ABSTRACT
Hyperosmolar hyperglycaemic state (HHS) is a life-threatening metabolic complication of type 2 diabetes (T2DM) that often presents with neurological symptoms. A 74-year-old man with known T2DM presented to the emergency department with collapse, left-sided weakness and slurred speech (National Institutes of Health Stroke Scale (NIHSS) 3) and a biochemical profile consistent with HHS. When he further deteriorated (NIHSS 20), he was managed for concurrent ischaemic stroke. All his symptoms fully resolved after 24 hours, which coincided with establishment of normoglycaemia. Subsequent magnetic resonance imaging (MRI) of the head revealed a tiny parietal lobe infarct. Two further cases of HHS mimicking ischaemic stroke have been reported with symptoms and imaging findings resolving with treatment of HHS. Our case demonstrates how HHS can also accentuate symptoms of a minor stroke, highlighting the importance of excluding ischaemic stroke in HHS patients with neurological dysfunction. We recommend consideration of early MRI and/or computed tomography angiography in this cohort, especially in those appropriate for intervention.
KEYWORDS: hyperosmolar hyperglycaemic state, type 2 diabetes, stroke, neuroradiology
Introduction
Hyperosmolar hyperglycaemic state (HHS) is a metabolic emergency seen in patients with type 2 diabetes mellitus (T2DM). It is a life-threatening condition with mortality of up to 20%, most commonly affecting older patients or those with multiple comorbidities.1 Neurological dysfunction is often present and can be the only presenting symptom. Correction of hyperosmolality and hyperglycaemia will typically fully resolve presenting symptoms.2 This case demonstrates the risk of occult ischaemic stroke in patients with HHS and neurological symptoms.
Case presentation
A 74-year-old man presented to his local emergency department (ED) having been found collapsed with a left-sided weakness and slurred speech. His past medical history included T2DM, hypertension, obstructive sleep apnoea, asthma and previous bladder cancer.
On arrival at the ED, his Glasgow Coma Scale (GCS) was 14 and National Institutes of Health Stroke Scale (NIHSS) score of 3 (1 point for dysarthria and 2 points for left leg weakness). His systemic examination was normal aside from hypertension (220/104 mmHg). His arterial blood gas results showed pH 7.37, partial pressure of carbon dioxide (pCO2) 5.63 kPa, partial pressure of oxygen (pO2) 8.6 kPa, bicarbonate of 24 mmol/L, glucose 38 mmol/L and base excess of 0 mmol/L. His urinary ketones were 0.9 mmol/L and he had a normal computed tomography (CT) of the brain. Subsequent blood tests confirmed serum glucose 51 mmol/L and serum osmolality of 322 mOsm/kg. He was promptly treated for HHS and hypertension.
He further deteriorated with reduced GCS 10 (E3, V2, M4) during the consultant review 5 hours later. His NIHSS had increased to 20 with reduced consciousness, inability to answer questions or follow commands, left hemiparesis, hemianopia, sensory neglect and dysarthria. The patient also became febrile with clinical evidence of pneumonia. An urgent stroke team opinion was requested.
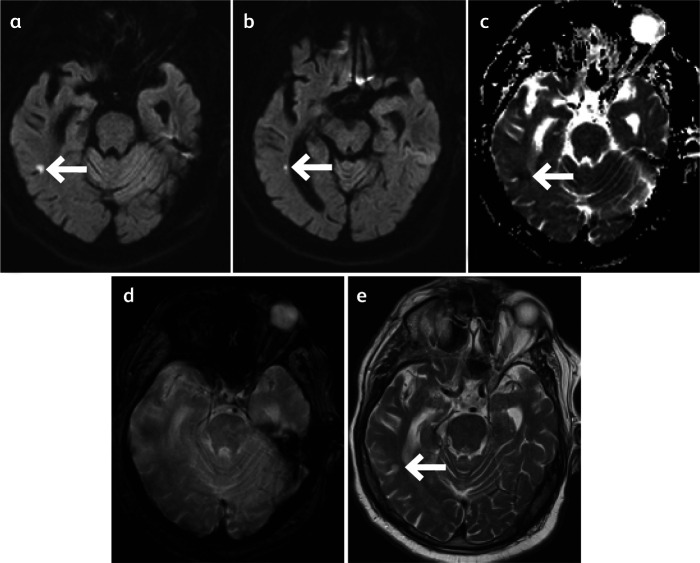
He was treated with aspirin empirically; he was not thrombolysed on admission and did not undergo CT cerebral angiography after stroke team review as it was felt that his initial presentation and subsequent deterioration was more likely to be related to HHS and pneumonia. He also received intravenous antibiotics and glyceryl trinitrate infusion following transfer to the stroke unit. His neurological deficits fully resolved after normoglycaemia was established after 24 hours of admission. However, the magnetic resonance imaging (MRI) of the head on day 4 of admission confirmed a tiny infarct at the right parieto-occipital region (Fig 1).
Fig 1.
Magnetic resonance imaging of the head of a patient with ischaemic stroke and hyperosmolar hyperglycaemic state. a) Diffusion-weighted imaging. b) Diffusion-weighted imaging. c) Apparent diffusion coefficient. d) Gradient echo. e) T2-weighted imaging.
The patient was discharged following a week-long admission with an insulin regimen as per the specialist diabetic nurses and stroke clinic follow-up. He has been fully independent following discharge and with no recurrence of stroke symptoms at 6 months' follow-up with improved glycaemic control. Outpatient CT angiography did not show stenosis in the intracranial vessels, or arch of the aorta or carotid arteries. He also had a normal 7-day prolonged cardiac monitoring.
Discussion
HHS is an acute metabolic complication of type 2 diabetes, defined as marked hyperglycaemia resulting in serum osmolality >320 mOsm/kg without ketosis. Relative insulin deficiency and subsequent increase in counter-regulatory hormones leads to increased gluconeogenesis and glycogenolysis, and reduced uptake of glucose by peripheral tissues. Resulting severe hyperglycaemia leads to osmotic diuresis and profound dehydration.2 HHS symptoms may develop insidiously over several days, notably polydipsia, polyuria, lethargy and altered mental state.3,4 Common triggers are inadequate insulin therapy and illness such as infection, cerebrovascular disease and myocardial infarction.2 Timely correction of hyperosmolality, hyperglycaemia and electrolyte abnormality is key to survival.2 Without prompt treatment, HHS can lead to complications such as cerebral oedema, electrolyte derangement, myocardial infarction, stroke, seizures and central pontine myelinolysis.2
Despite neurological dysfunction being an established manifestation of HHS, there is limited research on the spectrum of neurological dysfunction. The most common presenting symptom is altered sensorium.5 Symptoms can range from lethargy to confusion and coma, with a demonstrated linear relationship between serum osmolality and obtundity.3 Others may present with focal neurology including hemianopia, hemiparesis, and focal or generalised seizures.3,4 A retrospective case series investigating the variation in neurological complications of HHS had shown that coma was the most common presentation, followed by seizures and hemichorea-hemiballismus.6
The two previous case reports of HHS mimicking stroke feature transient neurological dysfunction with transient abnormal MRI findings (Table 1).7,8 The first one described a 55-year-old man presenting with right homonymous hemianopia in the context of HHS.7 MRI of the brain revealed a subcortical lesion in left occipital lobe. However, both symptoms and MRI findings resolved on correction of HHS. Similarly, another report described an 67-year-old woman presenting with left middle cerebral artery syndrome.8 Investigations revealed severe hyperglycaemia. She was managed for HHS and received thrombolysis. MRI / magnetic resonance angiography / magnetic resonance perfusion at 6 hours showed a small abnormal area in the left posterior temporal lobe but no evidence of large vessel occlusion. Treatment of HHS led to resolution of all neurological symptoms within 24 hours of admission. Subsequent MRI at day 5 revealed no evidence of stroke or other condition consistent with her clinical presentation.It is theorised that either hyperosmolality and hyperviscosity result in hypoxic-ischaemic injury or ‘release’ of free radicals and the electroencephalography and the MRI findings are the result of reduced energy demand from inactive brain tissue.7,8 This, in turn, leads to reduced perfusion rather than tissue at risk of infarction. However, a mechanism by which HHS causes metabolic change in such tissue is not known.8
Table 1.
Summary table of reported cases of HHS mimicking stroke
| Case report | Presentation | Imaging | Clinical course | Discussion |
|---|---|---|---|---|
| Bala et al 20207 | A 55-year-old man with a background of T2DM. 72 hours' right homonymous hemianopia BGL 27.6 mmol/L and hyperosmolality. |
MRI: subcortical lesion of left occipital lobe. EEG: no abnormality. |
Both symptoms and MRI changes (repeat scan at 3 months) resolved following normalisation of glucose. | Theorised that hyperglycaemia and hyperosmolality can result in hypoxic-ischaemic injury to the brain and consequent release of free radicals. |
| Shah et al 20148 | A 67-year-old woman with a background of T2DM, hypertension, hyperlipidaemia and previous ischaemic lacunar stroke. Sudden onset left MCA syndrome: global aphasia, left gaze deviation and right hemiplegia (NIHSS 32). BGL 45.8 mmol/L. |
CT of the brain: intracerebral haemorrhage excluded. MRI / MRA / MR perfusion at 6 hours: no diffusion restriction or large vessel occlusion. Small area of increased time to peak and mean transit time with normal cerebral blood volume in left posterior temporal lobe. Non-specific EEG findings (slowing) likely representing hyperglycaemia. MRI with DWI (day 5): no evidence of stroke or pathology consistent with left MCA syndrome. |
Thrombolysis given within 3 hours. Treated with IV fluids, nicardipine (for blood pressure control) and insulin. Symptoms improved with reduction of blood glucose: NIHSS of 17 at glucose 23.3 mmol/L. Within 24 hours of admission, full resolution of symptoms. |
Resolution of symptoms correlated with normalisation of blood glucose. MR and clinical findings not in keeping with seizure or post-ictal Todd's paresis. MR perfusion findings in conjunction with EEG findings suggest an area of lower perfusion due to lower energy demand, not hypoperfusion. However, the mechanism by which hyperglycaemia causes this is unknown. |
| Our case | A 74-year-old man with a background of T2DM, hypertension, obstructive sleep apnoea, asthma and previous bladder cancer. Collapse, left-sided weakness and slurred speech. BGL 51.0 mmol/L. |
CT of the brain at admission: normal. CT of the brain at deterioration: normal (haemorrhage excluded). MRI with DWI (day 4): tiny right parieto-occipital region infarct. |
Deterioration early in admission with NIHSS worsening from 3 to 20. Treated for HHS with IV fluids and insulin. Treated for hypertension with GTN infusion. Treated for stroke with aspirin. IV antibiotics (for pneumonia). NIHSS 0 at day 2 of admission. |
HHS can accentuate stroke symptoms. HHS can also mimic stroke but important to exclude concurrent ischaemic stroke given prothrombotic state. If possible, urgent CT angiography / MRI could aid thrombolysis decision. |
BGL = blood glucose level; CT = computed tomography; DWI = diffusion-weighted imaging; EEG = electroencephalography; GTN = glyceryl trinitrate; HHS = hyperosmolar hyperglycaemic state; IV = intravenous; MCA = middle cerebral artery; MR = magnetic resonance; MRA = magnetic resonance angiography; MRI = magnetic resonance imaging; NIHSS = National Institutes of Health Stroke Scale; T2DM = type 2 diabetes mellitus.
Where neurological symptoms predominate, the diagnosis of HHS can be difficult to differentiate clinically from stroke.5 Importantly, our case highlights that the diagnoses are not mutually exclusive. Early studies around HSS and seizures often attributed patients with focal neurological deficits to Todd's paresis.8 However, at post-mortem, some of these patients had evidence of cerebral ischaemia.9 Those with HHS are at an increased risk of stroke due to the presence of diabetes and the acute prothrombotic state created by HHS.10,11 Therefore, lateralising or deteriorating neurology should not always be attributed to HHS but should prompt consideration of concurrent cerebral ischaemia, particularly if the deficits develop after initiating treatment for HHS. This will enable successful management of both pathologies, particularly for time sensitive interventions such as thrombolysis and thrombectomy. In cases similar to our patient, further urgent imaging (such as CT angiography or MRI of the brain) will help guide whether reperfusion therapy is required, since such intervention should not be taken lightly in view of the bleeding risk associated with hyperglycaemia.12
Furthermore, our case demonstrates the confounding relationship between HHS and stroke through the ability of HHS to accentuate stroke symptoms ie a minor stroke resulted in significant neurological deficit out of keeping with the size of infarct. Subsequent treatment of HHS then rapidly improved neurological function.
Conclusion
This case study of a 74-year-old patient presenting with HHS and lateralising neurology demonstrates the complex interplay between HHS and stroke. We propose that urgent MRI or CT angiography should be performed where possible in all patients with HHS and lateralising or deteriorating neurology. This will ensure appropriate management of both conditions and enable consideration for time-sensitive interventions such as thrombolysis and thrombectomy.
References
- 1.Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014;37:3124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ 2019;365:l1114. [DOI] [PubMed] [Google Scholar]
- 3.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care 2001;24:131–53. [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectr 2002;15:28–36. [Google Scholar]
- 6.Misra U, Kalita J, Bhoi S, Dubey D. Spectrum of hyperosmolar hyperglycaemic state in neurology practice. Indian J Med Res 2017;146:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bala MI, Chertcoff A, Saucedo M, et al. Teaching neuroimages: nonketotic hyperglycemic hyperosmolar state mimicking acute ischemic stroke. Neurology 2020;95:e2600–1. [DOI] [PubMed] [Google Scholar]
- 8.Shah NH, Velez V, Casanova T, Koch S. Hyperglycemia presenting as left middle cerebral artery stroke: a case report. J Vasc Interv Neurol 2014;7:9–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Norris JW, Hachinski VC. Misdiagnosis of stroke. Lancet Lond Engl 1982;1:328–31. [DOI] [PubMed] [Google Scholar]
- 10.Luitse MJA, Biessels GJ, Rutten GEHM, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 2012;11:261–71. [DOI] [PubMed] [Google Scholar]
- 11.Scott AR. Joint British Diabetes Societies (JBDS) for Inpatient Care, JBDS hyperosmolar hyperglycaemic guidelines group . Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med J Br Diabet Assoc 2015;32:714–24. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N, D´valos A, Eriksson N, et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol 2010;67:1123–30. [DOI] [PubMed] [Google Scholar]