Significance
Chimeric antigen receptors (CARs) are engineered, artificial T cell receptors that can redirect cytotoxic immune T cells to eliminate cancer. Previous reports describe the benefit of less differentiated naive T cell subtypes for the purpose of CAR therapy. Here we test CD81, a T cell costimulator that preferentially activates naive T cells, for CAR engineering. We show that CD81 costimulation of naive T cells prior to CAR transduction can lead to enhanced CAR expression in this T cell subset.
Keywords: CAR T cells, CD81, naive T cells, adoptive immunotherapy
Abstract
Adoptive cellular therapy using chimeric antigen receptors (CARs) has revolutionized our treatment of relapsed B cell malignancies and is currently being integrated into standard therapy. The impact of selecting specific T cell subsets for CAR transduction remains under investigation. Previous studies demonstrated that effector T cells derived from naive, rather than central memory T cells mediate more potent antitumor effects. Here, we investigate a method to skew CAR transduction toward naive T cells without physical cell sorting. Viral-mediated CAR transduction requires ex vivo T cell activation, traditionally achieved using antibody-mediated strategies. CD81 is a T cell costimulatory molecule that when combined with CD3 and CD28 enhances naive T cell activation. We interrogate the effect of CD81 costimulation on resultant CAR transduction. We identify that upon CD81-mediated activation, naive T cells lose their identifying surface phenotype and switch to a memory phenotype. By prelabeling naive T cells and tracking them through T cell activation and CAR transduction, we document that CD81 costimulation enhanced naive T cell activation and resultantly generated a CAR T cell product enriched with naive-derived CAR T cells.
Genetic manipulation of T cells has enabled adoptive T cell therapy to be translated to the clinic (1–10). Chimeric antigen receptor (CAR) therapy has evoked recent enthusiasm upon mediating curative outcomes in aggressive, refractory B cell malignancies (1–7, 11–15), leading to Food and Drug Administration approval (16–18). The process of ex vivo transduction and expansion of T cells to express CARs influences the phenotype, function, and ultimate fate of the final CAR T cell product (19–23). Preclinical data in animal models indicate that selecting specific T cell subsets for CAR transduction improves efficacy (21, 22, 24–26). Naive-derived T cells have been shown to exhibit greater replicative capacity, persistence, and antitumor function, compared with both effector- and memory-derived T cells (19, 20, 27). Naive CD4+ T cells, specifically, have a critical role in enhancing the cytotoxic effect of the CD8+ cooperating central memory cell subset (21). Furthermore, the translational CAR experience demonstrates that the presence of cells consistent with the naive and early memory phenotype in premanufactured T cell products correlates with successful clinical responses in both pediatric and adult B cell leukemia (28–30). Here, we explore if selective activation of naive T cells can result in skewing of transduction toward this specific T cell subset without the need for physical subset sorting.
CAR constructs rely on intrinsic costimulatory signals, such as the intracellular domains of CD28 or 41BB, for efficacy (1–9). Here we focus on exogenous costimulatory signals necessary to induce proliferation and permit viral-mediated gene transfer. Prior to CAR transduction and antigen encounter, the majority of T cells are in a state of rest. Resting T cells mandate primary and costimulatory signals for activation (31, 32). CD28 is best known for its ability to costimulate T cells (33–38) and along with CD3 activation renders T cells susceptible to viral transduction (1, 39). CD81 is a member of the tetraspanin family that physically associates with CD4 and CD8 on the surface of T cells. CD81 was shown to have independent costimulatory properties and, when used with anti-CD3 and -CD28 antibodies, preferentially activates naive T cells as compared with effector and memory T cells, despite conserved surface CD81 expression across T cell subsets (40). Tetraspanins have no known cell-surface ligands, and therefore antibodies are used to engage and stimulate them. CD81 is the only tetraspanin whose complete three-dimensional structure has been solved (41). Moreover, the crystal structure of 5A6, the anti-CD81 antibody used in our study, in complex with CD81 has also been most recently solved (42). These authors demonstrate that engagement by this antibody changes the conformation of the large extracellular loop of the CD81 molecule. This conformational change may affect the interaction of CD81 with its associated CD4 and CD8 molecules.
Here, we costimulate purified T cells with CD81 as a proof of principle to illustrate that the in vitro activation window prior to CAR transduction can be leveraged to favor transduction of a specific T cell subset.
Results
Addition of CD81 Costimulation Enhances Activation of Naive T Cells.
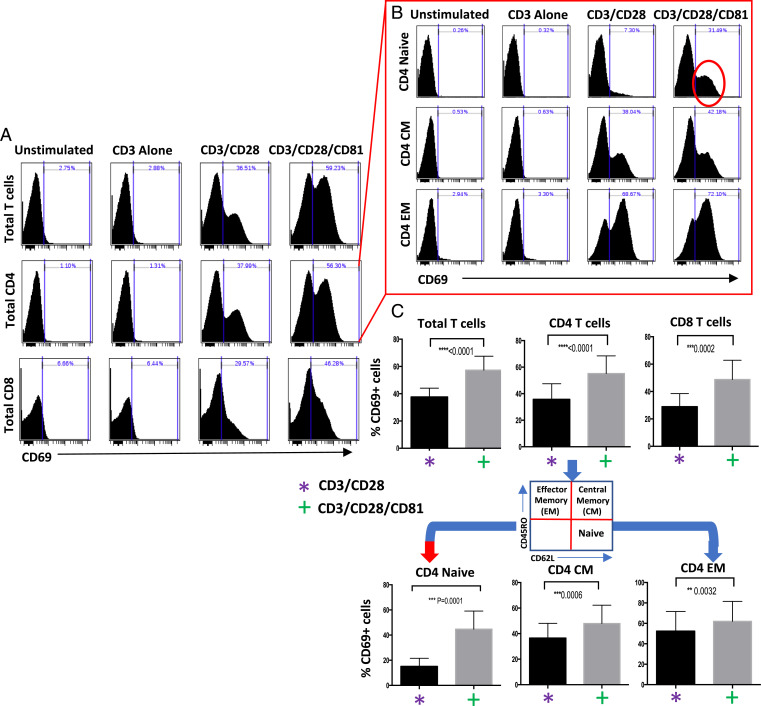
To interrogate the effect of CD81 costimulation on different T cell subsets, we isolated T cells from healthy donors and stimulated them with various activation conditions: antibodies to CD3, CD3/CD28, or CD3/CD28/CD81. We observe that adding CD81 costimulation to that of CD3/CD28 significantly enhances CD4 and CD8 T cell activation, as evident by increased CD69 expression (Fig. 1A). We dissected the differential activation effects on naive (CD62L+/CD45RO−), central memory (CD62L+/CD45RO+), and effector memory (CD62L−/CD45RO+) T cell compartments using flow cytometry. We establish that the engagement of CD81 enhances the activation of central memory and effector memory populations, but the effect is most pronounced in naive CD4 T cells (Fig. 1 B and C) despite known uniform CD81 expression across T cell subsets (40). This effect is not solely explained by increased cumulative costimulation, as supported by titration experiments demonstrating a specific CD81 effect (SI Appendix, Fig. S1).
Fig. 1.
CD81 costimulation enhances activation of T cells, with maximal effect seen in naive CD4 T cells. Isolated T cells were activated using CD3, CD3/CD28, or CD3/CD28/CD81 for 24 h. (A and B) Activation of (A) CD4 and CD8 T cells and (B) CD4 naive (CD62L+/CD45RO−), central memory (CM) (CD62L+/CD45RO+), and effector memory (EM) (CD62L−/CD45RO+) T cells was measured by levels of CD69 expression. (C) Summary data of seven experiments include the gating strategy, identify the significance of the CD81 effect in CD4 (<0.0001) and CD8 T cells (<0.0002), and highlight the specific CD81-mediated activation advantage in naive T cells (<0.0001). Error bars illustrate mean with standard deviation (SD).
CD81 Costimulation of Isolated Naive T Cells Induces a Shift toward Memory Phenotype.
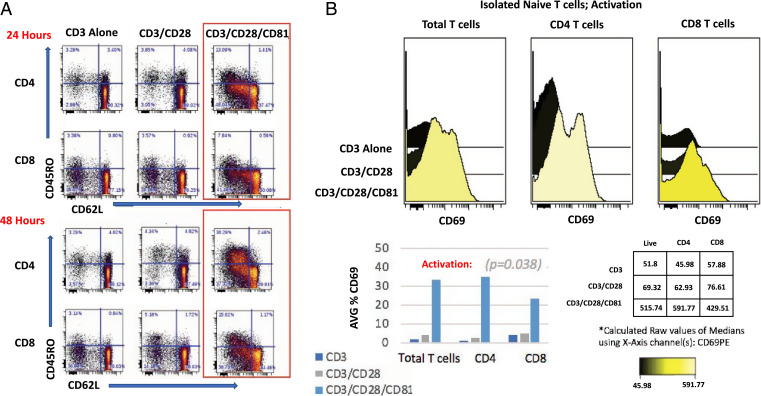
We hypothesized that enhancing activation of naive T cells using CD81 costimulation would improve CAR transduction efficiency of this T cell subset. To interrogate the specific contribution of CD81 stimulation to the fate of the input naive T cells, we first isolated naive T cells (CD62L+/CD45RO−) and took them through the process of activation and CAR transduction. Since the starting population was composed of purified naive T cells, we were able to document that the activation process induced phenotypic changes characteristic of effector memory T cells as soon as 24 h after costimulation (Fig. 2A). The CD81-costimulated naive-derived T cells yielded a higher degree of activation, as shown by their increased expression of CD69 (Fig. 2B).
Fig. 2.
CD81 costimulation enhances activation of isolated naive T cells. (A) Isolated naive T cells were activated as indicated for 24 and 48 h. Flow-cytometry plots illustrate change over time of naive (CD62L+/CD45RO−), CM (CD62L+/CD45RO+), and EM (CD62L−/CD45RO+) markers on CD4 and CD8 activated, naive-derived T cells. (B) CD69 expression is shown for CD4 and CD8 isolated naive T cells following 48 h of activation (time point of retroviral transduction). These data are representative of four experiments with average CD69 expression prior to CAR transduction across experiments illustrated. For activation, we employed the nonparametric Friedman test and exact test for repeated-measures experiments. Our null hypothesis (H0) is that there is no difference in median activation between groups. Our data reject the null hypothesis, indicating a statistically significant difference in activation (P = 0.038) for cells treated with additional CD81 costimulation.
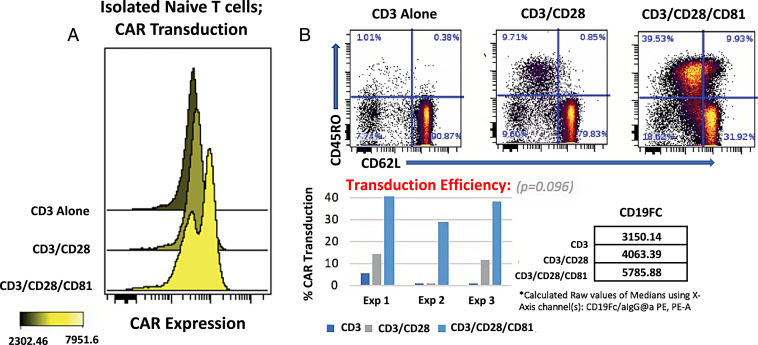
CD81 Costimulation Enhances CAR Transduction in Naive T Cells.
We demonstrate that inputting T cells with enhanced activation through CAR transduction yields a T cell product with increased CAR expression. In specific, activating naive T cells with additional CD81 costimulation achieved greater transduction efficiency as compared with CD3 or CD3/CD28 activation conditions (Fig. 3A and SI Appendix, Fig. S2A). Furthermore, the naive-to-memory phenotype shift seen with CD81-mediated T cell activation was further enhanced upon CAR gene transduction (Fig. 3B and SI Appendix, Fig. S2B).
Fig. 3.
CD81 costimulation improves CAR transduction efficiency. (A) Transduced CAR T cells were detected by staining with a fluorescent CD19–Fc fusion protein. (B) CAR T cells derived from CD81-costimulated naive T cells were costained with the indicated memory subset markers. These data are representative of three experiments, with transduction efficiency across experiments illustrated. For CAR transduction, we employed the nonparametric Friedman test and exact test for repeated-measures experiments. Our null hypothesis (H0) is that there is no difference in median transduction between groups. Our data reject the null hypothesis, indicating a statistically significant difference in transduction (P = 0.096) for cells treated with additional CD81 costimulation.
Tracking CAR Transduction of Naive T Cells within the Total T Cell Pool.
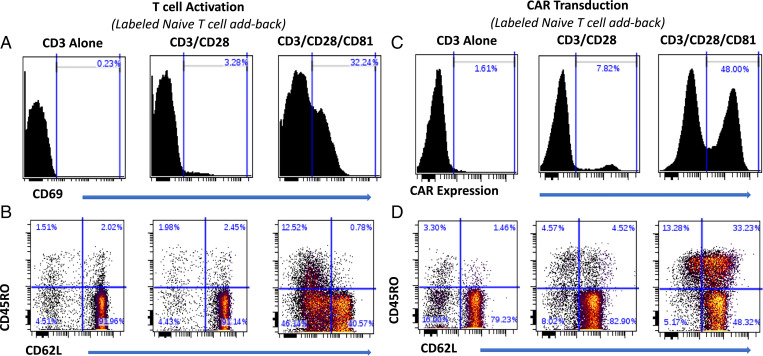
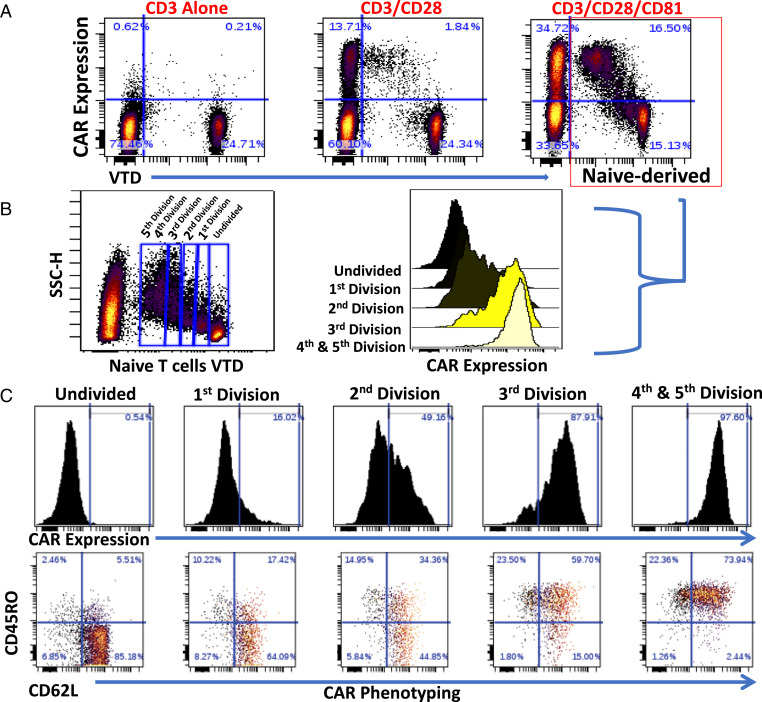
Activating naive T cells in isolation may not represent the phenomenon occurring when all T cell subsets are interacting together. We therefore investigated the potential of CD81 costimulation to preferentially activate and transduce naive T cells within a pool of all T cell subsets. Because, as shown above, naive T cells lose their surface signature throughout activation, we first isolated naive T cells, labeled them with violet tracking dye (VTD), and returned them to the parental T cell pool replete with effector and memory T cells. We then activated this replete T cell pool with the costimulation conditions under study (Fig. 4 A and B) and transduced the resulting activated T cells with the CAR (Fig. 4 C and D). CD81 costimulation remarkably enhanced CAR transduction of both the labeled naive-derived T cells and the unlabeled T cells (Fig. 5A).
Fig. 4.
CD81 costimulation enhances activation, induces surface phenotype changes, and improves CAR transduction efficiency of labeled naive T cells added back to their derived T cell pool. Isolated naive T cells were labeled using VTD and added back to the replete T cell pool, and then activated with the CD3, CD3/CD28, or CD3/CD28/CD81 antibodies, followed by lentiviral CD1928z CAR transduction. (A and B) CD69 expression (A) and parallel CD62L and CD45RO surface phenotyping (B) of the labeled naive T cell addback, shown at 48 h (time point of retroviral transduction). (C) CAR transduction of labeled naive T cells, as determined by CD19–Fc expression. (D) Parallel CAR T cell CD62L and CD45RO phenotyping, shown 7 d following initial T cell activation.
Fig. 5.
Naive-derived T cells that have undergone increased proliferation have greater CAR expression and increased surface phenotype remodeling. (A) CAR expression of VTD-labeled naive added back as in Fig. 4C. (A, Right) Naive-derived T cells costimulated with CD81 are outlined in red. (B and C) Analysis of CD81-costimulated, activated, and CAR transduced naive addback. (B, Left) VTD labeling of naive-derived cells distinguishes cells undergoing zero to five divisions. (B, Right) CAR expression across cells undergoing zero to five divisions. (C, Upper) CAR expression. (C, Lower) CD62L and CD45RO expression of the indicated cell divisions.
Naive-Derived T Cells That Undergo Cell Division Have Increased CAR Expression and Increased Phenotypic Shift.
To assess the impact of cellular division on CAR transduction efficiency, we analyzed VTD-labeled naive cells that have undergone successive cellular divisions. We costimulated a mixed T cell population that included VTD-labeled naive T cells with CD3, CD28, and CD81, followed by CAR transduction. We gated cell populations that have undergone increasing cellular divisions (Fig. 5B), and performed a linked analysis of CAR transduction and surface marker expression (Fig. 5C). We demonstrate that cells that have undergone increased divisions have the greatest CAR expression. Furthermore, with increased cell divisions and CAR transduction, we observed increased surface marker remodeling with a specific increase in memory markers (Fig. 5C), as was seen with isolated naive T cells (Fig. 3B).
Discussion
Adoptive T cell transfer using CARs has become an integral treatment strategy for advanced, refractory hematologic malignancies. Viral-mediated genetic engineering techniques have proven essential to effective CAR scalability. The CAR clinical experience to date revealed that varying CAR intracellular domains render differential T cell functions. CD28 induces an earlier peak in in vivo CAR activity in contrast to 41BB, which facilitates long-term persistence and immune memory (43). Independent of CAR endogenous domains, T cells require in vitro stimulation, traditionally using CD3 and CD28 costimulation, to permit efficacious viral-mediated gene transduction (1, 39). Little attention has been placed on the impact of varying costimulation signals at the stage of CAR transduction. We hypothesized that this period of in vitro T cell stimulation influences the phenotype of the final transduced T cell product and can be manipulated to skew transduction toward desired T cell subsets.
We introduce CD81, used as a T cell costimulator, to the process of viral-mediated CAR transduction. The addition of CD81 to CD3 and CD28 T cell stimulation introduces a more robust activation of naive T cells, compared with CD3 and CD28 stimulation. We demonstrate that stimulation signals that induce enhanced activation of a specific T cell subset enhance CAR transduction of that specific T cell population. We found that combined stimulation of CD3/CD28/CD81 preferentially activates naive T cells, compared with CD3/CD28. These triple-activated naive T cells switched their phenotype to a memory signature with ongoing evolution toward a more distinctive memory phenotype with CAR transduction. Importantly, the T cell product derived from these CD81-costimulated naive T cells achieved greater transduction efficiency, with greater levels of CD19 CAR expression.
We specifically chose to investigate CD81 due to its unique capacity to preferentially activate naive T cells (40), a subset previously shown to be desirable for adoptive cell therapy (19, 20). Alternative antibodies targeting ligands that have a role in manipulating T cell signaling pathways (i.e., CTLA4, PD-1, 41BB, ICOS, OX40) might have other unique effects on specific T cell subsets that could be further harnessed toward the goal of selective CAR T cell transduction. Additionally, utilizing antibodies that impact T cell function during this ex vivo window, prior to the infusion of CAR T cells, is an opportunity to manipulate T cell function without introducing the added risk of systemic antibody-mediated toxicity.
Overall, through tailored activation and fate mapping spanning CAR transduction, we demonstrate that the process of in vitro activation and CAR transduction impacts the resultant CAR T cell phenotype. We additionally demonstrate the proof of principle that activation can be manipulated to drive CAR transduction toward a desired T cell subset.
Materials and Methods
T Cell Purification.
T cells were isolated from healthy donors in accordance with ethical guidelines and with informed written consent. T cells were purified using magnetic bead negative selection. Unsorted pan-T cells were isolated using the Dynabeads Untouched Human T Cell Kit (Thermo Fisher Scientific; 11344D) and isolated naive T cells were purified using the Naive Pan T Cell Isolation Kit (Miltenyi Biotec; 130-097-095). All T cell purifications were performed according to the manufacturers’ guidelines.
T Cell Activation and Phenotyping.
Purified T cells (1 × 106) were differentially stimulated using anti-CD3 antibody (clone OKT3; eBioscience; 16-0037085; 0.1 μg/mL), anti-CD28 (clone 28.2; eBioscience; 16-0289-85; 2.5 μg/mL), and/or CD81 (clone 5A6; generated in the laboratory of S.L.; 2.5 μg/mL). Antibodies were then cross-linked using goat F(ab)2 anti-mouse immunoglobulin G (IgG) (Jackson ImmunoResearch; 115-006-062; 4 μg/mL). Unstimulated T cells were used as a control. T cells were cultured in RPMI1640 with l-glutamine (Corning Cellgro; 10-040-CV) supplemented with 10% heat-inactivated donor calf serum (HyClone; GE Healthcare Life Sciences; SH30109.03), l-glutamine (Mediatech; 25-005-Cl), and penicillin:streptomycin (Gemini Bio-Products; 400-109) and incubated for 24 and 48 h. Following incubation, cells were washed with phosphate-buffered saline containing 1% bovine serum albumin and blocked by 50 μg of whole-mouse IgG (Jackson ImmunoResearch; 015-000-003) for 20 min on ice (to prevent unbound goat anti-mouse cross-linkers from nonspecifically binding to mouse anti-human phenotyping antibodies). Following blocking, cells were washed and then stained for surface expression of CD4 (Pacific Blue; BD Pharmingen; 558116), CD8 (fluorescein isothiocyanate; BD; 347313), CD62L (APC; BD Pharmingen; 559772), CD45RO (PE-Cy7; BD; 337168), and CD69 (PE; BD; 341652).
Flow Cytometry.
Flow cytometry was performed using an LSR II flow cytometer (BD Biosciences). Cytobank software was used to analyze data generated from flow cytometry.
Retroviral CAR Transduction.
The 1928z SFG CAR construct (44) and its stable producer cell line, 293GP-GLV9, were used in this study (45), generously shared by Renier Brentjens, Memorial Sloan Kettering Cancer Center, New York, NY. The 293GP-GLV9 retroviral producer line was cultured in Dulbecco’s modified Eagle’s medium (GIBCO) supplemented with 10% heat-inactivated calf serum. Isolated human T cells were maintained in RPMI1640 with l-glutamine (Corning Cellgro; 10-040-CV) supplemented with 10% heat-inactivated calf serum (HyClone; GE Healthcare Life Sciences; SH30910.02), l-glutamine (Mediatech; 25-005-Cl), and penicillin:streptomycin (Gemini Bio-Products; 400-109). T cells were activated using CD3 (0.1 μg/mL), CD28 (2.5 μg/mL), and/or CD81 (2.5 μg/mL) -specific antibodies, followed by cross-linking with goat F(ab)2 anti-mouse IgG (Jackson ImmunoResearch; 115-006-062; 4 μg/mL), as described (16). Following 48 h of stimulation, activated T cells were transferred to retronectin-coated (Takara Bio) non–tissue-culture plates. Filtered supernatant from the 293GP-GLV9 1928z producer line was added to the T cells and retronectin-coated plates were centrifuged at 3,200 rpm for 60 min on 3 consecutive days. Cells were cultured with recombinant human IL-2 (PeproTech; 200-02; 100 IU/mL). Gene transduction was assessed using flow cytometry 7 d following initial T cell activation.
CAR T Cell Detection.
CAR T cell-surface expression was measured by binding of a recombinant fusion protein containing the extracellular region of human CD19 protein linked to the mouse IgG2a Fc domain, generated in-house by Chiung Chi Kuo, followed by goat anti-mouse IgG2a (Southern Biotech) and flow cytometry analysis.
Phenotyping CAR Transduced T Cells.
Transduced T cells were blocked with mouse IgG1 (BD Biosciences; 349040) to bind to excess cross-linking antibody. CAR T cells were detected as described followed by a second blocking step with whole-mouse IgG (Jackson ImmunoResearch; 015-000-003), used to prevent nonspecific binding of mouse anti-human phenotyping antibodies to the secondary goat anti-mouse IgG2a used for CAR detection. CD4, CD8, CD62L, and CD45RO antibodies were used to identify naive T cells (CD62L+/CD45RO−), central memory cells (CD62L+/CD45RO+), and effector memory cells (CD62L−/CD45RO+).
Tracking Naive T Cells through Retroviral Transduction.
Naive T cells were isolated from healthy donors and purified using the Naive Pan T Cell Isolation Kit (Miltenyi Biotec; 130-097-095). Naive T cells were labeled with VTD (CellTrace Violet Cell Proliferation Kit; Invitrogen, Thermo Fisher Scientific; C34557), and then returned to the replete pool of donor-derived unsorted T cells. T cells were activated and transduced to express 1928z CAR as described, followed by cell phenotyping and CAR transduction analysis using flow cytometry. VTD labeling permitted assessment of proliferation and surface phenotype changes through retroviral transduction of cells of naive origin.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute, US Public Health Service R35CA19735305 (to R.L.), and by St. Baldrick’s Foundation (Scholar Award to L.S.).
Footnotes
Reviewers: T.F., University of Colorado Anschutz Medical Campus; M.T., University of Birmingham; and M.W., Monash University.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910844119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Wang X., Rivière I., Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther. Oncolytics 3, 16015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee D. W., et al. , T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385, 517–528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentjens R. J., et al. , CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude S. L., et al. , Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude S. L., Shpall E. J., Grupp S. A., Chimeric antigen receptor T-cell therapy for ALL. Hematology (Am. Soc. Hematol. Educ. Program) 2014, 559–564 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Maude S. L., Teachey D. T., Porter D. L., Grupp S. A., CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125, 4017–4023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster S. J., et al. , Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Liu J., Zhong J. F., Zhang X., Engineering CAR-T cells. Biomark. Res. 5, 22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casati A., et al. , Clinical-scale selection and viral transduction of human naïve and central memory CD8+ T cells for adoptive cell therapy of cancer patients. Cancer Immunol. Immunother. 62, 1563–1573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perica K., Curran K. J., Brentjens R. J., Giralt S. A., Building a CAR garage: Preparing for the delivery of commercial CAR T cell products at Memorial Sloan Kettering Cancer Center. Biol. Blood Marrow Transplant. 24, 1135–1141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter D. L., Kalos M., Zheng Z., Levine B., June C., Chimeric antigen receptor therapy for B-cell malignancies. J. Cancer 2, 331–332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter D. L., Levine B. L., Kalos M., Bagg A., June C. H., Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude S. L., et al. , Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer J. N., Rosenberg S. A., Chimeric antigen receptor-modified T cells in CLL. N. Engl. J. Med. 365, 1937–1938, author reply 1938 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalos M., et al. , T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadelain M., CD19 CAR T cells. Cell 171, 1471 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Schuster S. J., et al. ; JULIET Investigators, Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Locke F. L., et al. , Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 20, 31–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinrichs C. S., et al. , Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. U.S.A. 106, 17469–17474 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinrichs C. S., et al. , Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 117, 808–814 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommermeyer D., et al. , Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30, 492–500 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restifo N. P., Dudley M. E., Rosenberg S. A., Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerkar S. P., et al. , Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J. Immunother. 34, 343–352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klebanoff C. A., et al. , Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 17, 5343–5352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff C. A., Gattinoni L., Restifo N. P., Sorting through subsets: Which T-cell populations mediate highly effective adoptive immunotherapy? J. Immunother. 35, 651–660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang A., et al. , The stoichiometric production of IL-2 and IFN-γ mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci. Transl. Med. 4, 149ra120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L., et al. , Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das R. K., Vernau L., Grupp S. A., Barrett D. M., Naïve T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 9, 492–499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh N., Perazzelli J., Grupp S. A., Barrett D. M., Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci. Transl. Med. 8, 320ra3 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Fraietta J. A., et al. , Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babcock S. K., Gill R. G., Bellgrau D., Lafferty K. J., Studies on the two-signal model for T cell activation in vivo. Transplant. Proc. 19, 303–306 (1987). [PubMed] [Google Scholar]
- 32.Shaw A. S., Dustin M. L., Making the T cell receptor go the distance: A topological view of T cell activation. Immunity 6, 361–369 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Bromley S. K., et al. , The immunological synapse and CD28-CD80 interactions. Nat. Immunol. 2, 1159–1166 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Thompson C. B., et al. , CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. U.S.A. 86, 1333–1337 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuosto L., Acuto O., CD28 affects the earliest signaling events generated by TCR engagement. Eur. J. Immunol. 28, 2131–2142 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Schwartz R. H., A cell culture model for T lymphocyte clonal anergy. Science 248, 1349–1356 (1990). [DOI] [PubMed] [Google Scholar]
- 37.Garlie N. K., et al. , T cells coactivated with immobilized anti-CD3 and anti-CD28 as potential immunotherapy for cancer. J. Immunother. 22, 336–345 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Levine B. L., et al. , Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 159, 5921–5930 (1997). [PubMed] [Google Scholar]
- 39.Bilal M. Y., Vacaflores A., Houtman J. C., Optimization of methods for the genetic modification of human T cells. Immunol. Cell Biol. 93, 896–908 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sagi Y., Landrigan A., Levy R., Levy S., Complementary costimulation of human T-cell subpopulations by cluster of differentiation 28 (CD28) and CD81. Proc. Natl. Acad. Sci. U.S.A. 109, 1613–1618 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman B., et al. , Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell 167, 1041–1051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Susa K. J., Seegar T. C., Blacklow S. C., Kruse A. C., A dynamic interaction between CD19 and the tetraspanin CD81 controls B cell co-receptor trafficking. eLife 9, e52337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long A. H., et al. , 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brentjens R. J., et al. , Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 13, 5426–5435 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Ghani K., et al. , Efficient human hematopoietic cell transduction using RD114- and GALV-pseudotyped retroviral vectors produced in suspension and serum-free media. Hum. Gene Ther. 20, 966–974 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.