Abstract
Pearson syndrome (PS) is a multisystem mitochondrial cytopathy arising from deletions in mitochondrial DNA. Pearson syndrome is a sporadic disease that affects the hematopoietic system, pancreas, eyes, liver, and heart and the prognosis is poor. Causes of morbidity include metabolic crisis, bone marrow dysfunction, sepsis, and liver failure in early infancy or childhood. Early diagnosis may minimize complications, but suspicion of the disease is difficult and only mitochondrial DNA gene testing can identify mutations. There is no specific treatment for PS, which remains supportive care according to symptoms; however, hematopoietic stem cell transplantation may be considered in cases of bone marrow failure.
We herein describe the clinical and genetic characteristics of four patients with PS. One patient presented with hypoglycemia, two developed pancytopenia, and the final patient had hypoglycemia and acute hepatitis as the primary manifestation. All patients had lactic acidosis. Additionally, all patients showed a variety of clinical features including coagulation disorder, pancreatic, adrenal, and renal tubular insufficiencies. Two patients with pancytopenia died in their early childhood. Our experience expands the phenotypic spectrum associated with PS and its clinical understanding.
Keywords: mitochondrial DNA, mitochondrial DNA deletion syndrome, Pearson syndrome
1. Introduction
Pearson syndrome (PS, MIM#557000) is a mitochondrial DNA (mtDNA) deletion disease that causes multisystem mitochondrial cytopathy, arising from a severe deficiency in mitochondrial energy in various organs. PS manifests clinically as bone marrow failure, macrocytic sideroblastic anemia, pancreatic exocrine dysfunction, lactic acidosis, adrenal insufficiency, tubulopathy, and growth retardation. The prognosis is poor, and death typically occurs in infancy or early childhood owing to metabolic disorders or severe infections.[1]
Three overlapping phenotypes comprise mtDNA deletion syndrome: PS, Kearns–Sayre syndrome (KSS), and progressive external ophthalmoplegia (PEO).[2] Patients may also present with Leigh syndrome.[3] Children diagnosed with PS eventually develop the typical features of KSS,[4] which present as a classic triad of pigmentary retinopathy, PEO, and cardiac conduction block with at least one additional feature: cerebellar ataxia, sensorineural hearing loss, endocrinopathy, and muscle weakness. PEO is characterized by ptosis, ophthalmoplegia, and oropharyngeal weakness.[3] The overall global prevalence of mtDNA disease is 1:5000, with single, large-scale deletions comprising 10% of total disease composition.[5,6]
In this study, we evaluated the clinical and genetic characteristics of four children with PS. Our experience expands the phenotypic spectrum associated with PS and its clinical understanding.
2. Patients and methods
We reviewed the medical records of four patients (3 Korean and 1 Kazakhstani) to analyze clinical symptoms and the results of biochemical and imaging studies. Informed consent was obtained from all patients and the study was approved by the Institutional Review Board for Human Research of Asan Medical Center (IRB number: 2021-0712).
We performed mtDNA amplification using long-distance polymerase chain reaction (PCR) with various combinations of primers for molecular analysis. Genomic DNA was isolated from peripheral blood using a PUREGENE DNA isolation kit (Gentra, Minneapolis, MN). We then amplified 7 parts of the mitochondrial DNA by PCR to identify any large genome deletions (Table 1). The amplifications were performed in 30 cycles, each cycle consisting of denaturation at 95°C for 30 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 45 seconds. We performed the PCR analysis in reaction volumes of 20 μL using GoTaq Long PCR Master Mix (Promega, Madison, WI). After amplification, PCR mixtures were run on 1.2% agarose gel in the presence of ethidium bromide.
Table 1.
Sequences of primers used for the PCR reaction.
| Positions | Sense (5’-to-3’) | Antisense (5’-to-3’) | Size (bp) |
| m.5385_m.15958 | gaggcctaacccctgtcttt | agctttgggtgctaatggtg | 7574 |
| m.14837_m.6661 | tgaaacttcggctcactcct | attccgaagcctggtaggat | 8393 |
| m.2480_m.8095 | aaatcttaccccgcctgttt | cgggaattgcatctgttttt | 5616 |
| m.591_m.9397 | cctcctcaaagcaatacactg | gtggccttggtatgtgcttt | 8807 |
| m.9397_m.1430 | gtggccttggtatgtgcttt | tgctaaatccaccttcgacc | 8632 |
| m.7908_m.14268 | acgagtacaccgactacggc | agaggggtcagggttgattc | 6361 |
| m.14081_m.2688 | gcataattaaactttacttc | ggcaggtcaatttcactggt | 5176 |
After identifying large deletions of the mitochondrial genome in the specific PCR product, DNA sequencing was performed using the same primers covering the specific deletion region using the BigDye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA), according to manufacturer's instructions. Electrophoresis and analysis of the reaction mixtures were performed with the ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Targeted exome sequencing (4813 OMIM genes) was performed. Exomes were captured using the TruSight One Panel (Illumina Inc.), which enriches a 12 Mb region spanning 4813 genes. Sequencing was performed using the Next Seq platform (Illumina Inc.) and reads were aligned to the reference genome, hg19, using BWA (v.0.7.12, MEM algorithm). Mean depth of coverage was 90 × (>10 × = 98%).
Overall survival was assessed by Kaplan–Meier analysis using SPSS (SPSS for Windows, version 27.0, SPSS, Chicago, IL).
3. Results
All patients were born after term pregnancy except one, who was delivered at 36 weeks 5 days gestation. No positive family history of mtDNA disease was observed. The follow-up period was 3.44 ± 3.25 years (range, 9 months to 9 years). The clinical features of each patient at presentation are described in Table 2.
Table 2.
Characteristics at birth and clinical presentations.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
| Age at presentation/sex | 2 m/male | 2 y 4 m/female | 5 m/male | 1 m/female |
| Gestation age/birth weight | 38 wks/2.9 kg | 39 wks/2.69 kg | 40 wks/3.4 kg | 36 + 5 wks/2.26 kg |
| Presentations | Pancytopenia and BM failure Lactic acidemia | Hypoglycemia Lactic acidemia Adrenal insufficiency Exocrine pancreatic insufficiency Macrocytic anemia | Pancytopenia and BM failure Lactic acidemia Distal RTA Exocrine pancreatic insufficiency Adrenal insufficiency Hypothyroidism | Hypoglycemia Acute hepatitis Lactic academia Macrocytic anemia Proximal RTA |
| Developmental delay | (–) | (+) | (+) | (+) |
| Growth profile at diagnosis | Height -0.34 SD Weight -0.75 SD | Height -1.6 SD Weight -1.1 SD | Height -3.3 SD Weight -3.8 SD | Height -0.53 SD Weight 0.68 SD |
| Age at diagnosis | 10m | 3y 9m | 3y 4m | 1y 6m |
| Genetic analysis | m.9497_13734 4,236bp deletion | m.12113_14421 2,309bp deletion | m.8926_13321 4,394bp deletion | m.8420_15573 7,154bp deletion |
BM = bone marrow, RTA = renal tubular acidosis, SD = standard deviation.
3.1. Clinical features before diagnosis
3.1.1. Patient1
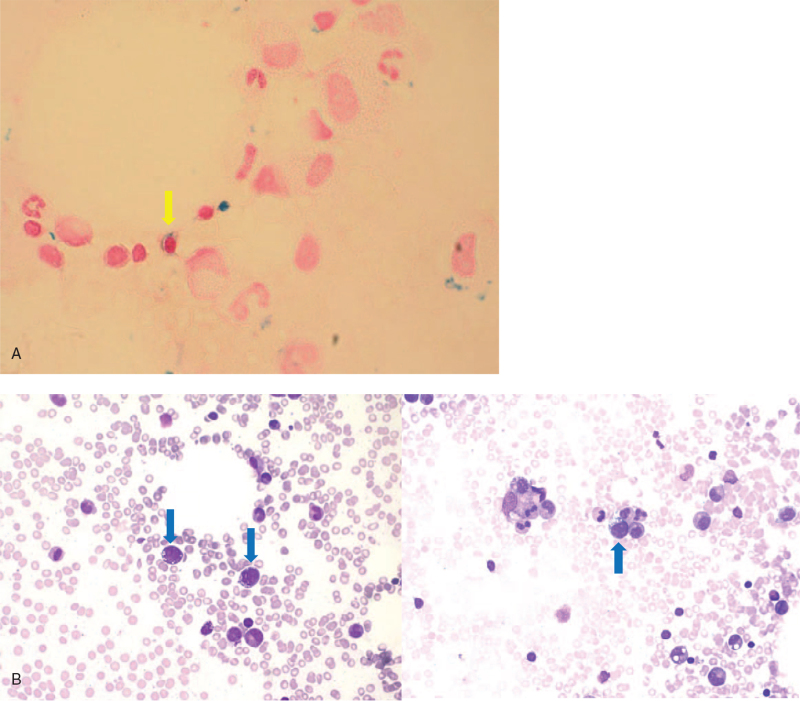
Pancytopenia was found in a 2-month-old Korean male who had a fever for 5 days. His white blood cell (WBC) count was 4000/μL, absolute neutrophil count was 440/μL, hemoglobin (Hb) was 3.2 g/dL, and platelet count was 115,000/μL. He was born at a gestational age of 38 weeks, weighing 2.9 kg. Bone marrow (BM) aspiration and biopsy revealed the atypical cells (6.6%) with medium-to-large size, oval-shaped nuclei, conspicuous nucleoli, and cytoplasmic vacuolization, but without a specific pathologic diagnosis. Pancytopenia persisted, and BM biopsy was performed again at 9 months of age, which revealed myelodysplastic syndrome, refractory anemia with excess blasts-2 (MDS RAEB-2). Blasts (13.6%) with cytoplasmic vacuolization, ring sideroblasts (2%), and sideroblasts (15%) were observed in the BM (Fig. 1). The patient had also suffered with diarrhea and persistent lactic academia (4.5–4.8 mmol/L).
Figure 1.
BM aspiration of Patient 1. (A) Prussian blue iron stain (×1000 magnification). Yellow arrow indicates sideroblast. (B) Wright's stain (×400 magnification). Blue arrows indicate blast with cytoplasmic vacuolization.
3.1.2. Patient 2
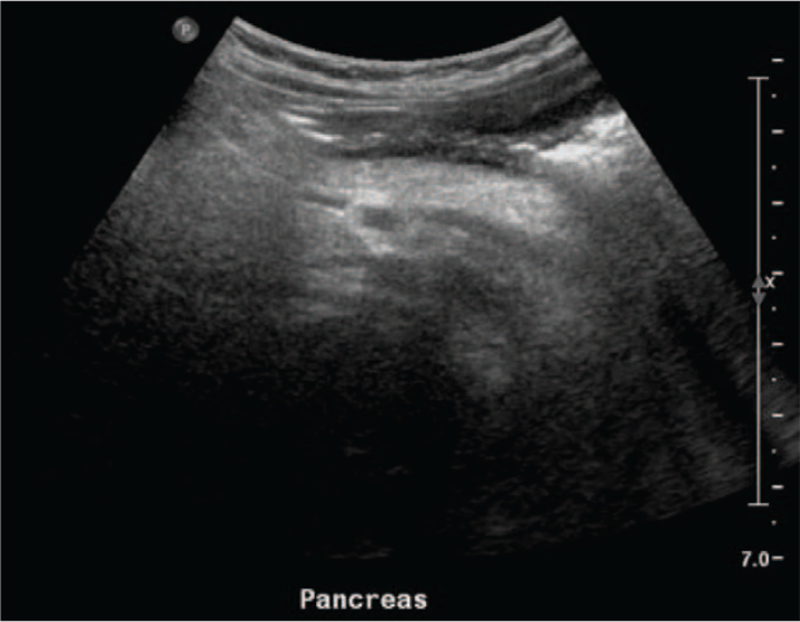
The Korean patient was born after 39 weeks of gestation. She received neonatal intensive care for 4 days to manage the aftermath of a seizure. From the age of 2 years, she had suffered from recurrent ketotic hypoglycemic episodes. Serum glucose level was low at 23 mg/dL. Her lactic acid was 2.5 to 3.6 mmol/L. Abdomen ultrasonography showed diffusely increased parenchymal echogenicity of the pancreas (Fig. 2).
Figure 2.
Abdomen ultrasonography of Patient 2 revealing diffusely increased parenchymal echogenicity of the pancreas without focal lesion, suggesting fatty infiltration of the pancreas.
3.1.3. Patient 3
A Kazakhstani boy presented with persistent anemia from age 5 months, which progressed into pancytopenia at age 2.5 years. His WBC count was 2300/μL, Hb was 7.2 g/dL, and platelet count was 50,000/μL. BM biopsy revealed hypoplastic BM. He had received a packed red blood cell (RBC) transfusion every month for 2 years, and suffered from recurrent vomiting and diarrhea with worsening general weakness. In addition, hypokalemia (2.3 mmol/L), hyponatremia (130 mmol/L), hypophosphatemia (1.6 mg/dL), hypomagnesemia (1.4 mg/dL), and metabolic acidosis (pH 7.286 and bicarbonate 10.8 mmEq/L) induced by renal tubular acidosis were observed. This patient has been described previously by Lee et al.[7]
3.1.4. Patient 4
A Korean female was born after 36 weeks gestation as the second twin. Her sibling was healthy with the exception of an atrial septal defect. At 1 month after birth, she was admitted to a hospital with a fever and lethargy. Laboratory investigations showed macrocytic anemia (Hb 6.5 g/dL, mean corpuscular volume 106.7 fL, mean corpuscular hemoglobin concentration 34.2%). At 15 months of age, vomiting, poor oral intake developed and lethargy reoccurred with mild metabolic acidosis (pH 7.356 and bicarbonate 11.8 mmEq/L). Vomiting, poor oral intake, and lethargy redeveloped again at 16 months. Her WBC, Hb, and platelet counts were 4800 /μL, 7.8 g/dL, and 82,000 /μL, respectively. Her aspartate aminotransferase was 2653 IU/L, alanine aminotransferase was 809 IU/L, total bilirubin was 8.5 mg/dL, and direct bilirubin was 7.7 mg/dL. Hypoglycemia (18 mg/dL) and metabolic acidosis (pH 7.356 and bicarbonate 11.8 mmEq/L) were also observed. Her prothrombin time was prolonged at 2.15 international normalized ratio and activated partial thromboplastin time was 68.8 seconds.
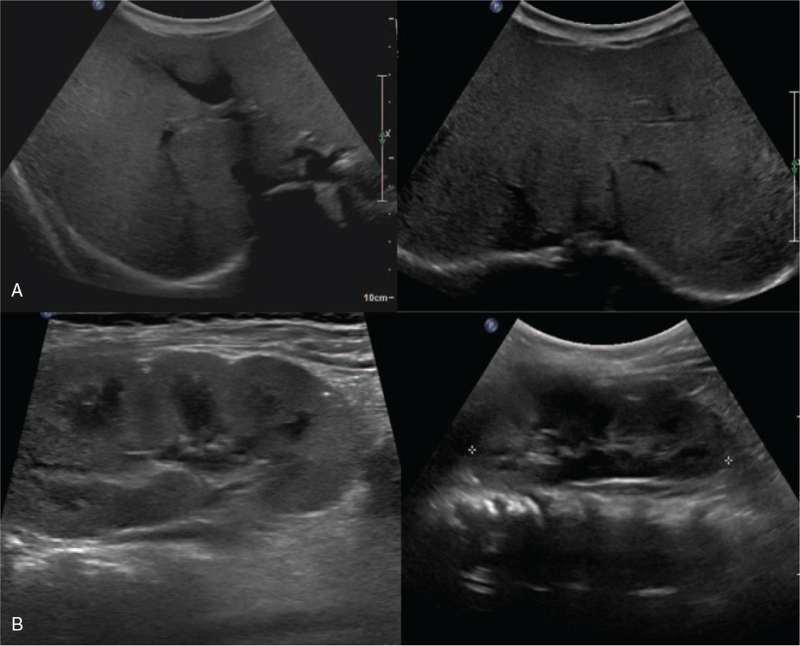
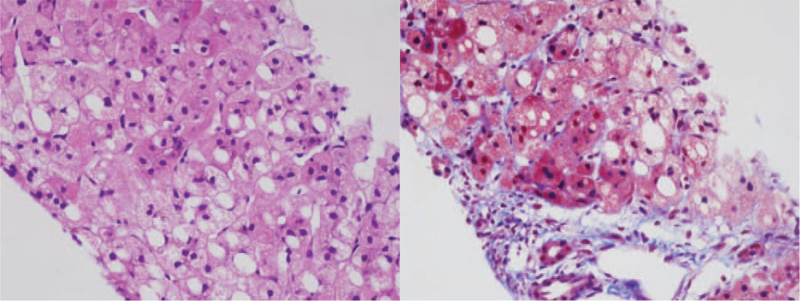
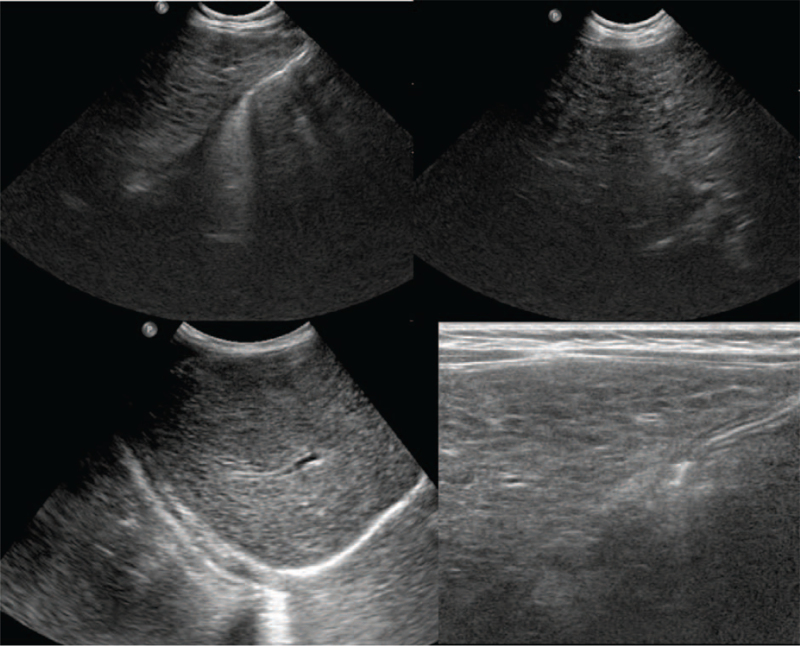
Abdomen ultrasonography showed diffuse increased echogenicity with mild hepatomegaly, and kidney ultrasonography revealed small cysts in both kidneys (Fig. 3). Liver biopsy indicated diffuse microvesicular and macrovesicular steatosis with a ballooning change of hepatocytes and periportal and perisinusoidal fibrosis consistent with mitochondrial hepatopathy (Fig. 4).
Figure 3.
(A) Abdomen ultrasonography of Patient 4. Diffuse increased echogenicity with mild hepatomegaly, possible infiltrative disease involving the liver. (B) Kidney ultrasonography. Small cysts in both kidneys. This is a nonspecific finding, but the possibility of renal manifestation of mitochondrial disease is considered.
Figure 4.
Liver biopsy of Patient 4. (A) hematoxylin and eosin stain (×400 magnification). Diffuse microvesicular and macrovesicular steatosis, ballooning change, and oncocytic change are noted. (B) Masson trichrome (×400 magnification). Periportal fibrosis and perisinusoidal fibrosis are highlighted.
3.2. The results of genetic analysis
Genomic testing for the 4813 OMIM genes (Online Mendelian Inheritance in Man, https://omim.org) was performed using the TruSight One Panel, which enriches a 12-Mb region spanning 4813 genes, for Patients 3 and 4. There was no pathogenic variant relevant to the patients’ clinical manifestations. Whole-exome sequencing was performed for Patients 3 and 4, for which no pathogenic variant was identified.
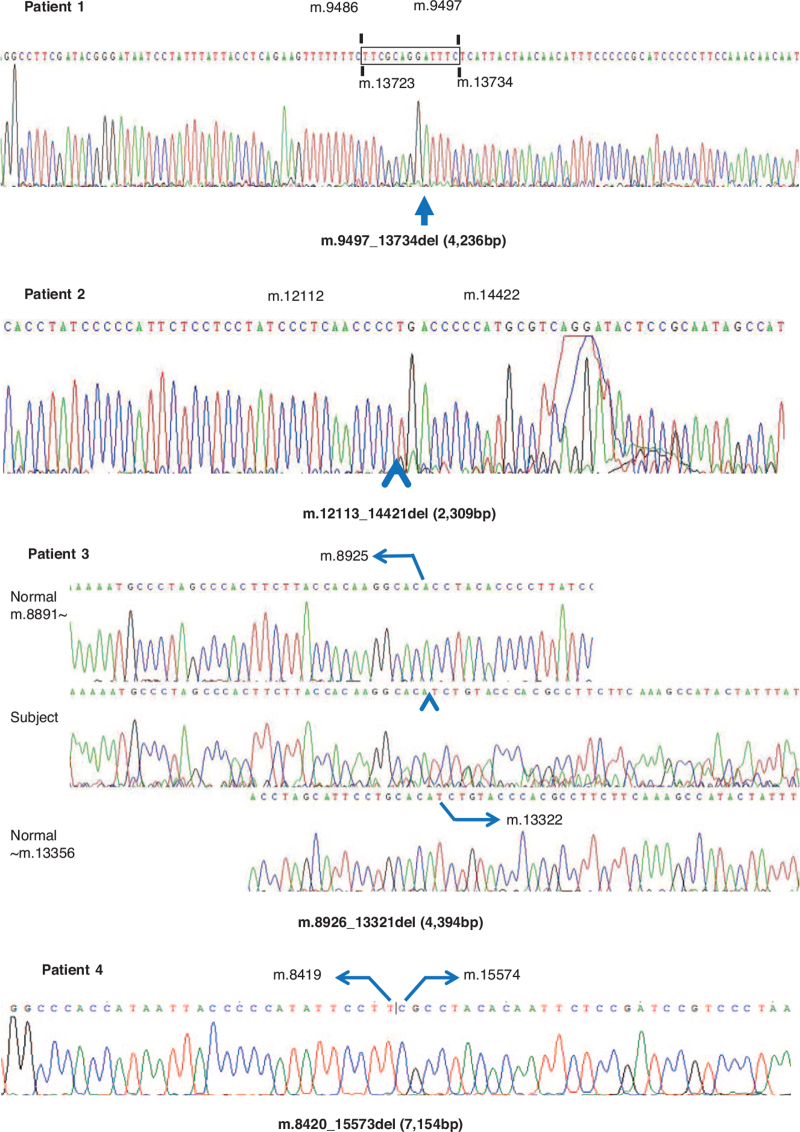
Mitochondrial genomic deletion was detected in all patients; a 4236 bp (m.9497_13734) deletion in Patient 1, a 2309 bp (m.12113_14421) deletion in Patient 2, a 4394 bp (m.8926_13321) deletion in Patient 3 and a 7154 bp (m.8420_15573) deletion in Patient 4 (Fig. 5).
Figure 5.
The results of mitochondrial DNA partial sequences.
3.3. Clinical course
We observed recurrent pancytopenia in Patient 1 who received allogeneic hematopoietic stem cell transplantation (HSCT) at 10 months of age. The conditioning regimen included busulfan (4.4 mg/kg 8, 7, 6, and 5 days before infusion), cyclophosphamide (60 mg/kg 3 and 2 days before infusion), and anti-thymocyte globulin (0.5 mg/kg 3 days before infusion and 2 mg/kg 2, and 1 day before infusion). We used T-cell depleted hematopoietic cell transplantation by CD34+ selection and had planned cyclosporine infusion for 2 months to prevent graft-versus-host disease. We infused 8.77 × 106/kg CD34-selected stem cells in preparation; however, the patient died of pulmonary hemorrhage and metabolic acidosis after 1 month.
Patient 2 failed to thrive despite normal growth hormone provocative test results and showed stunted growth velocity (2–3 cm/year). At age 9 years, her intelligence quotient score was 52, and at age 11 years, visual disturbance at night developed and pigment retinopathy was detected. At the final evaluation at the age of 12 years, she had been treated with hydrocortisone to prevent hypoglycemic crisis since age 6 years and exogenous pancreatic enzyme supplementation for pancreatic insufficiency folate for macrocytic anemia and folate deficiency.
Patient 3 presented with pancreatic insufficiency, adrenal insufficiency, and hypothyroidism. He received treatment in the forms of pancreatic enzyme, hydrocortisone, fludrocortisone, thyroxine, and parenteral nutrition support. Furthermore, he required oral electrolyte supplements such as calcium carbonate, sodium chloride, and sodium bicarbonate for renal tubular acidosis. At the age of 3 years, he developed impairment of renal function associated with hypertension. The patient died at the age of 5 years while waiting to receive an HSCT.
Patient 4 had presented with macrocytic anemia, which improved after receiving two packed RBC transfusions; however, thrombocytopenia remained with no transfusion (platelet count 62,000–140,000 /μL). She underwent percutaneous endoscopic gastrostomy, and was administered electrolyte, bicarbonate, and vitamin supplements with nutritional support. Follow-up abdomen ultrasonography at age 3 years revealed surface nodularity with coarse echogenicity of the liver with tiny nodules, suggesting liver cirrhosis with related nodules (Fig. 6). At this age, her weight and height were within the 25th to 50th percentile and her language development was delayed.
Figure 6.
Abdomen ultrasonography. Surface nodularity with coarse echogenicity of the liver. Multiple small hypoechoic nodules scattered in both hepatic lobes suggesting liver cirrhosis with cirrhosis-related nodules.
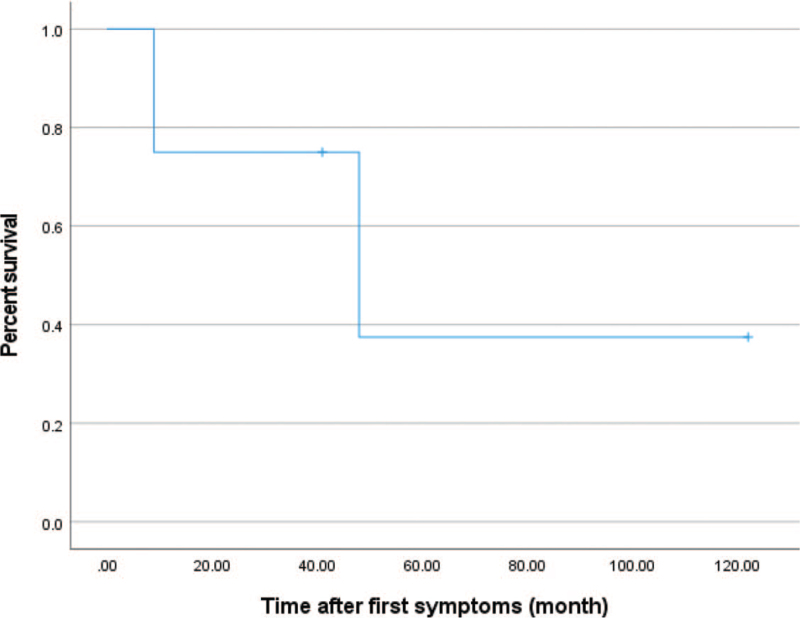
The mean time interval between first symptom onset and hospital visit was 1.3 ± 0.77 (0–2) years. The mean follow-up period was 3.5 ± 3.2 (0.75–9) years, and the age at the latest evaluation was 5.5 170 ± 4.4 (0.96–12.8). Patient 1 died aged 11 months and Patient 3 died aged 5 years. Kaplan–Meier analysis was used to describe the overall survival of the four patients (Fig. 7).
Figure 7.
Kaplan–Meier curves for the overall survival probability after first symptom onset.
4. Discussion
The global prevalence of childhood-onset mitochondrial disease is estimated to range from 5 to 15 patients per 100,000.[8] Pearson syndrome is caused by mtDNA deletions and is an extremely rare disease, with <100 cases reported worldwide.[9]
Pearson syndrome was first described in bone marrow precursors and exocrine pancreatic dysfunction.[10] It has since expanded to be recognized as a multi-organ mitochondrial cytopathy, of which the main characteristic features are macrocytic anemia alone or combined with thrombocytopenia or granulocytopenia, renal tubulopathy, pancreas insufficiency, failure to thrive, lactic acidosis, and hyperlipidemia with liver steatosis.[11]
In the current study, all patients showed multisystemic manifestations. All patients presented with lactic acidosis, and BM dysfunction varied in degrees of severity, ranging from pancytopenia to transient anemia, and various endocrine abnormalities were observed. Pancreatic dysfunction has been detected in 12.7% of infants and in up to 18% of patients at age 4 years,[12] primarily caused by fibrosis, fatty acid infiltration, and acinar dilatation/atrophy.[13] Two patients showed suspicious pancreatic insufficiency with low amylase and lipase levels and pancreatic lipomatosis on ultrasound evaluation. Adrenal insufficiency (2 patients), hypoglycemia (2 patients), and hypothyroidism (1 patient) were also observed. Diabetes mellitus has been found in 10% to 27% of patients with PS,[4,14] of which developed in our patients at 5.5 ± 4.4 (0.96–12.8) years of age. Liver failure has also been observed in 33% of PS cases,[15] and notably, one of our patients had liver cirrhosis (Table 3).
Table 3.
Frequency of clinical features in 4 patients and previously reported patients with PS.
| Clinical features | Patients affected, n (%) | ||
| Index case | Reported case | Reference | |
| Lactic acidemia | 4/4 (100%) | 10/33 (30%) | Manea et al[11] |
| Anemia | 4/4 (100%) | 32/33 (97%) | Manea et al[11] |
| Developmental delay | 3/3 (100%) | 8/11 (73%) | Broomfield et al[2] |
| Thrombocytopenia/neutropenia | 3/4 (75%) | 17/21 (81%) | Rotig et al[15] |
| Adrenal insufficiency | 2/4 (50%) | 2/11 (18%) | Farruggia et al[4] |
| Renal tubulopathy | 2/4 (50%) | 9/21 (43%) | Rotig et al[15] |
| Hypoglycemia | 2/4 (50%) | 5/33 (15%) | Manea et al[11] |
| Pancreatic insufficiency | 2/4 (50%) | 10/55 (18%) | Hsiu-Fen Lee et al[12] |
| Liver involvement | 1/4 (25%) | 7/21 (33%) | Rotig et al[15] |
| Ocular involvement | 1/4 (25%) | 6/11 (55%) | Farruggia et al[4] |
| Hypothyroidism | 1/4 (25%) | 1/11 (9.1%) | Broomfield et al[2] |
| Diabetes mellitus | 0/4 (0%) | 2/11 (18%) | Farruggia et al[4] |
| Heart involvement | 0/4 (0%) | 3/11 (27%) | Broomfield et al[2] |
The preceding and subsequent numbers refer to the number of affected and total patients, respectively. For example, 1/4 means that one in four patients showed the feature.
Neurological manifestations are also typical of PS, presenting as hypotonia, developmental delay, ataxia, and tremor. Additionally, more than 50% of patients show developmental delay before the age of 4 years, which was similar to our patients.[2,12] This syndrome can also transition into KSS,[3] with symptoms including pigmentary retinopathy with rod-cone dystrophy, PEO including ptosis, and at least one of the following: cardiac conduction abnormality, cerebellar ataxia, or high cerebrospinal fluid protein content.[2,3] Nyctalopia is an early symptom of KSS, which appeared in one of our patients who had developed impaired night vision and ptosis. Thus, patients with PS should be monitored for ophthalmologic and cardiac manifestations. Patients with KSS typically die in young adulthood,[3] and those with PS have a worse prognosis, with reports suggesting a higher risk of premature death than other mtDNA deletion syndromes. Approximately 22% of patients survive until 18 years compared with 73% of patients with other clinical phenotypes.[2]
Identifying PS in acutely ill infants or children is challenging given the variable multisystemic manifestations. Nuclear genome sequencing, such as targeted or whole-exome sequencing, has wide applications in genetic diagnostics for pediatric patients.[16,17] These tests can increase early detection of genetic disease, which will allow for improved patient care and prognosis.[16] However, these nuclear genome tests do not include mtDNA; therefore, early diagnosis of mtDNA deletion syndromes such as PS is challenging without clinical suspicion.
Additionally, Diamond-Blackfan anemia (DBA) and PS have similar clinical presentations, including early-onset anemia, non-hematologic manifestations, sporadic genetic occurrence, and occasional spontaneous hematologic improvement.[9] In a study by Gagne et al, 8 (4.6%) of 173 DNA samples submitted for DBA genetic studies were found to have large mtDNA deletions, and 2 patients were eventually diagnosed with PS.[9] Therefore, mtDNA deletion testing should be considered in pediatric patients with progressive, unexplained symptoms involving several unrelated organs.
Supportive therapy is the mainstay for PS, such as transfusions, nutritional support, and medications to combat symptoms. Moreover, allogeneic HSCT could be considered for patients with severe BM failure. The first patient with PS to receive a HSCT was reported by Faraci et al.[1]: the patient was diagnosed at the age of 4 months with BM, renal, and pancreas involvement, and received a HSCT twice at age 7 years; the second HSCT was performed due to rejection. Hematological manifestations completely disappeared, but complications including pulmonary aspergillosis and graft-versus-host disease occurred. Disease progression occurred after the second HSCT, and the patient died at 22 months after the second HSCT.[1] There are four cases of allogeneic HSCT in patients with PS in the literature.[1,4,18,19,20] Two patients received unrelated cord blood transplantation with resolved hematologic and non-hematologic features. But in other cases, HSCT resulted in critical complications and acute myeloid leukemia. In our study, one patient received HSCT due to BM failure, but he died due to complications.
There is speculation over whether disease severity correlates with mtDNA deletion location and size. In our patients, there was no strong correlation between mtDNA deletion size and phenotype; however, the patient with longer deletions had earlier symptom onset. Grady et al. reported that mtDNA deletion size was significantly correlated with age at onset (P = .0008). They noted that a deletion size greater than 5 kbp led to earlier symptom presentation. They also revealed that mtDNA deletion size is a predictor of disease progression.[21] Another report also suggested that patients who had longer deletions had earlier disease onset.[22] Furthermore, longer deletions were observed in patients with KSS than in patients with CPEO.[22] Conversely, Lopez-Gallardo et al. reported that deletion size was associated with the age of onset but not the phenotype after combined analysis of patients with CPEO and KSS.[23]
In conclusion, mtDNA genetic testing should be considered when a patient presents with lactic acidemia that indicates energy production, multiple organ aberrations including hematologic abnormalities, and features of progressive disease. Our report expands the understanding of the clinical spectrum and natural course of this extremely rare but fatal disease. Early identification may help provide conservative management to the affected patients in the early disease state and improve the clinical course. HSCT is a feasible option for BM failure, but further studies are needed to minimize its complications.
Acknowledgments
We are deeply grateful to the patients and their families for providing their clinical information.
Author contributions
Data curation: Ji Soo Son, Go Hun Seo, Yoon-Myung Kim.
Investigation: Gu-Hwan Kim, Hee Kyung Jin, Jae-sung Bae.
Supervision: Ho Joon Im, Han-Wook Yoo, Beom Hee Lee.
Validation: Ho Joon Im.
Writing – original draft: Ji Soo Son.
Writing – review & editing: Ho Joon Im, Beom Hee Lee.
Footnotes
Abbreviations: BM = bone marrow, DBA = Diamond-Blackfan anemia, Hb = hemoglobin, HSCT = hematopoietic stem cell transplantation, KSS = Kearns-Sayre syndrome, mtDNA = mitochondrial DNA, PCR = polymerase chain reaction, PEO = progressive external ophthalmoplegia, PS = Pearson syndrome, RBC = red blood cell, WBC = white blood cell.
How to cite this article: Son JS, Seo GH, Kim YM, Kim GH, Jin HK, Bae Js, Im HJ, Yoo HW, Lee BH. Clinical and genetic features of four patients with Pearson syndrome: An observational study. Medicine 2022;101:5(e28793).
This study was supported by grants from the National Research Foundation of Korea (NRF-2018M3A9H1078335) and intramural grants from the Asan Institute for Life Sciences, AMC (nos. 2019-756).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Faraci M, Cuzzubbo D, Micalizzi C, et al. Allogeneic bone marrow transplantation for Pearson's syndrome. Bone Marrow Transplant 2007;39:563–5. [DOI] [PubMed] [Google Scholar]
- [2].Broomfield A, Sweeney MG, Woodward CE, et al. Paediatric single mitochondrial DNA deletion disorders: an overlapping spectrum of disease. J Inherit Metab Dis 2015;38:445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goldstein A, Falk MJ. Mitochondrial DNA Deletion Syndromes. In: Adam MP, Ardinger HH, Pagon RA, et al, editors. GeneReviews((R)). Seattle (WA)1993. [Google Scholar]
- [4].Farruggia P, Di Cataldo A, Pinto RM, et al. Pearson Syndrome: A Retrospective Cohort Study from the Marrow Failure Study Group of A.I.E. O P (Associazione Italiana Emato-Oncologia Pediatrica) JIMD Rep 2016;26:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders--past, present and future. Biochim Biophys Acta 2004;1659:115–20. [DOI] [PubMed] [Google Scholar]
- [6].Lamont PJ, Surtees R, Woodward CE, Leonard JV, Wood NW, Harding AE. Clinical and laboratory findings in referrals for mitochondrial DNA analysis. Arch Dis Child 1998;79:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee Y, Kim T, Lee M, et al. De Novo Development of mtDNA Deletion Due to Decreased POLG and SSBP1 Expression in Humans. Genes (Basel) 2021;12:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Primers 2016;2:16080. [DOI] [PubMed] [Google Scholar]
- [9].Gagne KE, Ghazvinian R, Yuan D, et al. Pearson marrow pancreas syndrome in patients suspected to have Diamond-Blackfan anemia. Blood 2014;124:437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pearson HA, Lobel JS, Kocoshis SA, et al. A new syndrome of refractory sideroblastic anemia with vacuolization of marrow precursors and exocrine pancreatic dysfunction. J Pediatr 1979;95:976–84. [DOI] [PubMed] [Google Scholar]
- [11].Manea EM, Leverger G, Bellmann F, et al. Pearson syndrome in the neonatal period: two case reports and review of the literature. J Pediatr Hematol Oncol 2009;31:947–51. [DOI] [PubMed] [Google Scholar]
- [12].Lee HF, Lee HJ, Chi CS, Tsai CR, Chang TK, Wang CJ. The neurological evolution of Pearson syndrome: case report and literature review. Eur J Paediatr Neurol 2007;11:208–14. [DOI] [PubMed] [Google Scholar]
- [13].Lee WS, Sokol RJ. Mitochondrial hepatopathies: advances in genetics and pathogenesis. Hepatology 2007;45:1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Farruggia P, Di Marco F, Dufour C. Pearson syndrome. Expert Rev Hematol 2018;11:239–46. [DOI] [PubMed] [Google Scholar]
- [15].Rotig A, Bourgeron T, Chretien D, Rustin P, Munnich A. Spectrum of mitochondrial DNA rearrangements in the Pearson marrow-pancreas syndrome. Hum Mol Genet 1995;4:1327–30. [DOI] [PubMed] [Google Scholar]
- [16].Akesson LS, Eggers S, Love CJ, et al. Early diagnosis of Pearson syndrome in neonatal intensive care following rapid mitochondrial genome sequencing in tandem with exome sequencing. Eur J Hum Genet 2019;27:1821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Powis Z, Farwell Hagman KD, Speare V, et al. Exome sequencing in neonates: diagnostic rates, characteristics, and time to diagnosis. Genet Med 2018;20:1468–71. [DOI] [PubMed] [Google Scholar]
- [18].Hoyoux C, Dresse MF, Robinet S, et al. Cord blood transplantation in a child with Pearson's disease. Pediatr Blood Cancer 2008;51:566. [DOI] [PubMed] [Google Scholar]
- [19].Tumino M, Meli C, Farruggia P, et al. Clinical manifestations and management of four children with Pearson syndrome. Am J Med Genet A 2011;155A:3063–6. [DOI] [PubMed] [Google Scholar]
- [20].Nishimura A, Hirabayashi S, Hasegawa D, et al. Acquisition of monosomy 7 and a RUNX1 mutation in Pearson syndrome. Pediatr Blood Cancer 2021;68:e28799. [DOI] [PubMed] [Google Scholar]
- [21].Grady JP, Campbell G, Ratnaike T, et al. Disease progression in patients with single, large-scale mitochondrial DNA deletions. Brain 2014;137:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamashita S, Nishino I, Nonaka I, Goto YI. Genotype and phenotype analyses in 136 patients with single large-scale mitochondrial DNA deletions. J Hum Genet 2008;53:598. [DOI] [PubMed] [Google Scholar]
- [23].Lopez-Gallardo E, Lopez-Perez MJ, Montoya J, Ruiz-Pesini E. CPEO and KSS differ in the percentage and location of the mtDNA deletion. Mitochondrion 2009;9:314–7. [DOI] [PubMed] [Google Scholar]