Abstract
Population immunity (herd immunity) to SARS-CoV-2 derives from two sources: vaccinations or cases of infection with the virus. Infections can be diagnosed as COVID-19 and registered, or they can be asymptomatic, oligosymptomatic, or even full-blown but undiagnosed and unregistered when patients recovered at home. Estimation of population immunity to SARS-CoV-2 is difficult and remains a subject of speculations. Here we present a population screening for SARS-CoV-2 specific IgG and IgA antibodies in Polish citizens (N = 501) who had never been positively diagnosed with or vaccinated against SARS-CoV-2. Serum samples were collected in Wrocław (Lower Silesia) on 15th and 22nd May 2021. Sera from hospitalized COVID-19 patients (N = 22) or from vaccinated citizens (N = 14) served as positive controls. Sera were tested with Microblot-Array COVID-19 IgG and IgA (quantitative) that contain specific SARS-CoV-2 antigens: NCP, RBD, Spike S2, E, ACE2, PLPro protein, and antigens for exclusion cross-reactivity with other coronaviruses: MERS-CoV, SARS-CoV, HCoV 229E Np, HCoV NL63 Np. Within the investigated population of healthy individuals who had never been positively diagnosed with or vaccinated against SARS-CoV-2, we found that 35.5% (178 out of 501) were positive for SARS-CoV-2-specific IgG and 52.3% (262 out of 501) were positive for SARS-CoV-2-specific IgA; 21.2% of the investigated population developed virus-specific IgG or IgA while being asymptomatic. Anti-RBD IgG, which represents virus-neutralizing potential, was found in 25.6% of individuals (128 out of 501). These patients, though positive for anti-SARS-CoV-2 antibodies, cannot be identified in the public health system as convalescents due to undiagnosed infections, and they are considered unaffected by SARS-CoV-2. Their contribution to population immunity against COVID-19 should however be considered in predictions and modeling of the COVID-19 pandemic. Of note, the majority of the investigated population still lacked anti-RBD IgG protection (74.4%); thus vaccination against COVID-19 is still of the most importance for controlling the pandemic.
Introduction
COVID-19 is an acute respiratory disease caused by the novel coronavirus SARS-CoV-2, also known as 2019-nCoV. The World Health Organization (WHO) characterized COVID-19 as a pandemic on March 11th 2020. Since then, the COVID-19 pandemic has affected most aspects of life globally, including drastic lockdowns to reduce the death toll [1–3]. Extraordinary efforts have been focused on developing drugs and vaccines that could help the situation. A universal anti-COVID-19 drug has still not been developed, and the major strategy for controlling the pandemic is population immunity, with the key role of SARS-CoV-2 targeting vaccines [4].
Population immunity to SARS-CoV-2 derives from two sources: vaccinations or cases of infection with the virus. The latter ones can be diagnosed as COVID-19 and registered, or they can be asymptomatic, oligosymptomatic, or even full-blown but undiagnosed and unregistered when patients recovered at home. COVID-19 symptoms that are typical to many other flu-like and cold infections make it impossible to identify this disease without specific diagnostics. For this reason there is an unknown fraction of society that has already achieved immunity to COVID-19 but its extent is unknown. Attempts to estimate this fraction in each society is difficult, and it remains a subject of speculation, sometime extreme [5, 6].
Here we present the results of population screening for SARS-CoV-2 specific antibodies in Polish citizens (N = 501) who had never been positively diagnosed with or vaccinated against SARS-CoV-2. Blood samples were collected in Wrocław (Lower Silesia) on 15th and 22nd May 2021, which was shortly after the third main wave of the disease in Poland that was observed approximately between 15th February and 30th April 2021, reaching more than 35 000 diagnosed cases per day at its peak (out of almost 38 million citizens).
Overall statistics of COVID-19 for the whole country at 15th May were as follows:
Total number of all infected with SARS-CoV-2 during the pandemic: 7.5% of population (2 851 911/71 609 total infected/died).
Vaccinated with at least 1 dose: 30.3% of population, which includes full vaccination: 11.9% of population
Overall statistics of COVOD-19 for Lower Silesia at 15th May were as follows:
Total number of all infected with SARS-CoV-2 during the pandemic: 7.3% of population (210 353/4634 total infected/died).
Vaccinated with at least 1 dose: 31.9% of population, which includes full vaccination: 11.8% of population
Material and methods
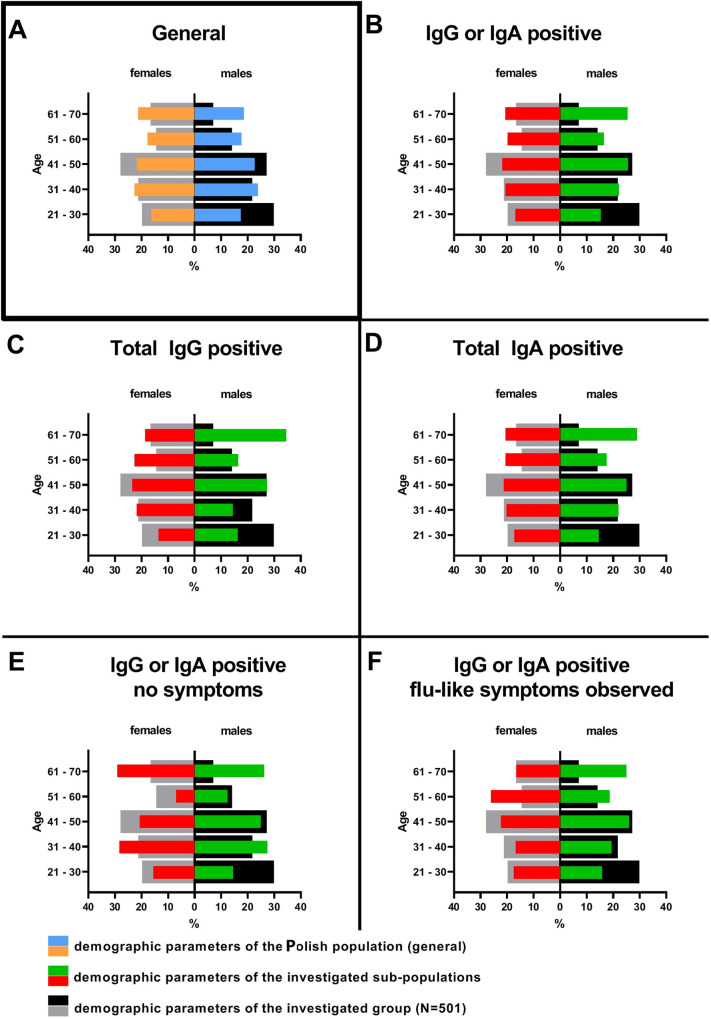
Blood samples were collected in Wrocław (Lower Silesia) on 15th and 22nd May 2021, from healthy individuals (over 17 years old, N = 501), who had not been vaccinated against COVID-19 with any kind of vaccine, nor had they ever been diagnosed positively for SARS-CoV-2 infection. These participants were recruited by open announcements in press, TV, and hospital website; the recruited group might contain both those who did not manage to vaccinate yet for any reason (e.g. the youngest participants were not eligible for prompt vaccination) or decided to avoid vaccination deliberately. The positive control was the group of patients hospitalized due to severe COVID-19 (non-vaccinated) 10–30 days after estimated start of the infection (N = 22) and individuals vaccinated against SARS-CoV-2 at least 2 months after the second dose (never diagnosed positively for SARS-CoV-2 infection) (N = 14). All patients were interviewed for possible flu-like and cold-type infections within the period of the COVID-19 pandemic, comorbidities, and for their occupation as potentially linked to frequency of social interactions. All reads and data are fully available for further analyses in the S1 Table. General comparison of demographics parameters within the investigated group S1 Table to those of the whole society in the country (https://stat.gov.pl/obszary-tematyczne/ludnosc/ludnosc/struktura-ludnosci,16,1.html) is presented in Fig 1A. It revealed some statistically significant difference between the whole population and the investigated group. This was probably resulting from inclusion/exclusion criteria that were applied as well as from potential accessibility of the study for potential participants (located in a big city).
Fig 1. Demographics in sub-populations immune to SARS-CoV-2.
Blood samples
Blood was collected into standard clotting tubes (BD SST II Advance), left for 1 hour at room temperature (RT) to clot and serum was separated from the clot by centrifugation (15 min, 2000 g, RT), then stored at -20°C for further use.
Bioethics statements
The experiments were approved by the local Commission of Bioethics of the Regional Specialist Hospital in Wrocław (approval number: KB/02/2020, policy No. COR193657). Individual conversation to each participant was completed, during which all information about the study was provided and written informed consent was obtained from the participant. The written informed consent form had been reviewed and approved by the local Commission of Bioethics of the Regional Specialist Hospital in Wrocław within the study approval (approval number: KB/02/2020).
Serological diagnostic tests
Microblot-Array COVID-19 IgG and Microblot-Array COVID-19 IgA (TestLine Clinical Diagnostics s.r.o); cat no. CoVGMA96, LOT 0100060496 and cat. no. CoVAMA96, LOT 0100066601); the arrays contain a combination of selected parts of the specific antigens of SARS-CoV-2: NCP, RBD, Spike S2, E, ACE2, PLPro protein, and antigens for exclusion cross-reactivity with other endemic coronaviruses: MERS-CoV, SARS-CoV, HCoV 229E Np, HCoV NL63 Np.
Statistics
Data were analyzed by ANOVA and the Kruskal–Wallis test with the Statistica 8.0 software package (www.statsoft.pl).
Results and discussion
Within the investigated population of healthy citizens who had never been positively diagnosed with or vaccinated against SARS-CoV-2, we found that 35.5% (178 out of 501) were positive for SARS-CoV-2-specific IgG and as many as 52.3% (262 out of 501) were positive for SARS-CoV-2-specific IgA (Table 1). These positive patients (with the exception of 2 individuals) did not demonstrate any cross-reactivity to other coronaviruses; thus their immune response to SARS-CoV-2 is specific and it apparently results from immunization with SARS-CoV-2. Taking the high rate of SARS-CoV-2-specific IgA, more than a half of the investigated population has had a contact (or infection) with the virus. Interestingly, 106 patients positive for SARS-CoV-2-specific IgG or IgA declared no flu-like infection or cold within the time of the COVID-19 pandemic; thus, 21.2% of the investigated population developed virus-specific antibodies while being asymptomatic (S1 Table).
Table 1. Results of qualitative testing for SARS-CoV-2-specific antibodies in healthy individuals (N = 501), non-vaccinated and never diagnosed positively for SARS-CoV-2 infection.
| antibody classes | Number of participants |
|---|---|
| IgG and IgA | 144 |
| IgA | 118 |
| IgG | 34 |
| NONE | 205 |
These results in general are in line with the current epidemiological model developed by ICM University of Warsaw [7], where the percentage of recoveries in the population on April 1 was estimated at about 35% or 45%. Indeed, our study that was conducted later, during 15–22 May, revealed that half of the population may have been affected by SARS-CoV-2, as demonstrated by the unexpectedly high contribution of individuals positive for anti-SARS-CoV-2 IgA. IgA antibodies were identified in blood serum, but overall serum levels of IgA are consistent with IgA secretion to mucosal surfaces. IgA in the ciliary mucosa of the respiratory epithelium are the first defense line and can be detected at the early stage of infection. The IgG is generated later in the infection course. This may suggest that within the investigated group of participants a considerable fraction had been infected shortly before testing, regardless they noticed any symptoms or not.
Analysis of demographic parameters within defined groups, like those positive for IgG and/or IgA, revealed a trend for older population to be overrepresented, but the differences to the parameters within the group of all participants was not significant (Fig 1B–1D). Similarly, in these participants we have not found significant differences between demographics in the sub-groups with or without flu-like symptoms noted during the pandemics who were positive for SARS-CoV-2 specific IgG or IgA (Fig 1E–1F). Thus, in this study we are not able to indicate a demographic parameter that might predispose for asymptomatic COVID-19.
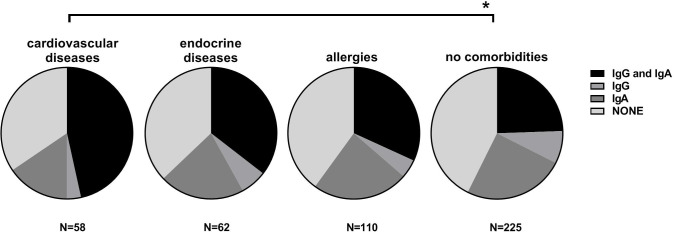
Comorbidities were also analyzed for possible correlation to increased incidence of unregistered infections with SARS-CoV-2. We found that participants with cardiovascular diseases (p<0.01) demonstrated higher frequency of anti-SARS-CoV-2 antibodies, particularly IgA (Fig 2). This suggests that health disorders commonly known to increase the risk of full-blown COVID-19 may also increase the risk of oligosymptomatic infections. Other health disorders were low-represented in the investigated group and no further correlations were noted.
Fig 2. Correlation between comorbidities and antibody responses to SARS-CoV-2.
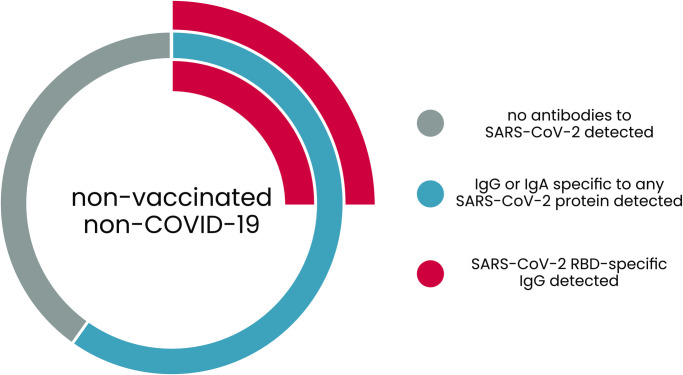
The virus-specific antibodies that confirm an individual’s contact or infection with the virus, do not necessary provide an effective protection from infections in the future. Anti-RBD IgG is considered the most significant fraction of virus-neutralizing antibodies, and it was found in 25.6% (128 out of 501) individuals, while 74.4% of the investigated population lacks anti-RBD IgG protection (Fig 3). In general, nucleocapsid protein was found the most immunogenic protein of the virus, since antibodies of that specificity were found in the largest group of participants (Table 2). Second represented specificities were those targeting spike protein (receptor binding domain or domain S2), while all other investigated proteins were of very low immunogenicity, found only in very few individuals (Table 2).
Fig 3. Fraction of the investigated population immune to SARS-CoV-2: Patients positive for IgG and IgA.
Table 2. Protein specificity of anti-SARS-CoV-2 antibodies detected in healthy individuals non-vaccinated and never diagnosed positively for SARS-CoV-2 infection.
Number of individuals demonstrating indicated specificity was presented.
| anti-NCP | anti-RBD | anti-S2 | anti-E | anti-ACE2 | anti-PLPro | |
|---|---|---|---|---|---|---|
| IgG | 136 | 128 | 135 | 1 | 1 | 3 |
| IgA | 182 | 136 | 121 | 14 | 20 | 14 |
NCP- nucleocapsid protein, RBD-receptor binding domain within Spike protein, S2- domain S2 within Spike protein, E- envelope protein, ACE2- angiotensin-converting enzyme 2, PLPro- papain-like protease.
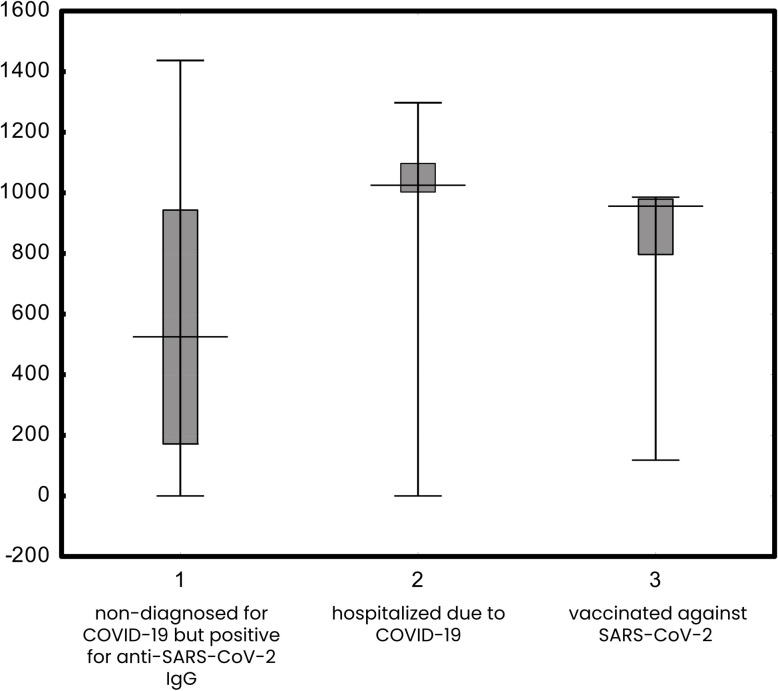
We further compared quantitative levels of the virus-specific anti-RBD IgG in the fraction of healthy population identified as positive for anti-SARS-CoV-2 IgG, in COVID-19 patients hospitalized due to a severe course of the disease, and in individuals vaccinated against SARS-CoV-2. Median values were 525.12 U/ml, 1025.13 U/ml, and 954.93 U/ml, respectively, thus antibody levels were higher in the hospitalized patients or in the vaccinated individuals. These median values with quartiles and min-max values are presented in Fig 4.
Fig 4. IgG specific to SARS-CoV-2 RBD in (1) non-diagnosed for COVID-19 but positive for anti-SARS-CoV-2 IgG, (2) in patients hospitalized due to COVID-19, (3) and in vaccinated against SARS-CoV-2.
To some extent, mucosal immunity can be provided by SARS-CoV-2-specific IgA. Within the group of SARS-CoV-2 IgA-positive patients, anti-RBD IgA was found in 136 individuals, which makes approximately 27.2% of the tested population.
This study has revealed a significant though still not sufficient fraction of the population of people who have developed natural immunological protection against COVID-19, apparently due to an asymptomatic, oligosymptomatic, or moderate (not-requiring hospitalization) course of the disease. Due to undiagnosed infections, they cannot be identified in the public health system as convalescents, being considered unaffected by SARS-CoV-2. This hidden fraction of immune individuals may really contribute to population immunity against COVID-19, improving the overall pandemic conditions and predictions [8]. On the other hand, this fraction seems to be insufficient for effective population immunity, since the majority of the investigated population still lacked anti-RBD IgG or IgA protection. Thus vaccination against COVID-19 is still of the utmost importance as the major tool for controlling the disease.
Practical aspect of serological screening in the population is its contribution to the epidemiological efforts to control the pandemic. Many countries are running serological screening in citizens, including school children and other groups. Given the results of this study, screening for SARS-CoV-2 specific antibodies reveals the hidden (unregistered) fraction of population that has already developed immunity to SARS-CoV-2. It may efficiently contribute to modeling of pandemics scenarios and help in planning the most applicable means of controlling virus transmission within a society.
Supporting information
Excel file available for further analyses.
(XLSX)
Acknowledgments
The authors are deeply grateful to all participants who agreed to act as serum donors in this study. Thank you for your help, support, understanding, and for your desire to contribute to scientific solutions to combat the disease.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Research and Development Center in Poland, grant no. SZPITALE-JEDNOIMIENNE/48/2020. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Finkel Y., Mizrahi O., Nachshon A. i e. al., „The coding capacity of SARS-CoV-2,” Nature, tom 589, nr 7840, pp. 125–130, 2021. doi: 10.1038/s41586-020-2739-1 [DOI] [PubMed] [Google Scholar]
- 2.„WHO,” 2020. [Online]. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. [Data uzyskania dostępu: 04 06 2021].
- 3.Li Y. D., Chi W. Y., Su J. H., Ferrall L., Hung i T C. F. Wu C. , „Coronavirus vaccine development: from SARS and MERS to COVID-19,” J Biomed Sci. tom 27, nr 1, p. 104, 2020. doi: 10.1186/s12929-020-00695-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashte S., Gulbake A., El-Amin S. F. III i e. al., „COVID-19 vaccines: rapid development, implications, challenges and future prospects.,” tom 34, pp. 711–733, 2021. doi: 10.1007/s13577-021-00512-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerowitz E. A., Richterman A., Bogoch I. I., Low i M. Cevik N., „Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2,” Lancet Infect Dis., tom 21, nr 6, pp. e163–e169, 2021. doi: 10.1016/S1473-3099(20)30837-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S. W., Cornforth D. M., Dushoff i J J. Weitz S., "The time scale of asymptomatic transmission affects estimates of epidemic potential in the COVID-19 outbreak,” Epidemics, tom 31, p. 100392, 2020. doi: 10.1016/j.epidem.2020.100392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ICU University of Warsaw, „ICM EPIDEMIOLOGICAL MODEL,” 2021. [Online]. Available: https://covid-19.icm.edu.pl/en/current-forecasts/. [Data uzyskania dostępu: 02 06 2021].
- 8.Fontanet i S A. Cauchemez, „COVID-19 herd immunity: where are we?,” Nat Rev Immunol., tom 20, nr 10, pp. 583–584, 2020. doi: 10.1038/s41577-020-00451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file available for further analyses.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.