Abstract
Background
The efficacy of methadone in reducing morbidity and mortality associated with opioid use disorder is supported by a wealth of evidence, yet methadone retention is often poor. While crystal methamphetamine (methamphetamine) use has been recently increasing in many countries, the effect of frequency of methamphetamine use on methadone discontinuation has not been investigated. We aimed to examine whether frequency of methamphetamine use is associated with increased rates of methadone discontinuation among individuals on methadone.
Design
Two harmonized ongoing open prospective cohort studies of community-recruited people who use illicit drugs with semi-annual follow-ups between 2014 and 2018.
Setting
Vancouver, Canada.
Participants
A community recruited sample of people who use drugs.
Intervention
A time-varying variable of self-reported methamphetamine use frequency within the past six months.
Measurements
The primary outcome was time to discontinuation of methadone, defined as reporting not being on methadone at the time of a follow-up interview during the study period. We employed multivariable extended Cox regression analysis to examine the relationship between frequency of methamphetamine use and time to methadone discontinuation after adjusting for potential confounders.
Findings
Of 875 eligible participants who contributed 2319 person-years of follow-up, 284 (32.5 %) discontinued methadone at least once during follow-up and 135 (15.4 %) reported more than weekly methamphetamine use at study baseline. In a multivariate analysis, in comparison to no use, ≥weekly use of methamphetamine remained independently associated with methadone discontinuation (adjusted hazard ratio [aHR] = 1.38, 95 % CI = 1.03–1.85).
Conclusions
A significant proportion of participants on methadone in this study reported more than weekly crystal methamphetamine use, which was associated with an increased risk of methadone discontinuation. Closer follow up, education, and treatment of methamphetamine use may be needed for this group to improve methadone retention.
Keywords: Opioid use disorder (OUD), Opioid agonist therapy (OAT), Methadone, Methamphetamine, Methadone retention, Polysubstance use
1. INTRODUCTION
The United States (US) and Canada are facing an overdose crisis, resulting in devastating morbidity and mortality (Centre for Disease Control, 2020; Special Advisory Committee on the Epidemic of Opioid Overdoses, 2021). Increasing contamination of the illicit drug supply since around 2014, most notably with illicitly manufactured fentanyl, has been implicated as a major contributor to rising overdose deaths across Canada and the US (BC Centre for Disease Control, 2019, 2017; Centre for Disease Control, 2020; DEA Intelligence Report, 2015). In British Columbia, Canada, fentanyl was detected in 86 % of overdose deaths in 2020 (BC Government Coroners Service, 2021). A recent study found that approximately 50 % of 590 community-recruited people inject drugs in Vancouver had a urine drug test positive for fentanyl, with a half of those testing positive for fentanyl were unknowingly being exposed (Hayashi et al., 2021).
Opioid agonist therapy (OAT) with methadone or buprenorphine is first-line treatment for opioid use disorder (OUD) in Canada (CIHR (Canadian Institutes of Health Research, 2018). OAT is a vital treatment for OUD and has been shown to be superior to withdrawal management in treatment retention and reducing opioid use, overdose, morbidity, and all-cause mortality (British Columbia Centre on Substance Use, 2017.; Fairbairn et al., 2008; Hser et al., 2016; Larochelle et al., 2018; Sordo et al., 2017; Wakeman et al., 2020). In British Columbia, provincial OUD guidelines recommend that anyone with a DSM-5 diagnosis of OUD currently using illicit opioids or at risk of relapse should be offered OAT including methadone, providing there are no contraindications (British Columbia Centre on Substance Use and B.C. Ministry of Health, 2017.). Methamphetamine and other stimulant use is not considered a contraindication or barrier to methadone treatment (British Columbia Centre on Substance Use and B.C. Ministry of Health, 2017). Longer duration on methadone has been shown to be associated with numerous positive outcomes and reduced risk of death, yet methadone retention rates 6–12 months after treatment initiation range widely from 38 to 74 % (Bao et al., 2009; Dolan et al., 2005; Gibson et al., 2008; Hubbard et al., 2003; Klimas et al., 2018; Morin et al., 2017; Sordo et al., 2017; Timko et al., 2016; Zhang et al., 2003).
Crystal methamphetamine is a highly purified form of d-methamphetamine, a potent central nervous system stimulant, which is associated with an increased incidence of dependence compared to lower purity amphetamine types (Courtney and Ray, 2014; McKetin et al., 2006). Recent reports suggest increasing prevalence of methamphetamine use across Canada and the US, including amongst those who use opioids (Ellis et al., 2018). Furthermore, methamphetamine use is also linked to rising fatal overdose rates, with detection in illicit drug overdose deaths increasing from 14 % in 2012 to 37 % in 2019 in the Canadian province of British Columbia (BC Coroners Service, 2020). In the US, the age-adjusted rate of psychostimulant-involved deaths increased by 33 % between 2016 and 2017, and approximately half of these deaths also involved opioids in 2017 (Kariisa et al., 2019). Additionally, US hospital admissions and healthcare expenditures related to illicit amphetamine use are increasing substantially, with costs rising from $436 million in 2003 to $2.17 billion by 2015 (Winkelman et al., 2018).
Stimulant use while on OAT has been shown to have variable effects, with some studies demonstrating an association with increased rates of ongoing illicit heroin use and decreased OAT retention (DeMaria et al., 2000; Eastwood et al., 2019; Franklyn et al., 2017; Heidebrecht et al., 2018; Oviedo-Joekes et al., 2015; Wang et al., 2017; Williamson et al., 2007). The majority of these studies, however, have focused on crack cocaine rather than crystal methamphetamine, which will be referred to as methamphetamine hereafter. Considering its rising prevalence, there is a relative lack of research examining methamphetamine use and its effect on OAT-related outcomes. To date, no large, prospective studies have been published in the US or Canada specifically estimating the effect of methamphetamine use on methadone retention. Additionally, the effects of frequency of methamphetamine use and route of administration on methadone retention rates have never been explored.
The focus of overdose prevention research and interventions to date has been on addressing fentanyl exposure and less so on methamphetamine use. In fact, few studies have examined the effect of methamphetamine use in North America on retention in OAT since the rise of fentanyl in the illicit drug market. Given the key role OAT has in reducing the risk of overdose in a drug market with increasingly lethal concentrations of fentanyl, it is important that the effect of methamphetamine use on OAT retention is better understood in order to inform public health responses and addiction treatment (BC Coroners Service, 2020; Stone et al., 2018). Therefore, this study sought to examine the effect of methamphetamine use frequency and route of administration on methadone discontinuation. Our hypothesis was that increasing frequency of methamphetamine use would be associated with increased rates of methadone discontinuation. We have focused on methadone because buprenorphine is prescribed and administered according to different protocols, and because methadone is the most commonly prescribed form of OAT in our study setting (BC Centre for Disease Control, 2021).
2. METHODS
2.1. Study procedures
The Vancouver Injection Drug Users Study (VIDUS) and the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS) are active open prospective cohort studies of adults who use drugs in Vancouver, Canada. Participants for these cohorts are recruited through word of mouth, street outreach and referrals largely in the Downtown Eastside neighbourhood of Vancouver, which is characterized by high rates of illicit drug use (Campbell and Neil Culbert, 2009). These cohorts have been described in detail in previous studies (Strathdee et al., 1998; Wood and Kerr, 2006). VIDUS enrols HIV-negative persons who report injecting an illicit drug at least once and ACCESS enrols HIV-positive individuals who report using an illicit drug (other than, or in addition to, cannabis) in the month prior to enrolment. In both cohorts, participants must be aged 18 years or older, reside in the greater Vancouver region and provide written informed consent. The study instruments and follow-up procedures for each study are harmonized to permit combined analyses. At baseline and semi-annually thereafter, participants complete an interviewer-administered questionnaire obtaining socio-demographic data as well as information on health care utilization, drug use patterns and risk behaviours. Participants receive a $40 (CDN) honorarium for each study visit. The University of British Columbia/Providence Health Care Research Ethics Board provides ethical approval for both studies.
We used data collected between March 2014 and November 2018, to capture the rise of fentanyl in the local illicit drug market in 2014 (BC Centre for Disease Control, 2017). The observations were restricted to ACCESS and VIDUS cohort participants who were currently on methadone at their first visit during the study period and who completed at least one subsequent follow-up visit.
2.2. Study variables
The primary outcome was time to discontinuation of methadone, defined as reporting not being on methadone at the time of a follow-up interview during the study period. The estimate for date of discontinuation was defined as the midpoint between the first report of methadone discontinuation and the last report of currently being on methadone. Additionally, we restricted the event of methadone discontinuation to those who discontinued and did not switch to other types of OAT available in our setting (i.e., buprenorphine/naloxone, slow-release oral morphine.)
The primary explanatory variable was frequency of methamphetamine use in the past six months, including the use of non-injection methamphetamine, injection methamphetamine, and the combination of co-injected opioids and methamphetamine (known locally as “goofballs.”) This time-varying variable had three categories based on the frequency of methamphetamine use within the past six months, specifically: “more than weekly use”, “no more than weekly use” and “not having used any methamphetamine.” These categorizations were used to help differentiate regular use of methamphetamine from sporadic and infrequent use.
Based on our clinical experience and existing research, we considered secondary explanatory variables that might confound the relationship between methamphetamine use and methadone discontinuation (Al-Tayyib et al., 2017; Bao et al., 2009; Franklyn et al., 2017; Heidebrecht et al., 2018; O’Connor et al., 2020; Oviedo-Joekes et al., 2015; Reist, 2010). These included the following socio-demographic characteristics: age (continuous; per year increase); self-identified gender (male vs. non-male); self-identified ancestry (white vs. non-white); HIV serostatus (positive vs. negative); incarceration (yes vs no in the last six months); living in the Downtown Eastside (yes vs no in the last six months); and homelessness (yes vs no in the last six months). Substance-use variables other than methamphetamine referred to ≥ daily use vs. < daily use and included: illicit opioid use (either via injection or non-injection, including use of street opioids [e.g., heroin] and non-medical use of prescription opioids), cocaine or crack use (either via injection or non-injection) and alcohol use. All variables except for age and gender referred to activities within in the past six months were time-varying variables. We hypothesized that those who discontinued methadone would likely re-engage in illicit opioid use, therefore, we lagged the variable of illicit opioid use to a prior study follow-up so that illicit opioid use that was a consequence of, rather than a contributor to, methadone discontinuation was excluded. We also controlled for methadone new initiates (yes vs. no) as we hypothesized this group would be more likely to discontinue if they were not yet at a therapeutic dose (Klimas et al., 2018). New initiates of methadone were defined as a participant reporting currently being on methadone and reporting not being on methadone in an immediately preceding study visit and were treated as a time-varying variable.
2.3. Statistical analysis
First, we calculated an incidence rate and 95 % confidence interval (CI) of methadone discontinuation using the Poisson distribution for the entire sample, as well as stratified by baseline methamphetamine use. We also compared the baseline sample characteristics stratified by methadone discontinuation at some point during the study period, using the Pearson’s Chi-squared test (for binary variables) and Wilcoxon Rank Sum test (for continuous variables).
We then used Kaplan–Meier methods to determine the probability of methadone discontinuation over time during follow-up, stratifying the sample by baseline methamphetamine use frequency as defined above (Kaplan and Meier, 1958). We used the log-rank test to compare the survival distributions of the three methamphetamine use frequency groups with no methamphetamine use being the reference category. We also used bivariable and multivariable extended Cox regression model incorporating recurrent events to estimate the relative hazard of recurrent methadone discontinuation associated with crystal methamphetamine use adjusting for potential confounders. Participants who discontinued methadone or switched to another form of OAT and then restarted on methadone at a subsequent follow up visit were considered at risk for discontinuation again from that subsequent follow up visit and were included in the recurrent event model. Participants who switched to another form of OAT and never returned to methadone were censored at the time of the switch to another OAT. We fit a multivariable extended Cox model using an a priori-defined model building procedure. Specifically, we included all variables that were associated with recurrent methadone discontinuation in unadjusted analyses at p < 0.10 in an initial full multivariable model. Then, we used a stepwise approach to fit a series of reduced models. After comparing the value of the coefficient associated with methadone discontinuation in the full model to the value of the coefficient in each of the reduced models, we dropped the secondary variable associated with the smallest relative change. We continued this iterative process until the minimum change exceeded 5 %. A sub-analysis was conducted using an unadjusted extended Cox regression model to explore potential differences in the effect of methamphetamine use on methadone discontinuation by routes of administration (both injection and non-injection, non-injection only and injection only, respectively vs. no use). All p-values were two-sided and tests were considered statistically significant at p < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Sample characteristics
For this analysis, 875 participants on methadone agonist therapy who completed more than one interview were included between March 2014 and November 2018, contributing to 4075 visits with 2319 person-years of follow up. The median length of follow up per individual was 2.93 years (1st to 3rd quartile (Q1–Q3) = 1.43–3.98). The baseline characteristics of all participants stratified by methadone discontinuation status are presented in Table 1. As shown, the median age at baseline was 48 years (Q1–Q3 = 39–54), 495 (57.3 %) were male, 346 (39.5 %) were HIV positive, and 396 (45.6 %) reported white ancestry. Further, 588 (67.2 %) participants reported no methamphetamine use, 152 (17.4 %) reported no more than weekly use, 135 (15.4 %) reported more than weekly use. Of this sample, 784 (89.6 %) were enrolled in the cohorts prior to March 2014.
Table 1.
Baseline sample characteristics stratified by methadone discontinuation during follow-up among methadone patients in Vancouver, Canada (n = 875).
| Methadone status during follow-up | ||||
|---|---|---|---|---|
| Total | No Discontinuation | Discontinuation | ||
| n (%) | n (%) | n (%) | p-value | |
| Characteristic | n=875 | 591 (67.5) | 284 (32.5) | |
| Methamphetamine use* | 0.001 | |||
| No use | 588 (67.2) | 421 (71.2) | 167 (58.8) | |
| No more than weekly | 152 (17.4) | 91 (15.4) | 61 (21.5) | |
| More than weekly | 135 (15.4) | 79 (13.4) | 56 (19.7) | |
| Age (median Q1,Q3) | 48 (39.54) | 49 (41.55) | 44 (36.52) | <.001 |
| Male | 495 (57.3) | 336 (57.6) | 159 (56.6) | 0.770 |
| White | 396 (45.6) | 287 (48.9) | 109 (38.8) | 0.005 |
| Living in the DTES* | 544 (62.2) | 359 (60.7) | 185 (65.1) | 0.209 |
| HIV Positive | 346 (39.5) | 236 (39.9) | 110 (38.7) | 0.734 |
| Homelessness* | 152 (17.4) | 84 (14.2) | 68 (23.9) | <.001 |
| ≥Daily illicit opioids* | 236 (27.0) | 126 (21.3) | 110 (38.7) | <.001 |
| ≥Daily cocaine/crack* | 157 (17.9) | 114 (19.3) | 43 (15.1) | 0.134 |
| ≥Daily alcohol* | 68 (7.8) | 43 (7.3) | 25 (8.8) | 0.425 |
| Incarceration* | 46 (5.3) | 27 (4.6) | 19 (6.7) | 0.186 |
DTES: Downtown Eastside. Q: quartile.
Denotes behaviours/events in the past six months.
During follow-up, 496 (56.7 %) remained on methadone, 95 (10.9 %) switched to another type of OAT, 238 (27.2 %) discontinued their methadone treatment once, 42 (4.8 %) discontinued their methadone treatment twice, and 4 (0.5 %) discontinued their methadone treatment three times. Overall, an incidence rate of methadone discontinuation was 14.4 per 100 person-years (95 % confidence interval [CI] = 12.8–16.2). Stratified by methamphetamine use frequency, the incidence rate per 100 person-years of methadone discontinuation was 11.6 (95 % CI = 10.0–13.6) for no use, 16.9 (95 % CI = 12.9–22.0) for no more than weekly use, 23.9 (95 % CI = 19.2–29.7) for more than weekly use. Fig. 1 is a Kaplan-Meier curve depicting the probability of methadone retention over time. Compared to those who did not use methamphetamine at baseline, those who used methamphetamine had significantly lower survival probabilities (log rank p < 0.001 for more than weekly use, and p = 0.002 for no more than weekly use).
Fig. 1.
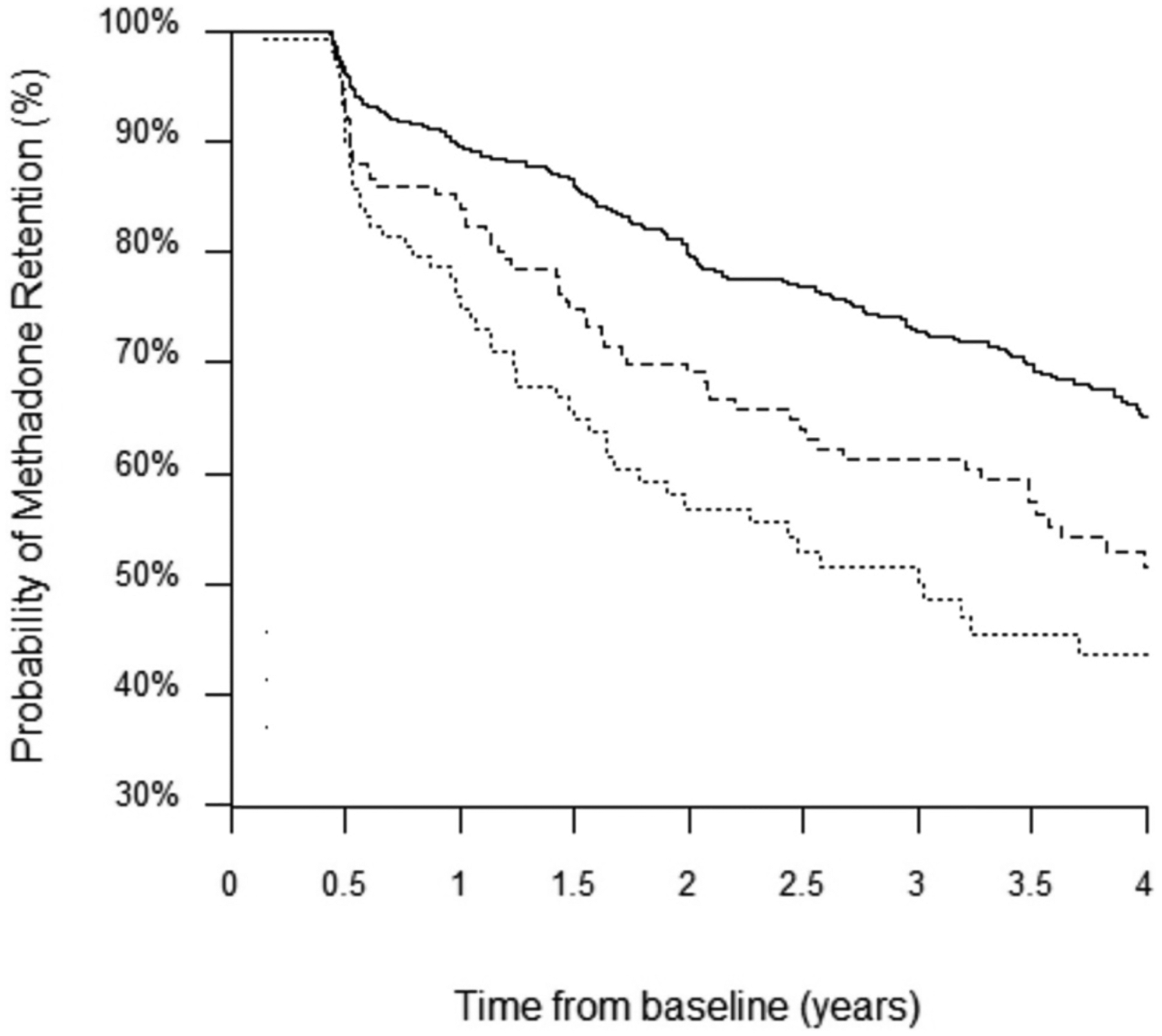
Kaplan–Meier curve depicting the probability of methadone retention over time stratified by baseline methamphetamine use frequencies.
Year(N): 0.5(803) 1.0(679) 1.5(594) 2.0(503) 2.5(448) 3.0(384) 3.5(329) 4.0(201) 4.5(22)
––– No use
----- No more than weekly use
…… More than weekly use
The results of the bivariable and multivariable extended Cox regression analyses are presented in Table 2. Compared to no methamphetamine use, the bivariable Cox regression model showed that compared to no use of methamphetamine, both no more than weekly use and more than weekly use (hazard ratio [HR] = 1.49, 95 % CI = 1.08–2.04, 2.17, 95 % CI = 1.63–2.88, respectively) were significantly associated with methadone discontinuation. After adjusting for the secondary explanatory variables in the multivariable model, only more than weekly use of methamphetamine remained independently associated with methadone discontinuation (adjusted hazard ratio [aHR] = 1.38, 95 % CI = 1.03–1.85).
Table 2.
Univariable and multivariable extended Cox regression analyses of the relationship between methamphetamine use and methadone discontinuation (n = 875).
| Methadone discontinuation | ||
|---|---|---|
| Characteristics | HR (95 % CI) | aHR (95 % CI) |
| Methamphetamine use* | ||
| No use | Reference | |
| No more than weekly use | 1.49 (1.08–2.04) | 1.06 (0.77–1.45) |
| More than weekly use | 2.17 (1.63–2.88) | 1.38 (1.03–1.85) |
| New initiate | 3.32 (2.56–4.33) | 2.67 (2.04–3.48) |
| Age (per year) | 0.95 (0.94–0.96) | 0.96 (0.95–0.98) |
| Male | 0.90 (0.70–1.16) | |
| White | 0.70 (0.54–0.90) | 0.69 (0.54–0.89) |
| Living in the DTES* | 1.56 (1.20–2.02) | 1.23 (0.94–1.61) |
| HIV positive | 0.98 (0.76–1.26) | |
| Homelessness* | 2.44 (1.85–3.21) | 1.55 (1.16–2.07) |
| ≥Daily illicit opioid use*, † | 2.49 (1.97–3.15) | 1.63 (1.28–2.08) |
| ≥Daily cocaine/crack use* | 0.86 (0.63–1.18) | |
| ≥Daily alcohol use* | 1.01 (0.66–1.55) | |
| Incarceration* | 1.41 (0.85–2.33) | |
HR: hazard ratio. aHR: adjusted hazard ratio. CI: confidence interval. DTES: Downtown Eastside.
Denotes behaviours/events in the past six months.
The variable of illicit opioid use was lagged to a prior study follow-up visit.
In a sub-analysis, compared to no methamphetamine use, all routes of administration of methamphetamine were significantly associated with methadone discontinuation: both injection and non-injection (HR = 1.97, 95 % CI = 1.40–2.77), non-injection only (HR = 1.85, 95 % CI = 1.20–2.86), and injection only (HR = 1.75, 95 % CI = 1.29–2.38).
4. DISCUSSION
In this prospective cohort study of 875 participants on methadone agonist therapy, more than weekly methamphetamine use was independently associated with higher rates of methadone discontinuation after adjusting for potential confounders including sociodemographic characteristics and other substance use patterns. Unmeasured confounders could exist as with any observational research; however, we tried to reduce this bias through adjustment of regression models using potential predictors of methadone discontinuation. As expected, the point estimates of hazard ratios for both categories of methamphetamine use substantially decreased towards the null after adjusting for potential confounders. These results indicate the presence of significant confounding effects by the covariates included in the adjusted model. In our sample of methadone patients, 32.5 % discontinued their methadone over the duration of this study, while additional 10.9 % switched to another type of OAT. All routes of administration of methamphetamine use were similarly significantly associated with methadone discontinuation when compared to no methamphetamine use.
The proportion of individuals on methadone who reported using methamphetamine at baseline (32.8 %) was higher than rates reported in literature examining methadone retention rates, which ranges from 5 % to 15.6 % (Banta-Green et al., 2009; Deck and Carlson, 2005; Liu et al., 2017; Peles et al., 2008; Proctor et al., 2015). This higher proportion likely reflects the rising prevalence of methamphetamine use in our study setting, with rates among all cohort participants as high as 36 % in a recent report (Bach et al., 2020). A similar pattern of rising prevalence has also been noted in other US settings (Kariisa et al., 2019; Winkelman et al., 2018). The methadone retention rate in this study (56.7 %) was substantially higher than a 12-month retention rate (32 %) for new methadone starts that has been reported in our study setting (Office of the Provincial Health Officer, 2017). This could be due to the high proportion (76.6 %) of our study sample having been on methadone prior to their baseline visit, as opposed to those who were new to or restarting methadone and therefore more likely to discontinue methadone. It may also represent bias given that participants included in our study were stable enough to present for at least one semi-annual interview.
To our knowledge, this is the largest prospective cohort study to specifically examine the relationship between methamphetamine use, including frequency and routes of administration, and methadone discontinuation, and the first to do so since fentanyl has become the predominant opioid in the illicit drug market most Canadian and the United States jurisdictions. The majority of past studies that have examined this relationship were retrospective, with only small numbers of individuals who were on methadone and using methamphetamine (O’Connor et al., 2020). A 2020 systematic review summarizing these past studies on methadone retention, four of which were retrospective, found that three of the five studies in the United States and Canada showed a significant association with methamphetamine use and reduced retention (Banta-Green et al., 2009; Deck and Carlson, 2005; O’Connor et al., 2020; Peles et al., 2008; Proctor et al., 2015). One prospective study from the United States did note a similar finding to ours, though this was published over ten years ago in 2008 and included 47 individuals who were using methamphetamine, based on UDS alone, out of 302 methadone patients (Peles et al., 2008). This study showed that a UDS positive for amphetamines at the time of admission to the program was associated with decreased retention at one year (Peles et al., 2008). Our study is novel in that it uses prospective data from a large sample size within the past five years since the rising rates of methamphetamine use and fentanyl exposure have been reported and it considers a more detailed and time-varying patterns of methamphetamine use (Bach et al., 2020; BC Coroners Service, 2020; Canadian Centre on Substance Use and Addiction, 2019; Centre for Disease Control, 2020). It also suggests a dose-dependent relationship between frequency of methamphetamine use and methadone discontinuation, while all routes of administration seemed to have similar associations. This study enhances our knowledge and understanding of the potential implications that these recent shifts in substance use patterns may have on treatment for substance use disorders.
Past literature has demonstrated that retention in methadone treatment is associated with numerous improved health and social outcomes (Dolan et al., 2005; Gibson et al., 2008; Hubbard et al., 2003; Sordo et al., 2017; Zhang et al., 2003). Given the potential relationship between at least weekly methamphetamine use and poorer methadone retention, our findings indicate that methamphetamine use should be explored during medical visits for methadone initiation and follow up, and that evidence-based patient education or behavioural interventions should be provided around at least weekly methamphetamine use and its possible detrimental effects on methadone treatment. Although we cannot conclude from this study that treatment for methamphetamine use increases methadone retention, given the many known harms and potential relationship with decreased retention, treatment for methamphetamine use disorder should be offered regularly for patients who engage in at least weekly methamphetamine use. However, there is currently no approved pharmacological treatment for stimulant use disorder and the available evidence-based treatments for methamphetamine use disorder are limited (Ronsley et al., 2020; Tardelli et al., 2020). Further research is needed to look into potential pharmacologic treatments to treat stimulant use disorder given the rise in methamphetamine use and its effects on opioid use disorder treatment. In addition, it is important that the reasons individuals continue to use stimulants while being on methadone are understood if health care providers are to help address this and improve methadone agonist therapy retention. Some of the reasons people use stimulants while on methadone were identified in a recent qualitative study, including countering the sedating effects of methadone, improving the ability to engage in survival activities, achieving intoxication from stimulants once stable on methadone and some individuals reported increased stimulant use to compensate for reduced stimulant intoxication while on methadone (McNeil et al., 2020).
There are several limitations in this study. This study was observational and therefore we cannot infer causation between methamphetamine use frequency and methadone discontinuation. Unmeasured confounders could exist as with any observational research; however, we tried to reduce this bias through adjustment of regression models using potential predictors of methadone discontinuation. Data used in the study was self-reported and therefore could be subject to reporting biases, however self-reported behavioural data has been shown to be generally accurate among adult drug-using populations (Darke, 1998). The prevalence and concentration of fentanyl in the illicit drug supply in our study setting has increased significantly since the end of the present study period in 2018, particularly since the coronavirus disease 2019 outbreak in 2020 (BC Government Coroners Service, 2021). Future research should investigate these recent changes and the effect on OAT retention. Additionally, the VIDUS and ACCESS cohorts are not random samples, and therefore, generalizability of the findings could be limited. The generalizability of our findings could be further limited by the fact that the majority of our study sample resided in the Downtown Eastside of Vancouver (62 % at baseline). This is a unique urban neighborhood characterized by high rates of substance use and homelessness but also a concentration of low-threshold treatment and harm reduction services for individuals with OUD (Campbell and Neil Culbert, 2009). For example, a range of treatment options from oral OAT to intensive treatment with injectable opioid agonist therapy with hydromorphone and diacetylmorphine and residential treatment services are provided in this neighborhood, which may have made the transition from methadone to other forms of OAT (if needed or desired) more possible compared to other settings, including rural settings that do not have the same concentration or intensity of treatment services for people who use drugs. Moreover, our study setting is characterized by low-threshold access to methadone through primary care physicians (British Columbia Centre on Substance Use and B.C. Ministry of Health, 2017). This may limit the generalizability of our findings to other settings including the US where access to methadone is more restricted and limited to specialized clinics. Lastly, given very different side effect profiles and prescribing systems, the results of this study cannot necessarily be extrapolated to other form of OAT such as buprenorphine or slow-release oral morphine. This study included only participants on methadone as it was the most commonly prescribed OAT in our study setting and accounts for more OAT prescribing than all other OAT types combined (BC Centre for Disease Control, 2021). Sample sizes for individuals on other forms of OAT such as buprenorphine/naloxone were also much smaller, resulting in the limited statistical power to perform multivariable analyses for other OAT. Examining the impacts of methamphetamine use on other forms of OAT remains an important area for future study.
5. CONCLUSION
Among this sample of participants on methadone in a Canadian setting, at least weekly methamphetamine use was independently associated with higher rates of methadone discontinuation. These findings highlight that closer follow up, evidence-based education, and treatment of methamphetamine use may be needed for those engaging in at least weekly use of methamphetamine use in order to improve methadone retention and reduce substance use related harms during the ongoing overdose crisis.
Highlights.
This study examined the effect of methamphetamine use on methadone discontinuation.
We found that at least weekly use was associated with methadone discontinuation.
Treatment of methamphetamine use disorder may help to improve methadone retention.
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. The study was supported by the US National Institutes of Health (NIH) (U01DA038886, R01DA021525). This research was undertaken, in part, thanks to funding from the Canadian Institutes of Health Research (CIHR) Canadian Research Initiative on Substance Misuse (SMN-139148). The work of LM is funded through the British Columbia Centre on Substance Use International Collaborative Addiction Medicine Research Fellowship (NIH grant R25-DA037756). PB receives funding from the Michael Smith Foundation for Health Research. KH holds the St. Paul’s Hospital Chair in Substance Use Research and is supported in part by the NIH grant (U01DA038886), a CIHR New Investigator Award (MSH-141971), a Michael Smith Foundation for Health Research (MSFHR) Scholar Award, and the St. Paul’s Foundation. MJM is supported by the NIH (U01DA0251525), a CIHR New Investigator Award, and a MSFHR Scholar Award. MJM’s institution has received an unstructured gift from NG Biomed, Ltd., to support his research. MJM is the Canopy Growth professor of cannabis science at the University of British Columbia, a position created by unstructured gifts to the university from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. MJM’s funder did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
REFERENCES
- Al-Tayyib A, Koester S, Langegger S, Raville L, 2017. Heroin and methamphetamine injection: an emerging drug use pattern. Subst. Use Misuse 52, 1051–1058. 10.1080/10826084.2016.1271432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Hayashi K, Milloy M-J, Nosova E, Kerr T, Wood E, Fairbairn N, 2020. Characterising the increasing prevalence of crystal methamphetamine use in Vancouver, Canada, from 2006–2017: a gender-based analysis. Drug Alcohol Rev 39 (7), 932–940. 10.1111/dar.13126. November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green CJ, Maynard C, Koepsell TD, Wells EA, Donovan DM, 2009. Retention in methadone maintenance drug treatment for prescription-type opioid primary users compared to heroin users. Addiction 104, 775–783. 10.1111/j.1360-0443.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- Bao Y-P, Liu Z-M, Epstein DH, Du C, Shi J, Lu L, 2009. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am. J. Drug Alcohol Abuse 35, 28–33. 10.1080/00952990802342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BC Centre for Disease Control, 2017. The BC public health opioid overdose emergency. Obs. Popul. Public Heal 1–17. 10.1002/gps.1662. [DOI] [Google Scholar]
- BC Centre for Disease Control, 2019. Prescription History and Toxicology Findings among People Who Died of an Illicit Drug Overdose in BC, pp. 2015–2017. [Google Scholar]
- BC Centre for Disease Control, 2021. Overdose Response Indicator Report
- BC Coroners Service, 2020. Illicit Drug Toxicity Deaths in BC. January 1st, 2010–January 31st, 2020, pp. 1–22. [Google Scholar]
- BC Government Coroners Service, 2021. Illicit Drug Toxicity Deaths in B.C, pp. 2010–2020. [Google Scholar]
- British Columbia Centre on Substance Use and B.C. Ministry of Health, 2017. A Guideline for the Clinical Management of Opioid Use Disorder
- Campbell Larry Boyd, Culbert Neil, L., 2009. A Thousand Dreams
- Canadian Centre on Substance Use and Addiction, 2019. Changes in Stimulant Use and Related Harms: Focus on Methamphetamine and Cocaine (CCENDU Bulletin), pp. 1–15. [Google Scholar]
- Centre for Disease Control, 2020. Synthetic Opioid Overdose Data
- CIHR (Canadian Institutes of Health Research), 2018. CRISM National Guidelines for the Clinical Management of Opioid Use Disorder, pp. 1–132. [Google Scholar]
- Courtney KE, Ray LA, 2014. Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143, 11–21. 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, 1998. Self-report among injecting drug users: a review. Drug Alcohol Depend 51, 253–258. 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- DEA Intelligence Report, 2015. National Heroin Threat Assessment Summary
- Deck D, Carlson MJ, 2005. Retention in publicly funded methadone maintenance treatment in two western states. J. Behav. Health Serv. Res 32, 43–60. 10.1007/BF02287327. [DOI] [PubMed] [Google Scholar]
- DeMaria PAJ, Sterling R, Weinstein SP, 2000. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. Am. J. Addict 9, 145–153. 10.1080/10550490050173217. [DOI] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD, 2005. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction 100, 820–828. 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Eastwood B, Strang J, Marsden J, 2019. Change in alcohol and other drug use during five years of continuous opioid substitution treatment. Drug Alcohol Depend 194, 438–446. 10.1016/j.drugalcdep.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Ellis MS, Kasper ZA, Cicero TJ, 2018. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug Alcohol Depend 193, 14–20. 10.1016/j.drugalcdep.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Fairbairn N, Wood E, Stoltz Janne, Li K, Montaner J, Kerr T, 2008. Crystal methamphetamine use associated with non-fatal overdose among a cohort of injection drug users in Vancouver. Public Health 122, 70–78. 10.1016/j.puhe.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Franklyn AM, Eibl JK, Gauthier GJ, Pellegrini D, Lightfoot NE, Marsh DC, 2017. The impact of cocaine use in patients enrolled in opioid agonist therapy in Ontario, Canada. Int. J. Drug Policy 48, 1–8. 10.1016/j.drugpo.2017.05.044. [DOI] [PubMed] [Google Scholar]
- Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S, 2008. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction 103, 462–468. 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Wood E, Dong H, Buxton JA, Fairbairn N, DeBeck K, Milloy M-J, Kerr T, 2021. Awareness of fentanyl exposure and the associated overdose risks among people who inject drugs in a Canadian setting. Drug Alcohol Rev 10.1111/dar.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidebrecht F, MacLeod MB, Dawkins L, 2018. Predictors of heroin abstinence in opiate substitution therapy in heroin-only users and dual users of heroin and crack. Addict. Behav 77, 210–216. 10.1016/j.addbeh.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, Liu D, Wakim P, Matthews AG, Hatch-Maillette M, Jelstrom E, Wiest K, McLaughlin P, Ling W, 2016. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 111, 695–705. 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RL, Craddock SG, Anderson J, 2003. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS). J. Subst. Abuse Treat 25, 125–134. 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P, 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc 53, 457–481. 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- Kariisa M, Scholl L, Wilson N, Seth P, Hoots B, 2019. Drug overdose deaths involving cocaine and psychostimulants with abuse potential - United States, 2003–2017. MMWR Morb. Mortal. Wkly. Rep 68, 388–395. 10.15585/mmwr.mm6817a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas J, Nosova E, Socías E, Nolan S, Brar R, Hayashi K, Milloy M-J, Kerr T, Wood E, 2018. Factors associated with discontinuation of methadone maintenance therapy (MMT) among persons who use alcohol in Vancouver, Canada. Drug Alcohol Depend 186, 182–186. 10.1016/j.drugalcdep.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann. Intern. Med 169, 137–145. 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gu J, Xu H, Hao C, Jiao M, Zhang X, Zhao Y, Andrew B, Hao Y, 2017. Club drugs and alcohol abuse predicted dropout and poor adherence among methadone maintenance treatment patients in Guangzhou, China. AIDS Care 29, 458–463. 10.1080/09540121.2016.1259452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Kelly E, McLaren J, 2006. The relationship between crystalline methamphetamine use and methamphetamine dependence. Drug Alcohol Depend 85, 198–204. 10.1016/j.drugalcdep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- McNeil R, Puri N, Boyd J, Mayer S, Hayashi K, Small W, 2020. Understanding concurrent stimulant use among people on methadone: a qualitative study. Drug Alcohol Rev 39, 209–215. 10.1111/dar.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin KA, Eibl JK, Franklyn AM, Marsh DC, 2017. The opioid crisis: past, present and future policy climate in Ontario, Canada. Subst. Abuse Treat. Prev. Policy 12, 45. 10.1186/s13011-017-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AM, Cousins G, Durand L, Barry J, Boland F, 2020. Retention of patients in opioid substitution treatment: a systematic review. PLoS One 15, e0232086. 10.1371/journal.pone.0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Provincial Health Officer, 2017. BC Opioid Substitution Treatment System Performance Measures 2014/2015 – 2015/2016 16
- Oviedo-Joekes E, Sordo L, Guh D, Marsh DC, Lock K, Brissette S, Anis AH, Schechter MT, 2015. Predictors of non-use of illicit heroin in opioid injection maintenance treatment of long-term heroin dependence. Addict. Behav 41, 81–86. 10.1016/j.addbeh.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Peles E, Linzy S, Kreek MJ, Adelson M, 2008. One-year and cumulative retention as predictors of success in methadone maintenance treatment: a comparison of two clinics in the United States and Israel. J. Addict. Dis 27, 11–25. 10.1080/10550880802324382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, Polukhina N, 2015. Predictors of patient retention in methadone maintenance treatment. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav 29, 906–917. 10.1037/adb0000090. [DOI] [PubMed] [Google Scholar]
- Reist D, 2010. Methadone Maintenance Treatment in British Columbia, 1996–2008: Analysis and Recommendations. Centre for Addictions Research of BC, University of Victoria, pp. 1996–2008. [Google Scholar]
- Ronsley C, Nolan S, Knight R, Hayashi K, Klimas J, Walley A, Wood E, Fairbairn N, 2020. Treatment of stimulant use disorder: a systematic review of reviews. PLoS One 15, e0234809. 10.1371/journal.pone.0234809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 357, j1550. 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Special Advisory Committee on the Epidemic of Opioid Overdoses, 2021. Opioid-Related Harms in Canada Public Health Agency of Canada, Ottawa. [Google Scholar]
- Stone AC, Carroll JJ, Rich JD, Green TC, 2018. Methadone maintenance treatment among patients exposed to illicit fentanyl in Rhode Island: safety, dose, retention, and relapse at 6 months. Drug Alcohol Depend 192, 94–97. 10.1016/j.drugalcdep.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PGA, Yip B, O’Shaughnessy MV, Montaner JSG, Schlechter MT, Hogg RS, 1998. Barriers to use of free antiretroviral therapy in injection drug users. J. Am. Med. Assoc 280, 547–549. 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- Tardelli VS, Bisaga A, Arcadepani FB, Gerra G, Levin FR, Fidalgo TM, 2020. Prescription psychostimulants for the treatment of stimulant use disorder: a systematic review and meta-analysis. Psychopharmacology (Berl.) 10.1007/s00213-020-05563-3. [DOI] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C, 2016. Retention in medication-assisted treatment for opiate dependence: a systematic review. J. Addict. Dis 35, 22–35. 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, Mcpheeters JT, Crown WH, 2020. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw. Open 3, 1–12. 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Min JE, Krebs E, Evans E, Huang D, Liu L, Hser Y-I, Nosyk B, 2017. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int. J. Drug Policy 49, 32–40. 10.1016/j.drugpo.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teesson M, 2007. The effect of baseline cocaine use on treatment outcomes for heroin dependence over 24 months: findings from the Australian Treatment Outcome Study. J. Subst. Abuse Treat 33, 287–293. 10.1016/j.jsat.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Winkelman TNA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G, 2018. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw. Open 1, e183758. 10.1001/jamanetworkopen.2018.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Kerr T, 2006. What do you do when you hit rock bottom? Responding to drugs in the city of Vancouver. Int. J. Drug Policy 17, 55–60. 10.1016/j.drugpo.2005.12.007. [DOI] [Google Scholar]
- Zhang Z, Friedmann PD, Gerstein DR, 2003. Does retention matter? Treatment duration and improvement in drug use. Addiction 98, 673–684. 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]


