study is a large population size of mostly English-speaking participants, including close to 190,000 patients. This allowed for comprehensive review of using health IT to collect FCH and to increase awareness of hereditary cancer syndromes through subsequent genetic testing and counseling.
In conclusion, the collection of a patient's FCH is essential for triaging patients to genetic testing and counseling. There have been promising data in the literature regarding the use of electronic and online methods of family health history collection. However, this is, to our knowledge, the first large systematic review and meta-analysis to address unique electronic FCH collection tools and encourage uptake of genetic testing and counseling through these tools. Our findings demonstrate that electronic FCH tools have high patient completion rates, acceptable time requirements, and high levels of user satisfaction and collection of complete health information. While Health IT for FCH collection holds the potential to improve detection rates of inherited cancer syndromes, additional studies are needed to measure the clinical efficacy through acceptance by patients and health care providers to increase genetic testing uptake, adaptation of the same tools in different clinical settings, and incorporation of the tools in EMRs. The long-term impact of such tools can be further evaluated by assessing uptake of cascade testing and risk-reducing surgeries.
Charlene Thomas
Travel, Accommodations, Expenses: Inovio Pharmaceuticals, Nektar, Pfizer
Kevin Holcomb
Research Funding: Fujirebio Diagnostics
Expert Testimony: Johnson and Johnson Inc
Melissa K. Frey
Research Funding: Invitae
No other potential conflicts of interest were reported.
SUPPORT
M.K.F. is supported by the following grant: NIH/NCATS Grant No. KL2-TR-002385. P.J.C. was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (Grant No. 1-UL1-TR002384-01). R.N.S. was supported by NCI K07CA216326, ROICA211723, and PCORI IHS-2017CS-9211.
AUTHOR CONTRIBUTIONS
Conception and design: Xuan Li, Ravi N. Sharaf, Eloise Chapman-Davis, Melissa K. Frey
Administrative support: Hannah Bergeron
Collection and assembly of data: Xuan Li, Ryan M. Kahn, Noelani Wing, Zhen Ni Zhou, Andreas Ian Lackner, Hannah Krinsky, Nora Badiner, Rhea Fogla, Hannah Bergeron, Becky Baltich Nelson, Ravi N. Sharaf, Melissa K. Frey
Data analysis and interpretation: Xuan Li, Ryan M. Kahn, Isabel Wolfe, Charlene Thomas, Paul J. Christos, Ravi N. Sharaf, Evelyn Cantillo, Kevin Holcomb, Melissa K. Frey
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Charlene Thomas
Travel, Accommodations, Expenses: Inovio Pharmaceuticals, Nektar, Pfizer
Kevin Holcomb
Research Funding: Fujirebio Diagnostics
Expert Testimony: Johnson and Johnson Inc
Melissa K. Frey
Research Funding: Invitae
No other potential conflicts of interest were reported.
REFERENCES
- 1.Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: An alternative to population-based screening J Clin Oncol 381398–14082020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer J Clin Oncol 353800–38062017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch BM, Kensaku K.Clinical decision support for genetically guided personalized medicine: A systematic review J Am Med Inform Assoc 20388–4002013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frey MK, Kahn RM, Lipkin K, et al. Prospective feasibility trial of a novel strategy of facilitated cascade genetic testing using telephone counseling and mailed saliva kit genetic testing. Gynecol Oncol. 2019;154:281. doi: 10.1200/JCO.19.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly MB, Pilarski R, Yurgelun MB, et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2020 J Natl Compr Canc Netw 18380–3912020 [DOI] [PubMed] [Google Scholar]
- 6.Provenzale D, Gupta S, Ahnen DJ, et al. Genetic/familial high-risk assessment: Colorectal version 1.2016, NCCN clinical practice guidelines in oncology J Natl Compr Canc Netw 141010–10302016 [DOI] [PubMed] [Google Scholar]
- 7.Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper Gynecol Oncol 146217–2242017 [DOI] [PubMed] [Google Scholar]
- 8.Benjamin C, Booth K, Ellis I.A prospective comparison study of different methods of gathering self-reported family history information for breast cancer risk assessment J Genet Couns 12151–1702003 [DOI] [PubMed] [Google Scholar]
- 9.Chin XW, Ang ZLT, Tan RYC, et al. Use of telephone intake for family history taking at a cancer genetics service in Asia J Genet Couns 291192–11992020 [DOI] [PubMed] [Google Scholar]
- 10. Ritchie JB, Allen CG, Morrison H, et al. Utilization of health information technology among cancer genetic counselors. Mol Genet Genomic Med. 2020;8:e1315. doi: 10.1002/mgg3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch BM, Wiley K, Pflieger L, et al. Review and comparison of electronic patient-facing family health history tools J Genet Couns 27381–3912018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aronson S, Mahanta L, Ros LL, et al. Information technology support for clinical genetic testing within an Academic Medical Center. J Pers Med. 2016;6:4. doi: 10.3390/jpm6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch HT, Snyder C, Stacey M, et al. Communication and technology in genetic counseling for familial cancer Clin Genet 85213–2222013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG.The measurement of observer agreement for categorical data Biometrics 331977159–174 [PubMed] [Google Scholar]
- 16. Wu RR, Orlando LA, Himmel TL, et al. Patient and primary care provider experience using a family health history collection, risk stratification and clinical decision support tool: A type 2 hybrid controlled implementation-effectiveness trial. BMC Fam Pract. 2013;14:111. doi: 10.1186/1471-2296-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgart LA, Vogel Postula KJ, Knaus WA, et al. Initial clinical validation of health heritage, a patient-facing tool for personal and family history collection and cancer risk assessment Fam Cancer 15331–3392015 [DOI] [PubMed] [Google Scholar]
- 18. Baumgart LA, Vogel Postula KJ, Walters SA, et al. Cancer risk assessment using an automated electronic patient-facing tool for the collection and evaluation of family history. J Clin Oncol. 2016;34(15 suppl):e13055. [Google Scholar]
- 19.Baer HJ, Schneider LI, Colditz GA, et al. Use of a web-based risk appraisal tool for assessing family history and lifestyle factors in primary care J Gen Intern Med 28817–8242013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guivatchian T, Koeppe ES, Baker JR, et al. Family history in colonoscopy patients: Feasibility and performance of electronic and paper-based surveys for colorectal cancer risk assessment in the outpatient setting Gastrointest Endosc 86684–6912017 [DOI] [PubMed] [Google Scholar]
- 21.Kallenberg FGJ, Aalfs CM, The FO, et al. Evaluation of an online family history tool for identifying hereditary and familial colorectal cancer Fam Cancer 17371–3802017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallenberg FGJ, IJspeert JEG, Bossuyt PMM, et al. Validation of an online questionnaire for identifying people at risk of familial and hereditary colorectal cancer Fam Cancer 14401–4102015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facio FM, Feero WG, Linn A, et al. Validation of my family health portrait for six common heritable conditions Genet Med 12370–3752010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritzlaff M, Yorczyk A, Robinson LS, et al. An internal performance assessment of CancerGene Connect: An electronic tool to streamline, measure and improve the genetic counseling process J Genet Couns 231034–10442014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn WF, Ropka ME, Pelletier SL, et al. Health Heritage©, a web-based tool for the collection and assessment of family health history: Initial user experience and analytic validity Public Health Genomics 13477–4912010 [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman N, Patel C, Chen DP.ChMP: A collaborative medical history portal AMIA Annu Symp Proc 2008859–8632008 [PMC free article] [PubMed] [Google Scholar]
- 27.Westman J.Efficacy of a touchscreen computer based family cancer history questionnaire and subsequent cancer risk assessment J Med Genet 37354–3602000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Bickmore T, Bowen DJ, et al. Acceptability and feasibility of a virtual counselor (VICKY) to collect family health histories Genet Med 17822–8302015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Everett J, Schradle K, Cameron H, et al. Effectiveness of tablet family history collection in a multidisciplinary pancreatic tumor clinic in identifying patients with risk factors for hereditary cancer. J Clin Oncol. 2017;35(15 suppl):e13020. [Google Scholar]
- 30.Hulse N, Ranade-Kharkar P, Post H, et al. Development and early usage patterns of a consumer-facing family health history tool AMIA Annu Symp Proc 2011578–5872011 [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson T, Seo J, Griffith J, et al. You don't have to keep everything on paper: African American women's use of family health history tools J Community Genet 4251–2612013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orlando LA, Buchanan AH, Hahn SE, et al. Development and validation of a primary care-based family health history and decision support program (MeTree) N C Med J 74287–2962013 [PMC free article] [PubMed] [Google Scholar]
- 33.O'Neill SM, Rubinstein WS, Wang C, et al. Familial risk for common diseases in primary care: The Family Healthware Impact Trial Am J Prev Med 36506–5142009 [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Sen A, Ruffin MT, IV, et al. Family history assessment: Impact on disease risk perceptions Am J Prev Med 43392–3982012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinstein WS, Acheson LS, O'Neill SM, et al. Clinical utility of family history for cancer screening and referral in primary care: A report from the Family Healthware Impact Trial Genet Med 13956–9652011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schultz M, Seo SB, Holt A, et al. Family history assessment for colorectal cancer (CRC) risk analysis—Comparison of diagram- and questionnaire-based web interfaces. BMC Med Inform Decis Mak. 2015;15:95. doi: 10.1186/s12911-015-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acheson LS, Zyzanski SJ, Stange KC, et al. Validation of a self-administered, computerized tool for collecting and displaying the family history of cancer J Clin Oncol 245395–54022006 [DOI] [PubMed] [Google Scholar]
- 38.Sweet KM, Bradley TL, Westman JA.Identification and referral of families at high risk for cancer susceptibility J Clin Oncol 20528–5372002 [DOI] [PubMed] [Google Scholar]
- 39.Rupert DJ, Squiers LB, Renaud JM, et al. Communicating risk of hereditary breast and ovarian cancer with an interactive decision support tool Patient Educ Couns 92188–1962013 [DOI] [PubMed] [Google Scholar]
- 40.Ozanne EM, Loberg A, Hughes S, et al. Identification and management of women at high risk for hereditary breast/ovarian cancer syndrome Breast J 15155–1622009 [DOI] [PubMed] [Google Scholar]
- 41.Slack WV, Kowaloff HB, Davis RB, et al. Evaluation of computer-based medical histories taken by patients at home J Am Med Inform Assoc 19545–5482012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doerr M, Edelman E, Gabitzsch E, et al. Formative evaluation of clinician experience with integrating family history-based clinical decision support into clinical practice J Pers Med 4115–1362014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braithwaite D, Sutton S, Mackay J, et al. Development of a risk assessment tool for women with a family history of breast cancer Cancer Detect Prev 29433–4392005 [DOI] [PubMed] [Google Scholar]
- 44.Weigl K, Tikk K, Hoffmeister M, et al. A web-based survey among adults aged 40–54 years was time effective and yielded stable response patterns J Clin Epidemiol 10510–182019 [DOI] [PubMed] [Google Scholar]
- 45.Brédart A, Kop JL, Antoniou AC, et al. Clinicians' use of breast cancer risk assessment tools according to their perceived importance of breast cancer risk factors: An international survey J Community Genet 1061–712018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellcross C, Hermstad A, Tallo C, et al. Validation of version 3.0 of the breast cancer genetics referral screening tool (B-RSTTM) Genet Med 21181–1842018 [DOI] [PubMed] [Google Scholar]
- 47. Skinner CS, Ahn C, Singal AG, et al. Outcomes associated with use of the cancer risk intake system among primary care safety-net patients identified as needing colorectal cancer screening. Prev Med Rep. 2019;16:101003. doi: 10.1016/j.pmedr.2019.101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harty EC, McIntosh JG, Bickerstaffe A, et al. The CRISP-P study: Feasibility of a self-completed colorectal cancer risk prediction tool in primary care Fam Pract 36730–7352019 [DOI] [PubMed] [Google Scholar]
- 49.Thomas SN, Hovick SR, Tan N, et al. How online family history tool design and message content impact user perceptions: An examination of family HealthLink Public Health Genomics 2153–662018 [DOI] [PubMed] [Google Scholar]
- 50.Qureshi N, Carroll JC, Wilson B, et al. The current state of cancer family history collection tools in primary care: A systematic review Genet Med 11495–5062009 [DOI] [PubMed] [Google Scholar]
- 51.de Hoog CLMM, Portegijs PJM, Stoffers HEJH.Family history tools for primary care are not ready yet to be implemented. A systematic review Eur J Gen Pract 20125–1332013 [DOI] [PubMed] [Google Scholar]
- 52.Cleophat JE, Nabi H, Pelletier S, et al. What characterizes cancer family history collection tools? A critical literature review Curr Oncol 25e335–e3502018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]