ABSTRACT
The geographic range and occurrence of tick species is dynamic. This has important public health implications due to important tick species that can transmit pathogens. This study presents a retrospective review of tick genera recovered from humans and submitted for identification in Alberta, Canada, over a 19-year period. The total number of ticks and proportion of genera were analyzed over time. Molecular testing for a number of pathogens associated with Ixodes scapularis and I. pacificus was conducted. A total of 2,358 ticks were submitted between 2000 and 2019, with 98.6% being acquired in Alberta. The number of ticks submitted increased significantly over time (p < 0.0001). Dermacentor ticks were the most abundant genus, followed by Ixodes and Amblyomma. There was a significant decrease in the proportion of Dermacentor ticks between 2013 and 2019 (p = 0.02), with a corresponding increase in the proportion of Ixodes ticks over the same time (p = 0.04). No statistically significant change in seasonality was identified. Borrelia burgdorferi was detected in 8/76 (10.5%; 95% CI 5.4–19.4%) of all I. scapularis and I. pacificus ticks submitted. This translated to a B. burgdorferi positivity of 0.35% (95% CI 0.15–0.68%) among all ticks received. Dermacentor species (especially D. andersoni) remains the most common tick feeding on humans in Alberta. Small numbers of vector species (including I. scapularis/pacificus) are encountered annually over widely separated geographic areas in the province. The risk of exposure to tick-borne pathogens (e.g. Lyme disease) in Alberta remains low.
KEYWORDS: Tick, ixodes, dermacentor, lyme, Borrelia burgdorferi, alberta
Introduction
Ticks constitute important vectors of several zoonotic pathogens. The distribution of ticks and pathogens they may transmit vary based on species, as well as geographic location [1]. Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti, and Powassan virus are agents of tick-borne zoonoses transmitted by species of Ixodes in North America [2,3].
Expansion of the geographic distribution of tick species is multifactorial, including climate change, abundance in source locations, and capacity to be spread by the host (such as northwards expansion via bird migration) [2,4,5]. One study of ticks from migratory birds found that up to 22 tick species had been introduced into Canada, some originating as far south as Brazil [6]. Once on Canadian soil, these ticks have the potential to spread through the ecosystem; and, in some instances, introduce novel zoonotic pathogens into local tick populations [7,8].
Tick surveillance in the province of Alberta, Canada, up until 2021 has been conducted via two methods. The primary methodology is passive surveillance, where ticks are submitted by veterinarians and the public themselves. The second methodology is active tick surveillance, in which ticks are collected from the environment, typically by drag sampling, and the locations to actively sample are often triggered by signals from passive tick surveillance. Veterinarians and the public are encouraged to submit ticks found in the environment or on humans or companion animals for identification. All submitted species of Ixodes ticks (except I. kingi and I. ochotonae) are tested for selected zoonotic pathogens, including B. burgdorferi. Passive surveillance of ticks from veterinary and environmental sources from 2013 to 2019 revealed a mean annual submission of 1,465 ticks per year (no travel outside the province). Of these, 10–11% each year were found to be I. scapularis or I. pacificus, with an average of 14.4% found to be positive for B. burgdorferi [9].
Active tick surveillance was last conducted in Alberta annually from 2014 to 2017 at various sites throughout the province. During this time, no ticks were identified. No active surveillance was conducted in 2018 and 2019, given insignificant findings on passive surveillance during those years [9].
Outside of these surveillance programs, clinicians have also been able to submit ticks extracted from human patients to diagnostic microbiology laboratories in Alberta since 2000. All ticks were identified to the genus (and, if possible, species); providing important clinically relevant information necessary for counseling patients with anxiety post-tick-bite. This subset of data was not captured locally by established passive surveillance programs, thus providing valuable information to complete data for all human and animal hosts for the province.
To better understand the epidemiology of ticks acquired from human hosts in Alberta, we undertook a retrospective review of tick identifications conducted by diagnostic microbiology laboratories in Alberta over a 19-year period. Of particular interest to clinicians and Public Health was to estimate the likelihood that a tick removed from a human host in Alberta carries B. burgdorferi, as this is a very common query from patients and the public that arises while awaiting identification of the tick that is often difficult to address.
Methods
Population and study design
The province of Alberta in Western Canada has a population of approximately 4.5 million; [10] and for the purposes of healthcare delivery, is divided into five zones: North, Edmonton, Central, Calgary, and South Zones [11]. Since 2000, clinicians have been able to submit ticks brought in by patients to diagnostic microbiology laboratories for identification [12]. Each submission is accompanied by a requisition requesting detailed history, including relevant travel. Ticks found in the environment or from non-human hosts were not accepted by diagnostic microbiology laboratories and instead redirected to other surveillance avenues [9].
Tick identification and pathogen detection
Each arthropod was identified using morphological identification keys [13]. All ticks identified as Ixodes spp. were then forwarded to the Provincial Public Health Laboratory for confirmation and, if necessary, for second confirmation by the Department of Biological Sciences at the University of Alberta.
All Ixodes spp. were subsequently sent to the National Microbiology Laboratory (Public Health Agency of Canada, Winnipeg, Manitoba) for pathogen testing. Specimens of I. scapularis and I. pacificus were tested for infection with Borrelia burgdorferi, Borrelia miyamotoi, Anaplasma phagocytophilum, and Babesia microti by real-time PCR as previously described [14]. Briefly, QIAGEN DNeasy 96 tissue kits (QIAGEN Inc., Mississauga, Ontario) were used for DNA extraction. A duplex screening assay was chosen to screen the samples for Borrelia spp. using the 23S rRNA real-time polymerase chain reaction (PCR) assay, and Anaplasma phagocytophilum using the msp2 real-time PCR assay [15]. Analysis for Babesia microti was conducted using the methods described by Nakajima et al. for the detection of the CCT eta gene [16]. All Borrelia spp.-positive samples were subsequently tested for B. burgdorferi using a confirmatory ospA real-time PCR assay, and Borrelia miyamotoi using an IGS real-time PCR assay. Borrelia miyamotoi-positive samples were further verified using the glpQ real-time PCR assay [14]. To account for possible contamination during extraction and the PCRs procedures, water or blank controls were included in all extractions and PCR, respectively.
Data extraction and statistical analysis
A retrospective review of all ticks submitted for identification to diagnostic microbiology laboratories in Alberta from 1 January 2000 to 31 December 2019 was conducted. Information was extracted from multiple laboratory information systems used across the province and compiled. All retrievable scans or copies of original requisitions (dating back to 2000) were obtained from electronic archiving for review to ensure no travel history was missed during data entry. Records indicating travel from outside the province (national or international) were analysed separately; any specimen without a travel history was assumed to have been acquired from within Alberta.
Data was extracted in multiple formats and tabulated in Microsoft Excel. Proportional comparisons over time were conducted using the sum of squares linear regression modeling with statistical comparison of non-parametric variables using Chi-square analysis regression modeling (StatPlus, AnalystSoft Inc, Alexandria, USA). Significance was set at p < 0.05. Confidence intervals (CIs; 95%) were calculated using Wilson’s method.
Results
Numbers of ticks submitted
Between 1 January 2000 and 31 December 2019, a total of 2,358 ticks were submitted for identification, of which 32 had travel history from outside of the province (Supplementary Table S1). Based on this review, 2,326 ticks were deemed to be acquired within Alberta. The most common identified genera were Dermacentor (91.7%; 95% CI 90.5–92.3%), Ixodes (5.8%; 95% CI 4.9–6.8%), and Amblyomma (1.9%; 95% CI 1.5–2.6%). In total, 17.6% (95% CI 16.4–19.5%) of the ticks could only be identified to the genus level (Table 1).
Table 1.
Identification of ticks deemeda to be acquired within Alberta submitted from 2000 to 2018.
| Genus (%) |
Number (% of total ticks) |
| Dermacentor | 2132 (91.7) |
| • Dermacentor spp – 338 (15.9) | |
| • D. andersonii – 1376 (64.5) | |
| • D. variabilis – 410 (19.2) | |
| • D. albipictus – 8 (0.4) | |
| Ixodes | 134 (5.8) |
| • Ixodes spp – 43 (32.1) | |
| • I. scapularis – 67 (50) | |
| • I. pacificus – 11 (8.3) | |
| • I. cookei – 4 (3.0) | |
| • I. angustus – 1 (0.7) | |
| • I. ricinus – 4 (3.0) | |
| • I. spinipalpis – 1 (0.7) | |
| • I. marxi – 1 (0.7) | |
| • I. muris – 2 (1.5) | |
| Amblyomma | 45 (1.9) |
| • Amblyomma spp – 22 (49.0) | |
| • A. americanum – 18 (40) | |
| • A. maculatum – 1 (2.2) | |
| • A. cajennense – 2 (4.4) | |
| • A. coelebs – 2 (4.4) | |
| Boophilus spp. | 3 (0.1) |
| Haemaphysalis leporispalustris | 1 (0.05) |
| Ornithodros spp. | 1 (0.05) |
| Rhipicephalus | 10 (0.4) |
| • Rhipicephalus spp. – 4 (40) | |
| • R. sanguineus – 5 (50) | |
| • R. pulchellus – 1 (10) | |
| Total | 2326 |
Based on the detailed review of requisitions submitted with accompanying tick specimen. Abbreviations: spp. – notes species.
Ticks submitted from within Alberta
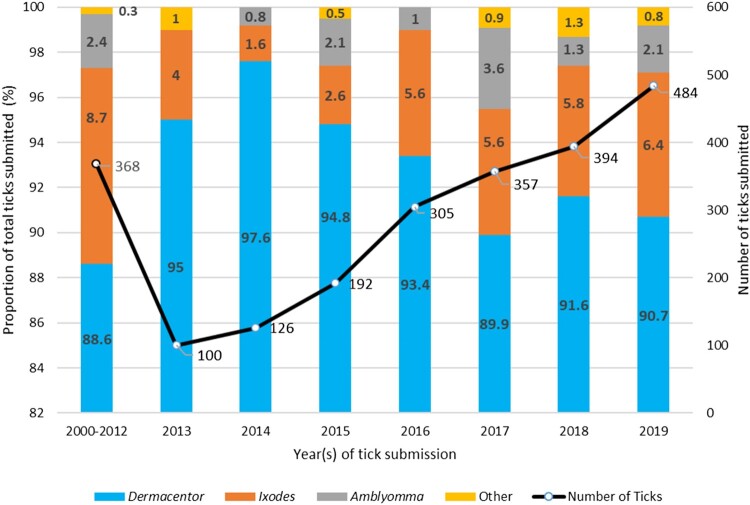
In total, 2,326 (98.6%; 95% CI 98.1–99.0%) ticks were presumed to be acquired within Alberta. Due to the transition from paper to electronic laboratory records in 2013, ticks submitted from 2000 to 2012 have been grouped together as a cohort as a result of data extraction differences and availability. Between 2013 and 2019, the number of ticks has increased by four-fold (p < 0.0001) every year (Figure 1). The most common ticks identified annually were Dermacentor spp. followed by Ixodes spp. There was a significant increase in the proportion of Ixodes spp. submitted over the time period (p = 0.04) with a corresponding decrease in the proportion of Dermacentor spp. (p = 0.02), but no statistically significant change was noted for Amblyomma spp. (p = 0.18) and other genera (p = 0.42). Complete data regarding duplicate tick submissions or presence of immature forms (nymphs or larvae) was not consistently available.
Figure 1.
Number of ticks submitted over time and proportional representation of genera in teach time category (n = 2,326 ticks). Data values in bold font represent proportions in the specific year(s) indicated. Data values that are not bolded represent numbers of ticks submitted in the year(s) indicated.
Geographic distribution of tick genera across the province
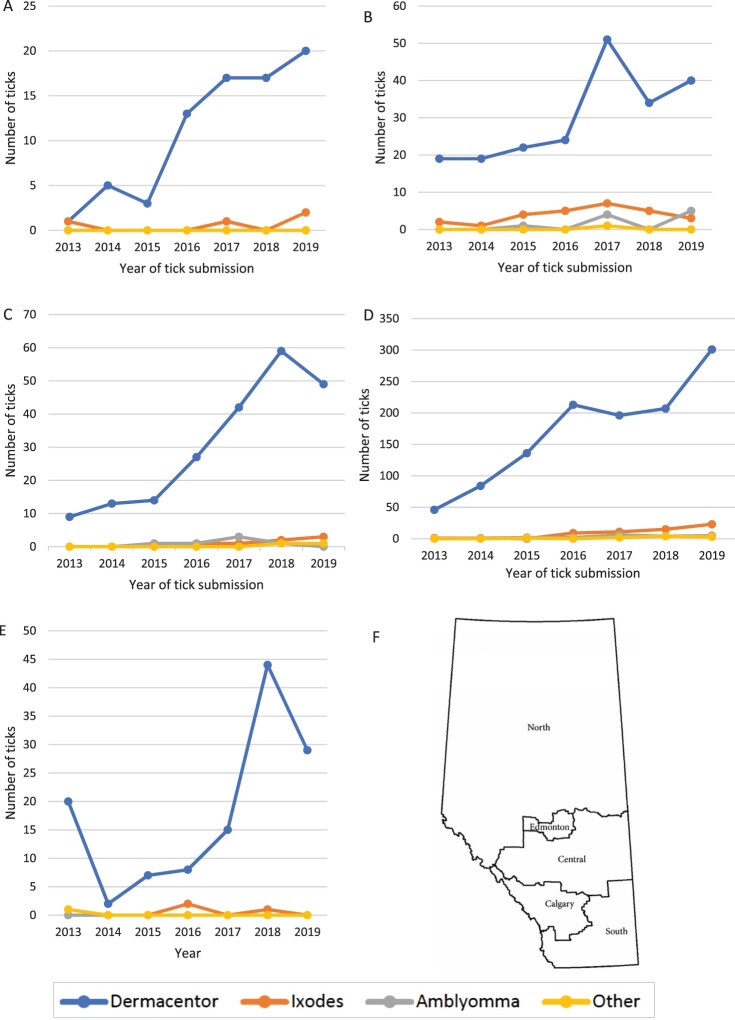
Data on Alberta Health zone of submissions was available for 1,958 (84.2%) of Alberta-acquired ticks from 2013 to 2019. A geographic representation of tick genera based on health zone unit designation is shown in Figure 2. Dermacentor spp. (n = 1806, 92.2%; 95% CI 91.0–93.3%) ticks were most common in all health zones annually. Amblyomma spp. (n = 36, 1.8%; 95% CI 1.3–2.5%) were only submitted from Edmonton, Central, and Calgary zones. Overall, 65.5% (95% CI 63.2–67.6%), 58.3% (95% CI 48.6–67.3%), and 55.5% (95% CI 39.6–70.5%) of ticks identified as Dermacentor spp., Ixodes spp., and Amblyomma spp. between 2013 and 2019 were from the Calgary Zone, representing approximately 31.1% of the population of Alberta (Supplementary Table S2) [10].
Figure 2.
Numbers of each tick genera submitted from each Alberta health region based from 2013 to 2019: (a) North Zone; (b) Edmonton Zone; (c) Central Zone; (d) Calgary Zone; (e) South Zone; (f) Map of Alberta showing health zones. a Figure adapted from Can Resp J 2016; 1382434:1–9.
Seasonality of tick submission
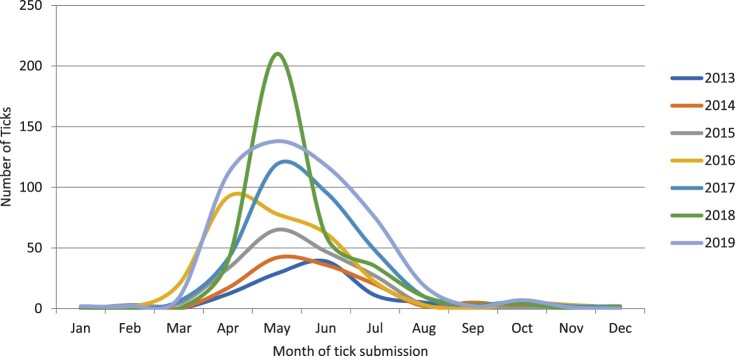
The month of tick submission was available for 1,876 (80.7%) of Alberta-acquired ticks. Predictably, most tick submissions centered around the spring (March, April, and May) and summer (June, July, and August) seasons (Figure 3). Between 2016 and 2019, tick submissions increased by 7–10 ticks per fall season (September, October, November) per year. This, however, was not found to be statistically significant (p = 0.94). The overall increase in tick numbers noted in winter (p = 0.46), spring (p = 0.19), or summer (p = 0.20) was also not significant.
Figure 3.
Seasonality of Alberta-acquired ticks submitted for identification from 2013 to 2018 (n = 1,876). Abbreviations: Jan – January; Feb – February; Mar – March; Apr – April; Jun – June; Jul – July; Aug – August; Sep – September; Oct – October; Nov – November; Dec – December.
Ixodes spp. ticks
Of the 2,326 ticks submitted from 2000 to 2019, 134 (5.8%; 95% CI 4.9–6.8%) were identified as Ixodes spp. Sixty-seven (50%; 95% CI 41.7–58.4%) were I. scapularis, eleven (8.2%; 95% CI 4.7–14.1%) I. pacificus, with the remainder identified as Ixodes spp. Molecular testing for B. burgdorferi/Anaplasma phagocytophilum DNA was conducted on 66/67 of the I. scapularis and 10/11 of the I. pacificus submissions. Of the ticks tested, 8/76 (10.5%; 95% CI 5.4–19.4%) (8/66 [12.1%; 95% CI 6.3–22.1%] I. scapularis and 0/10 I. pacificus ticks) were infected with B. burgdorferi. These ticks were identified between 2016 and 2019 (two in 2016; three in 2017; one in 2018; and two in 2019). Over this 4-year period, these positive I. scapularis ticks comprised 6.3–17.6% of the Ixodes spp. ticks submitted annually (Figure 2). Six of the B. burgdorferi positive ticks were from the Calgary zone and two from the Edmonton zone; the latter was also positive for Anaplasma phagocytophilum. Anaplasma phagocytophilum was also detected in three B. burgdorferi-negative I. scapularis ticks from the Calgary Zone (2019). None of the ticks tested were found to harbor Babesia microti or Borrelia miyamotoi.
Assuming the ticks identified in this sample represent an accurate cross section of species in Alberta, the probability of a tick being removed from a human host (with no travel history) carrying B. burgdorferi is estimated to be 0.35% (8/2,326; 95% CI 0.15–0.68%). This value refers to a tick feeding upon a human (at the point of submission before genus and species are known).
Discussion
This study summarizes overall numbers and the species distribution of ticks found on humans submitted to diagnostic laboratories over a 19-year surveillance period in Alberta, Canada. Analysis of 2,326 tick genera showed, in order of frequency, Dermacentor spp., Ixodes spp., and Amblyomma spp. This is in stark contrast to findings in Québec and Ontario, where Ixodes is the most abundant genus [17,18]. During the surveillance period, the annual proportions of Ixodes spp. and Amblyomma spp. ticks increased by 2.4% and 2.1%, respectively, and Dermacentor spp. decreased by 4.3%.
In comparison to passive surveillance from 2013 to 2019 outlined in the introduction, we received on average less ticks annually (264 ticks per year), of which a lower proportion (3.3%) were found to be able to transmit the agent of Lyme disease. B. burgdorferi was detected less frequently on average (4.8%) in the I. scapularis or I. pacificus ticks tested.
The overall proportion of B. burgdorferi positive I. scapularis ticks found in our study (12.1%) is lower than most other areas in Canada. A study evaluating Ixodes spp. ticks from Ontario and Quebec reported 33–41% positivity for B. burgdorferi [19]. In a cross-province Canadian study evaluating ticks from both human and animal sources, the range of B. burgdorferi positivity was 9.7–19% from the provinces of Quebec, Manitoba, New Brunswick, Newfoundland, Nova Scotia, Ontario, and Prince Edward Island [20]. Passive and active surveillance of tick populations in Saskatchewan found a rate of 12% [21]. Thus the B. burgdorferi positivity found in I. scapularis ticks from Alberta is consistent with that seen in other non-endemic areas of Canada, although studies used for this comparison included ticks combined from human, environmental, and veterinary sources. Similar epidemiologic studies evaluating human sources of ticks in Canada have been conducted in the provinces of Manitoba and Ontario. From 2008 to 2012, a surveillance study from Ontario found that 8.4-19.1% of I. scapularis submitted were infected with B. burgdorferi [17]. Another study evaluating I. scapularis from human sources from 2009 to 2016 found that 22.3–25% were infected with B. burgdorferi from Manitoba with a lower proportion (12.7–17.6%) positive from Ontario [22]. Both of these provinces are known to be endemic for reproducing I. scapularis populations.
The small but significant rise in the number of Ixodes spp. in our study (the majority of which are I. scapularis) is concerning in light of data supporting a doubling prevalence of tick-borne infections in the last decade [23]. This is likely explained by progressive northward expansion of I. scapularis from areas of established populations in the United States, estimated at 46 km/year in a study from Ontario, Canada [24]. Once populations of tick vectors are established, endemicity follows with the rapid emergence of B. burgdorferi in these tick populations over a period of several years [25]. This northward expansion is likely multifactorial, including climate change influencing favorable habitat conditions coupled with the increasing availability of hosts, and bird/host migration patterns [26].
Increased numbers of Ixodes spp. and Dermacentor spp. submissions in our study are not entirely explained by northward expansion, considering the projected timeline [27]. We hypothesize that the occurrence of an extensive wildfire in the areas of Fort McMurray and Wood Buffalo municipalities in northern Alberta between 1 May and 5 July 2016, was an important contributing factor [28]. This fire likely resulted in transient disruptions of local ecosystems leading to shifts in birds/tick host migration within the province with the rise in tick transfer, as well as the movement of tick populations to more metropolitan areas (Edmonton and Calgary Zones) [29–31]. This, along with the increasing involvement of healthcare providers in tick submissions, may explain the higher numbers in these locations. More studies are still required to discern the effect of environmental fires on tick populations [31].
The combination of this data set and ongoing provincial surveillance data (veterinary and environmental submissions) [9], supports the finding that most Ixodes spp. ticks are adventitious and reproducing populations of blacklegged ticks (BLTs) do not currently exist in Alberta. This is based on the absence of higher numbers of submissions of BLTs as well as the sporadic finding of B. burgdorferi positivity as compared to other endemic locales. Active annual surveillance activities in Alberta from 2014 to 2017 also did not find any I. scapularis or I. pacificus ticks in the environment [9]. Similar findings are also reported from the neighboring province of Saskatchewan, which partially shares comparable geographic terrain [21].
The lack of I. scapularis endemicity in the prairies (outside of Manitoba) supports the low frequency of ticks positive for Borrelia burgdorferi, calculated from our data to be 0.35%. This estimate likely represents an over-estimate given data for variables such as tick attachment time and efficiency of pathogen transfer were not available. Furthermore, this numeric is particularly useful for clinicians and public health teams when counseling patients or members of the public experiencing a tick-bite or exposure. While we acknowledge that only I. scapularis/I. pacificus were tested for B. burgdorferi, it is assumed that the other local species of Ixodes ticks would be negative for B. burgdorferi. This is a reasonable assumption given major clinical guidelines do not consider other ticks species as significant carriers for this bacterium [32–35]. Such patients generally experience a great deal of anxiety regarding Lyme disease given increased general societal and media awareness [36]. The determination of whether prophylaxis is provided should follow clinical guidelines [32].
A major limitation of this study is that of submission bias – not all ticks on human hosts are routinely submitted, and likely many are discarded after removal. Since 2013, the processes for tick submission to diagnostic laboratories in Alberta have been heavily promoted. Another important limitation is reliance on the provision of travel history on requisitions to determine if a tick was acquired within Alberta. It is not uncommon for requisitions to be incomplete, omit information, or be illegible (ranging from 6% to 32% in several studies) [37-40]. To mitigate this, an aggressive review of archived requisitions was conducted. Despite this, there is a likelihood that some ticks thought to be from within Alberta (based on lack of travel history) are actually imported, such as some listed in Table 1, not normally found in Alberta or Canada (Ixodes ricinus, Amblyomma cajennense, Amblyomma coelbs, and Rhipicephalus pulchellus). However, these constitute <0.5% of total ticks included. This may also suggest that long-distance travel and exposure to ticks such as I. ricinus A. cajennense, etc., is less common than short distance travels to areas where I. scapularis/I. pacificus may be endemic. A similar problem may exist for more common ticks that are also found within the province (e.g. D. variabilis). Lastly, we do not have complete data available on the number of multiple submissions or numbers of nymphs or larvae. While it is acknowledged these two variables are important in indicating the presence of non-adventitious tick populations, lack of BLT endemicity in Alberta is supported by the low proportion of ticks carrying B. burgdorferi, absence of Ixodes spp. on provincial active surveillance, and no locally acquired cases of Lyme disease in Alberta [34,41].
The major strength of this study is the long time period covered by the surveillance, allowing analysis of trends over time. While tick identification was carried out at different laboratories, identification and molecular testing of all Ixodes spp. was confirmed by a single reference center.
This study represents a retrospective window into a subset of data that is currently not captured in passive tick surveillance in Alberta – the geographic occurrence and prevalence of ticks from human hosts. As such, it provides valuable information to complete data for all hosts for the province. The major genera identified were Dermacentor, Ixodes, and Amblyomma. The progressive rise in tick submissions over time likely represents increased societal concerns about tick-borne infections (especially Lyme disease), however, the influence of climate change cannot be ruled out. The overall likelihood, of a tick in Alberta carrying the causative agent of Lyme disease is low, which is important to guide patient counseling as well as public health messaging and intervention. Vector species of Ixodes ticks will continue to be introduced into the province from neighboring areas. Passive surveillance programs like ours will play an important role in monitoring the occurrence of selected tick species and their implications for public health.
Author contributions
Jamil N kanji helps in methodology, formal analysis, data curation, writing – original draft, writing – review, and editing. Abraam Isaac helps in formal analysis, validation, resources, writing – original draft, writing – review, and editing. Daniel Gregson helps in investigation, data curation, writing – review, and editing. Monika Mierzejewski helps in formal analysis, data curation, writing – review, and editing. Danny Shpeley helps in investigation, data curation, writing – review, and editing. Pauline Tomlin helps in validation, investigation, data curation, writing – review, and editing. Michael Groeschel helps in investigation, data curation, writing – review, and editing. Robbin Lindsay helps in methodology, investigation, resources, writing – review, and editing. Lisa Lachance helps in writing – review, and editing. Kinga Kowalewska-Grochowska helps in conceptualization, methodology, validation, resources, writing – original draft, writing – review, and editing.
Supplementary Material
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was deemed an operational quality study by the research ethics board at the University of Alberta (study identifier Pro00102172).
Disclosure statement
All authors have filled out an ICMJE form to make any declarations. None of the authors have conflicts of interest relevant to this manuscript to declare.
References
- 1.Talbot B, Slatculescu A, Thickstun CR, et al. . Landscape determinants of density of blacklegged ticks, vectors of Lyme disease, at the northern edge of their distribution in Canada. Sci Rep. 2019 Nov 13;9(1):16652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchard C, Dibernardo A, Koffi J, et al. . Increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep. 2019 Apr 4;45(4):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) . Lyme disease [2020 December 15]. Available from: https://www.cdc.gov/lyme/index.html
- 4.Ogden NH, Mechai S, Margos G.. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Front Cell Infect Microbiol. 2013;29(3):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagurova I, Ludwig A, Ogden NH, et al. . Predicted northward expansion of the geographic range of the tick vectorAmblyomma americanumin North America under future climate conditions. Environ Health Perspect. 2019 Oct;127(10):107014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JD. Studies abound on how far north Ixodes scapularis ticks are transported by birds. Ticks Tick Borne Dis. 2016 Mar;7(2):327–328. [DOI] [PubMed] [Google Scholar]
- 7.Scott JD, Fernando K, Banerjee SN, et al. . Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol. 2001 Jul;38(4):493–500. [DOI] [PubMed] [Google Scholar]
- 8.Scott JD, Anderson JF, Durden LA.. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol. 2012 Feb;98(1):49–59. [DOI] [PubMed] [Google Scholar]
- 9.Government of Alberta . Tick surveillance [2020 December 15]. Available from: https://open.alberta.ca/publications/2369-0690
- 10.Government of Alberta . Population statistics [2020 June 18]. Available from: https://www.alberta.ca/population-statistics.aspx
- 11.Alberta Health Services . AHS in my zone [2020 September 28]. Available from: https://www.albertahealthservices.ca/zones/zones.aspx
- 12.Alberta Precision Laboratories . Test Directory: Tick identification. [2021 December 1]. Available from: https://www.albertahealthservices.ca/webapps/labservices/indexAPL.asp?id=8541&tests=&zoneid=1&details=true
- 13.Centers for Disease Control and Prevention (CDC) . Environmental Health Services (EHS): Pictorial Keys to Arthropods, Reptiles, Birds, and Mammals of Public Health Significance: Ticks [2021 November 14]. Available from: https://www.cdc.gov/nceh/ehs/publications/pictorial_keys.htm
- 14.Dibernardo A, Cote T, Ogden NH, et al. . The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasit Vectors. 2014 Apr 15;7:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtney JW, Kostelnik LM, Zeidner NS, et al. . Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42(7):3164–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima R, Tsuji M, Oda K, et al. . Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCT.ETA gene in 36 isolates. J Vet Med Sci. 2009 Jan;71(1):55–68. [DOI] [PubMed] [Google Scholar]
- 17.Nelder MP, Russell C, Lindsay LR, et al. . Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One. 2014;9(8):e105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koffi JK, Savage J, Thivierge K, et al. . Evaluating the submission of digital images as a method of surveillance for Ixodes scapularis ticks. Parasitology. 2017 Jun;144(7):877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott JD, Anderson JF, Durden LA, et al. . Prevalence of the Lyme Disease Spirochete, Borrelia burgdorferi, in Blacklegged ticks, Ixodes scapularis at Hamilton-Wentworth, Ontario. Int J Med Sci. 2016;13(5):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden NH, Trudel L, Artsob H, et al. . Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J Med Entomol. 2006 May;43(3):600–609. [DOI] [PubMed] [Google Scholar]
- 21.Chilton NB, Curry PS, Lindsay LR, et al. . Passive and active surveillance for Ixodes scapularis (Acari: Ixodidae) in Saskatchewan, Canada. J Med Entomol. 2019;57(1):156–163. [DOI] [PubMed] [Google Scholar]
- 22.Gasmi S, Ogden NH, Ripoche M, et al. . Detection of municipalities at-risk of Lyme disease using passive surveillance of Ixodes scapularis as an early signal: A province-specific indicator in Canada. PLoS One. 2019;14(2):e0212637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Vicente S, Tagliafierro T, Coleman JL, et al. . Polymicrobial nature of tick-borne diseases. mBio. 2019 Sep 10;10(5):e02055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clow KM, Leighton PA, Ogden NH, et al. . Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One. 2017;12(12):e0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni M, Narula I, Slatculescu A, et al. . Lyme disease emergence after invasion of the blacklegged tick, Ixodes scapularis, Ontario, Canada, 2010–2016. Emerg Infect Dis. 2019;25(2):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden NH, Ben Beard C, Ginsberg HS, et al. . Possible effects of climate change on Ixodid ticks and the pathogens they transmit: predictions and observations. J Med Entomol. 2020 Oct 28:58(4):1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPherson M, García-García A, Cuesta-Valero FJ, et al. . Expansion of the Lyme disease vector Ixodes scapularis in Canada inferred from CMIP5 climate projections. Environ Health Perspect. 2017 May 31;125(5):057008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simms CD. Canada's Fort McMurray fire: mitigating global risks. Lancet Glob Health. 2016 Aug;4(8):e520. [DOI] [PubMed] [Google Scholar]
- 29.Needleman RK, Neylan IP, Erickson T.. Potential environmental and ecological effects of global climate change on venomous terrestrial species in the wilderness. Wilderness Environ Med. 2018 Jun;29(2):226–238. [DOI] [PubMed] [Google Scholar]
- 30.Santos-Silva MM, Beati L, Santos AS, et al. . The hard-tick fauna of mainland Portugal (Acari: Ixodidae): an update on geographical distribution and known associations with hosts and pathogens. Experimental and Applied Acarology. 2011;55(1):85–121. [DOI] [PubMed] [Google Scholar]
- 31.Albery GF, Turilli I, Joseph MB, et al. . From flames to inflammation: how wildfires affect patterns of wildlife disease. Fire Ecology. 2021;17(1):23. [Google Scholar]
- 32.Infectious Diseases Society of America (IDSA) . Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease [2021 November 30]. Available from: https://www.idsociety.org/practice-guideline/lyme-disease/ [DOI] [PubMed]
- 33.Centers for Disease Control and Prevention (CDC) . Diseases Transmitted by Ticks [2021 November 30]. Available from: https://www.cdc.gov/ticks/diseases/index.html
- 34.Government of Canada . Surveillance of Lyme disease [2021 November 30]. Available from: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html
- 35.Canadian Paediatric Society (CPS) . Lyme disease in Canada: Focus on children [2021 November 30]. Available from: https://cps.ca/en/documents/position/lyme-disease-children [PMC free article] [PubMed]
- 36.Aenishaenslin C, Michel P, Ravel A, et al. . Factors associated with preventive behaviors regarding Lyme disease in Canada and Switzerland: a comparative study. BMC Public Health. 2015 Feb 25;15:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tapper MA, Pethick JC, Dilworth LL, et al. . Pre-analytical errors at the chemical pathology laboratory of a teaching hospital. J Clin Diagn Res. 2017 Aug;11(8):Bc16–bc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atay A, Demir L, Cuhadar S, et al. . Clinical biochemistry laboratory rejection rates due to various types of preanalytical errors. Biochem Med (Zagreb). 2014;24(3):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layfield LJ, Factor RE, Jarboe EA.. Clinician compliance with laboratory regulations requiring submission of relevant clinical data: A one year retrospective analysis. Pathol Res Pract. 2012 Nov 15;208(11):668–671. [DOI] [PubMed] [Google Scholar]
- 40.Cohen MD, Curtin S, Lee R.. Evaluation of the quality of radiology requisitions for intensive care unit patients. Acad Radiol. 2006 Feb;13(2):236–240. [DOI] [PubMed] [Google Scholar]
- 41.Government of Alberta . Public health disease management guidelines: Lyme disease [2021 November 30]. Available from: https://open.alberta.ca/publications/lyme-disease
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.