Abstract
Purpose
To determine whether handheld widefield OCT can be used to document retinopathy of prematurity (ROP) stage while using scleral depression to improve peripheral views.
Design
Prospective, observational study.
Participants
Consecutive neonates admitted to the neonatal intensive care unit in a single academic medical center who also met criteria for ROP screening and whose parents or guardians consented for them to undergo research imaging.
Methods
Scleral depression was combined with widefield OCT using an investigational 400-kHz, 55° field of view, handheld OCT during routine ROP screening from October 28, 2020, through March 3, 2021.
Main Outcome Measures
Acquisition of en face and B-scan imaging of the peripheral retina to assess early vitreoretinal pathologic features objectively, including the demarcation between vascularized and anterior avascular retina, the presence of early ridge formation, and small neovascular tufts.
Results
Various stages of ROP were detected using a rapid-acquisition OCT system. In 1 neonate, serial OCT imaging over a 5-week period demonstrated accumulation of neovascular tufts with progression to stage 3 ROP with extraretinal fibrovascular proliferation along the ridge. Videography of this technique is included in this report for instructional purposes.
Conclusions
Serial examinations using widefield OCT and scleral depression are feasible and may improve detection and documentation of ROP disease progression. Earlier detection of ROP-related proliferation may prevent vitreoretinal traction, retinal detachment, and blindness.
Keywords: OCT, Retinopathy of prematurity, Scleral depression
Abbreviations: NICU, neonatal intensive care unit; ROP, retinopathy of prematurity
The routine use of OCT in retinopathy of prematurity (ROP) has been limited, despite the advantages OCT provides in the care of patients with retinal diseases. In adults, the leading causes of blindness include age-related macular degeneration and diabetic retinopathy; in both, OCT is more sensitive than the clinical examination for the detection of severe disease, and it has become standard of care and has facilitated the transition toward quantitative and objective disease screening, diagnosis, and monitoring. This has resulted in opportunities for earlier treatment and improved visual outcomes.
In ROP, the standard of care remains the ophthalmoscopic examination, with subjective assessment of zone, stage, and plus disease. Prior applications of OCT in ROP have been limited by the field of view of the commercially available and investigational OCT systems. Thus, most early applications of OCT focused on posterior manifestations of ROP, rather than assessment of peripheral ROP stage.1, 2, 3, 4, 5, 6, 7 Previous work demonstrated that OCT may provide added information about foveal architecture,2 vitreous organization,6 3-dimensional manifestations of plus disease,7 and other clinical signs that we cannot appreciate using the clinical examination. In addition, Mangalesh et al1 recently demonstrated the potential for reduced neonatal stress using OCT rather than the clinical examination, which is a key potential benefit of screening with OCT.
Unlike in most applications of OCT, the primary pathologic features in ROP are peripheral; therefore, the most compelling case would be made if OCT could diagnose the stage of disease reliably, potentially replacing the ophthalmoscopic examination. Several groups have demonstrated that investigational OCT devices can achieve wider fields of view, roughly equivalent to the visualization provided by indirect ophthalmoscopy; however, these still cannot visualize the retinal periphery routinely.3,8 Given that clinical assessment of peripheral ROP with ophthalmoscopy often requires scleral depression, we tested in this study the hypothesis that scleral depression, combined with the use of a widefield OCT system, could provide accurate, objective, and rapid assessment of ROP stage.
Methods
Handheld OCT retinal imaging was performed on fully awake neonates in the neonatal intensive care unit (NICU) at Oregon Health & Science University from October 28, 2020, through March 3, 2021. Infants were included in the study if they met eligibility criteria for ROP screening (birth weight, ≤ 1500 g; gestational age, ≤ 30 weeks; or both) and if parent(s) consented for research imaging. Any infant whose parent(s) declined participation in the study was excluded and did not undergo research imaging; however, those infants still underwent ROP screening using traditional methods, including indirect ophthalmoscopy. Thirteen infants were included in this study, and the research OCT images were obtained at each visit during usual ROP screening. Birthweight, gestational age, gender, race or ethnicity, ROP zone by clinical assessment, and ROP stage by clinical assessment were recorded for all infants (Table 1). The imaging for the nonsedated infants was conducted with pharmacologic dilation with cyclopentolate 0.2% and phenylephrine 1%. After installation of anesthetic eye drops, an infant lid speculum was used, and scleral depression was performed in a manner similar to the clinical ROP examination. This study was approved by the institutional review board of Oregon Health & Science University in accordance with the tenets of the Declaration of Helsinki.
Table 1.
Demographic and Clinical Characteristics of Included Infants
| Patient No. | Birthweight (g) | Gestational Age (Weeks/Days) | Gender | Race or Ethnicity | Initial Retinopathy of Prematurity Examination Findings (Zone, Stage, Vasculature) | Highest Documented Retinopathy of Prematurity Stage (Zone, Stage, Vasculature) | Final Neonatal Intensive Care Unit Examination Findings (Zone, Stage, Vasculature) | No. of Serial Examinations |
|---|---|---|---|---|---|---|---|---|
| 1 | 518 | 24/4 | Male | Black/Hispanic | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | Both eyes: regressed ROP, ZII, preplus | 3 |
| 2 | 570 | 25/5 | Female | White/Hispanic | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | Both eyes: ZII, S1, preplus | 2 |
| 3 | 1012 | 27/2 | Male | White/Hispanic | Both eyes: regressed ROP, ZII, preplus | Both eyes: regressed ROP, ZII, preplus | Both eyes: regressed ROP, ZII, preplus | 3 |
| 4 | 500 | 25/3 | Male | White | Both eyes: ZII, S1, normal | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | 7 |
| 5 | 900 | 26/1 | Male | Black | Both eyes: ZII, S2, preplus | Right eye: ZII, S3, preplus Left eye: ZII, S2, preplus |
Both eyes: ZII, S1, normal | 5 |
| 6 | 835 | 24/5 | Male | White | Both eyes: ZII, S2, normal | Both eyes: ZII, S2, normal | Right eye: ZII, S1, normal Left eye: regressed ROP, ZII, normal |
6 |
| 7 | 642 | 24/3 | Male | Black | Both eyes: ZII, S2, normal | Both eyes: ZII, S2, normal | Right eye: ZII, S2, normal Left eye: ZII, S1, normal |
3 |
| 8 | 1053 | 29/6 | Male | White | Both eyes: incomplete vasculature, ZII, normal | Right eye: ZII, S1, normal Left eye: incomplete vasculature, ZII, normal |
Right eye: ZII, S1, normal Left eye: incomplete vasculature, ZII, normal |
2 |
| 9 | 1075 | 29 | Male | White | Both eyes: ZII, S1, preplus | Both eyes: ZII, S1, preplus | Both eyes: ZII, S1, preplus | 2 |
| 10 | 755 | 25/5 | Male | White | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | 2 |
| 11 | 698 | 25/5 | Male | White | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | Both eyes: ZII, S2, preplus | 2 |
| 12 | 885 | 27/3 | Female | White | Both eyes: ZII, S1, normal | Both eyes: ZIII, S1, normal | Both eyes: ZIII, S1, normal | 2 |
| 13 | 732 | 25/6 | Female | White | Both eyes: ZII, S1, normal | Both eyes: ZII, S1, normal | Both eyes: ZII, S1, normal | 1 |
ROP = retinopathy of prematurity; S = stage; Z = zone.
An investigational 400-kHz, 55° field of view, portable, handheld OCT system was used in this study.9 This portable, handheld OCT extends views to the peripheral retina of pediatric patients and displays cross-sectional (i.e., B-scan) and en face views in real time.10 With a single hand, the operator could hold the lens tube, allowing the operator to rest parts of their palm gently on the infant’s forehead and to manipulate the eye position using a depressor with their other hand (Video 1). The acquisition time of each volume was 120 ms with 400 A-scan per B-scan and 120 B-scans per volume, which provided real-time feedback in optimizing images. As soon as the target area on the ocular fundus was located, autofocusing with an electronically focus tunable lens could be performed in 1 second based on the brightness of the en face view.
The OCT images were reviewed in real time by the examiners (S.O., Y.J., and J.P.C.), and additional images were obtained, when needed, to acquire good-quality images of the retinal periphery for each infant at every session. OCT images were obtained of the temporal, inferior, nasal, and superior retinal periphery at every session. The ROP zone and stage associated with any OCT image were determined by the examiner using traditional examination with indirect ophthalmoscopy. RetCam imaging was performed in select infants to document clinical findings.
Results
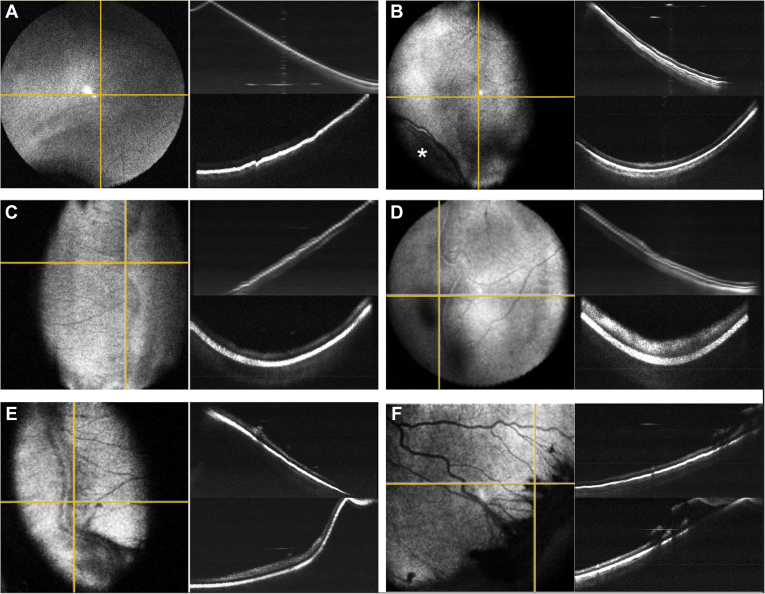
In this study, 13 awake neonates in the NICU underwent imaging during routine ROP screening, with serial imaging during the study period when possible. In total, 40 clinical visits were completed, and good-quality OCT images were obtained at each session to view the retinal periphery of each infant. Using the en face OCT view of the periphery with scleral depression, the extent of vascularization (zone) and any vascular abnormalities (stage) could be determined, as shown in Figure 1 and Video 2. In eyes that were fully vascularized, the scleral depressor could be visualized with retinal vessels extending within 1 disc area of the ora serrata (Video 1). In contrast, in eyes that demonstrated persistent avascular retina, or any degree of stage, the edge of the vascularized retina could be viewed en face (Fig 1, Video 2).
Figure 1.
Retinopathy of prematurity (ROP) peripheral pathologic features as seen with peripheral OCT en face imaging using scleral depression. A, Avascular retina without a clear vascular–avascular border (sometimes referred to as stage 0 ROP). B, C, Two examples of stage 1 ROP visible on en face OCT with (B) faint and (C) more pronounced demarcation lines without ridge formation. The asterisk indicates the scleral depressor location. D, E, Two examples of stage 2 ROP with (D) early and (E) later ridge formation without neovascularization. F, Stage 3 ROP with significant extraretinal fibrovascular proliferation along the ridge. The yellow horizontal lines correlate to top cross-sectional images; the yellow vertical lines correlate to the bottom cross-sectional images.
We observed a spectrum of vascular abnormalities ranging from avascular retina without a clear demarcation line (sometimes referred to as stage 0; Fig 1A) to extraretinal neovascularization (stage 3; Fig 1F). Even when comparing 2 neonates with clinical stage 1 ROP (Fig 1B, C), meaningful differences in OCT appearance were observed, including B-scan images. Figure 1B shows a faint demarcation line separating the vascularized and anterior avascular retina without dimension, whereas Figure 1C shows a more distinct demarcation line. Similarly, the images in Figure 1D, E show early stage 2 ROP (Fig 1D) compared with the prominent, more vascularized later stage 2 ROP (Fig 1E).
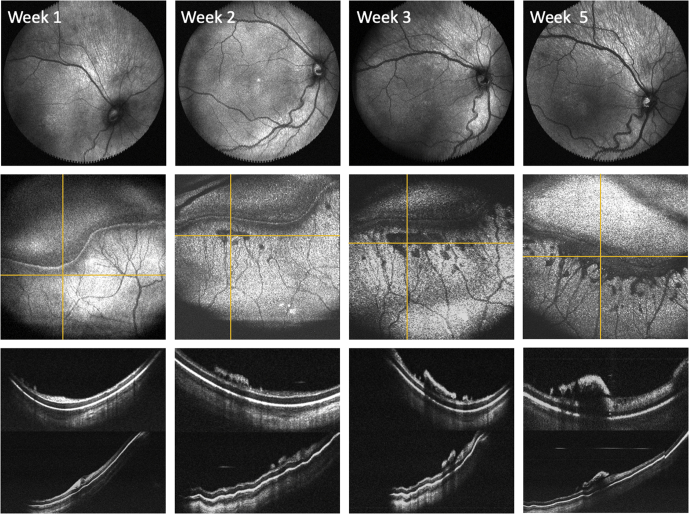
This technique can also be used to monitor the progression of ROP over time. Figure 2 and Video 3 show OCT en face images of the posterior pole and peripheral retina obtained with B-scan images of the peripheral pathologic features in 1 neonate over a 5-week period. This 1 example demonstrates 2 important concepts. First, although the anterior ridge slowly progressed into a typical stage 3 lesion by week 5, the serial OCT imaging demonstrated accumulation of so-called popcorn neovascular tufts (typically associated with stage 2 ROP) with clear and continuous progression of extraretinal fibrovascular proliferation along the ridge in the weeks prior. Second, the en face images centered on the posterior pole showed increased vascular dilation and tortuosity over time, correlating with the increased severity of peripheral disease.11
Figure 2.
Monitoring retinopathy of prematurity (ROP) disease progression in a preterm infant using OCT en face and cross-sectional views. Top row, Posterior en face views over a 5-week period demonstrating increased vascular tortuosity. Middle row, Serial peripheral en face views centered on the patient’s peripheral ridge showing increased severity of ROP disease over time. Bottom row, The yellow horizontal lines correlate to the respective cross-sectional images obtained (top image) posterior to the ridge and (bottom image) across the ridge.
Discussion
In this study, we demonstrated that peripheral ROP stage can be documented with OCT using scleral depression. Zone III is visualized clearly using this technique, and these far peripheral views provide objective documentation of the extent of vascularization, avascular retina, or both for preterm infants. Improved documentation and visualization of zone III before NICU discharge may help clinicians to better predict who is most at risk of complications and who may benefit from serial examination, prophylactic laser, or both. Although this study suggests that (1) stage runs a spectrum from avascular retina to extraretinal neovascularization and retinal detachment and (2) peripheral stage corresponds with posterior vascular changes in the spectrum of preplus and plus disease, further study is warranted using widefield imaging with a larger population.
Videography of this entire process is provided to demonstrate the usefulness of this imaging method at bedside with minimal disruption to the neonate or ROP screening process. We have found that incorporating OCT in our practice, especially when performed with scleral depression, improves our ability to visualize peripheral ROP, to teach trainees, to communicate findings with NICU staff and parents, and to visualize early vitreoretinal traction not visible with ophthalmoscopy.
Chen et al3 previously published spectral-domain OCT examples at the vascular–avascular junction with comparison with prior histology. The cross-sectional images in our report and those documented by Chen et al3 similarly contrast the hyperreflectivity of the inner retina in stage 1 ROP with a stage 2 ROP ridge, the latter characterized by increased inner retinal thickness and extension out of the plane of the retina. Both studies show the small neovascular tufts posterior to a ridge that are easily seen in OCT en face and B scan images as the first signs of proliferation.3 In this study, we further demonstrated the longitudinal changes in the posterior pole and peripheral retina over time and the coalescing of popcorn neovascularization and stage 2 ROP into stage 3 ROP with extraretinal neovascularization.
In this study, we demonstrated that with scleral depression, it may be feasible to use OCT in ROP practice, overcoming one of the limitations to its use. Although in this series we did not observe any retinal detachments, an added advantage of using OCT to detect extraretinal fibrovascular tissue is that it may be more sensitive for the detection of “flat” extraretinal neovascularization associated with aggressive ROP and earlier signs of vitreoretinal traction, which can be very difficult to appreciate clinically.
In this study, the combination of widefield OCT and scleral depression allowed detection and documentation of ROP stage and progression of peripheral pathologic features. As the cost of this technology comes down, we hope that reports like this demonstrating added clinical value may lead to routine use of OCT as part of ROP screening, earlier detection of progressive stage and extent of disease, fewer retinal detachments, and improved visual outcomes in ROP.
Manuscript no. D-21-00108.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form. Y.J.: Equity owner – Optos, Inc.
Y.J.: Equity owner – Seymour Vision
J.P.C.: Consultant – Boston AI Laboratories; Financial support – Genentech
Supported by the National Institutes of Health, Bethesda, Maryland (grant nos.: R01 EY19474, R01 EY031331, R21 EY031883, and P30 EY10572); and Research to Prevent Blindness, Inc., New York, New York (unrestricted departmental funding, a Career Development Award [J.P.C.], and a Career Advancement Award [Y.J.]).
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Oregon Health & Science University approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants’ parents or guardians provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Scruggs, Ostmo, Chiang, Jia, Huang, Jian, Campbell
Analysis and interpretation: Scruggs, Ni, Nguyen, Chiang, Jia, Huang, Jian, Campbell
Data collection: Scruggs, Ni, Nguyen, Ostmo, Jian, Campbell
Obtained funding: N/A; Study was performed as part of regular employment duties at OHSU. No additional funding was provided.
Overall responsibility: Scruggs, Ni, Nguyen, Ostmo, Chiang, Jia, Huang, Jian, Campbell
Supplementary Data
VidClip of visualization of the peripheral retina during ROP screening using scleral depression and OCT. En face views and cross-sectional scans are shown simultaneously in real time. The scleral depressor, seen prior to image acquisition, provides indentation throughout this process and facilitates view of peripheral vasculature. This video demonstrates the rapid acquisition time of the investigational portable handheld OCT system.
VidClip of documentation of various ROP stages detected by OCT using scleral depression. This video shows real time acquisition of OCT en face and cross-sectional scans of five preterm infants. The images are shown in increasing severity of ROP peripheral pathology. The videography clips of Patients 1-5 correspond with the images in Fig 1 B-F, respectively. Patients 1 and 2 demonstrate examples of stage 1 ROP, whereas patients 3 and 4 had clinical evidence of stage 2 ROP. Patient 5 had extraretinal fibrovascular proliferation, consistent with stage 3 ROP.
VidClip of OCT detection of peripheral ROP disease progression. OCT en face views centered on a patient’s peripheral ridge demonstrate increased severity of ROP disease over a five-week period. The adjacent cross-sectional images demonstrate an increase in “popcorn” neovascularization tufts and extraretinal fibrovascular tissue over this period. By week 5, the patient had evidence of stage 3 ROP and underwent treatment.
References
- 1.Mangalesh S., Sarin N., McGeehan B., et al. Preterm infant stress during handheld optical coherence tomography vs binocular indirect ophthalmoscopy examination for retinopathy of prematurity. JAMA Ophthalmol. 2021;139(5):567–574. doi: 10.1001/jamaophthalmol.2021.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinekar A., Mangalesh S., Jayadev C., et al. Retinal imaging of infants on spectral domain optical coherence tomography. Biomed Res Int. 2015;2015:782420. doi: 10.1155/2015/782420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X., Mangalesh S., Dandridge A., et al. Spectral-domain OCT findings of retinal vascular-avascular junction in infants with retinopathy of prematurity. Ophthalmol Retina. 2018;2(9):963–971. doi: 10.1016/j.oret.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangalesh S., McGeehan B., Tai V., et al. Macular OCT characteristics at 36 weeks’ postmenstrual age in infants examined for retinopathy of prematurity. Ophthalmol Retina. 2021;5(6):580–592. doi: 10.1016/j.oret.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman A.L., Tran-Viet D., Gustafson K.E., et al. Poorer neurodevelopmental outcomes associated with cystoid macular edema identified in preterm infants in the intensive care nursery. Ophthalmology. 2015;122(3):610–619. doi: 10.1016/j.ophtha.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legocki A.T., Zepeda E.M., Gillette T.B., et al. Vitreous findings by handheld spectral-domain OCT correlate with retinopathy of prematurity severity. Ophthalmol Retina. 2020;4(10):1008–1015. doi: 10.1016/j.oret.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maldonado R.S., Yuan E., Tran-Viet D., et al. Three-dimensional assessment of vascular and perivascular characteristics in subjects with retinopathy of prematurity. Ophthalmology. 2014;121(6):1289–1296. doi: 10.1016/j.ophtha.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell J.P., Nudleman E., Yang J., et al. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol. 2017;135(9):977–981. doi: 10.1001/jamaophthalmol.2017.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni S., Wei X., Ng R., et al. High-speed and widefield handheld swept-source OCT angiography with a VCSEL light source. Biomed Opt Express. 2021;12(6):3553–3570. doi: 10.1364/BOE.425411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jian Y., Wong K., Sarunic M.V. Graphics processing unit accelerated optical coherence tomography processing at megahertz axial scan rate and high resolution video rate volumetric rendering. J Biomed Opt. 2013;18(2):26002. doi: 10.1117/1.JBO.18.2.026002. [DOI] [PubMed] [Google Scholar]
- 11.Campbell J.P., Kim S.J., Brown J.M., et al. Evaluation of a deep learning-derived quantitative retinopathy of prematurity severity scale. Ophthalmology. 2021;128(7):1070–1076. doi: 10.1016/j.ophtha.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VidClip of visualization of the peripheral retina during ROP screening using scleral depression and OCT. En face views and cross-sectional scans are shown simultaneously in real time. The scleral depressor, seen prior to image acquisition, provides indentation throughout this process and facilitates view of peripheral vasculature. This video demonstrates the rapid acquisition time of the investigational portable handheld OCT system.
VidClip of documentation of various ROP stages detected by OCT using scleral depression. This video shows real time acquisition of OCT en face and cross-sectional scans of five preterm infants. The images are shown in increasing severity of ROP peripheral pathology. The videography clips of Patients 1-5 correspond with the images in Fig 1 B-F, respectively. Patients 1 and 2 demonstrate examples of stage 1 ROP, whereas patients 3 and 4 had clinical evidence of stage 2 ROP. Patient 5 had extraretinal fibrovascular proliferation, consistent with stage 3 ROP.
VidClip of OCT detection of peripheral ROP disease progression. OCT en face views centered on a patient’s peripheral ridge demonstrate increased severity of ROP disease over a five-week period. The adjacent cross-sectional images demonstrate an increase in “popcorn” neovascularization tufts and extraretinal fibrovascular tissue over this period. By week 5, the patient had evidence of stage 3 ROP and underwent treatment.