Abstract
Background:
Endoscopic resection bleeding (ERB) classification was proposed by the authors’ team to evaluate the severity of intraoperative bleeding (IB) during endoscopic submucosal dissection (ESD). This study aimed to evaluate the application of ERB classification and to analyze the risk factors of major IB (MIB) and postoperative bleeding (PB) associated with ESD for gastric neoplastic lesions.
Methods:
We retrospectively enrolled a total of 1334 patients who underwent ESD between November 2006 and September 2019 at The First Medical Center of Chinese People's Liberation Army General Hospital. All patients were divided into the non-MIB group (including ERB-0, ERB-controlled 1 [ERB-c1], and ERB-c2) and the MIB group (including ERB-c3 and ERB-uncontrolled [ERB-unc]) according to the ERB classification. Risk factors of major MIB and risk factors of PB were analyzed using a logistic regression model.
Results:
Among the 1334 patients, 773 (57.95%) had ERB-0, 477 (35.76%) had ERB-c1, 77 (5.77%) had ERB-c2, 7 (0.52%) had ERB-c3, and no patients had ERB-unc. The rate of PB in patients with IB classifications of ERB-0, ERB-c1, ERB-c2, and ERB-c3 were 2.20% (17/773), 3.35% (16/477), 9.09% (7/77), and 2/7, respectively. In multivariate analysis, proximal location (odds ratio [OR]: 1.488; 95% confidence interval [CI]: 1.045–3.645; P = 0.047) was the only significant risk factor of MIB. Chronic kidney disease (CKD) (OR: 7.844; 95% CI: 1.637–37.583; P = 0.010) and MIB (ERB-c3) (OR: 13.932; 95% CI: 2.585–74.794; P = 0.002) were independent risk factors of PB.
Conclusions:
Proximal location of lesions was a significant risk factor of MIB. Additionally, CKD and MIB (ERB-c3) were independent risk factors of PB. More attention should be paid to these high-risk patients for MIB and PB.
Keywords: Endoscopic resection bleeding classification, Endoscopic submucosal dissection, Gastric neoplasms
Introduction
Endoscopic submucosal dissection (ESD) has been widely accepted as the standard treatment for gastric neoplastic lesions with no evidence of lymphovascular invasion, including intraepithelial neoplasia and early gastric cancer (EGC). Although ESD enables a higher en bloc resection rate, it demands higher technical skills and a longer procedure time (PT) compared with the traditional endoscopic mucosal resection. Accordingly, ESD is accompanied by a relatively high risk of procedure-related adverse events, such as bleeding.[1,2] According to the previous studies, rates of intraoperative bleeding (IB) can range from 2.9% to 45.1%.[3,4]
However, the standards to evaluate the IB rate vary in different studies, causing major discrepancies and making the results incomparable. Although one study reported a grading method to define the degree of IB according to the amount of bleeding and hemostatic time,[5] it is much more difficult to accurately evaluate the amount of bleeding during endoscopic operations than during surgical operations, and the operator's experience makes a difference in controlling IB. To overcome these problems, Linghu[6] proposed a new classification of IB, termed the endoscopic resection bleeding (ERB) classification.
Previous studies have reported that the risk factors for IB are patient age, lesion location, the presence of gastric malignancy, prolonged PT, and endoscopist's experience.[4,5,7,8] However, those studies only defined the risk factors for overall or subjectively classified IB. Therefore, this study divided patients into two groups: the non-major IB (NMIB) group (including ERB-0, ERB-controlled 1 [ERB-c1], and ERB-c2) and the major IB (MIB) group (including ERB-c3 and ERB-uncontrolled [ERB-unc]) according to ERB classification, then we analyzed the characteristics of different ERB classification, risk factors of MIB, and risk factors of postoperative bleeding (PB).
Methods
Patients and ethical approval
We retrospectively reviewed a total of 1707 patients who underwent gastric ESD between November 2006 and September 2019 at The First Medical Center of Chinese People's Liberation Army (PLA) General Hospital. In cases with multiple synchronous lesions, the lesions that showed deeper invasion or were larger in diameter if the invasion depths were the same, were included. The inclusion criteria were as follows: (1) the pathological results of biopsy or ESD were intraepithelial neoplasia or EGC; (2) medical records were complete and endoscopic pictures were clear; (3) all patients were treated by ESD. Ultimately, a total of 1334 patients with 1334 lesions were enrolled for analysis and were divided into four groups according to the ERB classification. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of The First Medical Center of Chinese PLA General Hospital (No. S2017-010-02). Before ESD was performed, all patients signed informed consent.
ESD procedure and postoperative treatment
During the operation, the patient was placed in the left lateral position or supine position with the right shoulder elevated. The entire operation was performed under endotracheal intubation anesthesia, and the patient's respiratory rate, heart rate, and blood pressure were monitored. The specific steps of ESD included the following: (1) circumferential marking: after close observation and confirmation of the gastric lesion, a marking was made 3–5 mm away from the lesion boundary; (2) submucosal injection: a submucosal injection was performed around the lesion in the order of distal-to-proximal so that the mucosa was separated from the muscularis propria, and then the lesion was fully lifted; (3) circular incision: after the lesion was fully lifted, the lesion mucosa was cut approximately 3 mm outside the marked point; (4) submucosal dissection: during the dissection, submucosal injections were performed intermittently to ensure adequate submucosal lift; (5) wound treatment: electrocoagulation, hemostatic forceps, or argon plasma coagulation (APC) were used to treat the exposed blood vessels at the base of the ulcer, and if necessary, fibrin protein glue was sprayed to protect the wound.
After ESD, all patients remained fasting and were treated with a proton pump inhibitor (PPI) and other indicated treatments. Specifically, patients with large wounds or complications, such as perforation or aspiration pneumonia, were treated with antibiotics. In general, the patients were given fluid food 3 days later and the diet was gradually advanced if no obvious discomfort occurred, at this time patients began to take PPI orally instead of intravenous PPI for at least 4 weeks. The patient was discharged from the hospital when tolerating liquids or semi-liquids. Adverse events, such as PB, were closely observed after ESD. Patients were instructed to return to the hospital immediately if they noticed hematemesis or melena after discharge from the hospital.
ERB classification
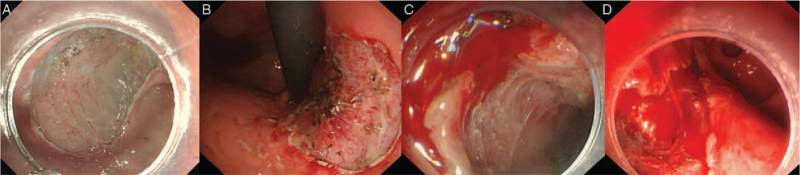
ERB classification includes three grades and five sub-grades, as follows: ERB-0, no bleeding is seen during gastric ESD due to timely pretreatment.[6] ERB-c, bleeding that can be controlled under endoscopy, which is divided into three sub-grades (ERB-c1 [minor bleeding that can be easily stopped by endoscopy, does not influence the patient's vital signs, and does not require blood transfusion], ERB-c2 [the bleeding volume is between c1 and c3]; and ERB-c3 [major bleeding, although the bleeding can be controlled under endoscopy, control of bleeding is technically difficult and a blood transfusion is required during or after gastric ESD]); and ERB-unc refers to uncontrollable bleeding under endoscopy, which must be stopped by surgery or vessel embolotherapy [Figure 1]. Endoscopic hemostasis methods include APC, electrocoagulation, hemostatic forceps, and hemostatic clips. The ERB classification was evaluated by two endoscopists who reviewed endoscopic pictures, endoscopic reports, endoscopic videos, and medical records. If there was a disagreement between reviewers, the consensus was reached by a third experienced physician.
Figure 1.
ERB classification: (A) ERB-0 means that no bleeding is seen during gastric ESD. (B) ERB-c1: minor bleeding. (C) ERB-c2: the bleeding volume is between c1 and c3. (D) ERB-c3: major bleeding. ERB: Endoscopic resection bleeding; ESD: Endoscopic submucosal dissection; ERB-c1: ERB-controlled1.
Definition
In this study, NMIB means no bleeding during ESD or bleeding that can be controlled by endoscopy during ESD without affecting postoperative vital signs and with no need for extra treatments, such as blood transfusion; MIB refers to bleeding that requires additional treatment after ESD, such as blood transfusion or must be treated by surgery or vessel embolotherapy. PB was defined as one of the following: apparent hematemesis or melena, unstable vital signs, or a >2 g/dL decrease in hemoglobin concentration after ESD.[7] The long location of lesions was divided into three parts (upper, middle, and lower) and the short location of lesions was divided into lesser curvature, greater curvature, anterior wall, posterior wall, and others.[9] According to the Pairs endoscopic classification,[10] macroscopic type was classified into elevated (type I, IIa, and IIa+IIc), flat (type IIb), and depressed (type IIc, IIc+IIa, and III). The PT was defined as the time required from marking the lesion margin to completing the removal of the lesions. En bloc resection indicates that the lesion was removed under an endoscope and a single specimen was obtained. In the present study, the average and the median number of gastric ESD cases per endoscopist were 98 and 88, respectively, thus we defined junior endoscopists as those who had performed <100 ESD procedures; whereas experienced endoscopists were defined as those who had performed ≥100 cases.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD) and analyzed using Student's t test or the Mann–Whitney test. Categorical variables are shown as numbers with percentages and were analyzed using a chi-squared test or Fisher exact test. The values with a P value < 0.10 in the univariate analysis were included in the multivariate logistic regression model. All statistical analyses were performed with SPSS v22.0 (IBM, Corp., Armonk, NY, USA). A P value < 0.05 was considered to be statistically significant.
Results
Patient-related characteristics in the NMIB and MIB groups
Of the 1334 patients, 773 (57.95%) had ERB-0, 477 (35.76%) had ERB-c1, 77 (5.77%) had ERB-c2, 7 (0.52%) had ERB-c3, and no patients had ERB-unc. The proportion of male patients who had ERB-0, ERB-c1, ERB-c2, and ERB-c3 was 73.09% (565/773), 84.28% (402/477), 90.91% (70/77), and 7/7, respectively (χ2 = 37.471, P < 0.001). No significant differences were found in age, history of smoking, and drinking, comorbidities, such as hypertension, diabetes mellitus, liver disease, heart disease, chronic kidney disease (CKD), and usage of aspirin or clopidogrel among different ERB classifications [Table 1].
Table 1.
Characteristics of patients undergoing gastric ESD among different ERB classifications.
| NMIB group | MIB group | ||||
| Characteristics | ERB-0 (n = 773) | ERB-c1 (n = 477) | ERB-c2 (n = 77) | ERB-c3 (n = 7) | P value |
| Age (years) | 61.0 ± 10.3 | 60.5 ± 10.3 | 61.8 ± 10.4 | 57.6 ± 13.7 | 0.360 |
| BMI (kg/m2) | 24.45 ± 3.31 | 24.31 ± 3.14 | 25.50 ± 3.44 | 22.78 ± 3.80 | 0.069 |
| Male | 565 (73.09) | 402 (84.28) | 70 (90.91) | 7/7 | <0.001 |
| Smoking | 291 (37.65) | 207 (43.40) | 36 (46.75) | 1/7 | 0.059 |
| Drinking | 278 (35.96) | 207 (43.40) | 38 (49.35) | 3/7 | 0.104 |
| Comorbidities | |||||
| Hypertension | 213 (27.55) | 120 (25.16) | 28 (36.36) | 1/7 | 0.178 |
| Diabetes mellitus | 104 (13.45) | 65 (13.63) | 14 (18.18) | 1/7 | 0.721 |
| Liver diseases | 38 (4.92) | 21 (4.40) | 4 (5.19) | 0 | 0.906 |
| Heart diseases | 54 (6.99) | 35 (7.34) | 5 (6.49) | 0 | 0.889 |
| CKDs | 10 (1.29) | 2 (0.42) | 1 (1.30) | 0 | 0.477 |
| Usage of anticoagulant and/or antiplatelet drugs | |||||
| Aspirin | 50 (6.47) | 24 (5.03) | 5 (6.49) | 1/7 | 0.664 |
| Clopidogrel | 6 (0.78) | 12 (2.52) | 0 | 0 | 0.147 |
Values were shown as mean ± standard deviation, n (%), or n. BMI: Body mass index; CKD: Chronic kidney disease; ESD: Endoscopic submucosal dissection; ERB: Endoscopic resection bleeding; ERB-c1: ERB-controlled1; IB: Intraoperative bleeding; MIB: Major intraoperative bleeding; NMIB: Non-major intraoperative bleeding.
Lesion-related characteristics in the NMIB and MIB groups
Lesion-related characteristics in the minor and MIB groups are shown in Table 2. With the increasing ERB classification, the rate of ulcer, submucosal adhesion, and EGC also gradually increased, as did the proportion of lesions located in the middle and upper part of the stomach. Macroscopic types were not significantly different among ERB-0, ERB-c1, ERB-c2, and ERB-c3 (χ2 = 10.312, P = 0.112). 5.69% (44/773), 11.53% (55/477), and 16.88% (13/77) of lesions in ERB-0, ERB-c1, and ERB-c2 group, respectively, involved the submucosa, whereas those in the ERB-c3 group were all limited to the mucosa layer (χ2 = 21.295, P < 0.001).
Table 2.
Lesion-related characteristics of patients undergoing gastric ESD among different ERB classifications.
| NMIB group | MIB group | ||||
| Characteristics | ERB-0 (n = 773) | ERB-c1 (n = 477) | ERB-c2 (n = 77) | ERB-c3 (n = 7) | P value |
| Lesion size (cm) | 1.45 ± 1.08 | 1.59 ± 1.05 | 1.73 ± 1.04 | 1.16 ± 0.62 | 0.005 |
| Ulcer | 0.003 | ||||
| Absence | 684 (88.49) | 389 (81.55) | 62 (80.52) | 5/7 | |
| Presence | 89 (11.51) | 88 (18.45) | 15 (19.48) | 2/7 | |
| Adhesion | <0.001 | ||||
| No | 724 (93.66) | 398 (83.44) | 60 (77.92) | 4/7 | |
| Yes | 49 (6.34) | 79 (16.56) | 17 (22.08) | 3/7 | |
| Location | <0.001 | ||||
| Upper | 180 (23.29) | 216 (45.28) | 45 (58.44) | 4/7 | |
| Middle | 168 (21.73) | 131 (27.46) | 16 (20.78) | 3/7 | |
| Lower | 425 (54.98) | 130 (27.25) | 16 (20.78) | 0 | |
| Macroscopic type | 0.112 | ||||
| Elevated | 572 (74.00) | 329 (68.97) | 51 (66.23) | 4/7 | |
| Flat | 93 (12.03) | 62 (13.00) | 13 (16.88) | 0 | |
| Depressed | 108 (13.97) | 86 (18.03) | 13 (16.88) | 3/7 | |
| Histological type | <0.001 | ||||
| EGC | 297 (38.42) | 247 (51.78) | 34 (44.16) | 4/7 | |
| HGIN | 315 (40.75) | 164 (34.38) | 33 (42.86) | 2/7 | |
| LGIN | 161 (20.83) | 66 (13.84) | 10 (12.98) | 1/7 | |
| Invasion depth | <0.001 | ||||
| Mucosa | 729 (94.31) | 422 (88.47) | 64 (83.12) | 7/7 | |
| SM | 44 (5.69) | 55 (11.53) | 13 (16.88) | 0 | |
Values were shown as mean ± standard deviation, n (%), or n. ERB: Endoscopic resection bleeding; EGC: Early gastric cancer; ESD: Endoscopic submucosal dissection; ERB-c1: ERB-controlled1; HGIN: High-grade intraepithelial neoplasia; LGIN: Low-grade intraepithelial neoplasia; MIB: Major intraoperative bleeding; NMIB: Non-major intraoperative bleeding; PT: Procedure time; SM: Submucosa.
Procedure-related characteristics in the NMIB and MIB groups
Table 3 shows that from ERB-0 to ERB-c3, the PT was gradually prolonged (t = 41.089, P < 0.001) and the specimen size also gradually increased (t = 89.601, P < 0.001), the rate of PT ≥ 120 min was also increased from ERB-0 to ERB-c3, whereas the en bloc resection rate was gradually decreased (P < 0.001). Additionally, the incidences of PB in patients with IB classifications of ERB-0, ERB-c1, ERB-c2, and ERB-c3 were 2.20% (17/773), 3.35% (16/477), 9.09% (7/77), and 2/7, respectively (χ2 = 26.105, P < 0.001). There was no significant difference in the endoscopic experience of endoscopists among cases of different ERB classifications (χ2 = 4.229, P = 0.238).
Table 3.
Procedure-related characteristics of patients undergoing gastric ESD among different ERB classifications.
| NMIB group | MIB group | ||||
| Characteristics | ERB-0 (n = 773) | ERB-c1 (n = 477) | ERB-c2 (n = 77) | ERB-c3 (n = 7) | P value |
| PT (min) | 36.11 ± 23.75 | 65.87 ± 53.57 | 97.56 ± 71.84 | 194.43 ± 153.07 | <0.001 |
| <120 min | 766 (99.09) | 414 (86.79) | 53 (68.83) | 2/7 | <0.001 |
| ≥120 min | 7 (0.91) | 63 (13.21) | 24 (31.17) | 5/7 | |
| Specimen size (cm) | 3.53 ± 1.41 | 4.03 ± 1.62 | 4.32 ± 1.57 | 4.74 ± 1.72 | <0.001 |
| En bloc resection | <0.001 | ||||
| No | 22 (2.85) | 60 (12.58) | 16 (20.78) | 2/7 | |
| Yes | 751 (97.15) | 417 (87.42) | 61 (79.22) | 5/7 | |
| Endoscopists | 0.238 | ||||
| Junior | 237 (30.66) | 145 (30.40) | 19 (24.68) | 0 | |
| Experienced | 536 (69.34) | 332 (69.60) | 58 (75.32) | 7/7 | |
| PB | <0.001 | ||||
| No | 756 (97.80) | 461 (96.65) | 70 (90.91) | 5/7 | |
| Yes | 17 (2.20) | 16 (3.35) | 7 (9.09) | 2/7 | |
Values were shown as mean ± standard deviation, n (%), or n. ESD: Endoscopic submucosal dissection; ERB: Endoscopic resection bleeding; ERB-c1: ERB-controlled 1; MIB: Major intraoperative bleeding; NMIB: Non-major intraoperative bleeding; PT: Procedure time; PB: Postoperative bleeding.
Univariate and multivariate analysis for predictors of major IB
In the univariate analysis, submucosal adhesion (P = 0.033), lesion location (long axis) (χ2 = 3.898, P = 0.034), and PT ≥ 120 min (P < 0.001) were significantly correlated with MIB. However, only proximal location (upper, odds ratio [OR]: 1.488; 95% confidence interval [CI]: 1.045–3.645; P = 0.047) and PT ≥ 120 min (OR: 19.033; 95% CI: 3.066–118.153; P = 0.002) were significantly related with MIB in the multivariate analysis [Table 4].
Table 4.
Univariate and multivariate analysis of major intraoperative bleeding among patients undergoing gastric ESD.
| Multivariate analysis | |||||
| Variables | NMIB group (n = 1327) | MIB group (n = 7) | P value | OR (95% CI) | P value |
| Patient characteristics | |||||
| Age (years) | 60.9 ± 10.3 | 57.6 ± 13.7 | 0.255 | ||
| Male | 1037 (78.15) | 7/7 | 0.358 | ||
| Usage of aspirin | 79 (5.95) | 1/7 | 0.352 | ||
| Usage of clopidogrel | 18 (1.36) | 0 | 1.000 | ||
| Hypertension | 361 (27.20) | 1/7 | 0.683 | ||
| Heart diseases | 94 (7.08) | 0 | 1.000 | ||
| Liver diseases | 63 (4.75) | 0 | 1.000 | ||
| Diabetes mellitus | 183 (13.79) | 1/7 | 1.000 | ||
| CKDs | 13 (0.98) | 0 | 1.000 | ||
| Lesion characteristics | |||||
| Ulcer | 192 (14.47) | 2/7 | 0.271 | ||
| Adhesion | 145 (10.93) | 3/7 | 0.033 | 0.477 (0.086–2.637) | 0.397 |
| Location (long axis), upper/middle/lower | 441/315/571 | 4/3/0 | 0.034 | 1.488 (1.045–3.645) | 0.047 |
| Location (short axis), LC/AW/GC/PW/others | 552/182/213/326/54 | 5/0/0/2/0 | 0.420 | ||
| Macroscopic type, elevated/flat/depressed | 952/168/207 | 4/0/3 | 0.159 | ||
| Histology type, EGC/HGIN/LGIN | 578/512/237 | 4/2/1 | 0.881 | ||
| Invasion depth, M/SM | 1215/112 | 7/0 | 1.000 | ||
| Lesion size ≥3 cm | 101 (7.61) | 0 | 0.534 | 1.000 | |
| Procedure characteristics | |||||
| PT ≥120 min | 94 (7.08) | 5/7 | <0.001 | 19.033 (3.066–118.153) | 0.002 |
| En bloc resection | 1129 (92.61) | 5/7 | 0.091 | 0.913 (0.148–5.640) | 0.922 |
| Specimen size ≥4 cm | 561 (42.28) | 5/7 | 0.347 | ||
| Endoscopists, junior | 401 (30.22) | 0 | 0.110 | ||
| Multiple lesions | 87 (6.56) | 2/7 | 0.074 | 1.465 (0.670–4.325) | 0.826 |
Values were shown as mean ± standard deviation, n (%), or n. AW: Anterior wall; CKD: Chronic kidney disease; CI: Confidence interval; EGC: Early gastric cancer; ESD: Endoscopic submucosal dissection; GC: Greater curvature; HGIN: High-grade intraepithelial neoplasia; IB: Intraoperative bleeding; LC: Lesser curvature; LGIN: Low-grade intraepithelial neoplasia; M: Mucosa; MIB: Major intraoperative bleeding; NMIB: Non-major intraoperative bleeding; OR: Odds ratio; PW: Posterior wall; PT: Procedure time; SM: Submucosa.
Characteristics and predictors of PB patients
A total of 42 patients had PB. The median occurrence time of PB was 2 days (range, 1–30 days) after gastric ESD. Of the patients, 97.62% (41/42) of patients had PB within 2 weeks after gastric ESD. Only one patient had PB 30 days after gastric ESD. The most common symptoms of patients with PB are hematemesis and melena. Of the 42 patients with PB, 27 underwent endoscopic hemostasis, including epinephrine injection (4 patients), hemostatic clip/forceps (1 patient), and APC or electrocoagulation (22 patients). The remaining 15 patients achieved hemostasis through conservative treatment. All patients with PB successfully stopped bleeding.
In the univariate analysis, PT ≥ 120 min (P = 0.031) and MIB (ERB-c3; P < 0.001) were significantly correlated with PB. In addition, the rate of patients with CKD in the PB group was higher than the non-PB group (P = 0.061). Multivariate analysis also showed that CKD (OR: 7.844; 95% CI: 1.637–37.583; P = 0.010) and MIB (ERB-c3; OR: 13.932; 95% CI: 2.585–74.794; P = 0.002) were independent risk factors of PB (Table 5).
Table 5.
Univariate and multivariate analysis of postoperative bleeding among patients undergoing gastric ESD.
| Multivariate analysis | |||||
| Variables | NPB (n = 1292) | PB (n = 42) | P value | OR (95% CI) | P value |
| Patient characteristics | |||||
| Age (years) | 60.9 ± 10.3 | 58.8 ± 11.6 | 0.288 | ||
| Male | 1008 (78.02) | 36 (85.71) | 0.234 | ||
| Usage of aspirin | 78 (6.04) | 2 (4.76) | 0.510 | ||
| Usage of clopidogrel | 17 (1.32) | 1 (2.38) | 0.440 | ||
| Hypertension | 352 (27.24) | 10 (23.81) | 0.622 | ||
| Heart diseases | 91 (7.04) | 3 (7.14) | 0.980 | ||
| Liver diseases | 60 (4.64%) | 3 (7.14) | 0.452 | ||
| Diabetes mellitus | 181 (14.01) | 3 (7.14) | 0.204 | ||
| CKDs | 11 (0.85) | 2 (4.76) | 0.061 | 7.844 (1.637–37.583) | 0.010 |
| Lesion characteristics | |||||
| Lesion size ≥3 cm | 99 (7.66) | 2 (4.76) | 0.594 | ||
| Location (long axis), upper/middle/lower | 433/306/553 | 12/12/18 | 0.703 | ||
| Location (short axis), LC/AW/GC/PW/others | 535/177/208/318/54 | 22/5/5/10/0 | 0.480 | ||
| Macroscopic type, elevated/flat/depressed | 926/163/203 | 30/5/7 | 0.980 | ||
| Histology type, EGC/HGIN/LGIN | 564/499/229 | 18/15/9 | 0.815 | ||
| Invasion depth, M/SM | 1183/109 | 39/3 | 0.766 | ||
| Procedure characteristics | |||||
| PT ≥120 min | 92 (7.12) | 7 (16.67) | 0.031 | 1.738 (0.625–4.833) | 0.289 |
| En bloc resection | 1198 (92.72) | 36 (85.71) | 0.090 | 0.618 (0.229–1.668) | 0.342 |
| Specimen size ≥4 cm | 542 (41.95) | 24 (57.14) | 0.115 | ||
| Endoscopists, junior | 386 (29.87) | 15 (35.71) | 0.054 | 2.119 (0.920–4.881) | 0.078 |
| MIB (ERB-c3) | 4 (0.31) | 2 (4.76) | <0.001 | 13.932 (2.585–74.794) | 0.002 |
| Multiple lesions | 86 (6.66) | 3 (7.14) | 0.756 | ||
Values were shown as mean ± standard deviation, n (%), or n. AW: Anterior wall; CKD: Chronic kidney disease; CI: Confidence interval; EGC: Early gastric cancer; ERB: Endoscopic resection bleeding; ESD: Endoscopic submucosal dissection; GC: Greater curvature; HGIN: High-grade intraepithelial neoplasia; IB: Intraoperative bleeding; LC: Lesser curvature; LGIN: Low-grade intraepithelial neoplasia; M: Mucosa; MIB: Major intraoperative bleeding; NPB: No postoperative bleeding; OR: Odds ratio; PW: Posterior wall; PB: Postoperative delayed bleeding; PT: Procedure time; SM: Submucosa.
Discussion
In this study, we found that the incidence of MIB was 0.52% and the incidence of PB gradually increased from ERB-0 to ERB-c3 (P < 0.001). Multivariate analysis indicated that proximal location (P = 0.047) was the only significant risk factor of MIB, whereas CKD (P = 0.010) and MIB (ERB-c3) (P = 0.002) were independent risk factors of PB.
Previous studies have reported the risk factors of IB during ESD. Oda et al[11] analyzed the relationship between IB and lesion location, lesion size, and findings of ulceration, but found that only location (upper and middle third) and lesion size (≥31 mm) were significantly associated with IB. Jeon et al[5] found that younger age and more proximal lesion locations were significant predictors of IB. Toyonaga et al[12] and Horikawa et al[13] also found that IB usually develops with lesions in the proximal location of the stomach. Our results are consistent with the aforementioned studies. Possible reasons are as follows: first, the proximal stomach is a difficultly accessible site for endoscopists, so lesions in this area are more difficult to treat; second, the diameter and number of the submucosal blood vessels in the proximal area are larger than in the other areas.[12]
In addition, we not only found that PT was significantly related to the incidence of MIB but also that it was related to the degree of bleeding during ESD. This result was consistent with the previous studies.[14–16] Larger lesions, difficult lesion locations, or a difficult dissection due to submucosal adhesion or ulcers may lead to prolonged PT. However, the more severe IB is, the more difficult it is to stop bleeding under endoscopy, and the longer the hemostasis time may also be. These findings may explain the gradually prolonged PT from ERB-0 to ERB-c3. Therefore, we believe that a longer PT may result from IB, not the reason for the IB.
In our analysis of the relationship between IB and PB, we found that PB increased with increased ERB classification. Moreover, IB classified as ERB-c3 (MIB) was an independent risk factor for PB (OR: 13.932; 95% CI: 2.585–74.794; P = 0.002). Okano et al[17] reported that the rate of PB was 8.13-fold higher in patients with IB compared with the patients without IB (P < 0.001). Park et al[4] divided the delayed bleeding into early delayed bleeding (EDB) and late delayed bleeding (LDB) and found the incidences of EDB and LDB in patients with IB were 3.14-fold (P < 0.001) and 1117.94-fold (P < 0.001) that of patients without IB. One possible reason is that the predilection sites for IB and PB are different. In this study, most IB (57.14%) occurred in the upper third of the stomach, whereas the majority of PB (42.86%) was found in the lower third. Tsuji et al[18] and Miyahara et al[16] reported that the lower part of the stomach is more prone to PB than the upper or middle part. So, the upper and middle parts of the stomach require more careful endoscopic hemostasis during gastric ESD, which may ultimately prevent PB.[11,16–19] Moreover, antral active peristalsis and bile reflux may contribute to the high incidence of PB in the lower part of the stomach.[18,19]
This present study also found that CKD is an independent risk factor of PB. Cheung et al[20] reported that the rate of bleeding in patients with CKD significantly increased. A propensity score-matched study conducted by Choi et al[21] found that stage 4 (OR: 5.79; 95% CI 1.52–22.0; P = 0.010) and stage 5 CKD patients (OR: 4.80; 95% CI 1.58–14.6; P = 0.006) had higher bleeding risks than non-CKD patients. Based on multivariate analysis, stage 4/5 CKD was a significant predictor for bleeding risk (OR: 4.99; 95% CI 1.32–18.8; P = 0.018). A meta-analysis also found that CKD was one of the independent risk factors of PB after ESD.[22] A possible reason for this conclusion is that renal dysfunction or the drugs used in CKD treatment may affect coagulation.
Prevention of bleeding is very important. The methods to prevent bleeding are as follows: first, when vessels are encountered during the procedure, they should be cut after preventative coagulation techniques; second, the amount of fluid should be sufficient to ensure the full lifting of the mucosa; third, reaching the appropriate deep submucosal layer in the process of submucosal dissection is one way to prevent bleeding, because the deep submucosal layer contains larger but fewer penetrating vessels and fibrotic tissue.[23] When hemorrhage occurs during ESD, it's important to immediately identify the exact bleeding point. Endoscopic instruments with water jet function are beneficial to maintain a good view during the process of gastric ESD, and to find the bleeding point.[24] Minor hemorrhage (such as bleeding classified as ERB-c1 and ERB-c2) can also be easily controlled using the cutting device alone by coagulation. Major hemorrhage (such as bleeding classified as ERB-c3) may require endoscopists to use hemostatic forceps or clips to ensure complete hemostasis.[25] It has been reported that the use of hemostatic forceps for preventative coagulation is an effective method to prevent IB.[26]
The limitations of this study include the following. First, this is a retrospective and single-center study, which meant that some data, such as the types of scope or device and submucosal injection fluid during ESD could not be collected. In addition, an ERB classification of IB could not be evaluated in all patients by reviewing endoscopic videos. Prospective multicenter studies are warranted to validate our results. Second, in our study, the incidence of MIB was too small to evaluate the risk factors by multivariate logistic regression analysis, and there were no patients with IB classified as ERB-unc, so the results may have some bias.
In conclusion, ERB classification can be used to objectively assess the degree of IB during ESD for gastric neoplastic lesions and it is also related to PB. Our multivariate analysis showed that the proximal location of lesions was a predictor of MIB. In addition, CKD and IB classified as ERB-c3 were the independent risk factors of PB. More attention should be paid to these high-risk patients for MIB and PB.
Funding
The present study was supported by a grant from the National Key Research & Development Program of China (No. 2016YFC1303601).
Conflicts of interest
None.
Footnotes
How to cite this article: Xu S, Chai N, Tang X, Linghu E, Wang S. Risk factors of major intraoperative bleeding and postoperative bleeding associated with endoscopic submucosal dissection for gastric neoplasms. Chin Med J 2022;135:309–316. doi: 10.1097/CM9.0000000000001840
Shanshan Xu and Ningli Chai contributed equally to this study.
References
- 1.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006; 64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Wang C. Long-term clinical efficacy and perioperative safety of endoscopic submucosal dissection versus endoscopic mucosal resection for early gastric cancer: an updated meta-analysis. Biomed Res Int 2018; 2018:3152346.doi: 10.1155/2018/3152346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akintoye E, Obaitan I, Muthusamy A, Akanbi O, Olusunmade M, Levine D. Endoscopic submucosal dissection of gastric tumors: a systematic review and meta-analysis. World J Gastrointest Endosc 2016; 8:517–532. doi: 10.4253/wjge.v8.i15.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SE, Kim DH, Jung HY, Lim H, Ahn JY, Choi KS, et al. Risk factors and correlations of immediate, early delayed, and late delayed bleeding associated with endoscopic resection for gastric neoplasms. Surg Endosc 2016; 30:625–632. doi: 10.1007/s00464-015-4250-6. [DOI] [PubMed] [Google Scholar]
- 5.Jeon SW, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK, et al. Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions. Surg Endosc 2008; 23:1974–1979. doi: 10.1007/s00464-008-9988-7. [DOI] [PubMed] [Google Scholar]
- 6.Linghu EQ. New classifications of intraoperative bleeding and muscularis propria injury in endoscopic resection. Chin Med J 2019; 132:1856–1858. doi: 10.1097/cm9.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang JS, Choi SR, Graham DY, Kwon HC, Kim MC, Jeong JS, et al. Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol 2009; 44:1370–1376. doi: 10.3109/00365520903194609. [DOI] [PubMed] [Google Scholar]
- 8.Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol 2009; 45:30–36. doi: 10.1007/s00535-009-0137-4. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 10.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58 (Supp 6):S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 11.Oda I, Gotoda T, Hamanaka H, Eguchi T, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2010; 17:54–58. doi: 10.1111/j.1443-1661.2005.00459.x. [Google Scholar]
- 12.Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc 2010; 18:S123–S127. doi: 10.1111/j.1443-1661.2006.00645.x. [Google Scholar]
- 13.Horikawa Y, Toyonaga T, Mizutamari H, Mimori N, Kato Y, Fushimi S, et al. Feasibility of knife-coagulated cut in gastric endoscopic submucosal dissection: a case-control study. Digestion 2016; 94:192–198. doi: 10.1159/000450994. [DOI] [PubMed] [Google Scholar]
- 14.Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survery by Osaka University ESD study group. Dig Endosc 2010; 23:73–77. doi: 10.1111 /j.1443-1661.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol 2012; 27:907–912. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyahara K, Iwakiri R, Shimoda R, Sakata Y, Fujise T, Shiraishi R, et al. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion 2012; 86:273–280. doi: 10.1159/000341422. [DOI] [PubMed] [Google Scholar]
- 17.Okano A, Hajiro K, Takakuwa H, Nishio A, Matsushita M. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc 2003; 57:687–690. doi: 10.1067/mge.2003.192. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol 2010; 16:2913–2917. doi: 10.3748/wjg.v16.i23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection - an analysis of risk factors. Endoscopy 2008; 40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]
- 20.Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc 2010; 71:44–49. doi: 10.1016/j.gie.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Choi YK, Ahn JY, Na HK, Jung KW, Kim DH, Lee JH, et al. Outcomes of endoscopic submucosal dissection for gastric epithelial neoplasm in chronic kidney disease patients: propensity score-matched case-control analysis. Gastric Cancer 2018; 22:164–171. doi: 10.1007/s10120-018-0848-4. [DOI] [PubMed] [Google Scholar]
- 22.Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc 2016; 84:572–586. doi: 10.1016/j.gie.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc 2012; 45:362–374. doi: 10.5946/ce.2012.45.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25 (Supp 1):71–78. doi: 10.1111/j.1443-1661.2012.01376.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujishiro M, Abe N, Endo M, Kawahara Y, Shimoda R, Nagata S, et al. Current managements and outcomes of peptic and artificial ulcer bleeding in Japan. Dig Endosc 2010; 22:S9–S14. doi: 10.1111/j.1443-1661.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 26.Oyama T, Tomori A, Hotta K, Miyata Y. Hemostasis with hook knife during endoscopic submucosal dissection. Dig Endosc 2010; 18:S128–S130. doi: 10.1111/j.1443-1661.2006.00620.x. [DOI] [PubMed] [Google Scholar]