Abstract
Background:
Certain types of hair products are more commonly used by Black women. Studies show hair products contain several endocrine disrupting chemicals that are associated with adverse health outcomes. As chemical mixtures of endocrine disruptors, hair products may be hormonally active, but this remains unclear.
Objective:
To assess hormonal activity of commonly used Black hair products.
Methods:
We identified 6 commonly used hair products (used by >10% of the population) from the Greater New York Hair Products Study. We used reporter gene assays (RGAs) incorporating natural steroid receptors to evaluate estrogenic, androgenic, progestogenic, and glucocorticoid hormonal bioactivity employing an extraction method using bond elution prior to RGA assessment at dilutions from 50–500.
Results:
All products displayed hormonal activity, varying in the amount and effect. Three samples showed estrogen agonist properties at levels from 12.5–20ng/g estradiol equivalent concentrations (EEQ). All but one sample showed androgen antagonist properties at levels from 20–25ng/g androgen equivalent concentrations (AEQ). Four samples showed antagonistic and agonistic properties to progesterone and glucocorticoid.
Significance:
Hair products commonly used by Black women showed hormonal activity. Given their frequent use, exposure to hormonally active products could have implications for health outcomes and contribute to reproductive and metabolic health disparities.
Keywords: Hair preparations, personal care products, estrogen receptor, androgen receptor, glucocorticoid receptor, progesterone receptor, hormone receptor assays
Introduction
Recent studies highlight a pressing racial/ethnic disparity in hair and personal care product use (1–3), with Black women generally using more hair oils, lotions, chemical relaxers, leave-in conditioners, and placenta-containing products (4). Of importance, data suggest that these products may contain hormonally active ingredients, such as endocrine disrupting chemicals (EDCs), including lower molecular weight phthalates and parabens (4, 5). Interestingly, nationally representative data from the United States shows that Black women have among the highest concentrations of these personal care product chemicals compared to other racial/ethnic groups(6, 7), with hair products potentially being an important source of hormonally active chemical exposure(4). Chemicals contained in hair products have been linked to hormonally mediated diseases that are more prevalent among Black women and girls, including early puberty (8), preterm birth (9), obesity (10), and diabetes (11). As such, determining whether ingredients in hair products could contribute to higher exposure to hormonally active chemicals and related diseases among Black women is of key importance to reducing these health disparities.
Understanding the drivers of hair product use as a link to higher hormonally active chemical exposures and hormone-mediated disease risk provides critical insight into why these disparities may exist. An important driver of hair product use among Black women is Eurocentric beauty standards that have impacted social and economic position from post-slavery and colonization to modern day (12). Specifically, straighter hair represented ideal beauty standards and higher social status, requiring Black women to assimilate to white culture (13). A number of Black pioneers developed hair maintenance systems that provided and maintained straighter hair, including Anna Turbo Malone, Elroy J. Duncan, and Madame C.J. Walker. Beyond rinse-out soaps to clean hair, these products included leave-in hair oils and stimulating lotions (13, 14). Today, Black hair care is a half-trillion dollar industry (12, 13), with Black women spending more money on hair styling products than any other ethnic group (13, 15). That said, even recent legislation in the United States has had to be enacted to ensure Black women can wear their hair in ways that could allow for less chemical exposure (16). Of concern, the use of these products may be placing Black women at increased exposure to toxic chemicals—including EDCs—due to these underlying social and political factors that are systemic drivers of beauty and economic status.
While some studies have documented individual chemicals and chemical classes contained in hair products (5, 17), few have evaluated the impact of these products—as chemical mixtures—on alterations to hormone systems (18). In a study by Myers et al., a number of hair and skin care products were assessed for hormone activity (19). Of the eight products assessed, only one product was a leave-in hair product, which showed estrogen activity. With leave-in hair products being commonly used by Black women (1–3), evaluating whether chemicals in these hair products could be agonist or antagonist of hormone receptors linked to diseases is key. Many of the EDCs identified in hair products are thought to bind to and alter hormone receptors including estrogen, progesterone, androgen, and glucocorticoid receptors (20). Of note, these specific hormone receptors are involved in numerous biological processes, which if altered, could contribute to the risk of diseases such as diabetes, cardiovascular disease, as well as adverse reproductive health outcomes (3, 21–24). Thus, hair product use could contribute to a mixture of chemical exposures that could perturb hormonal processes and affect disease risk.
While few studies have evaluated hair products as mixtures, a number of studies have evaluated the hormonal effects of individual chemicals present in hair products. For example, previous work has identified Black hair products as containing phthalates, parabens, alkylphenols, cyclosiloxanes, ethanolamines, and UV filters (5). Alkylphenols have estrogenic properties, with the ability to alter reproductive parameters in rodents (25). Similarly, cyclosiloxanes (26) and certain phenols, including certain parabens and UV filters (27–29) are also estrogenic. Parabens have anti-androgenic properties (30) and can disrupt the glucocorticoid receptor (31, 32), with implications for glucose and other metabolic pathways (33, 34). In addition, phthalates also have anti-androgenic properties and have been shown to be peroxisome-proliferator alpha and gamma agonists (35–37), impacting lipid and glucose metabolism. Together, these chemicals may operate in complex ways to perturb hormonal pathways.
Understanding whether commonly used Black hair products, as chemical mixtures, could bind to and alter hormone signaling will provide critical information to environmental exposure and associated hormone mediated disease disparities. Thus, the objective of this study was to evaluate the extent to which hair products, as EDC mixtures, could bind to and operate as agonists or antagonists of four hormone receptors: estrogen, androgen, progesterone, and glucocorticoid. We selected commonly used hair products based on data from the Greater New York Hair Products Study, which were previously shown to contain EDCs and have been linked to adverse health outcomes (2, 4). As previous studies found estrogenic chemicals (4, 5), and estrogenic activity (19), we hypothesized that these products would be able to bind to the estrogen receptor and act as agonists and included other hormone receptor analyses as exploratory analyses.
Materials and Methods
Selection of hair products
Hair products were selected from the Greater New York Hair Products Study, a cross-sectional study conducted in a population of 359 women from across the New York City metropolitan area between 2004–2006 (2, 4). We used convenience sampling techniques, identifying local churches, nail and hair salons, workplaces, restaurants/cafes, and laundromats as recruitment locations (2, 4). Women had to be over 18 years old, speak English, and self-identify as African-American (descendants of United States slaves), African-Caribbean (Black and born in the Caribbean or their descendants), Hispanic, or non-Hispanic white.
For this study, women completed a detailed survey about their current and past hair product use. The questionnaire was generated from detailed qualitative research data from two focus groups to identify names and categories of hair products, along with information for how to best ask and obtain information on hair product use, frequency, duration, and brand. Using qualitative data that was transcribed and evaluated for themes, a quantitative questionnaire was constructed and queried hair oil, lotion, leave-in conditioner, perm/relaxer, and other product use. In addition to current and past use, data on frequency, duration, and product brand was collected. To aid with recall of current and past use of products, a hair product label book was constructed featuring images of over 150 different products brand names. Additional details on the Greater New York Hair Products Study can be found elsewhere (2, 4).
Hair Product Selection
Among the products listed for current use, we identified 6 specific hair product brand names that had >10% use in the study population and had frequent reported use (i.e. daily or more than once per week) (4). The following products were purchased and sent for independent laboratory testing for hormone receptor assays in 2012: Sample 1, hair lotion; Sample 2, leave-in conditioner; Sample 3, leave-in conditioner; Sample 4, hair oil; Sample 5, root stimulator; Sample 6, hair oil. Label information from the products did not indicate the addition of estrogen or other hormones for most of the hair products. However, the label information did indicate the presence of placenta in one hair oil brand (Sample 6).
Extraction of hair products
Briefly, one gram of each product was extracted in ethyl acetate and cleaned up via solid phase extraction followed by evaporation and final suspension in 250 μL methanol. A more detailed extraction protocol is outlined within supplementary materials for methods on extraction of hair products.
Thiazolyl blue tetrazolium bromide (MTT) analysis
The Thiazolyl blue tetrazolium bromide (MTT) assay was used to control for cytotoxic effects in parallel to RGA analysis of test samples by measuring cellular metabolic activity as an indicator of cellular health. The detailed procedure can be found in supplementary materials for MTT analysis.
Reporter Gene Assay cell lines
The four mammalian reporter gene assay cell lines which were used in this study were previously established by Willemsen et al.(38). For the estrogen receptor assay, the estrogen responsive MMV-Luc cell line was created from MCF-7 cells by transfection with the MAR-Vit-Luc reporter plasmid, and is specific for estrogens and estrogenic compounds. The progestogen responsive TM-Luc cell line was generated using T47D cells (which express high levels of endogenous progestogen receptors) transfected with the MMTV-Luc plasmid, and is specific for progestogens and progestogenic compounds. The TGRM-Luc and TARM-Luc cell lines were co-transfected from the MMTV-Luc reporter plasmid and the RS-hGRα vector (codes for the human GR), or the pSV-AR0 vector (codes for the human AR) into T47D cells. TGRM-Luc and TARM-Luc cell lines are specific for glucocorticoids and progestogens, and for androgens and progestogens, respectively.
The cell lines were routinely cultured in Dulbecco’s modified Eagle medium (DMEM) and 10% fetal bovine serum, and grown in 75cm2 tissue culture flasks (Nunc, Roskilde, Denmark) at 37°C with 5% CO2 and 95% humidity. As phenol red is estrogenic, the MMV-Luc cell line was cultured in phenol red free cell culture medium. RGA analysis was carried out in media containing DMEM supplemented with 10% hormone depleted serum described in supplementary materials for methods on RGA cell lines.
Results
Thiazolyl blue tetrazolium bromide (MTT) analysis
Table 1 illustrates the cytotoxicity results for the six sample extracts at the concentrations used in the four RGA cell lines. The hair lotion (sample 1) induced cytotoxicity on the TM-Luc cells at 1/100 (15%) and 1/200 (10%) dilutions. One of the leave-in conditioners (sample 2) showed dose-dependent cytotoxic effects on all four cell lines, but was most potent in MMV-Luc cells, while the other leave-in conditioner (sample 3) induced cytotoxicity in all four cell lines, but not monotonically. One of the hair oils (sample 4) showed cytotoxic effects in TM-Luc, TARM-Luc and TGRM-Luc cells, but not MMV-Luc cells. While the other hair oil (sample 6), showed the most cytotoxicity in MMV-Luc cells, as well as inducing cytotoxicity in the other 3 cell lines. The root stimulator (sample 5) induced the most potent toxicity in TARM-Luc cells of all six samples, but did not lead to cytotoxicity in either MMV-Luc or TM-Luc cell lines.
Table 1.
Bond Elut: % toxicity of the six product sample extracts at 50, 100, 200 and 500 dilutions as assessed on the four RGA cell lines by MTT assay.
| Cell line | Sample | Extract dilutiona | Cell line | Sample | Extract dilutiona | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/50 | 1/100 | 1/200 | 1/500 | 1/50 | 1/100 | 1/200 | 1/500 | ||||
| MMV-Luc | 1 | 0 | 0 | 0 | - | TM-Luc | 1 | - | 15 | 10 | 0 |
| 2 | 30 | 15 | 0 | - | 2 | - | 10 | 0 | 0 | ||
| 3 | 15 | 15 | 0 | - | 3 | - | 20 | 10 | 0 | ||
| 4 | 0 | 0 | 0 | - | 4 | 20 | 10 | 0 | - | ||
| 5 | 0 | 0 | 0 | - | 5 | 0 | 0 | 0 | - | ||
| 6 | 90 | 20 | 15 | 0 | 6 | - | 20 | 0 | 0 | ||
| TARM-Luc | 1 | 0 | 0 | 0 | - | TGRM-Luc | 1 | 0 | 0 | 0 | 0 |
| 2 | 20 | 10 | 0 | - | 2 | 5 | 0 | 0 | - | ||
| 3 | 10 | 0 | 0 | - | 3 | 10 | 10 | 0 | - | ||
| 4 | 15 | 10 | 0 | - | 4 | 30 | 15 | 0 | - | ||
| 5 | 40 | 10 | 0 | 0 | 5 | 5 | 0 | 0 | - | ||
| 6 | 30 | 10 | 0 | 0 | 6 | 30 | 15 | 5 | 0 | ||
‘-’ indicates did not check dilution due to lack of toxicity at previous dilution
RGA results
The RGA assays tested the estrogenic, androgenic, progestogenic, and glucocorticoid activity of extracted samples. Dose-response curves and EC50 values were calculated for the relevant steroid hormone standards for each assay: 17β-estradiol (MMV-Luc), testosterone (TARM-Luc), progesterone (TM-Luc), and hydrocortisone (TGRM-Luc) (Supplemental Table 1, Supplemental Figure 1). Percent recoveries from the Bond Elut extractions for each RGA were 75–80% for estrogen, 75–80% for androgen, 60% for progestogen, and 100% for glucocorticoid (Supplemental Table 2). Assay results are summarized by product in Table 2 and are described in more detail below. We refer to hormonal activity as measured by luciferase activity induced in the cell line.
Table 2.
The agonistic, antagonistic, and partial agonist activities of 1g of product presented as hormonal equivalent concentrations relative to their respective positive controls.
| RGA | Sample 1 (Hair lotion) | Sample 2 (Leave-in conditioner) | Sample 3 (Leave-in conditioner) | Sample 4 (Hair oil) | Sample 5 (Root stimulator) | Sample 6 (Hair oil) |
|---|---|---|---|---|---|---|
| Estrogen | Agonist with additive response 12.5–20ng/g EEQ | No response observed |
Partial agonist:
Agonist 5–7.5ng/g EEQ Antagonist reducing EEQ by 20–25ng/g |
Antagonist reducing EEQ by 22.5–25ng/g | Agonist 15ng/g EEQ | Antagonist – difficult to estimate due to toxicity. reducing EEQ by 25ng/g (in all dilutions) |
| Androgen | No response observed | Antagonist reducing TEQ by 145ng/g | Antagonist reducing TEQ by 145ng/g | Antagonist reducing TEQ by 131ng/g | No response observed | Antagonist reducing TEQ by 145ng/g |
| Progesterone | Antagonist reducing PEQ by 75ng/g |
Partial agonist:
Agonist 250ng/g PEQ Antagonist reducing PEQ by 125ng/g |
Antagonist reducing PEQ by 200ng/g |
Partial agonist:
Agonist 50ng/g PEQ Antagonist reducing PEQ by 175–225ng/g |
No response observed | No response observed |
| Glucocorticoid | No response observed | Antagonist reducing GEQ by 2500ng/g | Antagonist reducing GEQ by 2000ng/g | Antagonist reducing GEQ by 15002000ng/g | Antagonist reducing GEQ by 500ng/g | No response observed |
Abbreviations: EEQ, Estrogen Equivalents of 17beta-estradiol; TEQ, Testosterone Equivalents of testosterone; PEQ, Progesterone Equivalents of progesterone; GEQ, Glucocorticoid Equivalents of hydrocortisol
The hair lotion, one of the leave-in conditioners (sample 3), and the root stimulator presented agonist effects in the estrogen receptor assay. The hair lotion (sample 1) induced an estrogenic agonist response equivalent to 17beta-estradiol at concentrations close to 12.5 – 20 ng/g of sample (Figure 1). One of the leave-in conditioners (sample 3) induced a partial agonist effect in the estrogen assay, but showed a reduced response when combined with the positive control 17beta-estradiol (Figure 1). The root stimulator (sample 5) induced agonist activity on its own that was equivalent to 17beta-estradiol at a concentration close to 15 ng/g of sample, but elicited a much higher response when spiked hormone was added (Figure 1), indicating a synergistic response in the MMV-Luc cells. The other leave-in conditioner (sample 2) did not induce any change in the estrogen assay (Figure 1). Both hair oils (samples 4 & 6), did not induce estrogenic responses in the MMV-Luc cells, but appeared to be estrogen antagonists by lowering the response of spiked samples (Figure 1).
Figure 1.
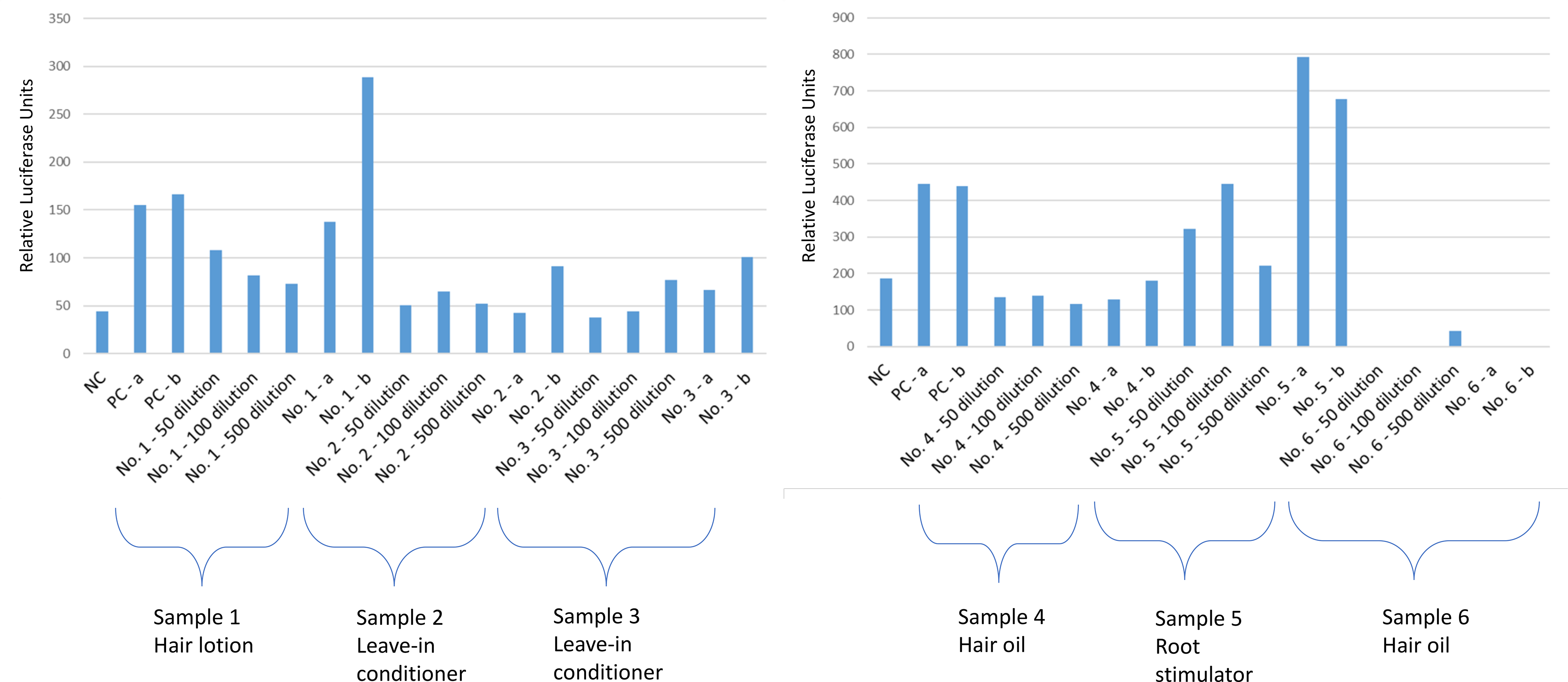
Response of the A) sample 1 (hair lotion), 2 (leave-in conditioner), 3 (leave-in conditioner), and B) sample 4 (hair oil), 5 (root stimulator), 6 (hair oil) as assessed by the estrogen responsive MMV-Luc reporter gene cell line and compared with the negative control. NC, negative control; PC, positive control (0.5 ng/mL E2); a, spiked with positive control before extraction; b, spiked with positive control after extraction.
The androgenic activity of samples was tested in the TARM-Luc cells. Both leave-in conditioners and hair oils showed antagonist activity in the androgen assay, blocking the action of free testosterone. Additionally, both leave-in conditioners and one of the hair oils (sample 6) induced strong antagonist activity in the androgen assay (Figure 2). The other hair oil (sample 4) also showed an antagonist effect. Neither the hair lotion nor the root stimulator showed an androgenic response (Figure 2).
Figure 2.
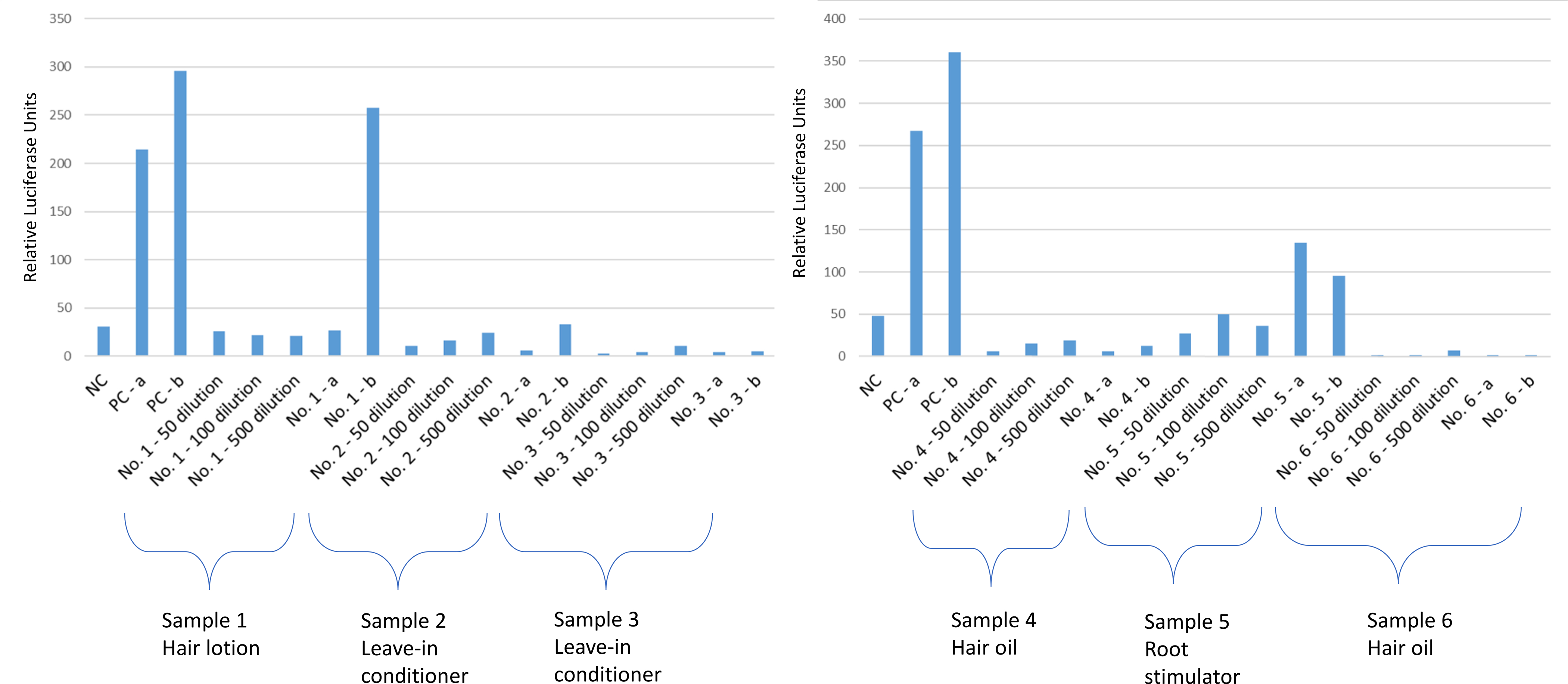
Response of the A) sample 1 (hair lotion), 2 (leave-in conditioner), 3 (leave-in conditioner), and B) sample 4 (hair oil), 5 (root stimulator), 6 (hair oil) as assessed by the androgen responsive TARM-Luc reporter gene cell line and compared with the negative control. NC, negative control; PC, positive control (2.9 ng/mL testosterone); a, spiked with positive control before extraction; b, spiked with positive control after extraction.
Figure 3 presents results for progesterone assays. Both leave-in conditioners, the hair lotion, and one of the hair oils (sample 4) induced antagonistic effects on the progesterone assays. For example, a leave-in conditioner (sample 2) was able to block the action of progesterone at concentrations of 75 ng/g of sample (Figure 3). In addition, this same leave-in conditioner, as well as one of the hair oils (sample 4), showed partial agonist properties. An example of this was seen for this hair oil, which was able to induce a progesterone agonist response equivalent to progesterone at concentrations of 50 ng/g of sample (Figure 3).
Figure 3.
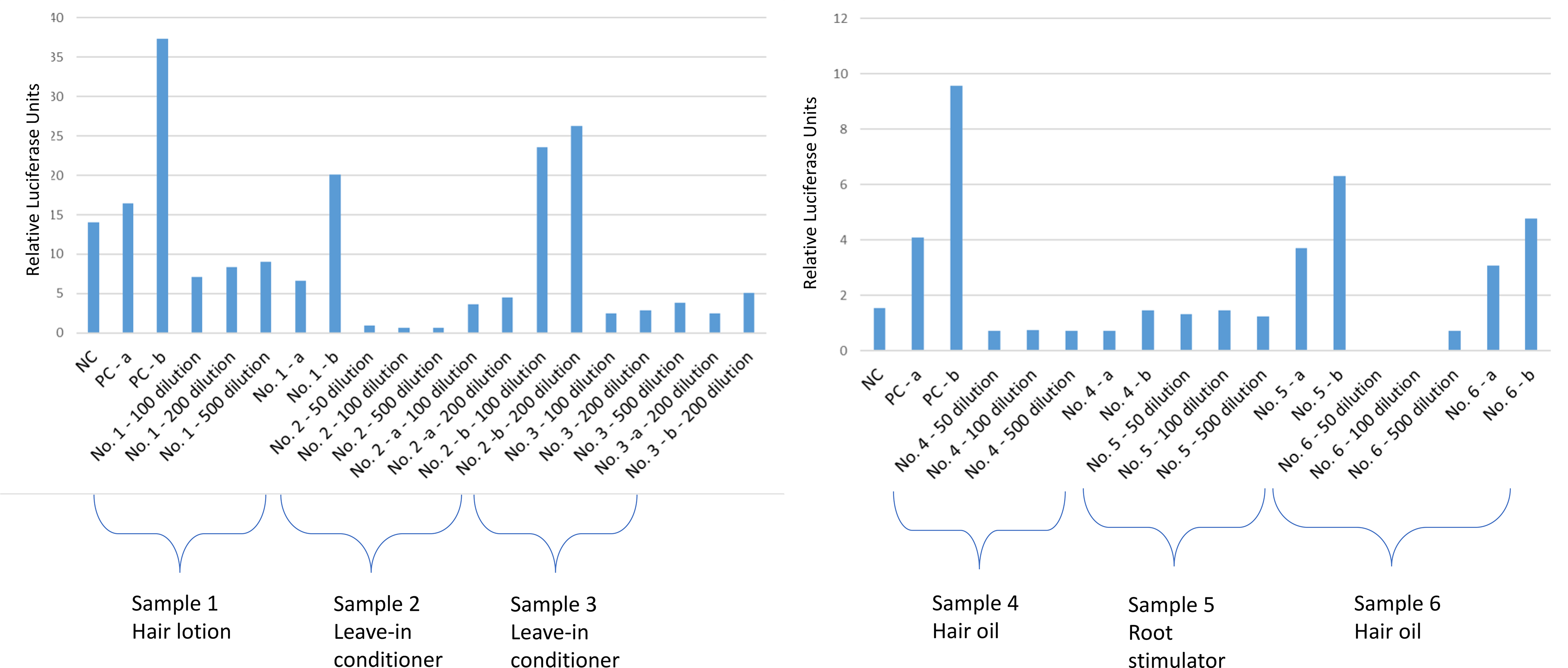
Response of the A) sample 1 (hair lotion), 2 (leave-in conditioner), 3 (leave-in conditioner), and B) sample 4 (hair oil), 5 (root stimulator), 6 (hair oil) as assessed by the androgen responsive TM-Luc reporter gene cell line and compared with the negative control. NC, negative control; PC, positive control (5 ng/mL progesterone); a, spiked with positive control before extraction; b, spiked with positive control after extraction.
In the glucocorticoid assay, both leave-in conditioners, as well as one hair oil (sample 4), and the root stimulator induced antagonistic effects (Figure 4). Both leave-in conditioners showed blocking action of free hydrocortisone at 2000–2500 ng/g of sample (Figure 4). One of the hair oils (sample 4) showed an antagonistic effect by blocking the free hydrocortisone response at 1500–2000 ng/g of sample (Figure 4). The root stimulator lowered the action of free hydrocortisone at concentrations up to 500 ng/g of sample (Figure 4). Conversely, the other hair oil (sample 6) and the hair lotion did not induce responses on the glucocorticoid assay.
Figure 4.
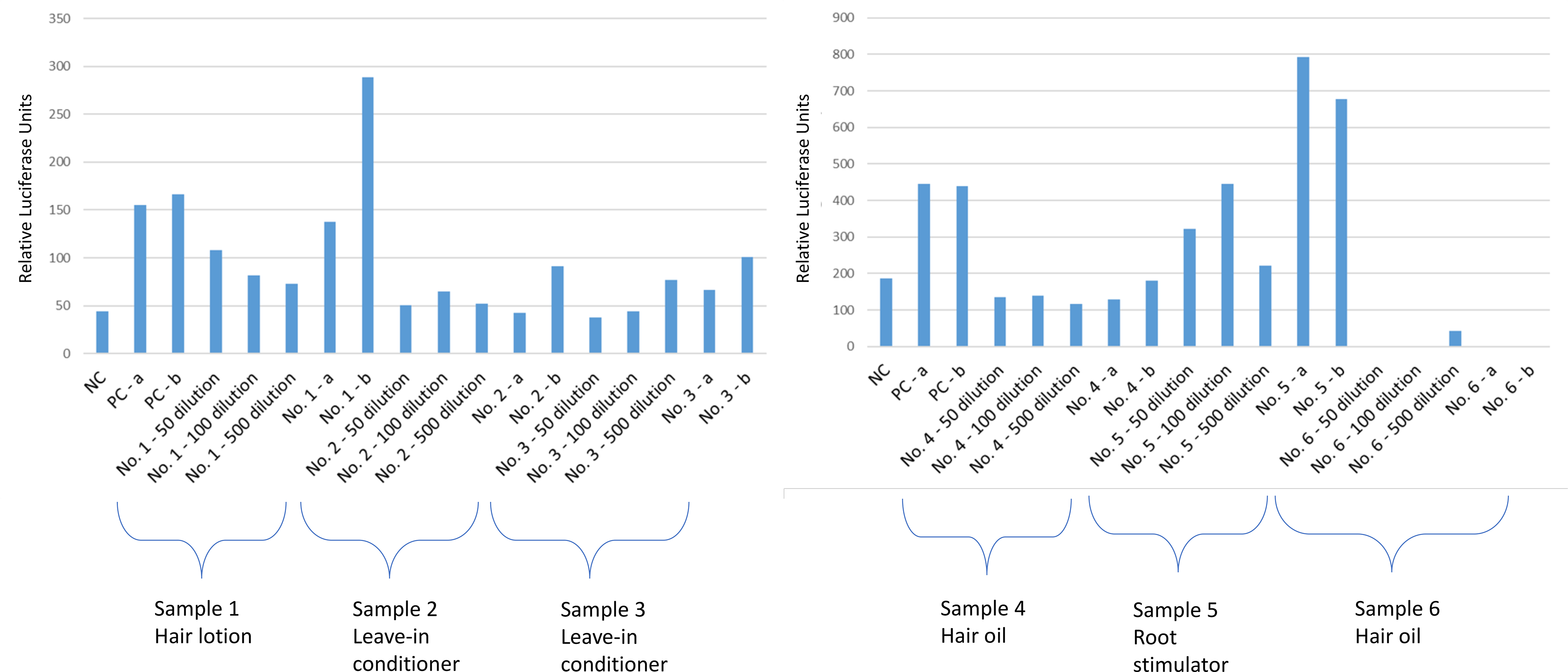
Response of the A) sample 1 (hair lotion), 2 (leave-in conditioner), 3 (leave-in conditioner), and B) sample 4 (hair oil), 5 (root stimulator), 6 (hair oil) as assessed by the androgen responsive TM-Luc reporter gene cell line and compared with the negative control. NC, negative control; PC, positive control (100 ng/mL cortisol); a, spiked with positive control before extraction; b, spiked with positive control after extraction.
Discussion
In this study of six leave-in hair products commonly used by Black women participating in the Greater New York Hair Products Study (2, 4), we found that all products elicited hormonal activity. In general, the type of hormone activity varied by product, with some displaying agonistic properties and others displaying both partial agonistic and antagonistic properties related to one or more of the hormone receptors. One of the leave-in conditioners (sample 3) and one of the hair oils (sample 4), displayed hormonal activity on all 4 hormone assays, potentially indicating that use of these products may be of particular concern in relation to hormonally-mediated disease. The hair lotion and root stimulator both displayed estrogenic activity, but did not show any androgenic activity, while all other samples acted as androgen antagonists. These findings suggest that products commonly used by Black women may have hormonal activity when each product is considered as a chemical mixture. To our knowledge this is one of the first studies to evaluate the hormonal activity of multiple commonly used Black leave-in hair products.
Few studies have assessed chemical content of Black hair products. One previous study by Helm et al. using a greater number of hair products from the same study population found that all of the hair products tested contained endocrine disrupting and asthma-associated chemicals (5). Specifically, the study found that these commonly used hair products contained 45 EDCs, including cyclosiloxanes, parabens, fragrance marker diethyl phthalate (DEP), and nonylphenols (5). In an earlier study in the same population, 50% of Black women reported using products containing hormones or placenta compared to only 7% of white women based on product label information (4). A separate study focusing more broadly on personal care products, found that a number of products, including the only tested hair product, displayed hormonal activity using a similar reporter gene assay model (19). Our study suggests the need to further evaluate hormonal activity in personal care products, particularly those used by individuals with higher use and a higher risk of many hormonally mediated adverse health outcomes.
Studies show that hair products are very commonly used by Black women, with reported usage of almost 100% for hair oils, >70% for hair lotions, and >50% for leave-in conditioners (2, 4). These products are often applied to the hair and scalp and not washed out for days or weeks, allowing chemicals in the product to accumulate on the scalp and potentially enter the body transdermally through porous scalp tissue (39). A recent study by Hsieh et al showed greater chemical exposure for leave-in products (40), it is unclear whether the findings from this work can extend to other Black hair care products. Helm et al. identified the presence of diethyl phthalate and methyl and propyl parabens (5), which could suggest repeated and cumulative exposures to these chemicals from hair product use. Worth noting, a biomonitoring study comprised of a representative sample of U.S. women participating in NHANES found that non-Hispanic Black women had significantly higher concentrations of methyl paraben, propyl paraben, and mono-ethyl phthalate—a metabolite of di-ethyl phthalate—chemicals found in hair products (4, 41).
Previous work has shown that mixtures of certain EDCs, such as phthalates, parabens, as well as UV filters can have additive anti-androgenic properties (42) or additive estrogenic properties (43). In the present study, we found that the root stimulator had additive agonist properties with the estrogen receptor. We also found that all other products had anti-androgenic properties. This is one of the first studies to look at progesterone and glucocorticoid properties of multiple leave-in Black hair care products, where each was evaluated as a mixture.
In the present study, leave-in conditioners and one of the hair oils had the highest degree of hormonal activity, with agonist properties for estrogen and progesterone and antagonist properties for androgen, progesterone, and glucocorticoid receptors. These findings suggest that leave-in hair products could be particularly harmful. Indeed, a recent study found that leave-in products were a source of higher mono-ethyl and mono-benzyl phthalate exposure (40). As such, re-formulation could potentially provide an opportunity for these leave-in products to become safer and reduce exposure to hormonally active chemicals and associated health outcomes.
While few studies have assessed the chemical content and hormonal activity of hair products for women in general and particularly for Black women, studies of hair product use more broadly have shown associations with hormonally-mediated conditions. For example, a previous study by our group involving the same study population found that hair oil use was associated with 40% odds of earlier age at menarche (2). In that study, associations with earlier age at menarche were stronger when hair oils were used for a longer duration of time. A separate study conducted by McDonald et al. using the same study questionnaire found that hair oil use was associated with a 2-fold increased odds of reaching menarche before age 11 (41). Products with estriol concentrations ranging from 9 to 24 mg/g are associated with premature development of secondary sexual characteristics and pre-pubertal gynecomastia (19). Furthermore, many of the chemicals found in these hair products(5) are linked to the disparate conditions of diabetes and obesity (44, 45). As such, more work is needed to determine whether exposure to hormonally-active hair products might contribute to these persistent health disparities.
The present study provides biologic plausibility for how exposure to higher levels of hair products might impact hormonally-mediated disease by perturbing normal hormone activity through agonistic or antagonistic processes. For example, several of the hair products were either estrogen agonist or antagonist based on an assay for 17-beta estradiol. There are three physiologic forms of estrogen—estrone, estradiol, and estriol—each with different relative potency, with estradiol being the most potent (46). Estrogen receptors (ER) are involved in estrogen signaling pathways, both stimulatory and inhibitory, found in neuroendocrine, skeletal, adipogenesis, and cardiovascular systems. Receptor activity is dependent on the two types of nuclear receptors, ERα and ERβ (21). Furthermore, each tissue has a unique threshold at which estrogen may mediate physiologic versus pathophysiologic processes (47). Because estrogen is related to several crucial pathways in the body, disruption in signaling can increase the risk of health conditions, such as breast and ovarian cancer (48, 49), as well as cardiovascular disease (50). Likewise, androgen receptors mediate the activity of androgens such as testosterone and dihydrotestosterone with biological actions that include development and maintenance of reproductive, musculoskeletal, cardiovascular, and immune systems (22). Glucocorticoid receptor (GR) activity is essential for regulating stress response. Glucocorticoid signaling affects a variety of organs in the body with their anti-inflammatory, anti-proliferative, pro-apoptotic, and anti-angiogenic properties (51). An imbalance in the regulation of GR activity in the heart may result in cardiovascular disease and similarly, imbalance in the lungs may exacerbate respiratory disease (3). Progesterone receptors (PR) with two common isoforms Pr-A and Pr-B, act as sensors for growth factor-induced signaling and are involved in pro-proliferative signaling in the breast and uterine quiescence during pregnancy (52). Along with estrogen receptors, PR are crucial in the female reproductive system and disruption may lead to breast cancer, uterine disease, and preterm labor (23, 24). Therefore, the present study’s findings may provide evidence for a group of hormonally active consumer products marketed to Black women that could potentially explain part of the increased risk of hormonally mediated diseases linked to these specific hormones.
Related to disparate hormonally mediated health conditions, estrogen drives the pubertal transition in humans and is involved in breast cancer and is comparatively a stronger estrogen than estriol (53). Black girls reach menarche earlier (8) and Black women have a higher mortality due to breast cancer, often due to a higher incidence of a subtype of the disease that is less responsive to hormone therapy treatment (54). Considering data evaluating hair products and these conditions, two studies found associations between hair products (e.g. hair dyes and relaxers) with an increased breast cancer risk (55, 56). In the present study, it is worth noting that while three of the products showed estrogen agonist properties, one of these 3 along with two others showed estrogen antagonist properties. These antagonist properties countered our original hypothesis, but could also have biological effects in women, including hot flashes (57) and weight gain (58). Future work is needed to understand the full scope by which these hormonally active consumer products might impact health and health disparities.
It is important to note that product regulation in the United States is less strict than in other settings. For example, chemicals commonly used in personal care products in the United States have been banned in the European Union (59). Some U.S. states, such as California, require warning information on products containing certain hormone-altering chemicals, including benzophenone, bisphenol A, and nonylphenol under Proposition 65 (5). However, due to U.S. federal exemptions for the labeling of incidental chemicals, some of these chemicals of concern do not appear on the product label, including fragrance ingredients such as phthalates (5, 37). Yet, these chemicals separately—and potentially as a mixture—could have important health implications, especially for populations that are more likely to frequently use these products. Based on the present findings of hormonal activity of these hair products and previous work showing that these chemicals are present in hair products, future work is needed to help provide a better understanding of how these policies may impact the development of safer products, with implications on EDC exposure reduction and potential decreases in EDC-associated adverse health outcomes.
This study has several limitations. First, we tested a small number of hair products identified as being commonly used from a study conducted in 2004–2006. Hair products and their formulations have changed over time. However, this previous study was the first to collect information on Black hair care product use, including details on product brand, type, and frequency of use (2, 4). Interestingly, more recent data still ranks some of these hair products within the top 20 products more commonly used by this demographic (60). That said, differences in formulations may have occurred over time, which could impact the hormonal activity of these products. Second, the hair products were quite toxic to the cell lines. However, we were able to improve the assays by adding an additional pre-treatment step to reduce contaminant toxicity. Third, this was an exposure assessment study to evaluate hormonal activity of hair products as product-specific mixtures. We are uncertain as to what specific ingredient may be driving the particular activity seen, as the product formulations may have changed since our laboratory analysis was conducted. Future work will need to evaluate both the individual components, as well as the mixture, to identify the most biologically active ingredients, or combination of ingredients, to help identify safer replacements. Additionally, the focus of this study was to evaluate hair products commonly used by Black women and not the full breadth of personal care products. While women use multiple personal care products at any given period, hair products are a particularly prevalent personal care product used by Black women and have been understudied with respect to their content and biological activity. Finally, while we measured different types of hair products, we only evaluated this in 6 different specific brands of hair products. Thus, this study’s findings may not be generalizable to other brands of hair products; instead, this should provide rationale for further study of hair and other products that may be more commonly used by Black women and other groups with higher exposure concentrations to personal care product chemicals.
Despite these limitations, our study has several notable strengths. First, this is one of the first studies to evaluate the hormonal activity of commonly used leave-in Black hair products assessed as mixtures. While data suggests that Black women may have higher body burdens of certain EDCs (6, 7), as well as higher prevalence of health conditions linked to these chemicals, few studies have looked at hair products to evaluate whether they could have biological activity. Second, we measured different types of hair products, including hair oils, leave-in conditioners, lotions, and root stimulators. While all hair products tested had hormonal activity, patterns differed by product and type. Third, this study provides novel data on the biological plausibility for how chemicals found in hair products may act together to alter hormonally mediated diseases, including those that are racially/ethnically disparate.
Conclusions
In the present study we found that commonly used hair products are hormonally active, with impacts on the estrogen, androgen, progesterone, and glucocorticoid receptors, providing critical information for understanding how leave-in hair products as a mixture could have a biological effect and potentially affect hormonally mediated disease risk. Previous findings from this same study population have shown that these products contain di-ethyl phthalate and parabens, chemicals known to be hormonally active (5). Given that hormonal activity of consumer products could contribute to disparities in related disease risk, re-formulation of these products could aid in reducing the disparity in exposure and associated health outcomes in Black women. Further work is needed to understand how these products may act as a source of disparate environmental chemical exposures and the resulting implications for adverse health outcomes in Black women.
Supplementary Material
Acknowledgements:
This study was funded in part by the March of Dimes, the American Diabetes Association Minority Postdoctoral Fellowship, and the National Institutes of Health (R01ES026166, T32ES007069, and Black Women’s Health Study R01CA058420). We would like to acknowledge Erika Rodriguez and Victoria Fruh for facilitating parts of this work.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Gaston SA, James-Todd T, Harmon Q, Taylor KW, Baird D, Jackson CL. Chemical/straightening and other hair product usage during childhood, adolescence, and adulthood among African-American women: potential implications for health. Journal of exposure science & environmental epidemiology. 2020;30(1):86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James-Todd T, Terry MB, Rich-Edwards J, Deierlein A, Senie R. Childhood hair product use and earlier age at menarche in a racially diverse study population: a pilot study. Annals of epidemiology. 2011;21(6):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34(9):518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James-Todd T, Senie R, Terry MB. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immigr Minor Health. 2012;14(3):506–11. [DOI] [PubMed] [Google Scholar]
- 5.Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448–58. [DOI] [PubMed] [Google Scholar]
- 6.Calafat AM, Ye X, Wong L-Y, Bishop AM, Needham LL. Urinary concentrations of four parabens in the US population: NHANES 2005–2006. Environmental health perspectives. 2010;118(5):679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang T, Saxena AR, Isganaitis E, James-Todd T. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environmental Health. 2014;13(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deardorff J, Abrams B, Ekwaru JP, Rehkopf DH. Socioeconomic status and age at menarche: an examination of multiple indicators in an ethnically diverse cohort. Ann Epidemiol. 2014;24(10):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burris HH, Hacker MR. Birth outcome racial disparities: A result of intersecting social and environmental factors. Semin Perinatol. 2017;41(6):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Jama. 2010;303(3):235–41. [DOI] [PubMed] [Google Scholar]
- 11.Demmer RT, Zuk AM, Rosenbaum M, Desvarieux M. Prevalence of diagnosed and undiagnosed type 2 diabetes mellitus among US adolescents: results from the continuous NHANES, 1999–2010. Am J Epidemiol. 2013;178(7):1106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson TA, Bankhead T. Hair It Is: Examining the Experiences of Black Women with Natural Hair. Open Journal of Social Sciences. 2014;02(01):86–100. [Google Scholar]
- 13.Teteh DK, Montgomery SB, Monice S, Stiel L, Clark PY, Mitchell E. My crown and glory: Community, identity, culture, and Black women’s concerns of hair product-related breast cancer risk . Cogent Arts & Humanities. 2017;4(1):1345297. [Google Scholar]
- 14.Byrd A, Tharps L. Hair Story: Untangling the Roots of Black Hair in America. New York, NY: St. Martin’s Press; 2001. [Google Scholar]
- 15.Mintel. Natural hair movement drives sales of styling products in US black haircare market 2015. [Available from: https://www.mintel.com/press-centre/beauty-and-personal-care/natural-hair-movement-drives-sales-of-styling-products-in-us-black-haircare-market.
- 16.Lee MS, Nambudiri VE. The CROWN act and dermatology: Taking a stand against race-based hair discrimination. Journal of the American Academy of Dermatology. 2020. [DOI] [PubMed] [Google Scholar]
- 17.Parlett LE, Calafat AM, Swan SH. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2013;23(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217(4):418.e1–.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers SL, Yang CZ, Bittner GD, Witt KL, Tice RR, Baird DD. Estrogenic and anti-estrogenic activity of off-the-shelf hair and skin care products. Journal of exposure science & environmental epidemiology. 2015;25(3):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakkiah S, Wang T, Zou W, Wang Y, Pan B, Tong W, et al. Endocrine Disrupting Chemicals Mediated through Binding Androgen Receptor Are Associated with Diabetes Mellitus. Int J Environ Res Public Health. 2017;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HR, Kim TH, Choi KC. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res. 2012;28(2):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev. 2016;37(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel AR, Hagan CR, Lange CA. Progesterone receptor action: defining a role in breast cancer. Expert Rev Endocrinol Metab. 2011;6(3):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst Rev. 2012(3):CD002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markey CM, Michaelson CL, Sonnenschein C, Soto AM. Alkylphenols and bisphenol A as environmental estrogens. Endocrine Disruptors–Part I: Springer; 2001. p. 129–53. [Google Scholar]
- 26.Fessenden RJ, Fessenden JS. Trends in Organosilicon Biological. Advances in Organometallic Chemistry. 1980:275. [Google Scholar]
- 27.Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from” modified” polystyrene. Environmental health perspectives. 1991;92:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Meeuwen J, Van Son O, Piersma A, De Jong P, Van Den Berg M. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicology and applied pharmacology. 2008;230(3):372–82. [DOI] [PubMed] [Google Scholar]
- 29.Prusakiewicz JJ, Harville HM, Zhang Y, Ackermann C, Voorman RL. Parabens inhibit human skin estrogen sulfotransferase activity: possible link to paraben estrogenic effects. Toxicology. 2007;232(3):248–56. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicology and applied pharmacology. 2007;221(3):278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Del Toro LVA, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environmental research. 2016;151:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klopčič I, Kolšek K, Dolenc MS. Glucocorticoid-like activity of propylparaben, butylparaben, diethylhexyl phthalate and tetramethrin mixtures studied in the MDA-kb2 cell line. Toxicology letters. 2015;232(2):376–83. [DOI] [PubMed] [Google Scholar]
- 33.Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinology and metabolism. 2017;32(2):180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Dalmazi G, Pagotto U, Pasquali R, Vicennati V. Glucocorticoids and type 2 diabetes: from physiology to pathology. Journal of nutrition and metabolism. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annual review of physiology. 2011;73:135–62. [DOI] [PubMed] [Google Scholar]
- 36.Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8(2):161–71. [DOI] [PubMed] [Google Scholar]
- 37.Wallack G Rethinking FDA’s Regulation of Cosmetics. Harv J on Legis. 2019;56:311. [Google Scholar]
- 38.Willemsen P, Scippo ML, Kausel G, Figueroa J, Maghuin-Rogister G, Martial JA, et al. Use of reporter cell lines for detection of endocrine-disrupter activity. Anal Bioanal Chem. 2004;378(3):655–63. [DOI] [PubMed] [Google Scholar]
- 39.Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93(6):289–93. [PubMed] [Google Scholar]
- 40.Hsieh CJ, Chang YH, Hu A, Chen ML, Sun CW, Situmorang RF, et al. Personal care products use and phthalate exposure levels among pregnant women. Sci Total Environ. 2019;648:135–43. [DOI] [PubMed] [Google Scholar]
- 41.McDonald JA, Tehranifar P, Flom JD, Terry MB, James-Todd T. Hair product use, age at menarche and mammographic breast density in multiethnic urban women. Environmental Health. 2018;17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orton F, Ermler S, Kugathas S, Rosivatz E, Scholze M, Kortenkamp A. Mixture effects at very low doses with combinations of anti-androgenic pesticides, antioxidants, industrial pollutant and chemicals used in personal care products. Toxicology and applied pharmacology. 2014;278(3):201–8. [DOI] [PubMed] [Google Scholar]
- 43.Evans RM, Scholze M, Kortenkamp A. Additive mixture effects of estrogenic chemicals in human cell-based assays can be influenced by inclusion of chemicals with differing effect profiles. PLoS One. 2012;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1–2):63–8. [DOI] [PubMed] [Google Scholar]
- 45.Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, et al. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res. 2000;8(3):211–8. [DOI] [PubMed] [Google Scholar]
- 46.Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47(4):269–75. [DOI] [PubMed] [Google Scholar]
- 47.Friedman AJ, Lobel SM, Rein MS, Barbieri RL. Efficacy and safety considerations in women with uterine leiomyomas treated with gonadotropin-releasing hormone agonists: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1990;163(4 Pt 1):1114–9. [DOI] [PubMed] [Google Scholar]
- 48.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6):s18–s24. [DOI] [PubMed] [Google Scholar]
- 49.Samtani R, Sharma N, Garg D. Effects of endocrine-disrupting chemicals and epigenetic modifications in ovarian cancer: a review. Reproductive Sciences. 2018;25(1):7–18. [DOI] [PubMed] [Google Scholar]
- 50.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. The American journal of cardiology. 2002;89(12):12–7. [DOI] [PubMed] [Google Scholar]
- 51.Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patil AS, Swamy GK, Murtha AP, Heine RP, Zheng X, Grotegut CA. Progesterone Metabolites Produced by Cytochrome P450 3A Modulate Uterine Contractility in a Murine Model. Reprod Sci. 2015;22(12):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Partsch CJ, Sippell WG. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update. 2001;7(3):292–302. [DOI] [PubMed] [Google Scholar]
- 54.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. Jama. 2015;313(2):165–73. [DOI] [PubMed] [Google Scholar]
- 55.Llanos AAM, Rabkin A, Bandera EV, Zirpoli G, Gonzalez BD, Xing CY, et al. Hair product use and breast cancer risk among African American and White women. Carcinogenesis. 2017;38(9):883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eberle CE, Sandler DP, Taylor KW, White AJ. Hair dye and chemical straightener use and breast cancer risk in a large US population of black and white women. International journal of cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. The Journal of steroid biochemistry and molecular biology. 2014;142:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Annals of internal medicine. 1995;123(9):673–5. [DOI] [PubMed] [Google Scholar]
- 59.Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. The lancet Diabetes & endocrinology. 2020;8(8):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sales growth of leading 20 ethnic hair care brands in th United States in 2014. Consumer Goods & FMCG - Cosmetics & Personal Care. Statista 2014. [Available from: https://www.statista.com/statistics/315501/sales-growth-top-ethnic-hair-brands-us/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


