ABSTRACT
Sri Lanka reported the last case of indigenous malaria in October 2012, and received malaria-free certification from WHO in September 2016. Malaria cases have since, shifted from indigenous to imported, and the country remains receptive and vulnerable to malaria. A case-based epidemiological study was conducted on all imported malaria cases reported in the country in 2015 and 2016 with the aim of profiling imported malaria to improve the effectiveness of the surveillance and case management system for malaria. Data were obtained from case reports of the Anti Malaria Campaign, hospital records and laboratory registers. Over the 2 years, 77 imported malaria infections were diagnosed in 54 Sri Lankans and 23 foreign nationals. A majority of the infections were reported among males (93%) in the age group of 21–50 years (85.8%), and all were recent travelers overseas. Most patients were detected by passive case detection, but 10% of cases were detected by Active Case Detection. Only 25% of patients were diagnosed within 3 days of the onset of symptoms. In 32% of patients, the diagnosis was delayed by more than 10 days after the onset of symptoms. Plasmodium falciparum infections manifested significantly earlier after arrival in Sri Lanka than did P.vivax infections. The majority of patients (74%) were diagnosed in the Western Province, which was not endemic for malaria. A third of patients were diagnosed in the private sector. The shift in the epidemiology of malaria infection from before to after elimination has implications for preventing the reestablishment of malaria.
KEYWORDS: Imported malaria, epidemiology, delayed diagnosis, case surveillance, prevention of reestablishment, receptivity, risk factors
Background
Malaria has been endemic in the dry zone of Sri Lanka. The countries largest epidemic was reported in 1934–1935 during which approximately 1.5 million individuals contracted the disease and 80,000 deaths were reported. With the commencement of malaria control activities comprising DDT spraying for adult mosquitoes, oiling of rivers and streams, application of Paris Green, automatic siphoning, treating infections and quinine prophylaxis, Sri Lanka greatly reduced its malaria burden reporting only 17 cases of malaria (of which 11 were imported) in 1963, but this fell short of elimination at that time. With the reduction in the number of cases, the malaria eradication program moved into the ‘maintenance’ phase, during which time DDT spraying was gradually withdrawn. After 1964, there was a slow increase in the number of cases culminating in a massive malaria resurgence during the period 1967–69 reporting up to 500,000 cases a year [1]. Fifty years later, the Anti Malaria Campaign (AMC) achieved remarkable success in controlling local transmission using key strategies, such as intensified parasitological surveillance focusing on early diagnosis and prompt treatment, entomological surveillance, selective vector control and enhanced health awareness and community engagement programs. This led to a significant reduction in malaria cases in the country from 1999 onwards [2] until zero cases were achieved by November 2012. Malaria-free certification by the World Health Organization (WHO) was received in September 2016. However, with continuing high receptivity, due to the high prevalence of mosquito vectors and vulnerability due to the high rate of malaria importation from other countries [3], it is imperative that a robust prevention of reestablishment (PoR) program is sustained in Sri Lanka.
Since 2008, all malaria cases reported in Sri Lanka were classified as imported, indigenous, introduced, imported, induced or relapsing [2]. The classification is based on rigorous investigation of all cases carried out by the AMC. Case classification is confirmed by an independent body, the Case Review Committee (CRC), a subcommittee of the Technical Support Group (TSG) of the AMC [4]. The TSG was appointed by the Director General of Health Services to fulfill the role of the National Malaria Elimination Task Force. Prior to 2012, the TSG supported the AMC with evidence-based strategic and technical advice on elimination. Since then, it continues to advice the AMC on the prevention of reestablishment of malaria.
Although indigenous transmission has been interrupted, approximately 50 imported malaria cases are being reported every year; imported malaria cases have the potential to reestablish local transmission due to the high receptivity that exists in previously malarious areas [3]. Over the past 8 years (2013 to 2020), 408 imported malaria infections and one introduced case of malaria have been reported in Sri Lanka [3,5]. All confirmed malaria cases have to be notified immediately to the Regional Malaria Officer (RMO) and AMC headquarters by telephone and also the Medical Officer of Health of the area in which the patient resides [6].
To inform strategy development during the PoR phase, the epidemiological features of imported malaria need to be characterized. The prevalence of malaria infection by age group, the proportion of infections caused by individual malaria species, the identity of the dominant mosquito vectors and their behavior, and knowledge of any social activities in the local population that would place specific groups especially at risk, diagnostic aspects, and information on patterns of drug and insecticide resistance are required to guide intervention strategies to prevent the resurgence of malaria. Thus, a case-based epidemiological survey of all imported malaria cases reported in the country in 2015 and 2016 was conducted. This paper documents the results of the survey in relation to the changing epidemiology of malaria in the country. As conventional control interventions practiced before malaria elimination are less likely to be effective with the shift in the epidemiology of the disease, the approaches to prevent the reestablishment of malaria may need to be revised and aligned with these changes.
Methods
In Sri Lanka, malaria cases are diagnosed by either passive case detection (PCD) or active case detection (ACD) [7–9]. In PCD, individuals presenting at medical institutions with symptoms that are clinically compatible with malaria are screened for malaria, while in ACD, public health staff of the AMC conduct screening of populations at risk either as reactive or proactive case surveillance. Reactive case detection is the active detection of malaria infections once a case or a cluster of cases are reported. This is done by blood screening for malaria of members of neighboring households through house-to-house visits in response to an index case. Proactive case detection is screening of known high-risk groups, such as security forces returning from United Nations peacekeeping missions or asylum seekers [8,9].
A case-based epidemiological survey on all imported malaria cases reported in the country from 2015 to 2016 was conducted. When a laboratory confirmed case of malaria is notified, re-confirmation of the diagnosis was carried out by the AMC using quality-assured microscopy and Rapid Diagnostic Tests (RDT). If there is a discrepancy in the results between microscopy and RDTs, a polymerase chain reaction (PCR) was carried out to confirm the diagnosis. A detailed case investigation was carried out using an interviewer-administered questionnaire. As malaria patients are treated as in-patients in hospital, data were also obtained from the Bed Head Ticket (BHT) and laboratory registers.
Data were entered in a SPSS database and a descriptive analysis of the variables was performed. The x2 test was used to compare qualitative variables. ANOVA and corresponding non-parametric tests were used to compare quantitative variables.
Results
Socio-demographic characteristics and risk factors for imported malaria
Seventy-seven imported malaria infections were diagnosed during the period. A majority of the infections (93.5%) were reported in males. Apart from a single case in a 15-year old, the rest were adults (mean age 38.17 years, range 15–66 years). The highest percentage (85.8%) of infection was reported among 21–50-year olds and all had a recent history of travel overseas to a malaria endemic country. Most imported cases originated from 14 countries (the highest numbers of eight, seven and six cases originating from Mozambique, Central African Republic and Uganda) located in Africa (n = 41, 53%) followed closely by 35 (46%) from Asia (the highest number of 28 cases originating from India) and one from Oceania. Plasmodium falciparum accounted for 45% (n = 35), P.vivax for 43% (n = 33), P.ovale for seven cases and one case each of P.malariae and P.knowlesi (Supplementary Table 1).
Seventy percent of infections (n = 54) were in Sri Lankan nationals, all of whom returned recently from malaria endemic countries. The main high-risk group was identified as security forces personnel returning from UN peacekeeping missions or military training (n = 16, 30%) and gem traders (n = 14, 26%), both returning from African destinations. Small-scale traders (10.4%) and pilgrims (2.6%) were the main high-risk groups returning from India. Of the 23 malaria infections in foreigners, 78% were traveling for occupational purposes, mainly to be employed as skilled or unskilled labor; most being residents of, and a few transiting through a malaria endemic country prior to arriving in Sri Lanka. Tourists visiting Sri Lanka (5.4%) were also identified as a high-risk group for importing malaria.
Almost a third of the Sri Lankan patients (30%) had obtained chemoprophylaxis from the AMC or a Regional Malaria Office prior to their departure from Sri Lanka, but they reported poor adherence to the drug regimen (Supplementary Table 1).
Mode of detection of malaria
As shown in Figure 1, a majority (90.9%) of the malaria cases were diagnosed by PCD. Of them, 71.4 % at health-care institutions when patients sought treatment usually for a febrile illness and were referred for malaria testing by a clinician, 9.1% when the patient visited a laboratory or clinician and him/herself requested a blood smear examination for malaria, and 10.4% incidentally during a blood picture examination that was requested by the treating physician on suspecting an illness other than malaria. Three cases (3.9%) (one P.falciparum case and two P.vivax cases) were detected by reactive case detection (RACD) through contact tracing and screening in relation to an index cases. All three cases were asymptomatic at the time of detection by microscopy. Four cases (5.2%) were detected by proactive case detection (PACD) during the course of screening identified high-risk individuals and groups. Two of them were members of a UN Peace Keeping Mission who were screened and detected at the airport on entry, and they both had symptoms on arrival. The other two cases were a foreign factory worker and an asylum seeker who were detected during the course of PACD screening, their groups having been identified as being at high risk; they were both asymptomatic at detection. All individuals diagnosed gave a history of recently returning from a malaria endemic country.
Figure 1.
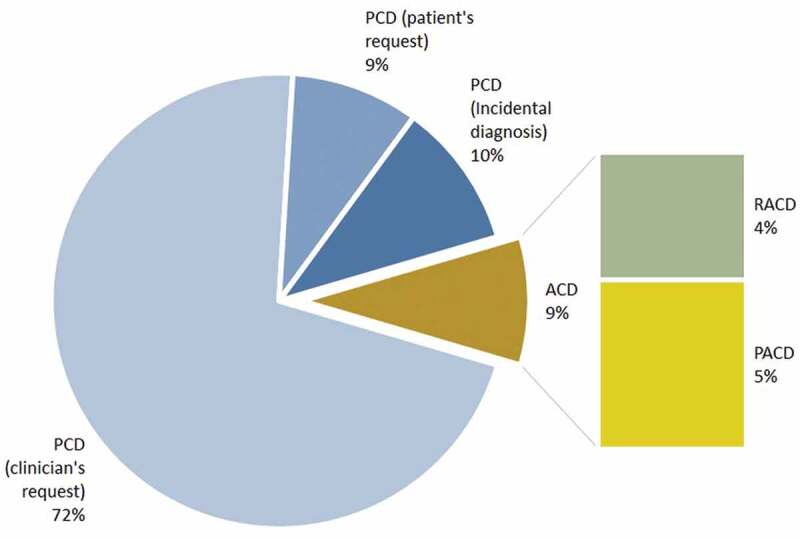
Title: Methods of detection of the 77 malaria patients (all imported cases) diagnosed in Sri Lanka in 2015 and 2016.
Legend: Passive case detection (PCD), Active case detection (ACD), Proactive case detection (PACD), Reactive case detection (RACD).
Onset of malaria illness in relation to date of arrival in Sri Lanka
The time that lapsed from the date of arrival in Sri Lanka to the first manifestation of symptoms in patients detected by PCD was analyzed. Eleven imported malaria patients of the 70 (15.7%) detected by PCD had symptoms on arrival in Sri Lanka and gave a history of developing symptoms even before their departure for Sri Lanka (Figure 2). In the rest (n = 59), the duration that lapsed between arrival in the country and the development of a symptomatic malaria illness ranged widely from 0 to 265 days (approximately 9 months) after arrival (Figure 2). There was a striking significant difference in the time between the arrival in Sri Lanka and the onset of the first malaria illness between Plasmodia species; P.falciparum infections manifested significantly earlier (median 3 days) than P.vivax infections (median 23 days). Seventy-nine percent (79%) of P.falciparum infections manifested within a week of arrival in the country, while 75% of P.vivax infections manifested after 1 week of arrival, and 32% after 2 months of arrival (Figure 2). The seven infections with P.ovale manifested later (median 51 days), and the P.malariae infection after 32 days of arrival. The P.knowlesi-infected patient was symptomatic on arrival.
Figure 2.
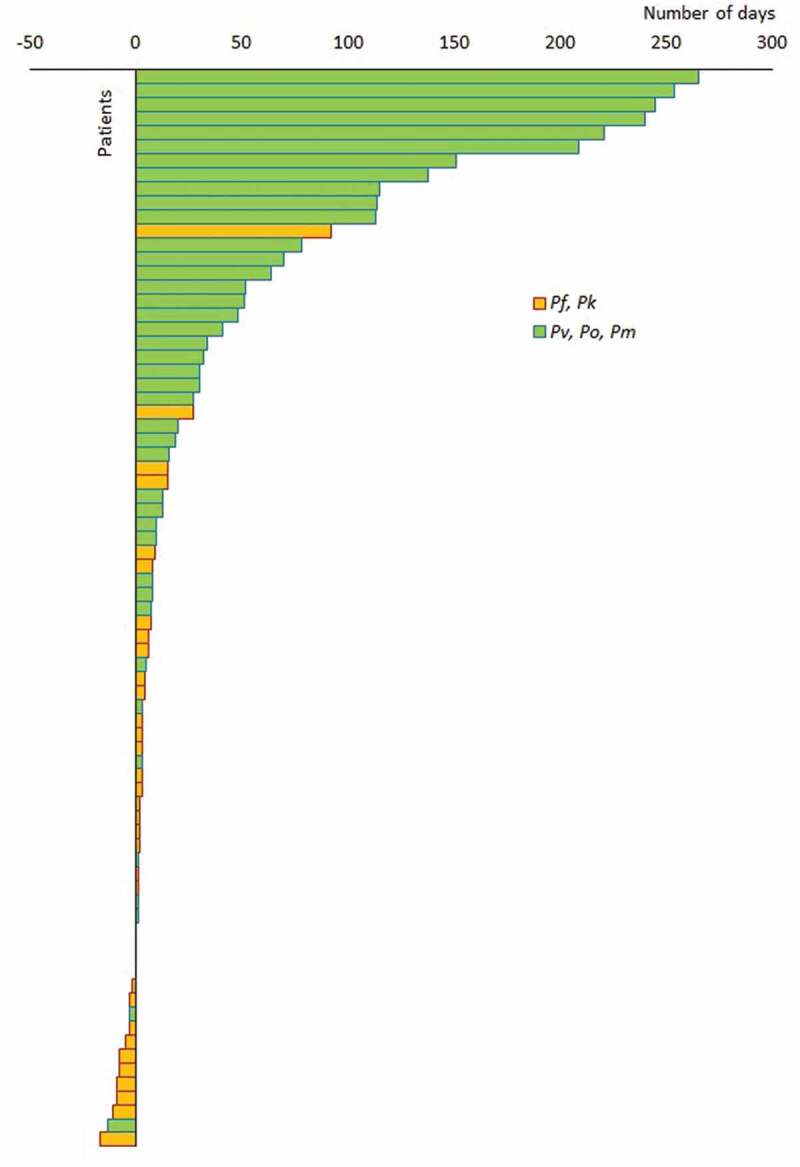
Title: Time from arrival in Sri Lanka to onset of malaria illness.
Bars represent the time that lapsed from the day of arrival in Sri Lanka to the onset of malaria illness, presented specieswise in 69 imported malaria patients detected by PCD. Negative values represent patients who developed symptoms of malaria before arrival in Sri Lanka. Patients are arranged in ascending order of time taken from arrival to the onset of illness.
Time to malaria diagnosis
The time to diagnosis from the onset of symptoms was analyzed in imported malaria patients detected by PCD. Only 25% of patients were diagnosed within 3 days of the onset of symptoms. In 32% of patients, the diagnosis was delayed more than 10 days from the onset of symptoms (Figure 3). The median time to diagnosis was 6.4 days. The period from the onset of symptoms to the first contact with the health system was, in most cases, short; most patients (51%) sought treatment at a health institution within a day of the onset of symptoms. However, the period between first contact with the health system to diagnosis was much longer ranging from 0 to 54 days (median 5 days). The patient who had a delay of 54 days in diagnosis had been treated repeatedly with antibiotics on the wrong assumption of it being due to other infections. This may have partially suppressed parasitaemias and alleviated symptoms leading to chronic malaria.
Figure 3.
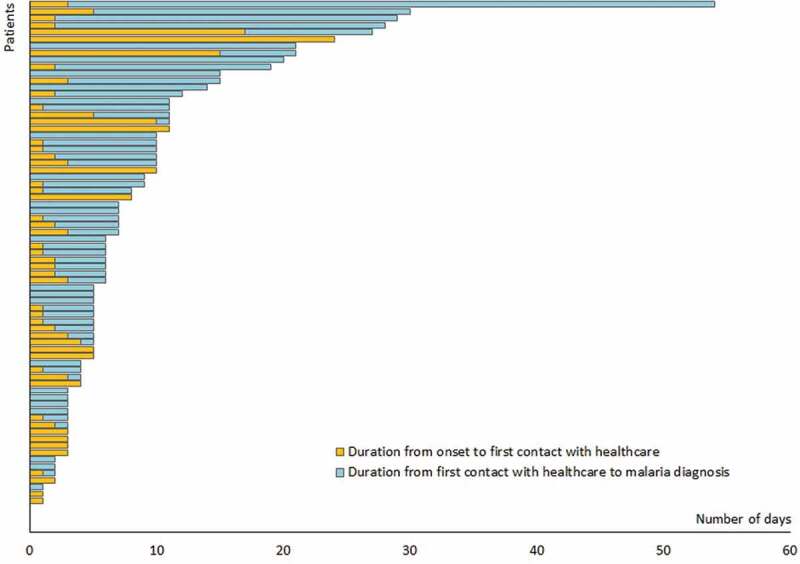
Title: Time to diagnosis.
Stacked bars represent the time to diagnosis of malaria, from the onset of symptoms to seeking healthcare (brown) and from the first contact with the health system to a diagnosis of malaria (blue) with the patients arranged in ascending order of the total time taken to diagnosis.
There were no significant differences in the time that patients took to present for treatment, i.e. the onset of symptoms to first contact with the health system (p > 0.2) or for the health system to diagnose malaria, i.e. from first contact with the health system to diagnosis (p > 0.4), between infections with the different plasmodium species (data not shown).
Among the imported malaria cases over the 2-year period, there were 15 classified as severe malaria patients on the basis of WHO criteria [10]. There was no significant difference in any of the parameters considered, i.e. time from the onset of symptoms to first contact with the health system and duration from first contact to diagnosis, between those diagnosed with uncomplicated and severe falciparum malaria. There were no malaria deaths during the study period, and none since 2008 in the country.
Geographical transition in areas where malaria is reported
Before malaria was eliminated, about two thirds of the country designated as the dry and intermediate zones based on rainfall patterns were endemic for malaria; the wet zone comprising the Western province and a part of the Southern Province were free of malaria transmission (Figure 4). After malaria was eliminated, and accompanying the shift from indigenous to imported malaria, there has been a major geographical transition in the areas where malaria is being detected and reported. Figure 4, based on data from 2008 onwards when the AMC began to classify malaria cases as imported and indigenous, shows this transition clearly. In Figure 4, during the period 2008–2012, a majority of infections were in dry zone provinces as shown by colors which correspond to those provinces in the map. From 2008–2012, most of the areas in Figure 4 are hatched indicating that they were indigenous cases. This is completely reversed during the period shown from 2012 to 2016. As the year of elimination was being approached and beyond, Figure 4 shows, moving from left to right, how the proportion of indigenous malaria gave way to imported malaria, and how, concurrently, where malaria cases were being reported to be moved from previously endemic provinces to previously non-endemic ones. The majority of imported malaria patients (74%) during the study period were diagnosed in the Western Province (wet zone) as compared to the period prior to elimination when a majority of malaria cases were reported from provinces in the dry zone (Figure 4).
Figure 4.
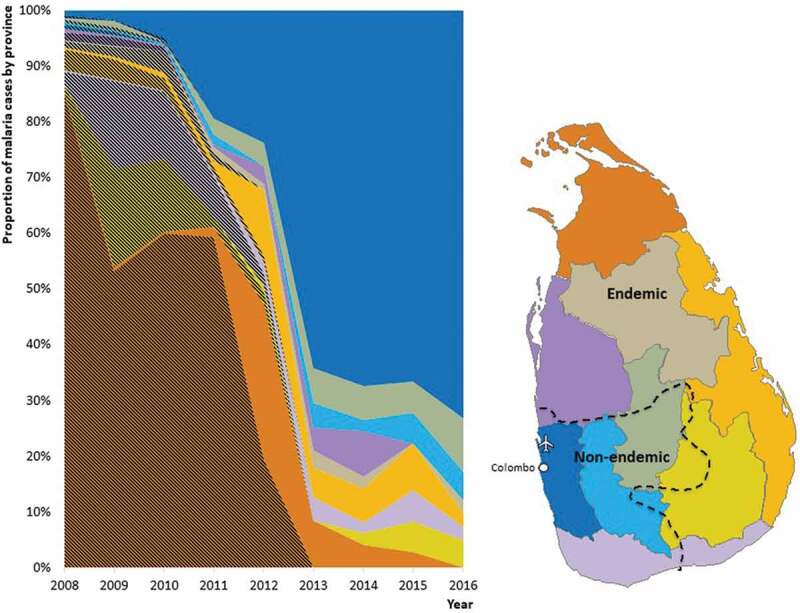
Title: Geographical transition in the areas where malaria is being detected and reported.
On the right is a map of Sri Lanka showing provinces shaded in different colors, and an interrupted line demarcating the previously endemic (to the right of the line) and previously non-endemic (to the left of the line) areas. The capital city of Colombo and the international airport have been shown in white. In the chart on the left, the proportion of yearly reported malaria cases in Sri Lanka from 2008 to 2016 are shown by province in colors corresponding to the colors of provinces in the map. The proportion of indigenous malaria cases are shown by hatched overlay on the color, and imported malaria cases in plain color (unhatched).
A third of the patients was diagnosed and treated in the private health sector. In all, including those diagnosed in the private health sector, confirmation of diagnosis was carried out by the AMC. All patients diagnosed with malaria were treated with quality-assured medicines based on the national treatment guidelines for malaria chemotherapy and management of patients with malaria [11].
Discussion
The findings of this study have some major implications for strategies to prevent the reestablishment of malaria after elimination. First, the case surveillance must include ‘active’ in addition to ‘passive’ case detection strategies since as much as 10% of malaria patients were detected by active surveillance strategies. Of the seven cases that were detected by ACD (both RACD and PACD), all but two were asymptomatic at the time of detection, and they would have remained undetected and had active case detection not been used. Besides, RACD, following the diagnosis of an imported malaria case by any other strategy, has led to identifying new high-risk groups, e.g. asylum seekers, gem miners, fishermen and pilgrims over the years [9,12,13] on whom Proactive Case Detection can be performed. Four cases diagnosed by PACD were, in fact, the result of screening high-risk groups, which is performed throughout the country, including at ports of entry.
Secondly, there is a conspicuous shift in the areas of the country from where malaria is being detected from before to after elimination. While malaria was endemic, the disease was contracted and reported from the dry zone constituting two thirds of the country, i.e. the districts of the Northern, Southern and North Central Provinces which still remains highly receptive (Figure 4); now imported malaria patients are being reported mostly from the Western Province in the wet zone, which comprises districts of Gampaha, Kalutara and Colombo – the city of Colombo being the country’s capital. The Colombo district is also the main business and economic hub of the country, hosts the country’s principal airport and is home to the highest concentration of private hospitals and laboratories, where a third of the malaria infections were detected. With the shift of malaria risk now to international travelers, and the more affluent population and tourists, the change in location from where malaria is being reported is not entirely unexpected. Fortuitously the Western Province in the wet zone happens to have very low or no receptivity due to the absence or low prevalence of malaria vectors, which considerably reduces the risk of onward transmission from imported malaria infections. This, however, is not an absolute safeguard from the risk of reestablishment because people travel widely between regions within the country, and any of these patients who were detected in the wet zone provinces may have been to other areas of the country while harboring a malaria blood infection. This has implications, in that the Western Province, which had not experienced malaria in the past, now needs to be strengthened in their capacity to deal with malaria.
Thirdly, due to the very low incidence of malaria in the country, clinicians rarely include malaria in the differential diagnosis of febrile illnesses by giving priority to investigations for other more prevalent infections, such as dengue, leading to unacceptable delays in malaria diagnosis. Only a quarter of the patients were diagnosed within 3 days of the onset of symptoms, and in 32% of patients, it took more than 10 days to be diagnosed with malaria. Such delays in the treatment of malaria may lead to severe and complicated malaria and therefore increased morbidity and mortality, but also increase the risk on onward transmission of the infection and the reestablishment of malaria in the country. This is a major challenge faced by all countries that are not endemic for malaria [14–17], but one, which countries with a high receptivity cannot afford to ignore. A major effort is being made by the AMC working jointly with medical professional bodies to alert clinicians on malaria, reminding them of the need to elicit a travel history as a clue to suspecting and testing for the disease. In addition to this, health education activities are being conducted for high-risk groups, including personnel of the armed forces and police and other identified high-risk groups, such as pilgrims and gem traders who frequently travel to malaria endemic countries, prior to their departure from the country.
There are other findings of this study which are of scientifically importance and which would also have implications for strategy development. With the transition from indigenous to imported malaria cases in Sri Lanka, there has been a major shift in both the age and sex distribution of malaria patients. Over 85% of imported malaria cases at present are reported in adult males in the 21–50-year-old age group, they being those who are most likely to travel overseas for business/occupation-related reasons. In the past, when malaria was endemic in the country females accounted for just less than half of malaria patients and the highest incidence rates were reported in the 1–4 and 10–14 year olds during the 1992–1994 period [18,19].
The study led to profiling of the high-risk groups for imported malaria, which should be the focus of active case surveillance. During the study period, the highest number of infections (n = 16) among Sri Lankan groups was from the armed forces and police returning from the United Nations (UN) peacekeeping missions from destinations, such as Central African Republic, Sudan and Liberia in Africa. Members of the armed forces and police constitute an important source of imported malaria on their return from the United Nations Peace Keeping Missions in malaria endemic countries. Therefore, the Ministry of Defense (MoD) is key partner of the AMC during this PoR phase. Prior to their departure on foreign missions, the AMC provides advice and guidance, as well as education on malaria risk reduction and preventive measures [20,21]. Again, on their return to Sri Lanka, they are screened at the airport, and follow-up screening is carried out at regular intervals. Thus, multiple case surveillance strategies have served their purpose over the past few years. There were also a large number of gem miners, resident in the city of Beruwela (Western Province), who travel frequently to Africa for the purposes of trading gems. The second largest source of imported malaria was from India, from where Sri Lankans return from trade activities, and from where migrant laborers arrive in Sri Lanka for employment [12]. Sri Lanka is heavily dependent on foreign labor mainly from India, China and Pakistan, being deployed in large-scale development projects. The AMC has established links with agencies that employ migrant labor to alert them on malaria and on actions to be taken in the event of a febrile illness among their employees.
The time of onset of malaria illness in relation to patients’ arrival in Sri Lanka ranged widely from even before arrival to almost 9 months after arrival and may reflect the biological characteristics of the different Plasmodia species. P.vivax, P.malariae and P.ovale infections arose significantly later after arrival in Sri Lanka than P.falciparum infections and the single P.knowlesi infection. Many P.vivax and P.ovale infections (seven cases reported over the 2-year period) are likely to have been due to relapses through the activation of latent hypnozoites as opposed to being primary infections, which P.falciparum infections would have been. The persistence in blood for long periods of time of P.malariae could also result in the delayed appearance of malaria illness.
Profiling and identifying high-risk groups will be central to improving the efficiency of the surveillance system, as well as to establishing and strengthening Intersectorial collaborations, which exist between the AMC and public health, medical, commercial and travel sectors. This analysis highlights the need to train local epidemiologists and physicians on malaria case diagnosis and case investigation. Epidemiological information on imported malaria such as defined here forms an important basis for strategy development in the POR phase.
Conclusions
The deployment of both active and passive case surveillance strategies will be required if all imported malaria cases are to be detected and treated to reduce the risk of malaria reestablishment in the country. The epidemiological profile of malaria has changed conspicuously with the shift from endemic to imported malaria with respect to age, sex and associated risk factors; the geographical areas within the country where malaria is being reported has changed markedly from previously endemic to previously non-endemic provinces, calling for strengthening of malaria diagnosis and treatment services and surveillance operations in previously non-endemic provinces. Imported malaria of different species manifest at different times after arrival in the country. Delays in malaria diagnosis occur when malaria ceases to be a high burden disease. These findings call for regular updating of the epidemiology of imported malaria to review and refine strategies to prevent the reestablishment of malaria.
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the Anti Malaria Campaign headquarters and regions for their continuous support. Financial assistance from the National Science Foundation (Grant No: RG/2014/HS/03) is gratefully acknowledged for assisting in data collection during the period 2015–2016.
Funding Statement
This study is funded by the National Science Foundation, Sri Lanka (grant no. RG/2014/HS/03) National Science Foundation, Sri Lanka .
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics approval and consent to participate
Ethical approval to report significant findings with regard to malaria patients in Sri Lanka has been obtained from the Ethics Review Committee, Faculty of Medicine, University of Colombo (ERC-14-165).
Consent for publication
The authors have not identified any patients by name.
Availability of data and material
The data and material are available with the first author.
Authors’ contributions
PD, SDF and RP planned the study. PD and RP collected the data. SDF, PD and ARW did the analysis. KM did further analysis. PD, SDF, KM and ARW wrote the draft paper. All authors contributed to and approved the final manuscript.
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Fernando P, Warusavithana S.. 100 years of malaria control efforts in Sri Lanka 1911-2011. (Published in 2011). Sri Lanka: M & S Associates. 2011. [Google Scholar]
- [2].Premaratne R, Wickremasinghe R, Ranaweera D, et al. Technical and operational underpinnings of malaria elimination from Sri Lanka. Malar J. 2019;18(1):1–12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karunasena VM, Marasinghe M, Koo C, et al. The first introduced malaria case reported from Sri Lanka after elimination: implications for preventing the re-introduction of malaria in recently eliminated countries. Malar J. 2019;18(1):1–10. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Datta R, Mendis K, Wickremasinghe R, et al. Role of a dedicated support group in retaining malaria-free status of Sri Lanka. J Vector Borne Dis. 2019;56:66–69. [DOI] [PubMed] [Google Scholar]
- [5].Anti Malaria Campaign, Sri Lanka. [cited 2021 Feb 1]. Available from: http://www.malariacampaign.gov.lk/en/.
- [6].Anti Malaria Campaign , Sri Lanka. Scope of work to be performed when a malaria patient is reported. 2016; [cited 19 December 2020]. Available from: http://www.malariacampaign.gov.lk/index.php/en/resources/guidelines.
- [7].Abeyasinghe RR, Galappaththy GNL, Gueye CS, et al. Malaria control and elimination in Sri Lanka : documenting progress and success factors in a conflict setting. PLoS One. 2012;7(8):e43162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wickremasinghe R, Fernando SD, Thillekaratne J, et al. Importance of active case detection in a malaria elimination programme. Malar J. 2014;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gunasekera WMKT , Pre maratne R, Fernando D, et al. A comparative analysis of the outcome of malaria case surveillance strategies in Sri Lanka in the prevention of re-establishment phase. Malaria J. 2021;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].World Health Organisation . Management of severe malaria. A practical handbook. 3rd ed. World Health Organisation, Geneva, Switzerland; 2012. [Google Scholar]
- [11].Anti Malaria Campaign, Sri Lanka ; Treatment guidelines. [cited 2020 Dec 12]. Available from: http://www.malariacampaign.gov.lk/precentation/TreatmentGuide.aspx.
- [12].Premaratne R, Ortega L, Navaratnasinghe J, et al. Malaria elimination from Sri Lanka: what it would take to reach the goal. WHO South-East Asia J Public Health. 2014;3:85–89. . [DOI] [PubMed] [Google Scholar]
- [13].Dharmawardena P, Premaratne R, Gunasekera K, et al. Characterization of imported malaria, the largest threat to sustained malaria elimination from Sri Lanka. Malar J. 2015;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tatarsky A, Aboobakar S, Cohen JM, et al. Preventing the reintroduction of malaria in Mauritius: a programmatic and financial assessment. PLoS One. 2011;6:e23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tatem AJ, Jia P, Ordanovich D, et al. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect Dis. 2017;17:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Muzrif MM, Weerasena M, Ranaweera AD, et al. August 2017. Delayed diagnosis of malaria risks reintroducing the disease to Sri Lanka. Sri Lanka Med Assoc (SLMA) News. accessed 2020 Dec 20. Available from: https://slma.lk/wp-content/uploads/2017/09/SLMA-August-2017-Draft-1.3.pdf [Google Scholar]
- [17].Ranaweera D, Rajapaksha RMJK, Silva P, et al. Plasmodium vivax malaria, HIV, tuberculosis co-infection in a Sri Lankan traveller: case management and challenges during the prevention of malaria reintroduction phase. Malaria J. 2018;17:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mendis C, Gamage-Mendis AC, de Zoysa APK, et al. Characteristics of malaria transmission in Kataragama. Sri Lanka: a focus for immuno-epidemiological studies. Am J Trop Med Hyg. 1990;42:298–308. [DOI] [PubMed] [Google Scholar]
- [19].Graves PM, Fernando D, Attanayake N.. 1995. Intensified malaria control programme in Sri Lanka with emphasis on primary care approach. Consultants report 1995.New Health Family Planning Project. Washington DC. IDA/World Bank. [Google Scholar]
- [20].Fernando SD, Dharmawardana P, Semege S, et al. The risk of imported malaria in security forces personnel returning from overseas missions in the context of prevention of re-introduction of malaria in Sri Lanka. Malaria J. 2016;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fernando SD, Booso R, Dharmawardana P, et al. The need for preventive and curative services for malaria when the military is deployed in endemic overseas territories: a case study and lessons learnt. Military Med Res. 2017;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and material are available with the first author.


