Abstract
Human blood components have been shown to enhance biofilm formation by gram-positive bacteria. We investigated the effect of human blood on biofilm formation on the inner lumen of needleless central venous catheter connectors by several gram-negative bacteria, specifically Enterobacter cloacae, Pseudomonas aeruginosa, and Pantoea agglomerans. Results suggest that a conditioning film of blood components promotes biofilm formation by these organisms in an in vitro system.
Evidence that biofilms can develop on intravascular devices, including central venous catheters (CVCs), has been well documented (7, 16). Colonization of the outer lumen of the catheter by microorganisms is usually the result of the catheter's proximity to skin flora. Colonization of the inner lumen of catheters (specifically by gram-negative rods) may be the result of a break in aseptic handling of the device prior to insertion or of the exposure of the end connectors to water, soil, or contaminated intravenous (i.v.) fluids. Both gram-positive and gram-negative bacteria have been isolated from biofilms on CVCs (6, 14, 15).
The initial event in the formation of a biofilm is the adhesion of the organisms to the surface. Material surfaces adsorb proteins or other organic materials when exposed in a fluid environment. These organic coatings, or conditioning films, have been shown to alter the material surface properties and affect microbial attachment (3, 18). It is standard practice for blood to be drawn through a CVC to ensure proper catheter placement (M. Bell, personal communication); although the catheter is flushed promptly afterwards, it would not be unreasonable to expect some adsorption of blood proteins to condition the exposed surfaces. The blood proteins fibrinogen and fibronectin affect the adhesion of gram-positive bacteria to biomaterials. For example, both of these purified plasma proteins enhanced the binding of Staphylococcus aureus to surfaces while inhibiting the binding of Staphylococcus epidermidis (9, 12) and gram-negative bacteria (1, 13, 19–21). Gram-negative bacteria, however, do colonize CVCs and other vascular access devices exposed to human blood components, resulting in bloodstream infections (23.2% of the central line-associated infections in intensive care units for 1986 to 1989 and 19.5% for 1990 to 1995) (10). This finding suggests that these organisms must not be completely inhibited by surfaces conditioned with whole blood.
The objective of this study was to evaluate the effect of human blood on the adhesion and biofilm formation of gram-negative organisms on the inner surface of CVC needleless connectors (NCs) in a laboratory model using a commercially available i.v. fluid as a nutrient.
In an earlier study, we reported the occurrence of biofilms on CVC NCs (6) and showed that biofilms on these devices were comprised of both gram-positive and gram-negative bacteria. Our purpose in the present study was to further investigate biofilm formation on NCs under controlled laboratory conditions in a model system and determine the effect of whole blood on the rate and extent of biofilm formation by selected clinically relevant gram-negative bacteria.
Bacterial strains and culture conditions.
The organisms used in this study, obtained from the stock culture collection of the Centers for Disease Control and Prevention, Dialysis and Medical Device Section (DMDS), were Enterobacter cloacae (DMDS 951225), Pseudomonas aeruginosa (DMDS 971406), and Pantoea agglomerans (DMDS 984322). Each frozen stock culture was initially inoculated onto Trypticase soy agar and incubated at 30°C for 24 h. Each culture was then transfered via swab to Butterfield Buffer (Becton Dickinson and Co., Cockeysville, Md), and a suspension equivalent to a McFarland 0.5 (∼108 CFU/ml) standard was prepared. This suspension was diluted 1:100, and 1 ml was used to inoculate 100 ml of lactated Ringer's solution and 5% dextrose, pH 5.35 (D5RL) (Baxter Healthcare Corporation, Deerfield, Ill.), for a final concentration of ∼104 CFU/ml and allowed to grow for 7 days at room temperature. These cultures in D5RL were transferred every 7 days by taking 1 ml (∼106 CFU/ml was reached during stationary phase) and adding it to a new 100-ml volume of D5RL for a final concentration of ∼104 CFU/ml.
Conditioning the NCs.
Five milliliters of fresh venous blood was collected into a 12-ml syringe. Three milliliters of blood was transferred from the syringe, through the same needle, into a 50-ml polypropylene clinical tube (Falcon; Becton Dickinson Labware) containing 27 ml of sterile physiological saline. This diluted (1:10) human blood was used the day it was drawn. Sterile NCs (ICU Medical, Inc.) were locked together in series (seven at a time) and connected to a 10-ml syringe on one end. Another 10-ml syringe containing diluted human blood was attached to the opposite end, and the blood was injected through the NCs into the opposite syringe, completely filling the NCs. After 1 h at room temperature, the blood was flushed once with physiological saline, pH 6.56 (Gibco BRL, Life Technologies).
Biofilm reactor model system design.
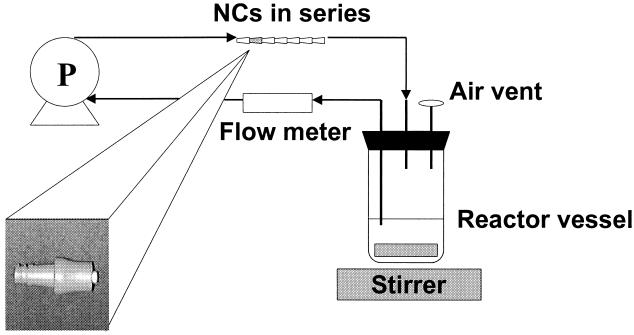
Figure 1 shows the design of the model system used for growing biofilms on NC inner lumens. Three independent recirculating tubing lines, each holding a set of seven NCs, were connected to each of seven reactors. Reactors contained 200 ml of D5RL and were inoculated with gram-negative bacteria (∼106 CFU/ml) that had been grown as pure cultures for 7 days on D5RL at room temperature. Two reactors were inoculated with P. aeruginosa (final concentration of 4.8 × 103 CFU/ml), two were inoculated with P. agglomerans (final concentration of 5.8 × 103 CFU/ml), and two were inoculated with E. cloacae (final concentration of 2.2 × 103 CFU/ml).
FIG. 1.
Diagram of biofilm receptor setup.
Batch mode studies.
For the purpose of the experiment, these systems were first operated in batch mode. Each system was closed without addition or removal of any component, with the exception of the gas phase (11). Reactors were placed on a stirring plate (Bamstead/Thermolyne, Dubuque, Iowa) set at 100 rpm, and the cultures were mixed at room temperature (21 to 25°C). Sterile silicone tubing (1.65-mm inside diameter; Cole Parmer, Niles, Ill.) from each reactor was connected to sets of NCs by means of a peristaltic pump (model no. 7553-80; Cole Parmer), and the cultures were recirculated at a flow rate of 1.0 ml min−1. For each organism tested, one reactor was used to test the human blood treatment, and a second one was used to test nonconditioned NCs. After 5 days of batch mode growth, the flow was stopped and a connector was removed from each line and processed for biofilm removal and quantification. Care was taken to ensure that the solution remained inside the connector during sampling. This sampling event was considered time zero, since it represented biofilm growth under batch conditions prior to continuous flow conditions.
Continuous flow studies.
Immediately following collection of the first connector, which had been exposed under batch mode conditions, all tubing lines were removed from the reactors and connected to a continuous feed of D5RL (1 ml/min) and a drain. The medium flowed continuously through the connectors to a waste container and was not recirculated. Connectors were removed on days 2, 5, 7, 9, and 13 (day 12 for E. cloacae) and processed for biofilm quantification. These sample results represented biofilm growth under continuous flow conditions.
Processing of NCs.
The connectors were removed from the lines and connected to two 5-ml syringes, one of them via a female-female luer coupling. One of the syringes contained 5 ml of phosphate-buffered saline (PBS). While still attached to the syringes, the outer surface of connectors was disinfected by immersion in 0.525% sodium hypochlorite (1:10 bleach) for 10 min and then immersion in a 0.12 M Na2S2O3 solution for 1 min to inactivate the sodium hypochlorite, followed by air drying. The PBS was gently flushed through the connector from one syringe to the other to ensure that those bacterial cells that were reversibly attached (4, 8) were removed, leaving on the surface the adherent bacterial biofilms. Following this outer surface disinfection procedure, connectors were removed from the syringes and the PBS flush was discarded. To remove and quantify inner lumen biofilms, connectors were cut in half transversely at their joint by using a flame-sterilized Handy Cut tool (Craftsman; Sears Roebuck and Co., Chicago, Ill.) and both halves were dropped into a tube containing 10 ml of fresh PBS. Biofilms were removed from the surface by three cycles of sonication for 30 s followed by vortexing for 30 s. The biofilm suspension was homogenized, and viable bacterial counts were quantified by plating serial dilutions onto R2A medium. Plates were incubated for 24 h at 30°C, and the bacteria were counted. The details of these methods are described elsewhere (6).
Statistical analysis.
CFU recovered from the inner lumens of the NCs were converted to logarithmic numbers and compared by applying a Wilcoxon test using Epi Info software (5). Subsets of the data were analyzed and compared under the assumption that biofilms growing in the inner lumens of NCs reached steady state after 5 days of continuous flow of the i.v. fluid. A biofilm is said to be at steady state when it is growing in an open system and the net accumulation of biomass approaches zero because the rate of growth in the biofilm approximates the rate of detachment of cells from the biofilm (2).
Biofilm log densities and standard deviations (SD) are presented in Fig. 2 through 4. SD varied depending on the organism. SD ranges for three replicates were 0.09 to 0.77 (average = 0.26), 0.03 to 1.53 (average = 0.34), and 0.27 to 1.66 (average = 0.79) for E. cloacae, P. aeruginosa, and P. agglomerans, respectively.
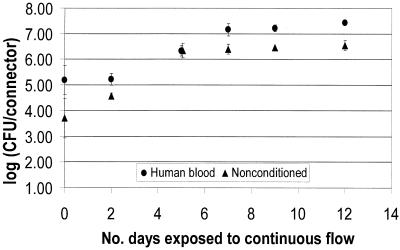
FIG. 2.
E. cloacae biofilm formation on NCs as a function of preconditioning.
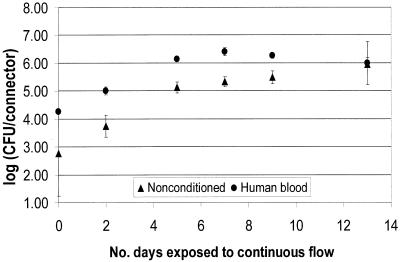
FIG. 4.
P. agglomerans biofilm formation on NCs as a function of preconditioning.
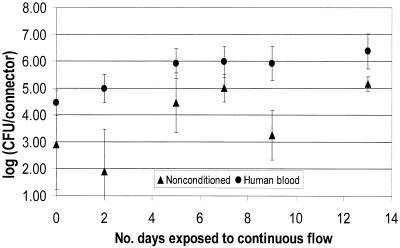
The results presented in Fig. 2 through 4 show that the numbers of CFU on the NC surface for all time points were higher for the conditioned surfaces for all three organisms. The initial colonization count of the inner lumen of the connectors after 5 days of batch growth (time zero in Fig. 2 through 4) was approximately 1 log higher for the nonconditioned surfaces. Wilcoxon two-sample tests were run to evaluate the effect of preconditioning the surfaces. For each organism, time zero counts for NCs conditioned with human blood were significantly higher than counts for nonconditioned NCs (P = 0.049). Further, the extent of biofilm formation over a 2-week continuous flow period was higher for conditioned surfaces than for nonconditioned surfaces. After day 5 of continuous flow, all biofilms appeared to have reached a steady state. However, over the next 7 or 8 days, the biofilms growing on nonconditioned surfaces did not reach the density levels of the biofilms growing on conditioned surfaces. The only exception was day 13 for the P. aeruginosa biofilms, where the means were very close, but the SD was large compared to the SD for the other days.
A Wilcoxon test of the data in Fig. 2 from days 7, 9, and 12 (biofilms at steady state) showed that human blood and nonconditioned NC biofilm counts were significantly different for all time points for E. cloacae (P = 0.0001). A Wilcoxon test of the data in Fig. 3 and 4 showed that at steady state (days 7 through 13), surfaces conditioned with human blood were significantly different from nonconditioned surfaces for all time points for P. aeruginosa (P = 0.007) and P. agglomerans (P = 0.001).
FIG. 3.
P. aeruginosa biofilm formation on NCs as a function of preconditioning.
Ideally, a system designed to model biofilm formation on an indwelling medical device would be one that simulated, as closely as possible, the conditions the device was exposed to in the patient (in vivo). The medical device utilized in this study was the NC, which is used to provide access to CVCs. For this study, we used a reactor system that utilized an i.v. fluid as the growth medium (D5RL) under continuously flowing conditions (1 ml/min) at ambient temperature. The organisms chosen were all clinically relevant gram-negative bacteria. The system was initially operated in batch mode for 5 days to allow for the initial colonization of the surfaces of the NCs. Once the system was switched to continuous flow (time zero in Fig. 2 through 4), the level of biofilm accumulation appeared to reach steady state after day 5 and remain stationary for the following 7 to 8 days. We assumed that our biofilms were at steady state during sampling days 7, 9, 12, and 13.
When a biofilm reactor is operated in batch mode or closed-system conditions, changes in chemical composition will occur until the limiting nutrient is depleted, even if the system is well mixed. A continuous-flow, or open-system, biofilm reactor is not in equilibrium, due to a constant replacement of media and biomass allowing the system to approach steady state. It is important to understand the difference between batch and open systems when modeling biofilms in vitro so that biofilm processes occurring on indwelling medical devices in vivo can be reproduced as accurately as possible. Medical device biofilms will normally simulate an open system, with a continuous exchange of nutrients and biomass. The use of freshly drawn human blood lends even greater credibility to the model by simulating the conditions to which these devices would be routinely exposed.
Our results showed that this system produced reproducible biofilms, as shown by the relatively small SD of our data, which according to Tilt and Hamilton (17) fall within the expected and acceptable range for repeatability precision of a protocol.
In conclusion, a model system for reproducibly growing and testing biofilms of clinically relevant gram-negative bacteria has been developed. Additional testing using other organisms, media, and devices, with the same basic system design, could support this concept for routine biofilm studies. Our results also show that whole blood, in contrast to specific blood proteins, enhanced the adhesion and biofilm formation of selected gram-negative bacteria. This study suggests that drawing blood through the same intravascular access line where fluids are being administered may enhance the rapid growth of gram-negative microorganisms that can colonize the access port and become a source of bloodstream infection in patients.
Acknowledgments
We acknowledge the helpful suggestions of Roger Bayston of the Biomaterials-Related Infection Group, Division of Microbiology and Infectious Diseases, University of Nottingham, and Matthew Arduino and Michael Bell of the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, for assistance with statistical analysis and helpful comments, respectively.
REFERENCES
- 1.Abraham S N, Beachey E H, Simpson W A. Adherence of Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983;41:1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Characklis W G. Process analysis. In: Characklis W G, Marshall K C, editors. Biofilms. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 50–51. [Google Scholar]
- 3.Characklis W G, McFeters G A, Marshall K C. Physiological ecology in biofilm systems. In: Characklis W G, Marshall K C, editors. Biofilms. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 341–394. [Google Scholar]
- 4.Costerton J W, Cheng K-J, Geesey G G, Ladd T I, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 5.Dean A G, Dean J A, Coulombier D, Brendel K A, Smith D C, Burton A H, Dicker R C, Sullivan K, Fagan R F, Arner T G. Epi Info, version 6: a word processing, database, and statistics program for epidemiology and microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 6.Donlan R M, Murga R, Bell M, Toscano C M, Carr J H, Novicki T J, Zuckerman C, Corey L C, Miller J M. Protocol for detection of biofilms on needleless connectors attached to central venous catheters. J Clin Microbiol. 2001;39:750–753. doi: 10.1128/JCM.39.2.750-753.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott T S J, Moss H A, Tebbs S E, Wilson I C, Bonser R S, Graham T R, Burke L P, Faroqui M H. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur J Clin Microbiol Infect Dis. 1997;16:210–213. doi: 10.1007/BF01709583. [DOI] [PubMed] [Google Scholar]
- 8.Escher A, Characklis W G. Modeling the initial events in biofilm accumulation. In: Characklis W G, Marshall K C, editors. Biofilms. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 445–486. [Google Scholar]
- 9.Espersen F, Wilkinson B J, Gahm-Hansen B, Thamdrup Rosdahl V, Clemmensen I. Attachment of staphylococci to silicone catheters in vitro. APMIS. 1990;98:471–478. [PubMed] [Google Scholar]
- 10.Fridkin S K, Welbel S, Weinstein R A. Magnitude and prevention of nosocomial infections in the intensive care unit. Infect Disease Clin N Am. 1997;11:479–496. doi: 10.1016/s0891-5520(05)70366-4. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt P, Drew S W. Liquid culture. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 224–247. [Google Scholar]
- 12.Herrmann M, Vaudaux P E, Pittet D, Auckendthaler R, Lew P D, Schumacher-Pedreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 13.Imai S, Okahashi N, Koga T, Nisizawa T, Hamada S. Ability of various oral bacteria to bind human plasma fibronectin. Microbiol Immunol. 1984;28:863–871. doi: 10.1111/j.1348-0421.1984.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 14.Maki D G. Infections caused by intravascular devices used for infusion therapy: pathogenesis, prevention, and management. In: Bisno A L, Waldvogel F P, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C.: ASM Press; 1994. pp. 155–212. [Google Scholar]
- 15.Maki D G, Goldman D A, Rhame F S. Infection control in intravenous therapy. Ann Intern Med. 1973;79:867–887. doi: 10.7326/0003-4819-79-6-867. [DOI] [PubMed] [Google Scholar]
- 16.Raad I I, Sabbagh M F, Rand K H, Sherertz R J. Quantitative tip culture methods and the diagnosis of central venous catheter-related infections. Diagn Microbiol Infect Dis. 1992;15:13–20. doi: 10.1016/0732-8893(92)90052-u. [DOI] [PubMed] [Google Scholar]
- 17.Tilt N, Hamilton M A. Repeatability and reproducibility of germicide tests: a literature review. J AOAC Int. 1999;82:384–389. [PubMed] [Google Scholar]
- 18.Vaudaux P E, Lew D P, Waldvogel F A. Host factors predisposing to and influencing therapy of foreign body infections. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C.: ASM Press; 1994. pp. 1–29. [Google Scholar]
- 19.Vercellotti G M, Lussenhop D, Peterson P K, Furcht L T, McCarthy J B, Jacob H S, Moldow C F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984;103:34–43. [PubMed] [Google Scholar]
- 20.Vercellotti G M, McCarthy J B, Lindholm P, Peterson P K, Jacob H S, Furcht L T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985;120:13–21. [PMC free article] [PubMed] [Google Scholar]
- 21.Woods D E, Straus D C, Johanson W G, Bass J A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981;143:784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]