Abstract
Introduction
Frontotemporal dementia (FTD) is a neurodegenerative disorder for which there is no effective pharmacological treatment. Recently, interneuron activity responsible for fast oscillatory brain activity has been found to be impaired in a mouse model of FTD with consequent cognitive and behavioral alterations. In this study, we aim to investigate the safety, tolerability, and efficacy of a novel promising therapeutic intervention for FTD based on 40 Hz transcranial alternating current stimulation (tACS), a form of non‐invasive brain stimulation thought to engage neural activity in a frequency‐specific manner and thus suited to restore altered brain oscillatory patterns.
Methods
This is a multi‐site, randomized, double‐blind, placebo‐controlled trial on 50 patients with a diagnosis of behavioral variant FTD (bvFTD). Participants will be randomized to undergo either 30 days of 1‐hour daily tACS or Sham (placebo) tACS. The outcomes will be assessed at baseline, right after the intervention and at a 3‐ to 6‐months follow‐up. The primary outcome measures are represented by the safety and feasibility of tACS administration, which will be assessed considering the nature, frequency, and severity of adverse events as well as attrition rate, respectively. To assess secondary outcomes, participants will undergo extensive neuropsychological and behavioral assessments and fluorodeoxyglucose (FDG)–positron emission tomography (PET) scans to evaluate changes in brain metabolism, functional and structural magnetic resonance imaging (MRI), resting and evoked electroencephalography, as well as blood biomarkers to measure changes in neurodegenerative and neuroinflammatory markers.
Results
The trial started in October 2020 and will end in October 2023. Study protocols have been approved by the local institutional review board (IRB) at each data‐collection site.
Discussion
This study will evaluate the safety and tolerability of 40 Hz tACS in bvFTD patients and its efficacy on gamma oscillatory activity, cognitive function, and brain glucose hypometabolism.
Keywords: 40 Hz, behavioral variant, brain stimulation, bvFTD, controlled clinical trial, dementia, FDG, frontotemporal dementia, FTD, gamma frequency, inhibitory interneurons, somatostatin, tACS, transcranial alternating current stimulation
1. INTRODUCTION
Frontotemporal dementia (FTD) is a devastating neurodegenerative disorder, primarily affecting the frontal and/or temporal lobes of the brain. 1 , 2 FTD is the second most frequent cause of presenile neurodegenerative dementia in individuals younger than 65 years of age. Currently, there is no effective pharmacological treatment that slows down the progression of FTD, which results in a poor prognosis, dependency on caregivers, and death occurring in ≈8 years after diagnosis. 1 FTD can be classified into three mainly clinical types: behavioral variant FTD (bvFTD), semantic dementia (SD), and progressive non‐fluent aphasia (PNFA), each one characterized by the presence of intracellular aggregation of neuronal proteins such as the microtubule‐associated protein tau (MAPT), 3 the transactive response DNA‐binding protein with molecular weight 43 kDa (TDP‐43), and the fused in sarcoma protein (FUS). Alteration in γ‐aminobutyric acid (GABA)ergic interneurons activity has been demonstrated in mouse models of FTD, suggesting a pathological substrate where a reduced interneuron inhibitory control over the activity of pyramidal neurons seems to lead to neurotoxicity and cellular death, with a resulting decrease of high‐frequency oscillatory brain activity in the gamma range (>35 Hz; normally generated by inhibitory interneurons activity). 4 , 5 Together with interneuron dysfunction, microglia impairment has been also investigated as a potential crucial component of FTD pathology, 6 , 7 and a link between neuroinflammation and FTD has been demonstrated since early stages of the disease. 8
As in FTD, evidence of decreased gamma activity (≈40 Hz) has been linked to parvalbumin‐positive (PV+) interneuron pathology in a mouse model of Alzheimer's disease (AD), suggesting that externally induced restoration of gamma activity could lead to a modulation of interneuron activity, neuroinflammatory changes involving microglia activity, and increased protein clearance. 5 Accordingly, optogenetics‐induced 40 Hz stimulation was able to restore gamma activity, with a parallel increase in microglial activation and subsequent significant reduction of amyloid beta (Aβ) and phosphorylated tau (p‐tau) deposition in a mouse model of AD as well as one of FTD tauopathy. 5 Current clinical trials conducted by our team are exploring the possibility of translating these findings in AD patients via a non‐invasive form of neurostimulation—transcranial alternating current stimulation (tACS) (NCT03880240, NCT03290326, NCT03412604)—ables to entrain cortical oscillatory activity at a specific frequency (ie, 40 Hz) and thus potentially modulating impaired interneuron activity, while at the same time promoting microglia activation and protein clearance. 9
Highlights
Transcranial alternating current stimulation (tACS) interacts with neural activity in a frequency‐specific manner.
Fast brain oscillations in the gamma band are altered in frontotemporal dementia (FTD) patients and mouse models.
We will evaluate the effect of tACS in bvFTD patients in a randomized, placebo‐controlled multicenter trial.
Sham or Real 40 Hz‐tACS will be applied via 1‐hour daily sessions for 6 weeks.
Clinical, cognitive, neuroimaging (positron emission tomography [PET], magnetic resonance imaging [MRI]), and electrophysiological changes will be evaluated.
Research in context
Systematic review: The authors reviewed the literature using traditional sources. Although there are no studies published evaluating the use of transcranial alternating current stimulation (tACS) on patients with frontotemporal dementia (FTD), tACS has been found to modulate several cognitive functions in the healthy and pathological brain, also including those significantly impaired in FTD. Recently, studies in Alzheimer's disease (AD) mouse models showed that externally inducing gamma activity (specifically at 40 Hz) could lead to a restoration of interneuron activity typically altered in AD, trigger neuroinflammatory changes involving microglia activation, and increase protein clearance. Similarly, interneuron activity is altered in FTD, resulting in decreased gamma‐band activity responsible for cognitive and behavioral alteration. Therefore, 40 Hz tACS could represent a safe and non‐invasive therapeutic option in FTD patients to slow down the pathophysiological cascade initiated by interneuron pathology, hopefully also counteracting associated cognitive deficits. The relevant articles are appropriately cited.
Interpretation: This is a multisite, randomized, double‐blind, placebo‐controlled trial to study the effects of 40 Hz tACS on patients with behavioral variant FTD (bvFTD). The intervention will consist of 30 days of 1‐hour daily tACS or Sham (= placebo) tACS. To evaluate the effects of tACS we will use extensive neuropsychological and behavioral assessments, fluorodeoxyglucose (FDG)–positron emission tomography (PET), functional and structural magnetic resonance imaging (MRI), resting and evoked electroencephalography (EEG), transcranial magnetic stimulation (TMS) measures of plasticity and excitability, and blood biomarkers, on top of strict adverse event monitoring.
Future directions: The aim of the trial is to provide a first step in the development of a novel intervention to treat FTD, demonstrating tACS safety and feasibility, potential mechanisms of action, target engagement, and thus informing the design of larger clinical trials.
External modulation of interneuron activity and induction of gamma oscillations could represent a promising therapeutic approach to slow the neurotoxicity and death of pyramidal cells, as well as to restore microglia activity with potential benefits on the removal of toxic aggregates also in FTD, where similar impairment in interneurons operating in the gamma frequency band have been recently documented. 4 , 10 , 11 Specifically, a 25% reduction of PV cells in the frontal cortex of TDP‐43 knock‐in mouse with a human‐equivalent mutation has been found, along with cognitive dysfunction similar to those characterizing FTD patients (ie, executive dysfunction and memory impairment). 11 Another study has shown that cortical hyperexcitability of pyramidal neurons (PNs) in layer 5 primarily originated from reduced GABAergic transmissions in TDP‐mutant mice (ie, great reduction in inhibitory postsynaptic currents [IPSCs] and evoked IPSCs). 4 In detail, the authors identified an alteration in the physiological circuitry involving somatostatin (SST) and PV interneurons, leading to reduced inhibition over PN whose hyperexcitability eventually lead to cellular death. Accordingly, by suppressing the activity of SST, the authors were able to restore the firing frequency of PNs in the gamma band and further reduce ubiquitin‐positive aggregates, ultimately reversing neuronal loss. Finally, gamma oscillations were found to be reduced between the frontal lobes of FTD patients, and such impairment has been related to cognitive dysfunction and functional deficits (eg, disinhibition) in patients. 12
tACS is a safe, non‐invasive brain stimulation technique that utilizes low‐amplitude alternating (sinusoidal) currents to modulate brain activity and entrain specific cortical rhythms depending on the applied stimulation frequency, 13 with effects outlasting the stimulation period. 14 Previous studies from our group have shown that enhancement of gamma oscillations in healthy volunteers via tACS is possible, and can lead to transient improvement of performance in visuomotor, working memory, or abstract reasoning tasks depending on the sites of stimulation. 15 , 16 , 17 , 18 Due to its safety 19 and controllability (in terms of stimulation frequency and the possibility of targeting almost any cortical region), tACS is considered as one of the most innovative techniques to modulate the healthy and pathological brain. 20 , 21 , 22 However, no studies have investigated the application of tACS in patients with FTD so far. Considering the documented safety profile of tACS in humans, 19 the evidence of altered gamma oscillations in FTD 12 and animal data on the impact of gamma induction on Aβ and p‐tau clearance in AD, tACS could represent a valid, safe, and noninvasive option for FTD patients to slow the pathophysiological cascade that leads to cognitive deficits. In addition, FTD is also characterized by significant atrophy and hypometabolism in the frontal and anterior temporal lobes, 23 correlated with disinhibited behavior and impaired language. Among the available neuroimaging markers, fluorodeoxyglucose–positron emission tomography (FDG‐PET) seems to represent the most validated and reliable one, with FTD patients showing spatially specific hypometabolism patterns co‐varying with atrophy distribution and cognitive and behavioral deficits. Even though bvFTD displays predominant prefrontal metabolic alterations, in later stages hypometabolic regions tend to converge to a more distributed pattern involving both the prefrontal and temporal lobes. 1 , 23 , 24 Assuming that metabolic changes are an expression of underlying interneuron pathology since they take place in the same regions, for this study, we will use hypometabolism FDG‐based maps to create a stimulation template maximizing the induction of gamma activity in the prefrontal and temporal lobe bilaterally, as the target guiding the tACS intervention.
The aim of this multisite, randomized, double‐blind, placebo‐controlled trial is to investigate the efficacy and safety of 40 Hz tACS in bvFTD patients. We hypothesize that gamma‐tACS is safe and well tolerated in bvFTD patients, and can enhance gamma oscillatory activity, improve cognitive function, and restore prefrontal and temporal lobe glucose hypometabolism, potentially constituting a novel therapeutic option for FTD patients.
2. METHODS
2.1. Study design
This multi‐site, randomized, double‐blind, placebo‐controlled study has been registered on Clinicaltrials.gov (NCT04425148). The study is coordinated by Emiliano Santarnecchi, PhD, PsyD, at the Berenson‐Allen Center for Non‐Invasive Brain Stimulation at Beth Israel Deaconess Medical Center (BIDMC), Boston, MA, USA. Participants will be recruited across three study sites represented by: (1) the Berenson‐Allen Center for Non‐Invasive Brain Stimulation at BIDMC, Boston, MA, USA; (2) the Non‐Invasive Brain Stimulation Unit at IRCSS Santa Lucia Foundation (SLF) in Rome, Italy, led by Giacomo Koch, MD, PhD; and (3) the FTD unit at Massachusetts General Hospital (MGH), Boston, Massachusetts, USA, led by Brad Dickerson, MD. Data collection will take place in parallel at BIDMC and SLF, following the same procedures and methods. All the neuropsychological and behavioral tests have been standardized in English and Italian. Both institutional review boards (IRBs; Boston and Rome) requested the informed consent form for this study in both languages (English and Italian). All laboratory tests are performed in Clinical Laboratory Improvement Amendments (CLIA)‐certified labs. In addition, the patients who will be recruited will be only fluently speaking the primary language referred to their country.
Participants will be randomized to undergo either 30 days (weekdays; 6 weeks) of 1‐hour tACS once a day or 30 days of 1‐hour Sham (placebo) tACS once a day (n = 30 visits) (Figure 1). The study outcomes will be assessed at baseline, right after the tACS intervention, and at multiple follow‐ups (in person at 3 months, phone screening at 6 months). Participants will undergo (1) neuropsychological and behavioral assessments to evaluate the cognitive, neuropsychiatric, and behavioral changes induced by the tACS treatment; (2) FDG‐PET scans to evaluate changes in brain metabolism possibly related to changes in cognition; (3) functional and structural magnetic resonance imaging (MRI) to measure changes in perfusion and functional connectivity; (4) resting electroencephalography (EEG), evoked transcranial magnetic stimulation (TMS) and tACS‐EEG to evaluate changes in synaptic transmission, as well as treatment effects on cortical reactivity, oscillatory activity, connectivity, and eventual biomarkers of responsiveness to tACS treatment; and (5) blood biomarkers to measure changes in neurodegeneration and neuroinflammation. The trial has been approved by the BIDMC IRB (2020P‐000457) and by the ethical committee at SLF (Protocol number: CE/PROG. 836). The investigators follow all federal regulations mandated by the U.S. Department of Health and Human Services (45 CFR 46) and the Declaration of Helsinki. The study is conducted in accordance with both U.S. and Italian (EU) GCP guidelines.
FIGURE 1.
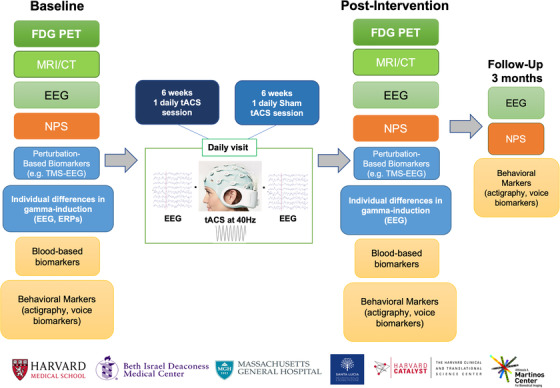
Study protocol. Patients will be randomized to one of two groups undergoing daily sessions of real tACS or Sham tACS for 6 weeks. Extensive assessments will be carried out at baseline as well as immediately after the intervention. A shorter version will be performed at a follow‐up visit (3 months later). Abbreviations: EEG, electroencephalography; NPS, neuropsychological assessment; TMS, transcranial magnetic stimulation; tACS, transcranial alternating current stimulation; ERPs, event‐related potentials; MRI, magnetic resonance imaging; CT, computed tomography; FDG‐PET, fluorodeoxyglucose‐positron emission tomography
2.2. Participants
We will enroll 50 patients with a diagnosis of probable bvFTD based on the International Consensus Clinical Diagnostic criteria 25 and on the most recent recommendations from the Neuropsychiatric International Consortium for Frontotemporal Dementia, 26 according to the following inclusion and exclusion criteria.
2.2.1. Inclusion criteria
Diagnosis of probable bvFTD:
Diagnosis will be based on an informed opinion by a consortium of neurologist(s) across data collection sites, based on previous imaging data (eg, PET, MRI), cognitive testing, and clinical notes. If needed, the patient will also get additional FDG‐PET imaging to determine the pattern of brain hypometabolism congruent with bvFTD;
Mini‐Mental State Examination (MMSE score) ≥18;
Frontotemporal Lobar Degeneration‐Modified Clinical Dementia Rating (FTLD‐CDR) total score of ≤1;
On stable medications related to cognition or behavior for >30 days such as acetylcholinesterase inhibitors, memantine, anti‐depressants, antipsychotic agents, other mood stabilizers, and benzodiazepines;
Age from 40 to 80 years;
Minimum of completed 8th‐grade education;
No history of intellectual disability;
The patient is able to comply with the study procedures in the view of the investigator;
Written informed consent must be obtained and documented (from the patient or, where jurisdictions allow it, from their substitute decisionmaker).
2.2.2. Exclusion criteria
Current or past history of any significant neurodegenerative disorder of the central nervous system other than FTD (eg, AD, Lewy body dementia, Parkinson disease, multiple sclerosis, progressive supranuclear palsy, normal pressure hydrocephalus, Huntington disease, any condition directly or indirectly caused by transmissible spongiform encephalopathy, Creutzfeldt‐Jakob disease, variant Creutzfeldt‐Jakob Disease, or new variant Creutzfeldt‐Jakob disease);
Current or past history of stroke (cortical stroke), intracranial brain lesions, previous neurosurgery, or head trauma that resulted in significant neurologic impairment;
Past or current history of major depression, bipolar disorder, psychotic disorders, or any other major psychiatric condition that is temporally distinct from FTD will be evaluated by the study MD;
Current history of poorly controlled migraines including chronic medication for migraine prevention;
History of seizures with the exception of a single seizure of benign etiology (eg, febrile seizure) in the judgment of the study MD;
History of fainting spells of unknown or undetermined etiology that might constitute seizures;
Chronic (particularly) uncontrolled medical conditions that may cause a medical emergency in the case of a provoked seizure (cardiac malformation, cardiac dysrhythmia, asthma, etc);
Metal implants in the head (except dental), pacemaker, medication pump, nerve stimulator, TENS unit, ventriculoperitoneal shunt, or cochlear implant, unless cleared by the study MD;
Contraindication for undergoing MRI or receiving TMS or tACS;
Any clinically significant hematological, endocrine, cardiovascular, renal, hepatic, gastrointestinal, or neurological disease. If the condition has been stable for at least the past year and is judged by the investigator not to interfere with the patient's participation in the study, the patient may be included;
>50 mSv of radiation exposure for research within the past year (PET imaging exclusion criteria);
Substance abuse or dependence within the past 6 months;
Medications will be reviewed by the responsible MD and a decision about inclusion will be made based on the following: The patient's past medical history, drug dose, history of recent medication changes or duration of treatment, and combination of CNS active drugs;
All female participants that are pre‐menopausal will be required to have a pregnancy test; any participant who is pregnant or breastfeeding will not be enrolled in the study;
Subjects who, in the investigator's opinion, might not be suitable for the study;
A hair style or head dress that prevents electrode contact with the scalp or would interfere with the stimulation (eg, thick braids, hair weave, afro, wig).
2.3. Randomization and masking
Subjects will be randomized at a ratio 1:1 stratified by sex, age, and screening severity (FTLD‐CDR) to either the Sham tACS group (n = 25) or the real tACS intervention group (n = 25). Randomization will be conducted by a pre‐determined member of the research team and applied across sites, ensuring that participants, care providers, investigators, and outcome assessors all remain blinded to the intervention at the time of each assessment. Stimulation and EEG templates for real and Sham tACS will not differ across sites and will be developed by the PI's team in collaboration with an industry partner Neuroelectrics Corporation (Cambridge, MA, USA). The “double‐blind mode” of the stimulation device will be used for blinding both patients and investigators. When the function is active, the operator can only monitor the impedance values of electrodes before the stimulation begins, whereas no information is displayed during stimulation. The same number of stimulating electrodes will be used for real and Sham tACS, and solutions for maximizing blinding across participants will be used. 27
2.4. Intervention
tACS will be administered using the Starstim Device, a 32‐channel device, which is also capable of EEG recording. tACS involves the administration of low‐amplitude (< 2 mA) sinusoidal electrical currents via scalp electrodes. Alternating current will be applied in the gamma frequency (40 Hz) range, and bilateral frontotemporal stimulation montage will be applied to target hypometabolic cortical regions, as defined via FDG‐PET imaging. Although tACS is usually administered via bipolar montages using two large electrodes, such montages have poor spatial specificity. Our group has been at the forefront of efforts using multifocal (multielectrode) montages that can deliver higher amplitude and more spatially specific stimulation patterns. 28 Consequently, stimulation will be applied to the target regions using multifocal (multielectrode) montages to maximize the induced electrical current in the regions of interest. Even though bvFTD patients display patterns of altered hypometabolism corresponding with their clinical profile (ie, predominant prefrontal alterations), hypometabolic regions tend to eventually converge to a distributed hypometabolism involving both prefrontal and temporal lobes. 1 , 23 , 24 A standardized template maximizing stimulation in the prefrontal and temporal lobe bilaterally will be used on every patient, attempting to increase metabolism in hypometabolic regions (prefrontal) while at the same time also exerting a possible effect on regions with still preserved metabolic activity (temporal cortices). The targeting template, developed by the PI and his team, will then be used to optimized electrode placement and stimulation parameters in collaboration with Neuroelectrics, maximizing the induced electric field over the target regions and minimizing it over the rest of the brain. Stimulation will be slowly ramped up/down at the beginning/end of each stimulation session to minimize skin sensation. Participants will be queried each day at the end of the stimulation visit to see if they experienced phosphenes. Sham stimulation will be delivered according to current standards. 19 Stimulation intensity will be increased up to the intensity used for real stimulation, held active for 2 minutes and then lowered down to zero. We will also collect information about subjective feeling during and after stimulation and the subject's guess regarding real/sham group assignment. 27
2.5. Outcome measures
2.5.1. Safety and tolerability assessments
The safety of tACS administration in FTD patients will be assessed considering the nature, frequency, and severity of adverse events (AEs), while feasibility will be evaluated considering the attrition rate, both in terms of patients completing the study and the number of visits completed. AEs, regardless of attribution to tACS or pre/post assessments, will be collected and recorded using a standard AE form. Participants will be asked, in an open‐ended way, about the presence of any such events daily. Intensity of each AE will be graded as mild, moderate, or severe. Any events that are serious or unexpected in nature, severity, or frequency as compared to the risks described in the study plan will be reviewed by the PI or designee (eg, a co‐investigator) to determine the relationship of the event to the study.
2.5.2. Neuropsychological and behavioral assessments
Participants will undergo a neuropsychological evaluation before, at the end of the intervention, and at 3‐month follow‐up from the tACS intervention. We hypothesize that tACS administration will increase global cognition, executive function, and language in the bvFTD population. To evaluate changes in global cognition we will use FTLD‐CDR, the Alzheimer's Disease Assessment Scale 13 (ADAS‐Cog 13), and the MMSE. 29 , 30 The Frontal Assessment Battery (FAB), the Trail‐making Tests A & B, Category fluency test (animals, fruits, vegetables), Verbal fluency test (F/A/S/L), and the NIH‐EXAMINER (Flanker subtest and Set‐shifting subtest) will be used to assess executive functions. 31 , 32 , 33 , 34 To evaluate neuropsychiatric symptoms, we will use the Neuropsychiatric Inventory‐Questionnaire (NPI‐Q), the Frontal Behavioral Inventory (FBI), and the Beck Depression Inventory (BDI). 35 , 36 , 37 The language domain will be assessed with the Multilingual Naming Test (MINT). The Activities of Daily Living Questionnaire (Weintraub) and the Functional Assessment Questionnaire (FAQ) will be used to assess daily function. 38 , 39 Finally, the Alzheimer's Disease Cooperative Study Clinical Global Impression of Change (ADCS‐CGIC) 40 will be used to evaluate tACS effects on subjective and objective behavioral and cognitive performance as reported by both patients and caregivers.
Sleep, nutrition and physical activity changes will be investigated with Community Healthy Activities Model Program for Seniors (CHAMPS), Mini Nutritional Assessment, Epworth Sleepiness Scale, and Pittsburgh Sleep Quality questionnaires. 41 , 42 , 43 , 44 At baseline we will also administer the test of Premorbid Functioning (TOPF) to investigate the patient's pre‐morbid cognitive and memory functioning. 45
2.5.3. 18FDG‐PET
Patients will undergo [18F]‐FDG‐PET/CT scans before and right after the tACS intervention. PET acquisition will be performed with the clinically validated radioligands for in vivo quantification of glucose metabolism ([18F]‐FDG) via static PET acquisition. The [18F]‐FDG dose is 5 mCi. The procedure will include static data acquisition in a time window of 30 to 60 minutes after injection.
2.5.4. MRI data collection
Structural and functional MRI data will be collected at baseline and at the end of tACS intervention.
Patients will undergo high‐resolution T1‐weighted structural scan, resting state functional MRI (fMRI), perfusion MRI via arterial spin labeling (ASL), diffusion tensor imaging (DTI), T2‐weighted gradient‐echo (T2* GRE), and fluid‐attenuated inversion recovery (FLAIR) sequences (total scan timing 60 minutes). MRI sequences have been harmonized across sites/scanners (ie, 3D T1‐weighted scan at 1 mm isotropic resolution) for group analysis.
2.5.5. Resting EEG, tACS‐EEG, TMS‐EEG
Resting EEG recording
Baseline resting state EEG and artifact recordings will be obtained with the eyes open for 5 minutes and then eyes closed for 5 minutes before and after the tACS treatment using a 64 channel EEG system. Subjects will be asked to briefly move their eyes, clench their jaws, and tense their foreheads so that the EEG artifacts associated with these movements can be recorded and similar artifacts removed from the remaining EEG recordings.
tACS‐EEG and Task‐EEG recording
To estimate the likelihood of induction of gamma in each participant, a shorter tACS‐EEG session will be carried out at baseline to see how each participant's brain responds to brief tACS bursts. The immediate response (eg, increase/decrease of gamma power) after short tACS stimulation blocks (up to 20 minutes) will be collected via EEG recording before/during/after tACS. Such a response will then be used to predict the response to the full tACS intervention. Specifically, tACS will be applied to up to four brain regions for each brain hemisphere, including a Sham stimulation block. Stimulation intensity will not exceed the limits suggested by tES safety guidelines, equal to 2 mA per stimulation electrode and 4 mA total injected current across all stimulating electrodes. Moreover, to collect information about the brain's ability to evoke gamma activity in response to stimuli different from tACS, we will monitor the amount of gamma activity induced by brief sensory stimulation (flickering lights, auditory stimulation) and cognitive tasks delivered using a regular desktop PC connected to the EEG system. To quantify the change in the way a participant's brain responds to tACS, the same tACS‐EEG recording session described above will be repeated at the end of the protocol.
Assessment of Motor Threshold
Resting motor threshold (RMT) will be determined before performing TMS and TMS‐EEG protocols by applying single pulses to the primary motor cortex‐M1. RMT will be defined as the minimum stimulus intensity that produces a motor evoked potential (MEP) of at least 50 μV in the hand muscles in at least 5 of 10 trials. MEPs will be measured by electromyography (EMG) during relaxation of the tested muscles. Determination of RMT will be used to set the intensity for single pulses as well as paired‐pulse protocols. The active motor threshold (AMT) will be defined as the minimum stimulus intensity that produces a MEP of at least 200 μV that is followed by a cortical silent period (absence of background EMG activity) in at least 50% of 10 trials. MEPs will be assessed during isometric contraction of the tested muscles at ≈20% of maximum voluntary contraction. Stimulation intensity will be set at 80% of AMT for the intermittent theta burst stimulation (iTBS) protocol.
Paired‐pulse TMS and plasticity assessments
To evaluate changes in synaptic transmission and cortical plasticity induced by tACS, patients will undergo different TMS protocols before and after treatment. Paired‐pulse TMS protocols will be used to evaluate in vivo the activity of different intracortical circuits in M1, such as short intracortical inhibition (SICI) and intracortical facilitation (ICF), reflecting GABAA‐ergic and glutamatergic neurotransmission; long intracortical inhibition (LICI) investigating GABAB activity, and short afferent inhibition (SAI) probing cholinergic neurotransmission. 46 , 47 , 48 , 49 , 50
iTBS will be used pre‐ and post‐tACS treatment to evaluate changes in long‐term potentiation (LTP)–like cortical plasticity in M1. 51 , 52 iTBS enhances cortical excitability for up to 1 hour inducing LTP‐like effects. These after‐effects are thought to reflect rTMS influence on the strength of glutamatergic synapses via the NMDA receptor, AMPA receptor, and calcium channels. 51 The stimulation will be delivered using a figure‐of‐eight coil over M1 and it consists of 2 second trains, each with bursts of three TMS pulses at 50 Hz repeated at intervals of 200 ms, with 8‐second pauses between trains (600 total pulses). Cortico‐motor reactivity will be assessed at M1 prior to and following at 5, 10, and 30 minutes of the iTBS stimulation by measuring peak‐to‐peak amplitude of MEPs induced in the hand muscles in response to single pulse TMS as measured by EMG. An index of modulation of motor cortical excitability will be calculated as the percentage change of mean MEP amplitude, post‐TMS relative to pre‐TMS, with positive values (MEP amplitude increase) reflecting facilitation of cortical excitability by TMS, and negative values (MEP amplitude decrease) representing suppression.
TMS‐EEG assessment
Participants will undergo TMS‐EEG protocols before and right after the intervention to investigate changes in cortical reactivity, connectivity, and oscillatory evoked activity. We will use a 64‐channel TMS compatible device to collect data. A masking noise system will be used to reduce auditory evoked‐potential artifacts. The stimulation will take place over multiple brain regions in the prefrontal and parietal lobes. TMS intensity will be set at 120% of each individual's resting motor threshold for single‐pulse TMS. Procedures for neuronavigation, TMS delivery, and EEG preprocessing/analysis will reflect validated procedures previously published by our group. 53 , 54 , 55
2.5.6. Behavioral biomarkers
Recent studies have explored the possibility of assessing neurodegenerative disorders such as AD and Parkinson disease 56 , 57 via automatic analysis of voice features. Combined with other methods such as actigraphy, 58 the speech analysis tool seems to have the potential to become a useful, non‐invasive, and relatively simple method for early dementia diagnosis, 59 prediction of disease signs and symptoms, as well as for monitoring for progression of disease or treatment‐related improvement. For these reasons, we will record each participant while performing various language tasks. Each task will be recorded to extract specific vocal features, including, for example, pause length and verbal reaction time, via a mobile device (ie, iPhone/iPad). Moreover, profound rest‐activity rhythm disturbances have been observed in FTD patients, such as sleep disruption (ie, increased nocturnal activity), decreased morning activity, and a consequent excessive daytime sleepiness. 60 Therefore, patients will be wearing an Actiwatch (Philips Respironics) for the entire duration of the study.
2.5.7. Blood biomarkers
Blood samples will be collected at baseline and at the end of the intervention to look for changes in neurodegeneration markers such as serum neurofilament light chain (Nfl), correlated with disease severity and progression, survival, and cerebral atrophy 61 as well as pro‐inflammatory markers 8 , 61 like RANTES, MCP‐1, IL‐5, IL‐6, IL‐10, and TNF. 62 Individual variability in the response to tES has been shown to depend on genetic factors, such as polymorphism of the BDNF gene (an indicator of neuronal survival and synaptic plasticity in the adult brain 63 ), and even more relevant for the present investigation, APOE status has been recently suggested as a mediator of microglia activation. 64 Therefore, we will explore also BDNF polymorphism and APOE status.
2.6. Primary outcome
The primary outcome measures are the safety and the feasibility of tACS administration in bvFTD patients. Safety will be measured in terms of frequency and severity of AEs, and feasibility will be quantified on the basis of attrition rate.
2.7. Secondary outcome measures
The secondary outcome measure for the neuropsychological evaluation includes changes in different cognitive tests and scales, administered at baseline and after the intervention. This includes ADAS‐Cog, FAB, TMT A & B, NIH‐EXAMINER, NPI‐Q, ADL (Weintraub), and ADCS‐CGIC. FDG‐PET scans will be performed at baseline and after the intervention to evaluate changes in brain metabolism. Participants will undergo resting EEG before, at the end of the intervention, and at 3 months follow‐up to assess the effects of tACS treatment and spectral power changes in the gamma frequency band, which is a secondary outcome. Changes in synaptic transmission will be evaluated with consideration of changes in different TMS paired‐pulse protocols. TMS/tACS‐EEG protocols will be performed at baseline and at the end of the intervention to evaluate the efficacy of tACS on cortical reactivity, oscillatory activity, and connectivity. It is important to note that given the natural progression of the disease that causes significant functional/clinical decrease in very short timeframes (ie, 2 weeks 65 ), clinical stabilization in the treatment group over the treatment period compared to a decrease in the sham arm will be considered a positive outcome.
2.8. Data collection
During the screening visit we will assess inclusion and exclusion criteria. If the participant is eligible to enter the study, he/she or the legally authorized representative will be asked to sign the written informed consent form. Demographic data, medical history, including medications and neurological examination, will be collected. Participants will then undergo neuropsychological and behavioral evaluation, FDG‐PET and MRI scans, resting EEG, blood samples, tACS‐EEG, and TMS‐EEG and TMS paired‐pulse protocols, and the same measures will be collected after the intervention. At the 3‐month follow‐up visit, we will collect data from neuropsychological and behavioral tests and resting EEG registration.
2.9. Data monitoring
The study will comply with the Declaration of Helsinki and good clinical practice (ICH‐GCP) guidelines. The investigators will be qualified by trainings, and experience will be assessed to ensure the proper conduct of the trial and to reduce variability across the different trial sites. The investigator at each study site will generate and maintain adequate records (eg, medical records, source documents, standard operating procedures) to enable monitoring of study procedures and to ensure data harmonization. Monitoring activity will be provided in each site to review the data entered into the Case Record Forms by investigational staff for completeness and accuracy. A data safety monitoring plan has been set up in a similar manner to what required for NIH‐funded trials. A data safety monitoring board (DSMB) has been appointed to provide independent safety review and guidance to the PI during the course of the study to ensure the ongoing safety of subjects. The board, composed by three members from EU and the United States, has reviewed the protocol and consent form(s), including plans for safety monitoring prior to study initiation. The DSMB will conduct regularly planned meetings to review safety data, participant recruitment and retention, risk versus benefit, unanticipated problems, and protocol violations in order to judge if the overall safety and feasibility of the trial remains acceptable. The DSMB will have access to de‐identified data via regularly scheduled reports and for reportable unexpected events. They will make recommendations to continue, modify, or terminate the study depending on the assessment of the data.
2.10. Sample size
Fifty (50) subjects will be randomized in a 1:1 ratio to tACS or sham groups (respectively 25 subjects in tACS group and 25 in the placebo group). No studies have investigated the effect of tACS in patients with FTD. The average sample size for tES studies in the literature is 15 participants per group, whereas the average sample size for tDCS studies on FTD patients is even lower. 66 , 67 Our sample of 25 per arm is significantly higher than the available published literature, and in line with our ongoing gamma‐tACS Alzheimer's disease trial (clinical.gov number NCT03290326). We estimated the sample size required to obtain a very conservative effect size of 0.35 (Cohen d; α = 0.05, β = 0.95) according to the ADAS‐Cog data obtained in previous AD trials; the result is n = 36, suggesting our sample of 50 patients will be enough to account for attrition and to reliably capture tACS effects.
2.11. Statistical analysis
A mixed‐model repeated‐measures analysis will be used for primary and secondary outcome measures: AEs, ADAS‐COG, ADL (Weintraub), Trial A&B, FAB, NPI, FTLD‐CDR, NIH‐EXAMINER, and ADCS‐CIGC. A repeated‐measures analysis modeling the change from baseline score at each scheduled post‐baseline visit (post‐intervention visits; follow‐up visits) as the dependent variable will be constructed. The null hypothesis is that the difference between tACS versus placebo at the acute follow‐up is equal to zero. No interim futility or efficacy analysis is planned in which treatment groups will be compared. PET and EEG analysis will be carried out according to the same statistical design.
3. DISCUSSION
Because there is no effective treatment available for FTD, the development of new interventional approaches is strongly needed to slow down the progression of the disease. By leveraging the experience accumulated by the PI Emiliano Santarnecchi over multiple currently active clinical trials on tACS in AD, where promising evidence on improvements of cognitive performance, activities of daily living, and brain oscillations have been documented (NCT03880240, NCT03290326, NCT03412604), and given several overlapping pathological substrates between AD and FTD, 4 , 5 , 11 tACS could be a promising therapeutic option for FTD patients. Currently there are no trials evaluating tACS effect on FTD patients. In this study we aim to investigate this possibility via an extensive assessment spanning from PET imaging to MRI, EEG, and blood biomarkers, in order to also disentangle the mechanisms of action of tACS. This study will provide the critical first step in the development of a novel intervention to treat FTD, demonstrating tACS effects on multiple levels, and thus informing the design of larger clinical trials.
Previous studies have investigated the effect of a different type of non‐invasive brain stimulation, that is, transcranial direct current stimulation (tDCS), a technique applying a constant (direct) electrical field aimed at increasing or decreasing cortical excitability in a frequency‐unspecific manner. 68 Of interest, no side/adverse effects were observed in FTD patients that received tDCS across multiple studies, whereas improvement of behavioral symptoms were found (ie, speech production, grammatical comprehension, naming). 65 , 66 , 67 However, no specific mechanism of action has been suggested for tDCS on FTD patients, apart from the increase of cortical excitability representing the primary effect of tDCS. Given the frequency‐specific alteration of oscillatory activity documented in FTD, the possibility to entrain such specific cortical rhythms constitutes a fundamental advantage of tACS with respect to tDCS. Gamma‐tACS has been used previously in healthy subjects and pathological conditions not only to modulate motor performances, perception, and abstract reasoning, but also to restore working memory and attention, cognitive functions typically impaired in FTD. 15 , 69 , 70 , 71 In light of the preclinical evidence of specific interneurons degeneration, related gamma frequency oscillation impairment, 4 , 11 and successful reductions of neurotoxic plaques in mice and the growth of tau proteins in a FTD mouse model, 5 targeting this neural population with 40 Hz tACS offers the possibility to directly act on a defined physio‐pathophysiological substrate in order to modify the progression of the disease and help clarify the link between gamma oscillations and FTD pathology.
A further advantage of 40 Hz tACS, compared to auditory or visual stimulation, which were used previously in AD and FTD models to entrain cortical rhythms, 65 is the possibility of targeting specific cortical regions and cognitive disturbances affected by the disease without being limited to stimulation only affecting visual or auditory cortices, and its possible efficacy on modulating the strength of gamma coherence between regions, which was found to be reduced in bvFTD patients. 71 , 72
Moreover, in this study, a multidisciplinary study team will allow us to collect one of the most comprehensive multimodal imaging, genetic, metabolic, cognitive, biomarker data set on bvFTD. Together with the neuropsychological and behavioral assessments we will collect FDG‐PET images to evaluate hypometabolism patterns and changes in brain glucose uptake and functional and structural MRI data (including fMRI, ASL, DTI) to measure brain volume alterations and functional connectivity changes; blood biomarkers to investigate the potential role of tACS in neurodegeneration and neuroinflammation; tACS/TMS‐EEG for the efficacy of tACS on cortical reactivity, oscillatory patterns, and connectivity at high temporal resolution; and paired‐pulse TMS measures to assess the synaptic transmission deficit. In addition, to investigate individual variability in the response to brain stimulation, 73 we will also collect EEG before and after each tACS treatment session, as well as before and after a brief burst of tACS delivered over multiple brain regions, to possibly identify novel markers characterizing brain responsiveness to tACS in FTD patients and then retrospectively identify “responders,” as well as to characterize individual trajectories of response to brain stimulation. Any potential future application of tACS in FTD patients (as well as AD) will likely benefit from TMS/tACS‐EEG metrics and paired‐pulse TMS measures defined during the study.
Finally, a recent preclinical animal study has shown how gamma band activity is directly responsible for arteriolar vasodilation and can in turn lead to an increase in blood oxygenation, 74 increasing the hope of potentially restoring the characteristic frontotemporal hypoperfusion and consequent hypometabolism of FTD patients. Accordingly, because glucose metabolism and synaptic activity (investigated through FDG‐PET) can be altered in dementias even before neurodegeneration and loss of neurons, and may be responsible for a positive feedback loop cascade in which neural dysfunction further promotes metabolic imbalance, oxidative stress, neuroinflammation, and disease progression, 75 , 76 targeting hypometabolism maps to guide tACS intervention could represent a further significant innovation of our trial, with the ability to potentially counteract the pathological mechanisms underlying FTD by acting on brain perfusion.
Even though personalizing tACS stimulation montages for each patient would seem the optimal strategy to treat every patient, it could potentially lead to noncomparable results in the current study. The pattern of hypometabolism in bvFTD typically affects the bifrontal lobes and eventually extends in the temporal ones at later stages of the disease, 1 , 23 , 24 suggesting the possibility of targeting both lobes even in the case of patients presenting a specific symptomatology (eg, prefrontal dysfunction in bvFTD). Consistently stimulating the same regions in all patients may lead to generalizable results for the greater patient population, and if the results are positive, subsequent studies could personalize the tACS montages. Therefore, to ease the transition of the treatment proposed here to larger populations of patients in case of success, for the present study we will optimize one tACS template to be applied to every patient, also maximizing future replicability of results. We will use biophysical modeling to optimize stimulation in the prefrontal and temporal lobe bilaterally, attempting to increase metabolism in hypometabolic regions (prefrontal) while at the same time also exerting a possible protective effect on “preserved” temporal lobe regions. Given the availability of FDG data on each patient, we will still have the possibility to retrospectively validate the accuracy of the stimulation montage against individual MRI/PET data. This will allow us to correlate behavioral and clinical effects with the degree of accuracy of the template stimulation solution for each participant, potentially explaining variance of responders/nonresponders and thus inform future trials on the need for personalized montages.
4. CONCLUSION
Forty Hz tACS could enhance gamma oscillations in bvFTD patients, modulate abnormal interneurons activity and potentially restore pathological hypoperfusion/hypometabolism in frontal and temporal regions. This study will provide a critical first step in the development of a novel intervention to treat FTD, demonstrating tACS potential mechanisms of action and target engagement, thus informing the design of larger clinical trials.
CONFLICT OF INTEREST
E. Santarnecchi reports that this grant is supported by the Alzheimer's Drug Discovery Foundation (ADDF), the Association for Frontotemporal Dementia (AFTD), and Treat FTD Fund via GA – 201902 – 2017902 (payment to the hospitals); he is supported by the Defense Advanced Research Projects Agency (DARPA) via HR001117S0030, the NIH (P01 AG031720‐06A1, R01 MH117063‐01, R01 AG060981‐01; all payments to the hospital); he received honoraria for an invited meeting/talks with payment to him.
D. Press is supported by NIH grants R01 MH117063, R01 AG060987‐01, R01AG060981, 1R01 MH111875‐01; ‐; he is also supported by Industry Sponsored Clinical Trials Aggregated Effort 1.8 CM 221AD301 Biogen ENGAGE and EMBARK Title: A Phase 3 Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study to evaluate the Efficacy and Safety of Aducanumab (BIIB037) in Subjects with Early Alzheimer's Disease. This study is restarting as an open‐label study (EMBARK) and he is the site PI; he is also PI for the JNJ‐63733657 Jansen Phase II trial Title: A Randomized, Double‐blind, Placebo‐controlled, Parallel‐group, Multicenter Study to Assess the Efficacy and Safety of JNJ‐63733657, an Anti‐Tau Monoclonal Antibody, in Participants with Prodromal Alzheimer's disease; he is on a DSMB for research in noninvasive brain stimulation with Timothy Wagner and Felipe Fregni at MGH.
B. Dickerson received a grant from NIH and consulting fees from Acadia, Alector, Arkuda, Biogen, Denali, Lilly, Merck, Novartis, Takeda, and Wave Lifesciences.
A. Connor received a payment contract from Harvard Medical School with payments made to her institution.
B. Wong received payment from NIH grants with payment made to her institution.
C. Shen received an NIH grant with payment made to his institution and consulting fees from Indiana University and Cardiovascular System, Inc.
G. Ruffini has received grants from the EU (ERC Galvani and FET Proactive Neurotwin), with payments to his institution; his company is the source of support (paying for traveling and lodging). He has a pending patent on brain stimulation in AD and in brain cancer; he holds stock in his company, Neuroelectrics; his company is the source of support (paying for traveling and lodging; he works and he is shareholder of Neuroelectrics, a company in the brain stimulation/neuromodulation business.
G. El Fakhri received NIH grants to MGH and he has an unrelated patent for PET imaging of membrane potential.
G. Koch received grants from ADDF and he has a patent for rotigotine in AD.
M. Assogna, G. Sprugnoli, J. Macone, S. Bonnì, I. Borghi, M. Hoffman, N. Grover, A. Martorana, and M. O'Reilly have nothing to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization ‐ ES, GS, GK, DP, BD; Funding acquisition ‐ ES; Data curation ‐ MA, BW, JM; Statistical modeling and design ‐ CS, ES; Data collection ‐ NG, BW, AM, SB, IB, AM, MO; Safety monitoring ‐ JM, AC; ES, GK GR; Project administration ‐ ES, GK, MH, JM; Brain Stimulation solution – ES, GK, GR; Supervision ‐ ES, JM, AC, GK, DP.
ACKNOWLEDGEMENT
The clinical trial is supported by the Alzheimer's Drug Discovery Foundation (ADDF), the Association for Frontotemporal Dementia (AFTD), and Treat FTD Fund via GA – 201902 – 2017902 (Study PI Emiliano Santarnecchi, PhD, PsyD, BIDMC, Boston, MA, USA; site PIs: Giacomo Koch, MD, PhD, SLF, Rome, Italy; Brad Dickerson, MD, MGH, Boston, MA, USA).
Assogna M, Sprugnoli G, Press D, et al. Gamma‐induction in frontotemporal dementia (GIFTeD) randomized placebo‐controlled trial: Rationale, non‐invasive brain stimulation protocol, and study design. Alzheimer's Dement. 2021;7:e12219. 10.1002/trc2.12219
Martina Assogna, Giulia Sprugnoli, Emiliano Santarnecchi, and Giacomo Koch contributed equally.
REFERENCES
- 1. Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet Lond Engl. 2015;386:1672‐1682. 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423‐434. 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M. Invited review: frontotemporal dementia caused by microtubule‐associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol. 2015;41:24‐46. 10.1111/nan.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang W, Zhang L, Liang B, et al. Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat Neurosci. 2016;19:557‐559. 10.1038/nn.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230‐235. 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerreiro . TREM2 and neurodegenerative disease. N Engl J Med. 2013;369:1564‐1570. 10.1056/NEJMc1306509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kleinberger G, Brendel M, Mracsko E, et al. The FTD‐like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. EMBO J. 2017;36:1837‐1853. 10.15252/embj.201796516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bright F, Werry EL, Dobson‐Stone C, et al. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol. 2019;15:540‐555. 10.1038/s41582-019-0231-z. [DOI] [PubMed] [Google Scholar]
- 9. Thomson H. How flashing lights and pink noise might banish Alzheimer's, improve memory and more. Nature. 2018;555:20‐22. 10.1038/d41586-018-02391-6. [DOI] [PubMed] [Google Scholar]
- 10. Murley AG, Rowe JB. Neurotransmitter deficits from frontotemporal lobar degeneration. Brain. 2018;141:1263‐1285. 10.1093/brain/awx327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White MA, Kim E, Duffy A, et al. TDP‐43 gains function due to perturbed autoregulation in a Tardbp knock‐in mouse model of ALS‐FTD. Nat Neurosci. 2018;21:552‐563. 10.1038/s41593-018-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes LE, Rittman T, Robbins TW, Rowe JB. Reorganization of cortical oscillatory dynamics underlying disinhibition in frontotemporal dementia. Brain. 2018;141:2486‐2499. 10.1093/brain/awy176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67:129‐143. 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasten FH, Dowsett J, Herrmann CS. Sustained aftereffect of α‐tACS lasts up to 70 min after stimulation. Front Hum Neurosci. 2016;10:245. 10.3389/fnhum.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santarnecchi E, Biasella A, Tatti E, Rossi A, Prattichizzo D, Rossi S. High‐gamma oscillations in the motor cortex during visuo‐motor coordination: a tACS interferential study. Brain Res Bull. 2017;131:47‐54. 10.1016/j.brainresbull.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 16. Santarnecchi E, Muller T, Rossi S, et al. Individual differences and specificity of prefrontal gamma frequency‐tACS on fluid intelligence capabilities. Cortex. 2016;75:33‐43. 10.1016/j.cortex.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 17. Santarnecchi E, Polizzotto NR, Godone M, et al. Frequency‐dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol. 2013;23:1449‐1453. 10.1016/j.cub.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 18. Santarnecchi E, Sprugnoli G, Bricolo E, et al. Gamma tACS over the temporal lobe increases the occurrence of Eureka! moments. Sci Rep. 2019;9:5778. 10.1038/s41598-019-42192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antal A, Alekseichuk I, Bikson M, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2017;128:1774‐1809. 10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front HumNeurosci. 2013;7:317. 10.3389/fnhum.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fröhlich F, Sellers KK, Cordle AL. Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation (tACS). Expert Rev Neurother. 2015;15:145‐167. 10.1586/14737175.2015.992782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vosskuhl J, Strüber D, Herrmann CS. Non‐invasive brain stimulation: a paradigm shift in understanding brain oscillations. Front Hum Neurosci. 2018;12. 10.3389/fnhum.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohnen NI, Djang DSW, Herholz K, Anzai Y, Minoshima S. Effectiveness and safety of 18F‐FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med Off Publ Soc Nucl Med. 2012;53:59‐71. 10.2967/jnumed.111.096578. [DOI] [PubMed] [Google Scholar]
- 24. Bickart KC, Brickhouse M, Negreira A, Sapolsky D, Barrett LF, Dickerson BC. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. J Neurol Neurosurg Psychiatry. 2014;85:438‐448. 10.1136/jnnp-2012-304656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J Neurol. 2011;134:2456‐2477. 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ducharme S, Dols A, Laforce R, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. 2020;143:1632‐1650. 10.1093/brain/awaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neri F, Mencarelli L, Menardi A, et al. A novel tDCS sham approach based on model‐driven controlled shunting. Brain Stimulat. 2020;13:507‐516. 10.1016/j.brs.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 28. Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual‐Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. NeuroImage. 2014;89:216‐225. 10.1016/j.neuroimage.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412‐2414. 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356‐1364. 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 31. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621‐1166. 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 32. Kramer JH, Mungas D, Possin KL, et al. NIH EXAMINER: conceptualization and development of an Executive Function Battery. J Int Neuropsychol Soc JINS. 2014;20:11‐19. 10.1017/S1355617713001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tombaugh T. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203‐214. 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 34. Tombaugh TN, Kozak J, Rees L. Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: fAS and Animal Naming. Arch Clin Neuropsychol;11:167‐177. [PubMed] [Google Scholar]
- 35. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty‐five years of evaluation. Clin Psychol Rev. 1988;8:77‐100. 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 36. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308‐2308. 10.1212/WNL.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 37. Kertesz A, Davidson W, Fox H. Frontal Behavioral Inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci J Can Sci Neurol. 1997;24:29‐36. 10.1017/S0317167100021053. [DOI] [PubMed] [Google Scholar]
- 38. Johnson N, Barion A, Rademaker A, Rehkemper G, Weintraub S. The activities of daily living questionnaire. Alzheimer Assoc Disord. 2004;18:8. [PubMed] [Google Scholar]
- 39. Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:32332‐32339. 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 40. Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(2):S22‐32. 10.1097/00002093-199700112-00004. Suppl. [DOI] [PubMed] [Google Scholar]
- 41. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193‐213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540‐545. [DOI] [PubMed] [Google Scholar]
- 43. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126‐1141. 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 44. Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutr Burbank Los Angel Cty Calif. 1999;15:116‐122. 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 45. Berg J‐L, Durant J, Banks SJ, Miller JB. Estimates of premorbid ability in a neurodegenerative disease clinic population: comparing the Test of Premorbid Functioning and the Wide Range Achievement Test. Clin Neuropsychol. 2016;30:547‐557. 10.1080/13854046.2016.1186224. [DOI] [PubMed] [Google Scholar]
- 46. Benussi A, Grassi M, Palluzzi F, et al. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Ann Neurol. 2020:ana.25677. 10.1002/ana.25677. [DOI] [PubMed] [Google Scholar]
- 47. Benussi A, Di Lorenzo F, Dell'Era V, et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89:665‐672. 10.1212/WNL.0000000000004232. [DOI] [PubMed] [Google Scholar]
- 48. Martorana A, Esposito Z, Di Lorenzo F, Giacobbe V, Sancesario GM, Bucchi G, et al. Cerebrospinal fluid levels of Aβ42 relationship with cholinergic cortical activity in Alzheimer's disease patients. J Neural Transm. 2012;119:771‐778. 10.1007/s00702-012-0780-4. [DOI] [PubMed] [Google Scholar]
- 49. Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503‐513. 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang Y‐Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201‐206. 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 52. Koch G, Di Lorenzo F, Bonnì S, Ponzo V, Caltagirone C, Martorana A. Impaired LTP‐ but not LTD‐Like cortical plasticity in Alzheimer's disease patients. J Alzheimers Dis. 2012;31:593‐599. 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- 53. Casula EP, Maiella M, Pellicciari MC, et al. Novel TMS‐EEG indexes to investigate interhemispheric dynamics in humans. Clin Neurophysiol. 2020;131:70‐77. 10.1016/j.clinph.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 54. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease. NeuroImage. 2018;169:302‐311. 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 55. Ozdemir RA, Tadayon E, Boucher P, et al. Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proc Natl Acad Sci. 2020;117:8115‐8125. 10.1073/pnas.1911240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. König A, Satt A, Sorin A, et al. Automatic speech analysis for the assessment of patients with predementia and Alzheimer's disease. Alzheimers Dement Diagn Assess Dis Monit. 2015;1:112‐124. 10.1016/j.dadm.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wroge TJ, Ozkanca Y, Demiroglu C, Si D, Atkins DC, Ghomi RH, Parkinson's disease diagnosis using machine learning and voice. 2018 IEEE Signal Process. Med. Biol. Symp. SPMB, Philadelphia, PA: IEEE; 2018, pp. 1‐7. 10.1109/SPMB.2018.8615607 [DOI] [Google Scholar]
- 58. Yakhia M, König A, van der Flier WM, Friedman L, Robert PH, David R. Actigraphic motor activity in mild cognitive impairment patients carrying out short functional activity tasks: comparison between mild cognitive impairment with and without depressive symptoms. J Alzheimers Dis JAD. 2014;40:869‐875. 10.3233/JAD-131691. [DOI] [PubMed] [Google Scholar]
- 59. López‐de‐Ipiña K, Alonso J‐B, Travieso CM, et al. On the selection of non‐invasive methods based on speech analysis oriented to automatic alzheimer disease diagnosis. Sensors. 2013;13:6730‐6745. 10.3390/s130506730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Merrilees J, Hubbard E, Mastick J, Miller BL, Dowling GA. Sleep in persons with frontotemporal dementia and their family caregivers. Nurs Res. 2014;63:129‐136. 10.1097/NNR.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meeter LH, Kaat LD, Rohrer JD, van Swieten JC. Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol. 2017;13:406‐419. 10.1038/nrneurol.2017.75. [DOI] [PubMed] [Google Scholar]
- 62. Katisko K, Solje E, Korhonen P, et al. Peripheral inflammatory markers and clinical correlations in patients with frontotemporal lobar degeneration with and without the C9orf72 repeat expansion. J Neurol. 2019. 10.1007/s00415-019-09552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ventriglia M, Zanardini R, Bonomini C, et al. Serum brain‐derived neurotrophic factor levels in different neurological diseases. BioMed Res Int. 2013;2013:901082. 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arranz AM, De Strooper B. The role of astroglia in Alzheimer's disease: pathophysiology and clinical implications. Lancet Neurol. 2019;18:406‐414. 10.1016/S1474-4422(18)30490-3. [DOI] [PubMed] [Google Scholar]
- 65. Benussi A, Dell'Era V, Cosseddu M, et al. Transcranial stimulation in frontotemporal dementia: a randomized, double‐blind, sham‐controlled trial. Alzheimers Dement N Y N. 2020;6:e12033. 10.1002/trc2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cotelli M, Manenti R, Petesi M, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis. 2014;39:799‐808. 10.3233/JAD-131427. [DOI] [PubMed] [Google Scholar]
- 67. Ferrucci R, Mrakic‐Sposta S, Gardini S, et al. Behavioral and neurophysiological effects of transcranial direct current stimulation (tDCS) in fronto‐temporal dementia. Front Behav Neurosci. 2018;12:235. 10.3389/fnbeh.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lefaucheur J‐P, Antal A, Ayache SS, et al. Evidence‐based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2017;128:56‐92. 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 69. Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer's disease. Neurology. 2000;54:2277‐2284. 10.1212/WNL.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 70. Stopford CL, Thompson JC, Neary D, Richardson AMT, Snowden JS. Working memory, attention, and executive function in Alzheimer's disease and frontotemporal dementia. Cortex. 2012;48:429‐446. 10.1016/j.cortex.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 71. Strüber D, Herrmann CS. Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: a review and perspective. Int J Psychophysiol. 2020;152:15‐25. 10.1016/j.ijpsycho.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 72. Hughes LE, Rowe JB. The impact of neurodegeneration on network connectivity: a study of change detection in frontotemporal dementia. J Cogn Neurosci. 2013;25:802‐813. 10.1162/jocn_a_00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krause B, Cohen Kadosh R. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci. 2014;8. 10.3389/fnsys.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mateo C, Knutsen PM, Tsai PS, Shih AY, Kleinfeld D. Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood‐oxygenation‐level‐dependent “Resting‐State” connectivity. Neuron. 2017;96:936‐948.e3. 10.1016/j.neuron.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shivamurthy VKN, Tahari AK, Marcus C, Subramaniam RM. Brain FDG PET and the diagnosis of dementia. Am J Roentgenol. 2015;204:W76‐85. 10.2214/AJR.13.12363. [DOI] [PubMed] [Google Scholar]
- 76. Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: ways and mechanisms of metabolic correction. J Neurosci Res. 2017;95:2217‐2235. 10.1002/jnr.24064. [DOI] [PubMed] [Google Scholar]


