ABSTRACT
Dengue is an arboviral infection that is hyperendemic in tropical and subtropical climates. Clinical manifestations of dengue can range from asymptomatic infection to severe infection with multi-organ failure. Dengue haemorrhagic fever (DHF) is a subcategory in dengue infection with a hallmark of plasma leak (ie critical phase). The plasma leak in DHF is selective (pleuroperitoneal spaces), transient and dynamic, and needs careful monitoring and meticulous fluid resuscitation. In addition, dengue fever may present with extended and unusual manifestations affecting any organ, including the heart, liver, kidney and brain. Studies on vaccine development and vector control are ongoing to prevent this infection of global importance. In this article, the clinicopathological features and management aspects of dengue are discussed.
KEYWORDS: Dengue haemorrhagic fever, Aedes, NS1, flavivirus, critical phase, plasma leak
Key points
Dengue is an arboviral infection commonly transmitted by the mosquito Aedes aegypti and it is hyperendemic in tropical and subtropical climates worldwide, with increasing incidence over recent years placing nearly half of the world's population at risk.
Most patients with dengue remain asymptomatic, while some develop an acute febrile illness that can range from an undifferentiated fever to dengue haemorrhagic fever (DHF)and shock.
Laboratory confirmation can be done either directly by detecting viral components in the blood or indirectly by serological measures; choice of test depends on the day of illness.
The clinical course has febrile, critical and recovery phases - plasma leakage is the hallmark of DHF, which occurs soon after the end of the febrile phase, while dengue fever bypasses the critical phase.
Plasma leakage in DHF is selective and transient, usually lasts for 24-48 hours and is dynamic in nature.
Use of blood products, steroids, immunoglobulin and N-acetylcysteine is controversial, but some studies show lifesaving benefits.
Extended and unusual presentations involving heart, liver, kidney, muscle and brain are recognised in dengue.
As conventional methods of vector control are less effective, newer techniques to reduce the mosquito vector density by gene technology are being tested.
Developing a vaccine for dengue is challenging as there are four serotypes; however, many tetravalent vaccine candidates are still in development.
Early detection of the critical period (onset of plasma leakage) and appropriate fluid management to match the plasma leak are the mainstays in management.
Introduction
Dengue is a febrile illness with clinical manifestations ranging from asymptomatic infection to severe infection with multi-organ dysfunction. It is one of the most important and fastest-growing mosquito-borne viral infections in the world today, and a disease of major public health concern owing to potential lethal outcomes of severe infection. Dengue is hyperendemic in tropical and subtropical climates worldwide, mostly in urban and semi-urban areas. Global incidence of dengue has grown exponentially in recent years and nearly half of the world's population is now at risk.1 There are an estimated 100-400 million new infections each year, although this number may be grossly under-reported as surveillance networks are not robust in most tropical countries. Fig 1 shows the global distribution of the infection, where tropical Asia and America show the highest density. Historically, the first description of dengue goes back to the early 19th century in the Caribbean islands long before germ theory, where breakbone fever had been described as Dandy fever. Later it was believed that dengue had been introduced to the New World from Africa during slave trading.2 Since then, dengue has passed through many milestones where the severity of the disease has changed to more severe forms and reached highest global attention, until the COVID-19 pandemic.
Fig 1.
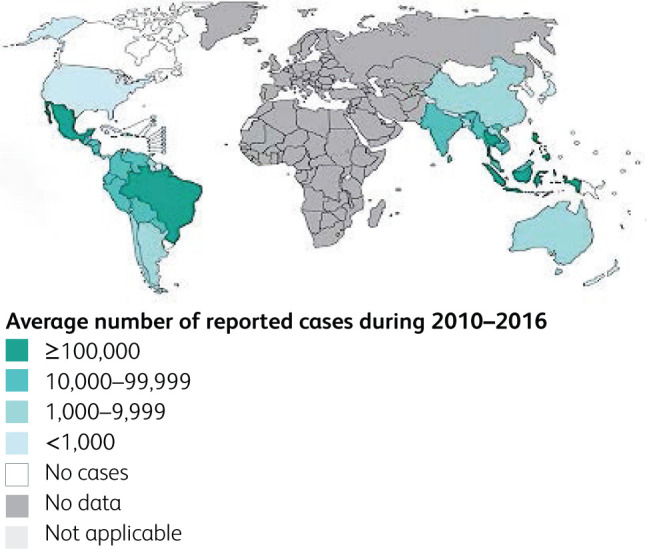
Distribution of suspected or confirmed dengue cases during 2010-2016 in the world (Panacea Biotec. n.d.). Map created by Control of Neglected Tropical Disease (NTD).3
Virological features
Dengue virus (DENV) is a small, spherical, single-stranded RNA virus with 10,700 bases. It belongs to the genus Flavivirus in the family Flaviviridae. West Nile virus, Zika virus and tick-borne encephalitis virus are other known members of the genus Flavivirus. DENV is made up of three structural and seven non-structural proteins. Depending on differences in the viral structural and non-structural proteins, there are four serotypes of dengue virus - DEN1 to DEN4. Due to mutations of the virus, the severity of the infection varies from time to time where genotypes have been described, eg A and B in DEN3. Infection with each serotype confers lifelong immunity for the causative serotype, but not for the other serotypes. On the contrary, reinfection with a different serotype causes severe disease. In a given region, periodic outbreaks occur due to different serotypes over decades, thus development of complete herd immunity for all four serotypes in the community is not achievable and the disease may remain without natural elimination.3–5
Immunopathogenesis
Dengue is transmitted from person to person via the bite of an infected mosquito. Of these, the primary vector is Aedes aegypti, which is a highly domestic mosquito, a day biter, breeding in water containers in peri-domestic areas. Its eggs could survive without desiccation in dried condition for months and, with the first opportunity of contact with water, the life cycle begins. Aedes albopictus is a secondary dengue vector, confined to a few regions in the world and is called ‘tiger mosquito’ due to its characteristic morphology.6 Fig 2 shows these two main Aedes mosquitoes and their unique body features.
Fig 2.
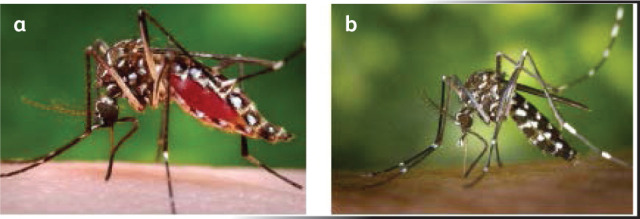
Aedes mosquitoes. Courtesy of Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD). (a) Aedes aegypti mosquito. (b) Aedes albopictus mosquito.
Three types of transmission cycle have been described with regard to DENV.7
Forest/enzoonotic cycle - Aedes mosquitos and low primates in the rain forests.
Rural/endemic cycle - occurring in small villages or islands where transmission is contained. Virus disappears with developing herd immunity over time.
Urban/epidemic/endemic cycle - in large urban areas in the tropics, periodic epidemics with multiple serotypes.
After a bite from an infected mosquito, initial viral replication occurs in subdermal Langerhans dendritic cells and then the virus migrates to regional lymph nodes. Viraemia occurs through circulating monocytes and macrophages and infects the solid organs and bone marrow.8
Like most viral infections, dengue is a self-limiting infection (Fig 3) from which the majority of patients recover without any complication - this is denoted as dengue fever (DF). Conversely, dengue haemorrhagic fever (DHF) is the severe form, characterised by increased vascular permeability leading to plasma leak and haemorrhagic tendency. The increased vascular permeability is short-lived and involves plasma leaking into peritoneal spaces, the pleural cavity and tissue plains called third spaces. This is likely to be due to the occurrence of an abnormal immune response with cytokine production, also named a cytokine storm. The abnormal immune response results in increased microvascular permeability without inflammation or vasculitis, and leads to altered thromboregulatory mechanisms.9 Antibody-dependent immune enhancement is a known phenomenon where there is an increased risk of DHF in the presence of pre-existing DENV antibodies for a different serotype, which are non-neutralising antibodies. The formed immune complexes are composed of non-neutralising DENV antibodies for a different serotype attached to current DENV that would have the ability to fix complement and bind to cell surface Fc receptors, facilitating viral entry into phagocytic cells (macrophages). This process is called opsonising. Inside the phagocytic cell, exponential viral replication takes place due to the opsonising effect and the result is the development of heavy viraemia. In cases of severe viraemia, the chance of severe DHF is high, even leading to shock - this is called dengue shock syndrome (DSS).
Fig 3.
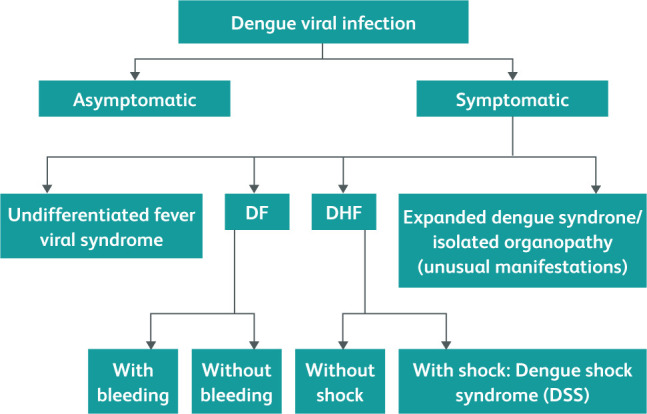
Natural course of dengue infection.10,11 Courtesy Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded edition. (SEARO Technical Publication Series No 60), 2011.11 DF, dengue fever; DHF, dengue haemorrhagic fever.
Differential diagnosis
Differential diagnosis in dengue fever is very broad and it varies according to the phase of the illness. During the febrile phase, the clinical picture is similar to those of common viral infections such as COVID-19, influenza, adenovirus, measles, rubella, enteroviral infections and bacterial infections like leptospirosis, rickettsial infections and typhoid fever. Connective tissue diseases such as systemic lupus erythematosus and Still's disease could closely mimic dengue infection at the onset. Certain malignancies such as acute leukaemia could closely mimic dengue fever.9 Therefore, detailed history taking - including travel to dengue-endemic areas, contact history and evolution of symptoms - is needed.
Classification
The World Health Organization (WHO) has had many classifications of dengue infection. The classification of 1997 describes subgroups of DHF and does not have much clinical application. The subsequent WHO 2009 classification divides dengue cases into two categories - non-severe and severe. The non-severe category is further subdivided into patients with warning signs and those without warning signs.10,11
The non-severe form of dengue is similar to an undifferentiated viral illness. A probable case of dengue is described as fever and two of the following criteria in a patient living in or has travelled to an endemic area:
nausea and vomiting
rash
aches and pains
positive tourniquet test
leucopenia.
This needs further confirmation with laboratory testing. In severe dengue, warning signs denote impending plasma leak progressing to hypovolaemic state, tissue hypoperfusion and bleeding. These warning signs include abdominal pain, persistent vomiting, bleeding manifestations, lethargy and restlessness, hepatomegaly and laboratory evidence of haemoconcentration, reflected as increasing haematocrit and a rapid drop in platelet count.
The above clinical classification is useful in the management of patients; however, to understand the whole picture of pathophysiology, both classifications are needed, and this has been attempted in the WHO document in 2011.11
Clinical manifestations
After an incubation period of 3-7 days, there is an abrupt onset of symptoms - mainly high fever, retro-orbital headache and body pain. Typically, the clinical course follows three phases - febrile, critical and recovery. Those who develop DHF go through all three phases; however, the dengue fever group does not go through the critical phase.
Febrile phase
This phase typically lasts for 3-7 days and manifests with a high spiking temperature, headache, arthralgia, myalgia, backache and anorexia. Occasionally, upper respiratory tract and gastrointestinal symptoms intervene. An ill look is common, and generalised flushing of skin blanching with pressure appears with or without morbilliform erythematous eruptions and islands of pallid areas (Fig 4). Cutaneous bleeding manifestations such as petechiae, purpura or ecchymosis may appear towards the latter part of the febrile phase. Tender right hypochondrium or mild hepatomegaly may be present. From the second day of fever, the complete blood count shows leucopenia, thrombocytopenia and rising haematocrit. Elevation of hepatic transaminases such as alanine transaminase (ALT) and aspartate transaminase (AST) is commonly observed. The temperature pattern could be biphasic.11,12
Fig 4.

Symptoms of dengue fever. a) Generalised flushing of skin and lips. b) Morbilliform erythematous eruptions and islands of pallid areas.
Critical phase
A proportion of patients will enter the critical phase, which is evidenced by systemic vascular leak, typically occurring with temporary defervescence. It is recognised by increased plasma concentration from rising haematocrit. The vascular leak occurs preferably into peritoneal spaces that could be detected early by ultrasound examination of the abdomen to find gall bladder wall oedema, and pericholecystic fluid collection.12 Indirectly, development of warning signs indicates entry into the critical phase. Initial physiological compensatory mechanisms of plasma leak will lead to narrowing of pulse pressure, but if it remains undetected or untreated the patient will decompensate, leading to severe shock and multi-organ dysfunction. Rising haematocrit more than 20% of baseline and hypoalbuminaemia are other indicators of the critical phase. The vascular leak may last for 24-48 hours and will be dynamic in nature, usually reaching its peak at 24 hours of onset (Fig 5). This phase is associated with increased risk of bleeding and liver dysfunction.12
Fig 5.
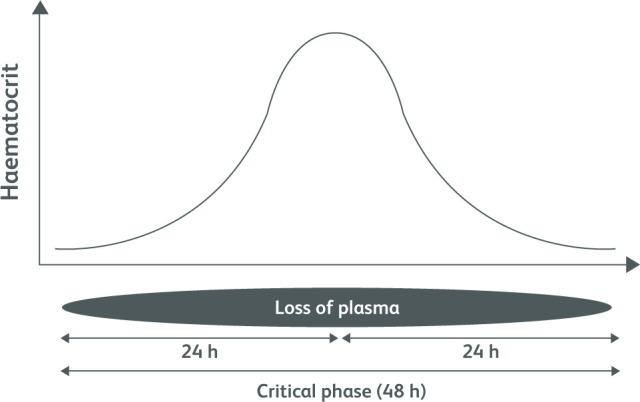
Diagrammatic representation of plasma leak during the critical phase of dengue haemorrhagic fever.11
Recovery phase
In this phase, the systemic vascular leak stops and extravasated third-space fluid starts getting reabsorbed. This phase is clinically recognised by the patient experiencing marked improvement of wellbeing and some develop an itchy rash. Also patients develop bradycardia, named recovery bradycardia. Haemodilution leads to a drop in haematocrit and a rapid rise in white cell count, followed by platelets.12 The patient develops polyuria, even leading to dehydration.
Other extended presentations
Unusual dengue or expanded dengue is described as multi-system involvement other than plasma leak.13–16
Neurological - Encephalitis, encephalopathy, neuropathies, Guillain-Barré syndrome
Gastrointestinal - Hepatitis, cholecystitis, pancreatitis, haemorrhagic liver necrosis
Renal - Nephritis
Cardiac - Myocarditis, pericarditis
Musculoskeletal - Myositis
Haematological - Haemophagocytic lymphohistiocytosis, immune thrombocytopenia
DENV RNA has been detected in most organs and tissues of the body in post mortem studies. This implies that the virus can infect the organ systems, leading to inflammation and dysfunction. The severity of myocarditis could vary and, in severe myocarditis, fatalities are inevitable. Similarly, haemorrhagic necrosis of the liver carries a bad prognosis. Hepatic ischaemia during prolonged shock and secondary bacterial sepsis are also contributory causes of the development of fulminant liver failure.15,16
Diagnostics
Laboratory confirmation of the diagnosis can be done either directly by detecting viral components in the blood or indirectly by serological measures. The choice of test depends on the timeline of the clinical presentation. During the early illness of the febrile phase, detection of viral components in the circulation is highly sensitive. Viral nucleic acid in serum can be detected by means of reverse transcriptase polymerase chain reaction (RT-PCR) assay or by detection of the virus-expressed soluble non-structural protein 1 (NS1) by means of enzyme-linked immunosorbent assay (ELISA).17 Serology to detect IgM and IgG has a place from the fifth day of illness and also helps in deciding between primary or secondary dengue infection. A high titre of haemagglutinin antibodies suggests secondary dengue infection.6,10
Management
The WHO guideline on clinical management of dengue provides details of decision making on either home-based or hospital-based management. Accordingly, at the beginning of the illness, a patient could fall into one of three groups: A, B or C. Those who meet the criteria of group A could be managed at home under supervision. Group C needs hospital admission.10 Furthermore, countries have their own dengue management guidelines.11 There is no specific antiviral drug for dengue, despite its grave threat to humanity.
Symptomatic management
During the febrile phase, patients are advised to maintain adequate oral intake of fluid and take paracetamol as an antipyretic. Use of non-steroidal anti-inflammatory medications should be avoided due to bleeding risks in the background of severe thrombocytopenia.18 Patients are advised to seek medical advice if they experience warning symptoms, which include persistent vomiting and diarrhoea, postural dizziness, bleeding manifestations or abdominal pain.
Fluid management
The mainstay of management of dengue fever is meticulous fluid resuscitation, particularly in the critical phase, where the plasma leak is matched with the rate of fluid administration. These recommendations are based on expert opinion and many assumptions.
Some guidelines recommend calculation of a fluid quota for the assumed 48-hour period of the critical phase, which involves maintenance fluid and 50 mL/kg fluid deficit (up to 50 kg) administered over the 48-hour period of the critical phase. Finally, this amounts to 4,600 mL for a 50 kg person for 48 hours. The rationale for fluid management in the critical phase of dengue fever is to keep the intravascular compartment adequately filled but avoiding overloading the patient. For this, the clinician should be very vigilant and fluid administration should be in stepwise increments or decrements based on clinical parameters, urine output and degree of haemoconcentration. The first-line therapy is crystalloids. Colloids (dextran 40, hetastarch) are the second-line fluid therapy where the response to crystalloid boluses is not adequate. The aim of fluid management is to maintain good perfusion and urine output of about 0.5 mL/kg/h. The guidelines provide algorithms to follow on the management of dengue shock. Additionally, correction of acidosis, blood sugar and calcium (ABC) is important during the clinical course of dengue.11,12
Blood products
Platelet transfusions are indicated in patients with severe haemorrhagic manifestations with thrombocytopenia or patients needing emergency surgery. However, the survival of transfused platelets is very short. There is evolving evidence in the use of blood transfusion in complicated dengue such as major bleeding, severe liver involvement or refractory acidosis.12,18
Steroids
Although there are some studies that describe the benefit of steroids in DHF, more data from large studies are needed to make recommendations on the use of steroids.11 However, clinical opinions favour the benefit of steroids in dengue myocarditis.12,15
Immunoglobulins
The use of immunoglobulin in dengue shock and severe thrombocytopenia is described in small study cohorts, but current data are insufficient to make recommendations.19
Other treatments
Liver involvement associated with dengue fever is commonly seen and may progress to fulminant hepatic failure. Meticulous use of N-acetylcysteine with rising transaminases is shown to be beneficial in some studies.20 Antibiotics are not routinely recommended, but secondary bacterial sepsis can occur due to leucopenia and immune paresis, even leading to severe septic shock, where appropriate antibiotics are indicated.21
Pregnancy and dengue
It is not uncommon for pregnant mothers to get dengue during different stages of gestation. Pregnancy carries a high risk of complications and clinical management needs multidisciplinary expert advice. The physiological changes of pregnancy should be considered when interpreting investigations and deciding on treatment. During labour, the blood loss should be estimated and should be replaced with fresh blood.11,12
Preventive measures
Vector control
Conventional vector control strategies have been less effective as the mosquito is highly domesticated. Other newer strategies of vector control, such as release of genetically modified male mosquitoes that sterilise the wild-type female population, could lead to reduction in vector density.22 Embryonic introduction of strains of the obligate intracellular bacterium Wolbachia into A aegypti, which makes the mosquito resistant to DENV, is an alternative.23 Personal protective methods such as adequate clothing and use of mosquito repellents have benefits.
Vaccine development
Vaccine development for DENV has been very challenging for many reasons. The vaccine has to cover all four serotypes, otherwise immune augmentation could occur. So, the hope is to produce a tetravalent vaccine. Furthermore, the pathophysiology of DENV is still to be elucidated and there is no animal model to recreate the human process. All these hinder the development of effective vaccine candidates. The first licensed vaccine Dengvaxia (CYD-TDV), authorised in 2015, had many problems and currently many researchers are working on this field for a better vaccine targeting all four serotypes.24
References
- 1.Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Furuya-Kanamori L, Wangdi K. Clinical predictors of severe dengue: a systematic review and meta-analysis. Infect Dis Poverty 2021;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smart WR. On dengue or dandy fever. Br Med J 1877;1:382–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol 2021;67:687–702. [DOI] [PubMed] [Google Scholar]
- 4.Gwee XWS, Chua PEY, Pang J. Global dengue importation: a systematic review. BMC Infect Dis 2021;21:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libraty DH, Young PR, Pickering D, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002;186:1165–8. [DOI] [PubMed] [Google Scholar]
- 6.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998;11:480–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman AL. Immunology and immunopathogenesis of dengue disease. Adv Virus Res 2003;60:397–419. [DOI] [PubMed] [Google Scholar]
- 8.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 2007;30:329–40. [DOI] [PubMed] [Google Scholar]
- 9.Hasan S, Jamdar SF, Alalowi M, Al Ageel Al Beaiji SM. Dengue virus: A global human threat: Review of literature. J Int Soc Prev Community Dent 2016;6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Regional office for South East Asia. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever, revised and expanded edition. New Delhi: World Health Organization South East Asia regional office, 2011. [Google Scholar]
- 11.Ministry of Health Sri Lanka, in collaboration with Ceylon College of Physicians. Guidelines on management of dengue fever and dengue haemorrhagic fever in adults. Sri Lanka: Ministry of Health, 2010. [Google Scholar]
- 12.Kularatne SAM. Dengue fever. BMJ 2015;351:h4661. [DOI] [PubMed] [Google Scholar]
- 13.Kularatne SAM, Pathirage MMK, Gunasena S. A case series of dengue fever with altered consciousness and electroencephalogram changes in Sri Lanka. Trans R Soc Trop Med Hyg 2008;102:1053–4. [DOI] [PubMed] [Google Scholar]
- 14.Dalugama C, Shelton J, Ekanayake M, Gawarammana IB. Dengue fever complicated with Guillain-Barré syndrome: a case report and review of the literature. J Med Case Rep 2018;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weerakoon KG, Kularatne SA, Edussuriya DH, et al. Histopathological diagnosis of myocarditis in a dengue outbreak in Sri Lanka, 2009. BMC Res Notes 2011;4:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kularatne SAM, Imbulpitiya IVB, Abeysekera RA, Waduge RN, Rajapakse RPVJ, Weerakoon KGAD. Extensive haemorrhagic necrosis of liver is an unpredictable fatal complication in dengue infection: a postmortem study. BMC Infect Dis 2014;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeling RW, Artsob H, Pelegrino JL, et al. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol 2010;8:Suppl:S30–8. [DOI] [PubMed] [Google Scholar]
- 18.Dalugama C, Gawarammana IB. Lessons learnt from managing a case of dengue hemorrhagic fever complicated with acute liver failure and acute kidney injury: a case report. J Med Case Rep 2018;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimaano EM, Saito M, Honda S, et al. Lack of efficacy of high-dose intravenous immunoglobulin treatment of severe thrombocytopenia in patients with secondary dengue virus infection. Am J Trop Med Hyg 2007;77:1135–8. [PubMed] [Google Scholar]
- 20.Dissanayake DMDIB, Gunaratne WMSN, Kumarihamy KWMPP, Kularatne SAM, Kumarasiri PVR. Use of intravenous N-acetylcysteine in acute severe hepatitis due to severe dengue infection: a case series. BMC Infect Dis 2021;21:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kularatne SAM, Ralapanawa U, Dalugama C, Jayasinghe J, Rupasinghe S, Kumarihamy P. Series of 10 dengue fever cases with unusual presentations and complications in Sri Lanka: a single centre experience in 2016. BMC Infect Dis 2018;18:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise de Valdez MR, Nimmo D, Betz J, et al. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci U S A 2011;108:4772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009;139:1268–78. [DOI] [PubMed] [Google Scholar]
- 24.Guy B, Saville M, Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum Vaccin 2010;6:9. [DOI] [PubMed] [Google Scholar]


