Abstract
It is well established that by modulating various immune functions, host infection may alter the course of concomitant inflammatory diseases, of both infectious and autoimmune etiologies. Beyond the major impact of commensal microbiota on the immune status, host exposure to viral, bacterial, and/or parasitic microorganisms also dramatically influences inflammatory diseases in the host, in a beneficial or harmful manner. Moreover, by modifying pathogen control and host tolerance to tissue damage, a coinfection can profoundly affect the development of a concomitant infectious disease. Here, we review the diverse mechanisms that underlie the impact of (co)infections on inflammatory disorders. We discuss epidemiological studies in the context of the hygiene hypothesis and shed light on the sometimes dual impact of germ exposure on human susceptibility to inflammatory disease. We then summarize the immunomodulatory mechanisms at play, which can involve pleiotropic effects of immune players and discuss the possibility to harness pathogen-derived compounds to the host benefit.
Introduction
The main functions of our immune system are to provide defenses against invasion by pathogens and tumor cells and to promote tissue homeostasis and repair. Through the process of immune tolerance, the immune system can distinguish self and nonself so that an immune response develops against nonself elements, while no harm is inflicted upon self. The disruption of tolerance may lead to the development of autoimmune diseases, which manifest by an attack on self-tissues as if they were foreign.
The efficiency of the immune system to defend against pathogens, but also the severity of its attacks against self after tolerance breakdown, are largely affected by intrinsic (for example, genetics) and extrinsic factors (for example, environmental cues or exposome), including exposure to pathogenic and/or commensal microorganisms. It is now well established that by modulating various immune functions, host infection may alter the course of concomitant inflammatory diseases, of both infectious and autoimmune etiologies. In fact, infections can either ameliorate or aggravate the clinical outcome of an inflammatory disorder. Numerous studies have contributed to identify the variety of mechanisms that govern the immune modulation by microorganisms during a defined pathology. In some cases, with helminths in particular, some of the molecules and pathways discovered are even being clinically tested as new therapeutic strategies against specific autoimmune diseases.
In this review, we aim to provide a comprehensive overview of the major mechanisms by which exposure to infectious agents throughout the host lifetime shapes the immune status.
Hygiene hypothesis: Is germ exposure always beneficial for the host?
In 1989, Strachan proposed for the first time the hygiene hypothesis for allergic diseases based on the fact that hay fever was less common in children with older siblings [1]. He reasoned that older children might have been less frequently exposed to microorganisms compared to their younger siblings and proposed that microbial exposure in early life could later protect against hypersensitivities. This hypothesis was supported by several epidemiological studies and has been extended not only to other allergic but also to autoimmune diseases. In the past few decades, the incidence of autoimmune and allergic diseases, such as asthma, atopic dermatitis, type 1 diabetes (T1D) and multiple sclerosis (MS) has indeed increased in more industrialized compared to less industrialized countries [2,3]. While several factors such as genetics, exposure to sun and vitamin D, and socioeconomic levels may in part explain this increase, a strong correlation with the decreased incidence of infectious diseases has been noted. For example, Sotgiu and colleagues reported a correlation between the increase of MS incidence and the eradication of malaria in Sardinia [4]. A lower risk to develop MS and T1D has also been linked to early exposure to a diverse microbial community. For example, naturally helminth-infected and/or treated MS patients showed less exacerbation and fewer magnetic resonance imaging changes compared to uninfected and placebo patients, respectively [5,6]. In addition, a study on a large cohort in Finland showed that children who spent their childhood with an indoor dog, which is thought to increase the probability of exposure to germs, had a reduced chance of developing T1D compared to children without an indoor dog [7]. In Gabon and Vietnam, 2 independent studies have found that schoolchildren infected with Schistosoma or Ascaris nematodes presented lower levels of allergen reactivity compared to their uninfected classmates [8,9]. These observations were supported by the finding that anthelminthic treatment of infected children resulted in an increased atopic reactivity [10]. A case–control study performed in Japan on 4,000 patients showed that the frequency of infection by Strongyloides stercoralis, a nematode parasite that infects the lungs and intestine, was lower in the group of patients with autoimmune liver diseases than in the control group [11]. Elsewhere, children reported to have taken antibiotics during infancy had a greater risk to develop inflammatory bowel disease [12], asthma, eczema, and hay fever later in life [13]. Taken together, these studies highlight a positive correlation between early exposure to microbes and reduced risk of autoimmune/allergic disorders.
Besides these beneficial effects, it has also been documented that exposure to microbes could induce or aggravate autoimmune/allergic disorders. Infection with Epstein–Barr virus (EBV) correlates with the development of systemic lupus erythematosus (SLE) and MS, as SLE and MS patients display higher seroprevalence of EBV compared to healthy controls [14,15] and some autoreactive CD4+ T cell clones can cross-react with EBV peptides [16]. Moreover, different types of pathogens like Acinetobacter and Pseudomonas aeruginosa (P. aeruginosa) have been associated with the induction of MS [17]. Several viruses have been correlated with T1D. For example, Coxsackie virus B4 can be detected in pancreatic but also intestinal biopsies of patients with T1D, especially after a recent onset of the disease [18].
Lastly, it has also become clear that coinfection can profoundly impact the clinical outcome of a concomitant infectious disease. A recent emblematic example is the Leishmania parasite-borne RNA virus (LRV) that infects and replicates within certain isolates of Leishmania parasites and has been correlated with treatment failure and metastasis of Leishmania lesions in patients [19,20]. Beyond LRV, EBV/malaria coinfection in children from sub-Saharan Africa can either cause an exacerbation of malaria or the development of EBV-related pathologies [21]. Moreover, a sero-epidemiological study among Tanzanian infants showed that those who developed severe malaria by 1 year of age were more likely to have been seropositive for filarial antigen earlier in life [22]. However, since the filarial and plasmodial parasites can share the same mosquito vector, a limitation of this work is that filarial seroreactivity may just be the sign of a higher risk of exposure to Plasmodium, independently from a direct impact of filarial parasites on malaria severity. In contrast, a study of Senegalese children showed that those who were mildly infected with Schistosoma haematobium had a lower density of Plasmodium falciparum compared to non-coinfected children [23]. In line with this observation, coinfection with Ascaris lumbricoides was associated with a reduction in cerebral malaria risk [24]. These differences may be explained by the nature of the helminth parasites and the age of children, as younger children may respond differently to coinfection.
In summary, multiple lines of evidence indicate that exposure to viruses, parasites, or bacteria can positively or negatively regulate the clinical outcome of concomitant autoimmune or infectious diseases. We will now discuss the major immune modulatory mechanisms that underlie this phenomenon.
Immunomodulation through type I interferons: Antiviral cytokines with high immunomodulatory potential
Almost all cells can produce type I interferons (IFN-I) and express their receptor. IFN-I play a pivotal role in antiviral defense, but since these cytokines have diverse effects on a wide variety of immune cells, they can differently modulate many pathologies. In the case of murine cutaneous leishmaniasis, copresence of LRV worsens the severity and metastasis of skin lesions by inducing the production of proinflammatory cytokines and chemokines such as IL-6, TNF-α, CCL5, CXCL10, and IFN-I via the activation of the TLR3-TRIF pathway in macrophages [25]. Interestingly, not only LRV but also coinfection with exogenous, nonparasite-borne viruses like lymphocytic choriomeningitis virus (LCMV) and Toscana virus increase Leishmania pathology due to IFN-I production in this mouse model. One of the effects of IFN-I is to down-regulate the expression of IFN-γ receptor that is normally required for Leishmania parasite control [26]. A similar detrimental role of IFN-I was recently reported during experimental P. aeruginosa infection. In this case, a filamentous bacteriophage produced by the bacteria exacerbates P. aeruginosa–mediated pathology through the induction of IFN-I via the TLR3-TRIF pathway, causing a reduction in TNF-α expression and altered bacterial phagocytosis by macrophages [27]. In addition to their impact on parasitic and bacterial infections, viral infections can change the prognosis of fungal diseases. A recent study in human macrophages has reported a virus-mediated enhancement of vomocytosis (a nonlytic extrusion leading to the expulsion of engulfed microbes by phagocytes) of the fungus Cryptococcus neoformans in an IFN-I–dependent manner [28].
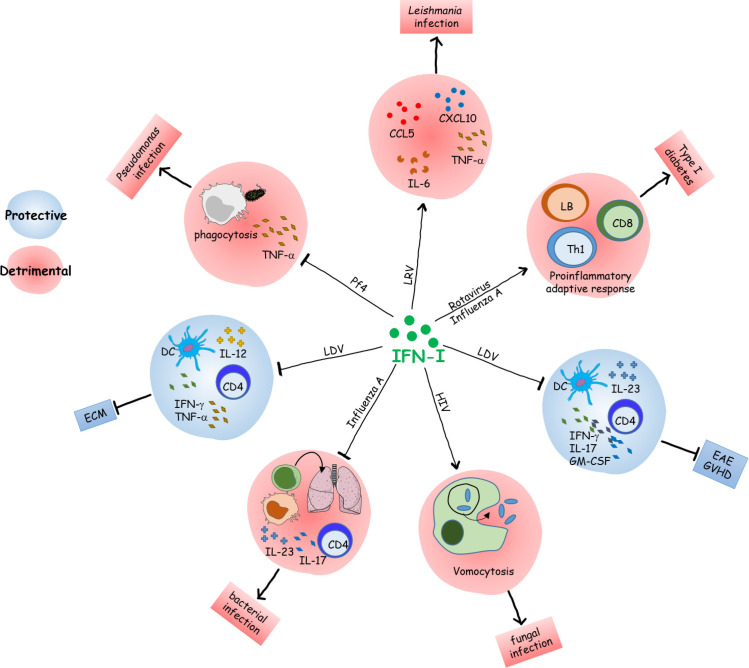
Another example illustrating the role of IFN-I is the infection by influenza (flu) virus, which affects millions of people around the world each year. Flu infection is often associated with secondary respiratory bacterial infections, which can dramatically worsen the clinical outcome of susceptible patients. Several studies in animal models have shown that previous or simultaneous influenza A virus infection enhances susceptibility to gram-negative and gram-positive bacterial pneumonia and that the pathology is promoted by IFN-I signaling. Mechanistically, IFN-I inhibits macrophage and neutrophil recruitment to the lung by altering the production of several chemoattractants like CXCL1 and CXCL2 through transcriptional and epigenetic modulation [29,30]. In addition, IFN-I suppresses type 17 immune responses by decreasing IL-17 and IL-23 production, and ROR-γt expression [31,32]. The deleterious impact of IFN-I was also observed on dendritic cells (DCs). In fact, concurrent pulmonary infection with influenza A virus is associated with an IFN-I–mediated decrease in MHC-I and MHC-II expression on DC, resulting in reduced activation of CD4+ and CD8+ T cells, and impaired clearance of Mycobacteria [33]. Such immune impairment could explain why a clinical study reported the reactivation of tuberculosis in some patients undergoing IFN-I treatment for chronic viral hepatitis [34]. Altogether, these results indicate that IFN-I can act negatively on a large scale by modulating a variety of innate and adaptive immune cells (Fig 1).
Fig 1. Functional modulation of immune cells by infection-mediated IFN-I.
Several immune functions can be regulated negatively or positively by IFN-I produced during concomitant pathologies, resulting in protective or detrimental phenotypes. DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis; ECM, experimental cerebral malaria; GVHD, graft versus host disease; IFN-I, type I interferon; LDV, lactate dehydrogenase-elevating virus; LRV, Leishmania parasite-borne RNA virus.
Conversely, in some situations, the immunomodulatory effects of IFN-I can play a protective role for the host. For example, coinfection with lactate dehydrogenase-elevating virus (LDV) protects mice against Plasmodium berghei ANKA-mediated experimental cerebral malaria, a vascular pathology that is typically caused by CD8+ effector T cells and is sustained by CD4+ Th1 responses. In this case, LDV induces the production of IFN-I, which promotes a decrease in the number of DC and in their capacity to produce IL-12, and consequently in their ability to induce Th1 responses. The critical role of IFN-I was confirmed as Ifnar1 KO mice display normal DC number and functions upon LDV infection [35]. These data are in line with the previous observations that (i) IFN-I elicits DC death by inducing the expression of certain proapoptotic proteins [36] and that (ii) IFN-I can regulate, in a dose-dependent manner, the transcription of IL-12p40 in DC by modifying the phosphorylation of STAT molecules [37].
IFN-I triggered by infection can also modulate the outcome of autoimmune disorders. Pane and colleagues suggested that rotavirus infection accelerates diabetes in nonobese diabetic (NOD) mice by inducing IFN-I release by plasmacytoid DC (pDC) through the TLR7/MDA5 pathways. Secreted IFN-I induces the activation of B and T cells, including autoreactive CD8+ T cells [38]. Accordingly, several studies have linked respiratory virus-induced IFN-I to T1D aggravation. For example, influenza A infection induces IFN-I production by pDC in mice and patients, which is associated with Th1-mediated T1D development [39,40]. While IFN-I seems to play an important role, other factors such as virus replication and cytolytic effects could contribute to T1D exacerbation [41]. Conversely, LDV infection protects mice against experimental autoimmune encephalomyelitis (EAE), a murine model of MS. In fact, LDV infection impairs IL-12 and IL-23 expression by DC through IFN-I signaling, leading to altered development of autoreactive CD4+ T cells [35]. In addition to this qualitative defect, LDV-derived IFN-I reduces the number of DC and T cells and is protective in a model of graft versus host disease [42].
Of note, the timing of IFN-I signaling is a key factor in the outcome of protective versus detrimental responses. Reports have suggested that early IFN-I production can elicit a protective response against Mycobacterium tuberculosis (Mtb) infection. In contrast, late IFN-I signaling suppresses the host-protective immune response [43]. Along this line, a retrospective study of Coronavirus Disease 2019 (COVID-19) patients found that early IFN-I treatment improves disease outcome, whereas late IFN-I administration is associated with increased mortality [44]. Based on these findings, we speculate that the timing of infection by the IFN-I–inducing pathogen should critically determine the outcome of the second pathology.
In conclusion, IFN-I has diverse, sometimes opposed, effects on immune and cellular responses (Fig 2). A moderate level of IFN-I is important to initiate an optimal immune response, while higher levels can be deleterious. Consequently, the type of pathogen, its capacity to induce low/high amounts of IFN-I, and in case of a secondary infection whether it occurs at the peak or resolution of IFN-I signaling are critical determinants of the fate of the immune response and the pathology.
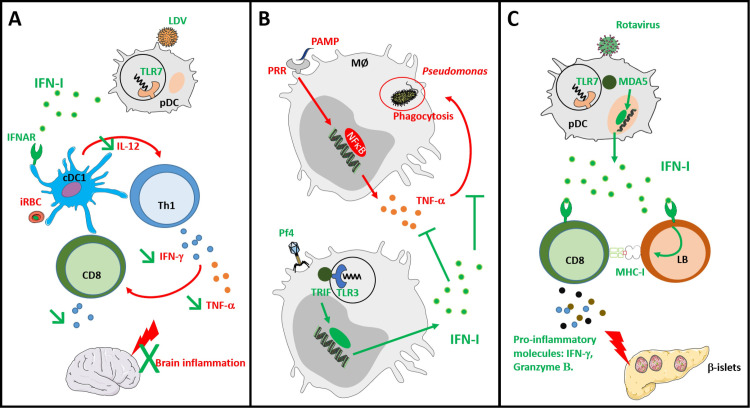
Fig 2. Examples of immunomodulation through infection-mediated IFN-I.
IFN-I display high modulatory potential as illustrated by their capacity to positively or negatively modulate infectious or autoimmune diseases and to shape the functions of innate and adaptive immune cells. (A) DCs present parasitic antigens to T cells, which induces cell migration to the brain and proinflammatory cytokine production causing cerebral malaria. LDV infection induces IFN-I production by pDC. Released IFN-I causes a quantitative and qualitative defect in DC and, consequently, a decreased inflammatory response leading to the protection against cerebral malaria. (B) Bacterial ligands stimulate NF-κB translocation leading to TNF-α production. TNF stimulates bacterial phagocytosis by macrophages. Phage RNA triggers IFN-I production through TLR3-mediated TRIF signaling. IFN-I inhibits TNF production and bacterial phagocytosis and prevents infection clearance. (C) Rotavirus RNA induces IFN-I by pDC through TLR7/MDA5 signaling. IFN-I induces lymphocyte activation, B cells up-regulate MHC-I expression and present autoantigens to CD8+ T cells, which, in turn, produce proinflammatory molecules and cause death of pancreatic β cells. (IFN-I production and modulatory functions are presented in green). DC, dendritic cell; IFN-I, type I interferon; LDV, lactate dehydrogenase-elevating virus; pDC, plasmacytoid DC; TNF, tumor necrosis factor.
Immunomodulation through tolerance to tissue damage and regulatory immunity
Tolerance to tissue damage (or disease tolerance)
During an infection, the host may undergo tissue damage directly caused by pathogen toxicity or by an inadequately resolved inflammatory response. Accordingly, a mechanism of tolerance is employed as a defense strategy to limit the negative impact of different forms of stress, thereby minimizing tissue damage. Failure to establish this tolerance can lead to a dramatic change in the clinical outcome of secondary infections, independently from pathogen burden. One example is the lethal coinfection of influenza virus and Legionella pneumophila. Interestingly, the use of attenuated bacteria, or of mice lacking the immune components induced during coinfection, like neutrophils, natural killer (NK) cells, or T and B cells (Rag2 KO), does not rescue them from mortality. Instead, mortality is associated with lung epithelial damage and a downregulation of genes involved in tissue repair. In this context, treatment with an epithelial growth factor contributing to tissue homeostasis and development increases survival [45]. This pioneer study revealed the impact of the loss of tolerance on infection-induced tissue damage and the importance of tissue repair for the clinical outcome of secondary infection. Similarly, selective inhibition of the membrane-tethered matrix metalloprotease MT1-MMP protects the tissue from damage and is correlated with a better clinical outcome during flu/Streptococcus pneumoniae mouse coinfection, without altering the immune response or cytokine expression [46].
Regulatory response in the context of coinfection
In addition to the immune-independent mechanisms of disease tolerance and tissue repair described above, regulatory immune responses are also critical processes that promote tissue repair and restore homeostasis. A hallmark cell type is the regulatory T cell (Treg), which, in addition to its immunosuppressive functions, plays an important role in tissue repair [47,48]. Interestingly, treatment with amphiregulin, a growth factor expressed by Treg, ameliorates survival during experimental influenza/L. pneumophila coinfection [45]. Yet, overall, the role of Treg and their regenerative properties during coinfection remains poorly understood. The role of immunosuppressive cytokines in controlling disease progression is well characterized. IL-10 is a hallmark suppressive cytokine, and multiple examples illustrate its regulatory functions. In a study on macaques and mice, Plasmodium infection attenuates intestinal inflammation caused by Salmonella typhimurium in an IL-10–dependent manner. This improvement is linked to a decrease in the expression of IL-12, IFN-γ, IL-17A, and in CXCL1-mediated neutrophil infiltration in the cecum, due to Plasmodium-induced IL-10 [49]. Although IL-10–mediated suppression of proinflammatory responses is beneficial in certain cases, it can also be detrimental. For example, ongoing, or previously resolved, helminth infections were reported to impair the efficacy of anti-influenza vaccination in various mouse studies. Helminth infection reduces the neutralizing capacity of influenza-specific antibodies via the expansion of CD49b+ LAG3+ Tr1 cells expressing IL-10 [50]. Moreover, helminth-mediated IL-10 expression was shown to dampen IFN-γ expression by CD8+ T cells, which exacerbates Toxoplasma gondii (T. gondii) infection [51]. Mechanistically, IL-10 can directly restrict CD8+ T cell activation and function by modifying cell surface glycosylation, which increases the antigenic threshold required for T cell activation [52]. Along the same line, blocking IL-10R or neutralizing IL-10 restores Th1 (IFN-γ, TNF-α) and Th17 (IL-17A, IL-17F) responses and ameliorates Mtb control that is blunted, respectively, by influenza A and helminth infection [53,54]. In contrast, deworming of helminth-coinfected patients is correlated with a significant decline of IL-10 level, without improvement of tuberculosis [55], showing that, in reality, coinfections involve a complex network of pro and anti-inflammatory cytokines, and likely other mechanisms beyond immunosuppression.
Regulatory response in the context of infection autoimmunity
Regarding the innate immune compartment, a recent study has shown that gamma herpesvirus protects against house dust mite–induced experimental asthma by promoting the replacement of embryonic resident alveolar macrophages by bone marrow–derived regulatory monocytes, which colonize the lungs and alter the ability of DC to trigger a specific Th2 response [56]. This indicates that some viruses could be protective through remodeling the immune microenvironment toward a more regulatory profile.
Concerning the role of regulatory adaptive cells, it was shown that helminth parasite–infected MS patients show a better disease outcome associated with an increase in CD4+ CD25+ Foxp3+ Treg cell frequency, IL-10 and TGF-β production, and a decrease in IL-12 and IFN-γ–secreting cells compared to noninfected patients [5,6,57]. A parasite modulation of the Smad7/TGF-β axis was proposed as a possible explanation. The protective role of Treg was observed in mice in a study where the clinical evolution of EAE was ameliorated by Plasmodium chabaudi infection [58], and adoptive transfer of Plasmodium-conditioned DC or CD4+ Treg was sufficient to ameliorate EAE through the suppression of autoreactive CD4+ T cell response [58,59]. In NOD mice, helminth parasites as well as their products prevent diabetes via the induction of CD4+ Treg and the production of IL-10 [60,61]. Mechanistically, parasite eggs induce Treg development in a TGF-β–dependent manner through the induction of TGF-β–activating α8 integrin, LAP, and galectin 1 and 3 expression on CD4+ T cells, in addition to the expression of IL-2 and IL-10 by DC [60]. However, IL-10–deficient NOD mice are protected against diabetes [62]. Moreover, IL-2 treatment reverses established disease in NOD mice by acting on local Treg but independently from IL-10 and TGF-β expression [63]. Thus, parasite-induced protection of NOD mice seems to be mediated by regulatory mechanisms that rely on Treg but are partially independent from IL-10 production. Finally, helminth-induced Treg modulate airway inflammation and inhibit asthma, by reducing antigen-specific immunoglobulin E (IgE) and pulmonary eosinophilia in mouse models of asthma [64,65]. Interestingly, protective pulmonary Treg are induced by helminth-activated CD1d high regulatory B cells (Breg) via IL-10 production [66,67]. A similar mechanism was found in several autoimmune experimental models such as lupus [68], arthritis [69], and EAE [70]. In the context of allergy, a clinical study on African children reported that IL-10 is induced by chronic schistosomiasis and that it is inversely correlated with reactivity in a house dust mite skin test, suggesting that IL-10 plays a central role in the helminth-mediated suppression of atopy [8].
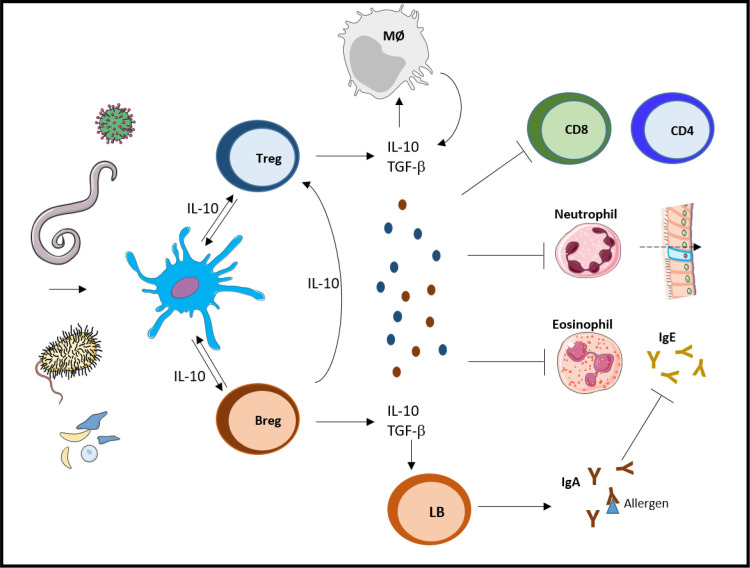
In summary, infection-induced Treg and Breg are often simultaneously useful in controlling inflammation, in large part through IL-10, but they may also have IL-10–independent functions and nonredundant roles (Fig 3).
Fig 3. Immune response modulation by pathogen-activated regulatory T and B cells.
Virus, bacteria, parasite, or pathogen-derived molecules could induce the development of a regulatory immune response by activating lymphocytes or DC, which stimulate Treg or Breg via the production of IL-10. Together, regulatory cells produce immunomodulatory cytokines like IL-10 and TGF-β, which inhibit CD4+ and CD8+ T cell differentiation and functions, chemokine-mediated neutrophil infiltration, eosinophil inflammatory response, and stimulate B cells to produce modulatory IgA, which neutralize allergen and control IgE. Consequently, the clinical outcome of a concomitant disease could be impacted positively or negatively by regulatory T and B cells, depending on the nature of the pathology. Breg, regulatory B cell; DC, dendritic cell; IgA, immunoglobulin A, IgE, immunoglobulin E; IL-10, interleukin 10; TGF-β, transforming growth factor beta; Treg, regulatory T cell.
Immunomodulation through type 1/type 2 immunity switching
Depending on the class of pathogen and the tissue microenvironment, activated immune cells can polarize toward phenotypically and functionally distinct cell populations, leading to the establishment of immune responses of different types, type 1 and 2 being the most common. Type 1 immunity relates to an inflammatory cytotoxic response mounted to fight intracellular pathogens and cancer cells. Type 2 immunity relates to responses that are critical for host resistance against helminthic infections, and it comprises immunosuppressive and tissue repair functions. Switching between type 1 and type 2 immunity can drastically change the way by which the immune system reacts to a specific pathological context and, thus, the course of disease. In humans and mice, Mtb elicits a proinflammatory type 1 response. Hence, patients coinfected with helminths, a strong driver of Th2 responses, develop a more protracted and severe tuberculosis [71,72]. Helminth coinfection leads to a decrease in Th1, Th17, and NK cell frequency, an increase in Th2 and Treg, and a modified profile of pro/anti-inflammatory cytokine production [53,71,73]. Unexpectedly, profiling of CD4+ T cells in Kenyan individuals showed that Schistosoma coinfection does not impair Mtb-specific Th1 cytokine production. In fact, coinfected individuals had a higher frequency of Mtb-specific CD4+ T cells expressing IFN-γ than Mtb-monoinfected individuals [74]. Differences in the stages of worm lifecycle, which can drive Th2 as well as Th1 responses, may explain these discordant results. The order of infection, a difficult parameter to define in humans, may also be involved. This hypothesis is supported by several murine coinfection studies. When T. gondii infection precedes helminth infection, a decrease in the expression of the transcription factor GATA3, in the production of IL-4, IL-13, as well as an increase in the production of IFN-γ by CD4+ T cells is observed [75]. In contrast, when helminth infection precedes T. gondii infection, mice display a defective IFN-γ and IL-12 production by CD8+ T cells and cDC1, correlated with an increased IL-4 and IL-10 production leading to an enhanced T. gondii cyst load in the brain of coinfected mice [51,76].
Beyond T cell responses, switching of macrophage polarization can also modulate the outcome of coinfection. For example, a helminth-promoted switch from M1 to alternatively activated macrophages via IL-4 signaling leads to decreased expression of several proinflammatory cytokines and chemokines such as IL-1β, TNF-α, IL-6, IL-12, CCL(1-2-4-11), and CXCL(2-9-10), and is associated with tuberculosis exacerbation [72,77–79]. In addition to the classical mechanisms of action (pro or anti-inflammatory response), a recent in vitro study shed light on an original mechanism by which functionally modulated macrophages could aggravate infection. Mtb infection was shown to increase HIV dissemination via the induction of permissive immunomodulatory macrophages and the formation, through an IL-10/STAT3 signaling pathway, of intercellular nanotubes, which facilitate HIV transmission between cells [80].
In the context of autoimmunity, MS patients treated with anthelminthic molecules show an increased clinical MS activity correlated with higher IFN-γ and IL-12 expression and a drop in the number of regulatory cells [57]. Conversely, in mice, helminth infection tends to reduce EAE severity by curtailing IFN-γ, IL-17, and IL-12 expression and enhancing IL-4 production via STAT6 signaling [57,81]. The same protective effect is observed in a murine model of rheumatoid arthritis where a preestablished Schistosoma infection causes a downregulation of the level of anticollagen IgG, IFN-γ, IL-17A, TNF-α, IL-1β, and IL-6 and an upregulation of IL-4 [82].
Altogether, these studies show that infection can modulate concomitant disease by switching the type of immune response. The lapse before disease induction is critical for determining the phenotype of the induced immune response and, consequently, the clinical outcome.
Immunomodulation through chemokine regulation and immune cell migration
By modulating the migration of immune cells to the local site of infection or inflammation, an infection can drastically affect the clinical evolution of the concomitant pathology. Teo and colleagues have shown that Chikungunya virus (CHIKV) coinfection protects mice against cerebral malaria by altering parasite-specific CD8+ T cells trafficking to the brain. In coinfected mice, splenic IFN-γ–mediated CXCL9 and CXCL10 production induces retention of CXCR3-expressing pathogenic CD8+ T cells in the spleen and prevents their migration to the brain [83]. Conversely, preinfection of mice with Plasmodium abolishes CHIKV-induced joint swelling due, in part, to altered migration of pathogenic CD4+ T cells to joints. Altered migration is partially dependent on CXCR3, in addition to an increased CD4+ T cell apoptosis in lymph nodes [84]. A lower CD8+ T cell infiltration in the brain was also reported during Schistosoma/Plasmodium coinfection. However, in this case, as IFN-γ expression is decreased, the lower brain infiltration is not due to an accumulation of IFN-γ–mediated chemokine in the spleen but rather to a Schistosoma-mediated lack of CD8+ T cell development and a limited impairment of the blood–brain barrier [85]. In another experimental study, Schistosoma infection disrupts the control of Helicobacter pylori colonization by inducing a misdirection of H. pylori-experienced CXCR3+ Th1 cells that fail to home to the stomach [86].
In addition to T cells, neutrophil migration is a process that is critical in the control of infections and may be affected by a coinfection. A preestablished gut-restricted helminth infection protects against P. aeruginosa coinfection by increasing neutrophil recruitment to the mouse lungs [87]. Mechanistically, helminth-induced IL-4 and IL-13 increase the transcription and activity of the lipoxygenase gene Alox15 [87], leading to the generation of the inflammatory lipid mediator hepoxilin A3 [88], which drives the migration of neutrophils across the mucosal epithelial barrier into the airspace [89].
During autoimmunity, LCMV infection in mice prevents T1D by stimulating an IFN-γ–mediated CXCL10 production, which attracts islet-infiltrated T cells back from the islets to the pancreatic draining lymph node [90]. By contrast, chemokine production plays a detrimental role during influenza A infection by inducing T cell, monocyte, and neutrophil trafficking to the brain, which causes the development of autoimmune encephalomyelitis in autoimmune-prone T-cell receptor transgenic 2D2 mice. RNA-seq analysis showed that several chemokines are upregulated in the central nervous system (CNS) of infected mice [91]. At the molecular level, binding of the flu viral RNA to TLR7 activates the MyD88/NF-κB pathway, which, in turn, enhances cell recruitment to the CNS [91]. Thus, the balance of chemokine production between lymphoid organs and the site of pathology, which is affected by the nature of the pathogen and the type of infection (systemic or local), is a key factor to determine cell migration. Targeting chemokine production could be helpful for ameliorating the clinical outcome of certain pathologies.
Modulation of humoral immunity
Perhaps less studied than cellular immunity, the modulation of humoral immunity also represents a mechanism by which an infection may impact concomitant diseases. In sub-Saharan Africa, infants are often coinfected with gamma herpesvirus, which infects B cells and is proposed to partially underlie the very slow acquisition of immunity to severe malaria in children. Using a mouse model of coinfection, Matar and colleagues have shown that acute, but not latent, gamma herpesvirus infection suppresses the antimalarial humoral response. In fact, coinfected mice are defective in generating malaria-specific IgG producing plasma cells. Interestingly, a viral protein causes a defect in germinal center maintenance by reducing the ability of B cells to communicate with follicular helper T cells (Tfh), probably by inducing the expression of the suppressive ligand PD-L1 [92]. Similar mechanisms were observed in mice sequentially infected with flu and S. pneumoniae. This coinfection results in a deadly phenotype and a reduced level of virus-specific IgG, IgM, and IgA, lower numbers of B and plasma cells, altered Tfh responses, and germinal center maintenance [93].
In the context of autoimmunity, through molecular mimicry and bystander activation, infections may lead to immune tolerance breakdown and cause the activation of B cells and the production of autoantibodies, leading to the development of autoimmune disorders [14,94]. Human studies have suggested that P. falciparum infection is correlated with an increased level of autoreactive IgG that may recognize antigens derived from brain tissue [95]. Interestingly, a more recent study in mouse malaria has shown that Plasmodium DNA, via synergistic activation of TLR9/IFN-γR, induces the development of a special population of “atypical” B cells, which are T-bet+ CD11c+ and produce anti-erythrocyte antibodies, causing the development of autoimmune anemia [96]. It remains to be established if the same B cell population could be the origin of the brain autoreactive IgG in cerebral malaria patients.
In the future, detailed analyses of the mechanisms of pathogen-mediated suppression or activation/differentiation of B and Tfh should be helpful to identify new target pathways and therapeutic molecules.
Immunomodulation through trained immunity
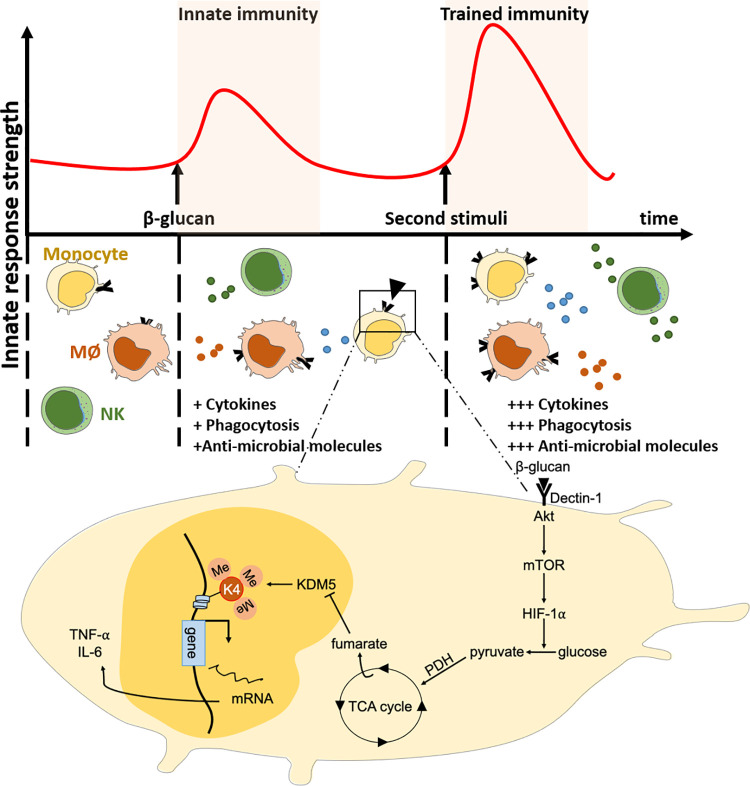
Conventionally, specific antigenic memory is the hallmark of adaptive immune T and B cells. Another type of nonspecific memory mediated by innate cells, called “trained immunity,” has been recently discovered [97] (Fig 4). Trained immunity is based on the functional reprogramming of innate immune cells through immunological, metabolic, and epigenetic modifications that occur upon encounter with certain pathogen-associated molecular patterns, and result in stronger immunity to a second infection by the same or distinct (cross-protection) pathogens, and last for a relatively long time (i.e., a few months and up to 1 year or longer in certain cases). One of the well-documented examples is trained immunity elicited by Candida albicans and the fungal cell wall component β-glucan. Exposure to β-glucan, or to a low dose of C. albicans, affords protection against lethal fungal reinfection in mice lacking functional T and B cells. Interestingly, preexposed monocytes display a functional reprogramming and produce more IL-6 and TNF-α in response not only to C. albicans, but also to other pattern recognition receptor ligands and bacterial stimulations [98]. Mechanistically, trained monocytes display an epigenetic activating change in the H3K4me3 profile of the related gene promoters through a dectin-1 signaling pathway [98,99]. Moreover, β-glucan–induced trained immunity confers protection against secondary nonfungal infection by modulating IL-32 production and increasing inflammatory and antimicrobial responses through a mechanism requiring monocytes/macrophages as well as IL-1 signaling [100,101]. Likewise, human PBMC exposed to Plasmodium-infected red blood cells show an increased production of IL-6 and TNF-α correlated with epigenetic remodeling, in response to secondary TLR stimulation. Interestingly, Plasmodium-infected Kenyan children present the same epigenetic modifications even after antimalaria treatment [102]. Thus, epigenetic changes may, at least partially, explain why the majority of multiply infected individuals are asymptomatic. In addition to natural infection, trained immunity is induced by vaccination. For example, antituberculosis vaccination is protective against Mycobacteria as well as against nontargeted pathogens [103,104]. Arts and colleagues reported that bacille Calmette-Guérin (BCG) vaccination promotes protection against yellow fever viremia. Protection is mediated by an epigenetic remodeling of monocytes, through Nod2 activation [103], which drives IL-1β and TNF-α production [105] and in turn contributes to enhance adaptive T cell responses [104]. Importantly, trained immunity is thought to be induced in the bone marrow. In fact, infectious challenge promotes myelopoiesis and educates hematopoietic stem cells to generate trained innate cells [106,107]. This observation is essential to help understand the duration of trained immunity considering the relatively fast turnover of monocytes. Consequently, the use of trained stem cells as a new type of nonspecific vaccination is enticing and under active scrutiny. During the COVID-19 pandemic, correlative data suggest that countries that never implemented a universal BCG vaccination policy display higher rate of mortality and reported cases of SARS-CoV2 infection, indicating that BCG may offer protection against the virus [108]. Overall, these studies highlight the novel concept of microbe-mediated nonspecific vaccines.
Fig 4. Infection-induced trained immunity.
Upon encounter with a pathogen and sensing of its specific ligands (for example, β-glucan), innate immune responses become activated to orchestrate immune defense. In addition, this first microbial stimulus leads to the development of a relatively long-term, metabolically and epigenetically controlled, innate memory response called trained immunity. Ligand recognition triggers a series of intracellular cascades that activates several metabolic pathways such as glycolysis and TCA. Certain metabolites derived from these processes such as fumarate can modulate enzymes involved in epigenetic remodeling such as the histone demethylase KDM5, leading to the modulation of histone methylation status of innate immune genes. Following a second stimulation with the same, or with an unrelated pathogen, the trained innate cells are able to respond more strongly and to control the infection more efficiently. HIF-1α, hypoxia-inducible factor 1α; IL-6, interleukin 6; KDM5, lysine-specific demethylase 5; mTOR, mechanistic target of rapamycin; NK, natural killer cell; PDH, pyruvate dehydrogenase; TCA, tricarboxylic acid; TNF-ɑ, tumor necrosis factor alpha.
In several contexts of autoimmune diseases, monocytes from patients display features consistent with a trained immunity phenotype, like increased cytokine production, and metabolic and epigenetic modifications. For example, during SLE and rheumatoid arthritis, isolated human monocytes display increased production of IL-1β, IL-6, and TNF-α [109,110], activated PI3K/mTOR and MAPK signaling pathways [111], high expression of CD80, CD86, and HLA-DR [112], suggesting a better capacity of presenting antigens to T cells, including the presentation of self-antigens to autoreactive T cells. The potentialization of the proinflammatory responses triggered by trained immunity supports the notion that training likely plays a deleterious role in the context of autoimmune diseases. Conversely, in a murine model of autoimmunity, injection of helminth extracts induces alternatively activated macrophages with an anti-inflammatory profile, correlated with the abrogation of the adaptive response and resistance to EAE [113]. However, a direct impact of helminth extracts on adaptive cells cannot be excluded. Overall, studies have started to reveal an association between autoimmunity in humans and a trained immunity phenotype; however, no direct causal link has been formally proven yet.
Currently, trained immunity stands as an appealing target to improve treatment and vaccination by inhibiting or activating the metabolic, epigenetic, and immune pathways involved in the innate training process.
Conclusions
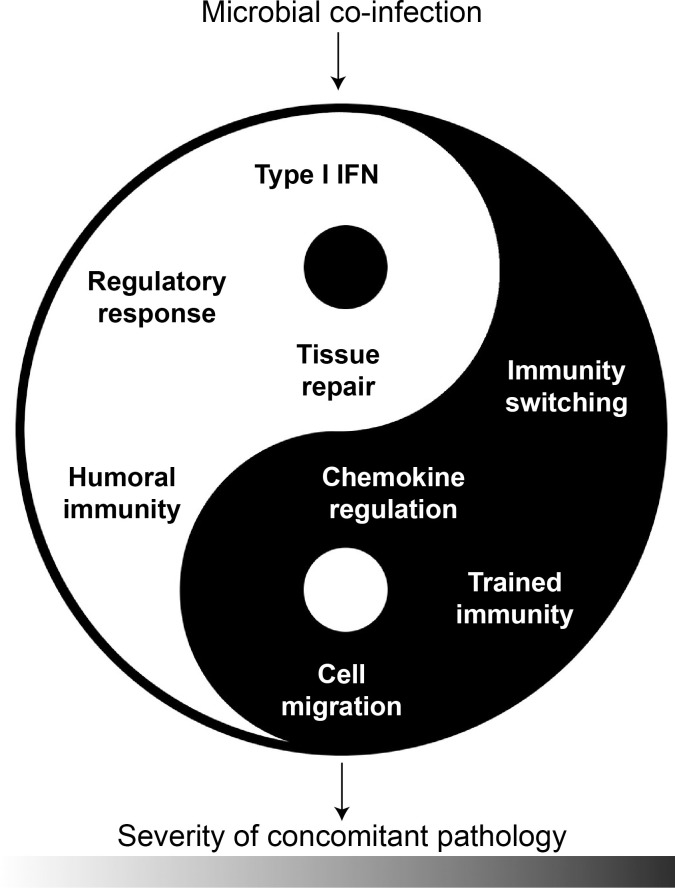
It is now well accepted that germ exposure, an element of the global “exposome,” can positively or negatively affect the clinical evolution of concomitant infectious or autoimmune pathologies. Although a causal link remains difficult to establish in humans because of the complexity of intrinsic and extrinsic factors that influence disease progression, experimental studies have demonstrated that a preestablished, simultaneous, or subsequent infection can either ameliorate or exacerbate a concurrent pathology. These studies have revealed the diverse immunological processes by which an infection modulates the clinical outcome of a concomitant disease, although in most cases, the actual effects remain hard to predict. As detailed above and summarized in Fig 5, the infection-induced immunomodulation mechanisms include the dual roles of IFN-I, various regulatory pathways and cells, modifications in tissue repair and tolerance to damage, changes in the polarization of immune cells, dysregulated chemoattraction and immune cell migration, and trained immunity.
Fig 5. Microbial (co)infection can positively or negatively change the clinical outcome of concomitant infectious and autoimmune diseases through a variety of mechanisms.
In the future, more investigations at the cellular, molecular, and genetic levels are needed to characterize microbe-derived molecules that carry these immunomodulatory effects. This would be the basis for the emergence and translational applications of microbial-based therapy. Currently, several clinical trials already aim to use microbes or microbe-derived products for the treatment of different types of infectious and autoimmune diseases (for review [114]). While some results are encouraging, this type of therapy is not yet mature, and more studies about the safety and efficacy are required.
Finally, it is important to highlight that the huge impact of infection on the host immune status has shed light on a weak spot of current vaccine development strategies. When living in areas with high incidence of helminth infection, children display a reduced H1N1-specific antibody response compared to those living in low incidence areas [115], possibly because vaccines that are developed and tested in the Western world may be less efficient in helminth-endemic areas because of the major impact of helminths on the immune system. In an era of renewed interest for large-scale vaccination, such data underscore the necessity of better evaluating the coinfection risks before implementing therapeutic or vaccine strategies in these endemic areas.
Acknowledgments
We thank Dr. Abdelhadi Saoudi for critical reading of the manuscript.
Funding Statement
Our research was supported by grants from ‘Institut National de la Santé et de la Recherche Médicale’ to NB, ‘Association pour la Recherche sur la Sclérose en Plaques’ (ARSEP) to NB, PIA PARAFRAP Consortium (ANR-11-LABX0024) to NB, ‘Agence Nationale pour la Recherche’ (ANR-18-CE15-0015, ANR-19-CE15-0008, ANR-19-CE15-0023) to NB, French Minister of Education, Research and Technology (MENRT PhD fellowship to AH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–60. doi: 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33. doi: 10.1016/S0140-6736(09)60568-7 [DOI] [PubMed] [Google Scholar]
- 3.Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18(2):105–20. doi: 10.1038/nri.2017.111 [DOI] [PubMed] [Google Scholar]
- 4.Sotgiu S, Angius A, Embry A, Rosati G, Musumeci S. Hygiene hypothesis: innate immunity, malaria and multiple sclerosis. Med Hypotheses. 2008;70(4):819–25. doi: 10.1016/j.mehy.2006.10.069 [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61(2):97–108. doi: 10.1002/ana.21067 [DOI] [PubMed] [Google Scholar]
- 6.Tanasescu R, Tench CR, Constantinescu CS, Telford G, Singh S, Frakich N, et al. Hookworm Treatment for Relapsing Multiple Sclerosis: A Randomized Double-Blinded Placebo-Controlled Trial. JAMA Neurol. 2020;77(9):1089–98. doi: 10.1001/jamaneurol.2020.1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtanen SM, Takkinen HM, Nwaru BI, Kaila M, Ahonen S, Nevalainen J, et al. Microbial exposure in infancy and subsequent appearance of type 1 diabetes mellitus-associated autoantibodies: a cohort study. JAMA Pediatr. 2014;168(8):755–63. doi: 10.1001/jamapediatrics.2014.296 [DOI] [PubMed] [Google Scholar]
- 8.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356(9243):1723–7. doi: 10.1016/S0140-6736(00)03206-2 [DOI] [PubMed] [Google Scholar]
- 9.Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, Liem HT, et al. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: A cross-sectional study. J Allergy Clin Immunol. 2006;118(6):1305–11. doi: 10.1016/j.jaci.2006.08.035 [DOI] [PubMed] [Google Scholar]
- 10.van den Biggelaar AH, Rodrigues LC, van Ree R, van der Zee JS, Hoeksma-Kruize YC, Souverijn JH, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189(5):892–900. doi: 10.1086/381767 [DOI] [PubMed] [Google Scholar]
- 11.Aoyama H, Hirata T, Sakugawa H, Watanabe T, Miyagi S, Maeshiro T, et al. An inverse relationship between autoimmune liver diseases and Strongyloides stercoralis infection. Am J Trop Med Hyg. 2007;76(5):972–6. [PubMed] [Google Scholar]
- 12.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2687–92. doi: 10.1038/ajg.2010.398 [DOI] [PubMed] [Google Scholar]
- 13.Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr Allergy Immunol. 2013;24(8):762–71. doi: 10.1111/pai.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarra SV, Leynes MS. Infections in systemic lupus erythematosus. Lupus. 2010;19(12):1419–24. doi: 10.1177/0961203310374486 [DOI] [PubMed] [Google Scholar]
- 15.Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y. Infections and autoimmunity—friends or foes? Trends Immunol. 2009;30(8):409–14. doi: 10.1016/j.it.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Jelcic I, Muhlenbruch L, Haunerdinger V, Toussaint NC, Zhao Y, et al. HLA-DR15 Molecules Jointly Shape an Autoreactive T Cell Repertoire in Multiple Sclerosis. Cell. 2020;183(5):1264–81 e20. doi: 10.1016/j.cell.2020.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes LE, Smith PA, Bonell S, Natt RS, Wilson C, Rashid T, et al. Cross-reactivity between related sequences found in Acinetobacter sp., Pseudomonas aeruginosa, myelin basic protein and myelin oligodendrocyte glycoprotein in multiple sclerosis. J Neuroimmunol. 2003;144(1–2):105–15. doi: 10.1016/s0165-5728(03)00274-1 [DOI] [PubMed] [Google Scholar]
- 18.Filippi CM, von Herrath MG. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: viruses, autoimmunity and immunoregulation. Clin Exp Immunol. 2010;160(1):113–9. doi: 10.1111/j.1365-2249.2010.04128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourreau E, Ginouves M, Prevot G, Hartley MA, Gangneux JP, Robert-Gangneux F, et al. Presence of Leishmania RNA Virus 1 in Leishmania guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J Infect Dis. 2016;213(1):105–11. doi: 10.1093/infdis/jiv355 [DOI] [PubMed] [Google Scholar]
- 20.Cantanhede LM, da Silva Junior CF, Ito MM, Felipin KP, Nicolete R, Salcedo JM, et al. Further Evidence of an Association between the Presence of Leishmania RNA Virus 1 and the Mucosal Manifestations in Tegumentary Leishmaniasis Patients. PLoS Negl Trop Dis. 2015;9(9):e0004079. doi: 10.1371/journal.pntd.0004079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matar CG, Jacobs NT, Speck SH, Lamb TJ, Moormann AM. Does EBV alter the pathogenesis of malaria? Parasite Immunol. 2015;37(9):433–45. doi: 10.1111/pim.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan JL, Seitz AE, Fried M, Lee KL, Metenou S, Morrison R, et al. Seroepidemiology of helminths and the association with severe malaria among infants and young children in Tanzania. PLoS Negl Trop Dis. 2018;12(3):e0006345. doi: 10.1371/journal.pntd.0006345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemaitre M, Watier L, Briand V, Garcia A, Le Hesran JY, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: additional evidence of the protective effect of Schistosomiasis on malaria in Senegalese children. Am J Trop Med Hyg. 2014;90(2):329–34. doi: 10.4269/ajtmh.12-0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbate JL, Ezenwa VO, Guegan JF, Choisy M, Nacher M, Roche B. Disentangling complex parasite interactions: Protection against cerebral malaria by one helminth species is jeopardized by co-infection with another. PLoS Negl Trop Dis. 2018;12(5):e0006483. doi: 10.1371/journal.pntd.0006483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science. 2011;331(6018):775–8. doi: 10.1126/science.1199326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi M, Castiglioni P, Hartley MA, Eren RO, Prevel F, Desponds C, et al. Type I interferons induced by endogenous or exogenous viral infections promote metastasis and relapse of leishmaniasis. Proc Natl Acad Sci U S A. 2017;114(19):4987–92. doi: 10.1073/pnas.1621447114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 2019;363(6434). doi: 10.1126/science.aat9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seoane PI, Taylor-Smith LM, Stirling D, Bell LCK, Noursadeghi M, Bailey D, et al. Viral infection triggers interferon-induced expulsion of live Cryptococcus neoformans by macrophages. PLoS Pathog. 2020;16(2):e1008240. doi: 10.1371/journal.ppat.1008240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119(7):1910–20. doi: 10.1172/JCI35412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schliehe C, Flynn EK, Vilagos B, Richson U, Swaminanthan S, Bosnjak B, et al. The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection. Nat Immunol. 2015;16(1):67–74. doi: 10.1038/ni.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186(3):1666–74. doi: 10.4049/jimmunol.1002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Er JZ, Koean RAG, Ding JL. Loss of T-bet confers survival advantage to influenza-bacterial superinfection. EMBO J. 2019;38(1). doi: 10.15252/embj.201899176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florido M, Grima MA, Gillis CM, Xia Y, Turner SJ, Triccas JA, et al. Influenza A virus infection impairs mycobacteria-specific T cell responses and mycobacterial clearance in the lung during pulmonary coinfection. J Immunol. 2013;191(1):302–11. doi: 10.4049/jimmunol.1202824 [DOI] [PubMed] [Google Scholar]
- 34.Sabbatani S, Manfredi R, Marinacci G, Pavoni M, Cristoni L, Chiodo F. Reactivation of severe, acute pulmonary tuberculosis during treatment with pegylated interferon-alpha and ribavirin for chronic HCV hepatitis. Scand J Infect Dis. 2006;38(3):205–8. doi: 10.1080/00365540500263268 [DOI] [PubMed] [Google Scholar]
- 35.Hassan A, Wlodarczyk MF, Benamar M, Bassot E, Salvioni A, Kassem S, et al. A Virus Hosted in Malaria-Infected Blood Protects against T Cell-Mediated Inflammatory Diseases by Impairing DC Function in a Type I IFN-Dependent Manner. mBio. 2020;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, et al. Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by PolyIC in vivo. PLoS ONE. 2011;6(6):e20189. doi: 10.1371/journal.pone.0020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci U S A. 1997;94(2):634–9. doi: 10.1073/pnas.94.2.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pane JA, Webster NL, Coulson BS. Rotavirus activates lymphocytes from non-obese diabetic mice by triggering toll-like receptor 7 signaling and interferon production in plasmacytoid dendritic cells. PLoS Pathog. 2014;10(3):e1003998. doi: 10.1371/journal.ppat.1003998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014;63(7):2538–50. doi: 10.2337/db13-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia CQ, Peng R, Chernatynskaya AV, Yuan L, Carter C, Valentine J, et al. Increased IFN-alpha-producing plasmacytoid dendritic cells (pDCs) in human Th1-mediated type 1 diabetes: pDCs augment Th1 responses through IFN-alpha production. J Immunol. 2014;193(3):1024–34. doi: 10.4049/jimmunol.1303230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capua I, Mercalli A, Pizzuto MS, Romero-Tejeda A, Kasloff S, De Battisti C, et al. Influenza A viruses grow in human pancreatic cells and cause pancreatitis and diabetes in an animal model. J Virol. 2013;87(1):597–610. doi: 10.1128/JVI.00714-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaignage M, Marillier RG, Uyttenhove C, Dauguet N, Saxena A, Ryffel B, et al. Mouse nidovirus LDV infection alleviates graft versus host disease and induces type I IFN-dependent inhibition of dendritic cells and allo-responsive T cells. Immun Inflamm Dis. 2017;5(2):200–13. doi: 10.1002/iid3.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreira-Teixeira L, Mayer-Barber K, Sher A, O’Garra A. Type I interferons in tuberculosis: Foe and occasionally friend. J Exp Med. 2018;215(5):1273–85. doi: 10.1084/jem.20180325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, et al. Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients. Cell Host Microbe. 2020;28(3):455–64 e2. doi: 10.1016/j.chom.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, et al. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340(6137):1230–4. doi: 10.1126/science.1233632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talmi-Frank D, Altboum Z, Solomonov I, Udi Y, Jaitin DA, Klepfish M, et al. Extracellular Matrix Proteolysis by MT1-MMP Contributes to Influenza-Related Tissue Damage and Mortality. Cell Host Microbe. 2016;20(4):458–70. doi: 10.1016/j.chom.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 47.Povoleri GAM, Nova-Lamperti E, Scotta C, Fanelli G, Chen YC, Becker PD, et al. Human retinoic acid-regulated CD161(+) regulatory T cells support wound repair in intestinal mucosa. Nat Immunol. 2018;19(12):1403–14. doi: 10.1038/s41590-018-0230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho J, Kuswanto W, Benoist C, Mathis D. T cell receptor specificity drives accumulation of a reparative population of regulatory T cells within acutely injured skeletal muscle. Proc Natl Acad Sci U S A. 2019;116(52):26727–33. doi: 10.1073/pnas.1914848116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mooney JP, Butler BP, Lokken KL, Xavier MN, Chau JY, Schaltenberg N, et al. The mucosal inflammatory response to non-typhoidal Salmonella in the intestine is blunted by IL-10 during concurrent malaria parasite infection. Mucosal Immunol. 2014;7(6):1302–11. doi: 10.1038/mi.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartmann W, Brunn ML, Stetter N, Gagliani N, Muscate F, Stanelle-Bertram S, et al. Helminth Infections Suppress the Efficacy of Vaccination against Seasonal Influenza. Cell Rep. 2019;29(8):2243–56 e4. doi: 10.1016/j.celrep.2019.10.051 [DOI] [PubMed] [Google Scholar]
- 51.Marple A, Wu W, Shah S, Zhao Y, Du P, Gause WC, et al. Correction: Cutting Edge: Helminth Coinfection Blocks Effector Differentiation of CD8 T Cells through Alternate Host Th2- and IL-10-Mediated Responses. J Immunol. 2017;199(8):3005. doi: 10.4049/jimmunol.1701193 [DOI] [PubMed] [Google Scholar]
- 52.Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, et al. Interleukin-10 Directly Inhibits CD8(+) T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity. 2018;48(2):299–312 e5. doi: 10.1016/j.immuni.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190(10):5161–8. doi: 10.4049/jimmunol.1203311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ring S, Eggers L, Behrends J, Wutkowski A, Schwudke D, Kroger A, et al. Blocking IL-10 receptor signaling ameliorates Mycobacterium tuberculosis infection during influenza-induced exacerbation. JCI Insight. 2019;5(10):e1265335. doi: 10.1172/jci.insight.126533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abate E, Belayneh M, Idh J, Diro E, Elias D, Britton S, et al. Asymptomatic Helminth Infection in Active Tuberculosis Is Associated with Increased Regulatory and Th-2 Responses and a Lower Sputum Smear Positivity. PLoS Negl Trop Dis. 2015;9(8):e0003994. doi: 10.1371/journal.pntd.0003994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. 2017;18(12):1310–20. doi: 10.1038/ni.3857 [DOI] [PubMed] [Google Scholar]
- 57.Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233(1–2):6–11. doi: 10.1016/j.jneuroim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 58.Farias AS, Talaisys RL, Blanco YC, Lopes SC, Longhini AL, Pradella F, et al. Regulatory T cell induction during Plasmodium chabaudi infection modifies the clinical course of experimental autoimmune encephalomyelitis. PLoS ONE. 2011;6(3):e17849. doi: 10.1371/journal.pone.0017849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thome R, Issayama LK, Alves da Costa T, Gangi RD, Ferreira IT, Raposo C, et al. Dendritic cells treated with crude Plasmodium berghei extracts acquire immune-modulatory properties and suppress the development of autoimmune neuroinflammation. Immunology. 2014;143(2):164–73. doi: 10.1111/imm.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39(4):1098–107. doi: 10.1002/eji.200838871 [DOI] [PubMed] [Google Scholar]
- 61.Mishra PK, Patel N, Wu W, Bleich D, Gause WC. Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol. 2013;6(2):297–308. doi: 10.1038/mi.2012.71 [DOI] [PubMed] [Google Scholar]
- 62.Rajagopalan G, Kudva YC, Sen MM, Marietta EV, Murali N, Nath K, et al. IL-10-deficiency unmasks unique immune system defects and reveals differential regulation of organ-specific autoimmunity in non-obese diabetic mice. Cytokine. 2006;34(1–2):85–95. doi: 10.1016/j.cyto.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 63.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–8. doi: 10.1084/jem.20100209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37(4):498–505. doi: 10.1111/j.1365-2222.2006.02629.x [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X, et al. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2007;120(1):8–18. doi: 10.1111/j.1365-2567.2006.02472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(5):1114–24 e8. doi: 10.1016/j.jaci.2010.01.018 [DOI] [PubMed] [Google Scholar]
- 67.Gao X, Ren X, Wang Q, Yang Z, Li Y, Su Z, et al. Critical roles of regulatory B and T cells in helminth parasite-induced protection against allergic airway inflammation. Clin Exp Immunol. 2019;198(3):390–402. doi: 10.1111/cei.13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184(9):4801–9. doi: 10.4049/jimmunol.0902385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186(10):5569–79. doi: 10.4049/jimmunol.1100284 [DOI] [PubMed] [Google Scholar]
- 70.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185(4):2240–52. doi: 10.4049/jimmunol.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147(1):45–52. doi: 10.1111/j.1365-2249.2006.03247.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208(9):1863–74. doi: 10.1084/jem.20091473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anuradha R, Munisankar S, Bhootra Y, Dolla C, Kumaran P, Nutman TB, et al. Anthelmintic Therapy Modifies the Systemic and Mycobacterial Antigen-Stimulated Cytokine Profile in Helminth-Latent Mycobacterium tuberculosis Coinfection. Infect Immun. 2017;85(4). doi: 10.1128/IAI.00973-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McLaughlin TA, Khayumbi J, Ongalo J, Tonui J, Campbell A, Allana S, et al. CD4 T Cells in Mycobacterium tuberculosis and Schistosoma mansoni Co-infected Individuals Maintain Functional TH1 Responses. Front Immunol. 2020;11:127. doi: 10.3389/fimmu.2020.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed N, French T, Rausch S, Kuhl A, Hemminger K, Dunay IR, et al. Toxoplasma Co-infection Prevents Th2 Differentiation and Leads to a Helminth-Specific Th1 Response. Front Cell Infect Microbiol. 2017;7:341. doi: 10.3389/fcimb.2017.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan IA, Hakak R, Eberle K, Sayles P, Weiss LM, Urban JF Jr. Coinfection with Heligmosomoides polygyrus fails to establish CD8+ T-cell immunity against Toxoplasma gondii. Infect Immun. 2008;76(3):1305–13. doi: 10.1128/IAI.01236-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aira N, Andersson AM, Singh SK, McKay DM, Blomgran R. Species dependent impact of helminth-derived antigens on human macrophages infected with Mycobacterium tuberculosis: Direct effect on the innate anti-mycobacterial response. PLoS Negl Trop Dis. 2017;11(2):e0005390. doi: 10.1371/journal.pntd.0005390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajamanickam A, Munisankar S, Bhootra Y, Dolla CK, Nutman TB, Babu S. Coexistent Helminth Infection-Mediated Modulation of Chemokine Responses in Latent Tuberculosis. J Immunol. 2019;202(5):1494–500. doi: 10.4049/jimmunol.1801190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rajamanickam A, Munisankar S, Dolla C, Menon PA, Nutman TB, Babu S. Helminth Coinfection Alters Monocyte Activation, Polarization, and Function in Latent Mycobacterium tuberculosis Infection. J Immunol. 2020;204(5):1274–86. doi: 10.4049/jimmunol.1901127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Souriant S, Balboa L, Dupont M, Pingris K, Kviatcovsky D, Cougoule C, et al. Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages. Cell Rep. 2019;26(13):3586–99 e7. doi: 10.1016/j.celrep.2019.02.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 2010;32(6):450–9. doi: 10.1111/j.1365-3024.2010.01207.x [DOI] [PubMed] [Google Scholar]
- 82.Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol. 2009;39(4):457–64. doi: 10.1016/j.ijpara.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 83.Teo TH, Howland SW, Claser C, Gun SY, Poh CM, Lee WW, et al. Co-infection with Chikungunya virus alters trafficking of pathogenic CD8(+) T cells into the brain and prevents Plasmodium-induced neuropathology. EMBO Mol Med. 2018;10(1):121–38. doi: 10.15252/emmm.201707885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teo TH, Lum FM, Ghaffar K, Chan YH, Amrun SN, Tan JJL, et al. Plasmodium co-infection protects against chikungunya virus-induced pathologies. Nat Commun. 2018;9(1):3905. doi: 10.1038/s41467-018-06227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang ML, Feng YH, Pang W, Qi ZM, Zhang Y, Guo YJ, et al. Parasite densities modulate susceptibility of mice to cerebral malaria during co-infection with Schistosoma japonicum and Plasmodium berghei. Malar J. 2014;13:116. doi: 10.1186/1475-2875-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhattacharjee S, Mejias-Luque R, Loffredo-Verde E, Toska A, Flossdorf M, Gerhard M, et al. Concomitant Infection of S. mansoni and H. pylori Promotes Promiscuity of Antigen-Experienced Cells and Primes the Liver for a Lower Fibrotic Response. Cell Rep. 2019;28(1):231–44 e5. doi: 10.1016/j.celrep.2019.05.108 [DOI] [PubMed] [Google Scholar]
- 87.Long SR, Lanter BB, Pazos MA, Mou H, Barrios J, Su CW, et al. Intestinal helminth infection enhances bacteria-induced recruitment of neutrophils to the airspace. Sci Rep. 2019;9(1):15703. doi: 10.1038/s41598-019-51991-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ivanov I, Kuhn H, Heydeck D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene. 2015;573(1):1–32. doi: 10.1016/j.gene.2015.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pazos MA, Pirzai W, Yonker LM, Morisseau C, Gronert K, Hurley BP. Distinct cellular sources of hepoxilin A3 and leukotriene B4 are used to coordinate bacterial-induced neutrophil transepithelial migration. J Immunol. 2015;194(3):1304–15. doi: 10.4049/jimmunol.1402489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113(1):74–84. doi: 10.1172/JCI17005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blackmore S, Hernandez J, Juda M, Ryder E, Freund GG, Johnson RW, et al. Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2017;114(30):E6107–E16. doi: 10.1073/pnas.1620415114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matar CG, Anthony NR, O’Flaherty BM, Jacobs NT, Priyamvada L, Engwerda CR, et al. Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity. PLoS Pathog. 2015;11(5):e1004858. doi: 10.1371/journal.ppat.1004858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolf AI, Strauman MC, Mozdzanowska K, Whittle JR, Williams KL, Sharpe AH, et al. Coinfection with Streptococcus pneumoniae modulates the B cell response to influenza virus. J Virol. 2014;88(20):11995–2005. doi: 10.1128/JVI.01833-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poole BD, Gross T, Maier S, Harley JB, James JA. Lupus-like autoantibody development in rabbits and mice after immunization with EBNA-1 fragments. J Autoimmun. 2008;31(4):362–71. doi: 10.1016/j.jaut.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bansal D, Herbert F, Lim P, Deshpande P, Becavin C, Guiyedi V, et al. IgG autoantibody to brain beta tubulin III associated with cytokine cluster-II discriminate cerebral malaria in central India. PLoS ONE. 2009;4(12):e8245. doi: 10.1371/journal.pone.0008245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rivera-Correa J, Guthmiller JJ, Vijay R, Fernandez-Arias C, Pardo-Ruge MA, Gonzalez S, et al. Plasmodium DNA-mediated TLR9 activation of T-bet(+) B cells contributes to autoimmune anaemia during malaria. Nat Commun. 2017;8(1):1282. doi: 10.1038/s41467-017-01476-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–61. doi: 10.1016/j.chom.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 98.Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12(2):223–32. doi: 10.1016/j.chom.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345(6204):1251086. doi: 10.1126/science.1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dos Santos JC, Barroso de Figueiredo AM, Teodoro Silva MV, Cirovic B, de Bree LCJ, Damen M, et al. beta-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: a Crucial Role for IL-32. Cell Rep. 2019;28(10):2659–72 e6. doi: 10.1016/j.celrep.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 101.Ciarlo E, Heinonen T, Theroude C, Asgari F, Le Roy D, Netea MG, et al. Trained immunity confers broad-spectrum protection against bacterial infections. J Infect Dis. 2020;222(11):1869–81. doi: 10.1093/infdis/jiz692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schrum JE, Crabtree JN, Dobbs KR, Kiritsy MC, Reed GW, Gazzinelli RT, et al. Cutting Edge: Plasmodium falciparum Induces Trained Innate Immunity. J Immunol. 2018;200(4):1243–8. doi: 10.4049/jimmunol.1701010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–42. doi: 10.1073/pnas.1202870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol. 2014;155(2):213–9. doi: 10.1016/j.clim.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23(1):89–100 e5. doi: 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 106.Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell. 2018;172(1–2):176–90 e19. doi: 10.1016/j.cell.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 107.de Laval B, Maurizio J, Kandalla PK, Brisou G, Simonnet L, Huber C, et al. C/EBPbeta-Dependent Epigenetic Memory Induces Trained Immunity in Hematopoietic Stem Cells. Cell Stem Cell. 2020;26(5):657–74 e8. doi: 10.1016/j.stem.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 108.Netea MG, Giamarellos-Bourboulis EJ, Dominguez-Andres J, Curtis N, van Crevel R, van de Veerdonk FL, et al. Trained Immunity: a Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell. 2020;181(5):969–77. doi: 10.1016/j.cell.2020.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kavai M, Szegedi G. Immune complex clearance by monocytes and macrophages in systemic lupus erythematosus. Autoimmun Rev. 2007;6(7):497–502. doi: 10.1016/j.autrev.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 110.Liote F, Boval-Boizard B, Weill D, Kuntz D, Wautier JL. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin Exp Immunol. 1996;106(1):13–9. doi: 10.1046/j.1365-2249.1996.d01-820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malemud CJ. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem. 2015;7(9):1137–47. doi: 10.4155/fmc.15.55 [DOI] [PubMed] [Google Scholar]
- 112.Zhu H, Hu F, Sun X, Zhang X, Zhu L, Liu X, et al. CD16(+) Monocyte Subset Was Enriched and Functionally Exacerbated in Driving T-Cell Activation and B-Cell Response in Systemic Lupus Erythematosus. Front Immunol. 2016;7:512. doi: 10.3389/fimmu.2016.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quinn SM, Cunningham K, Raverdeau M, Walsh RJ, Curham L, Malara A, et al. Anti-inflammatory Trained Immunity Mediated by Helminth Products Attenuates the Induction of T Cell-Mediated Autoimmune Disease. Front Immunol. 2019;10:1109. doi: 10.3389/fimmu.2019.01109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ryan SM, Eichenberger RM, Ruscher R, Giacomin PR, Loukas A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020;16(5):e1008508. doi: 10.1371/journal.ppat.1008508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Riet E, Adegnika AA, Retra K, Vieira R, Tielens AG, Lell B, et al. Cellular and humoral responses to influenza in gabonese children living in rural and semi-urban areas. J Infect Dis. 2007;196(11):1671–8. doi: 10.1086/522010 [DOI] [PubMed] [Google Scholar]