Abstract
Objective:
To determine if the mandibular condylar cartilage (MCC) will grow with and without mandibular anterior repositioning appliances with the administration of insulin-like growth factor (IGF-1) and transforming growth factor-β (TGF-β).
Materials and Methods:
Twenty-four growing New Zealand rabbits were divided into three groups: a group with saline injection in the temporomandibular joint, a group that received anterior positioning appliance, and a group that received injection of growth factors as well as mandibular repositioning appliance. Real-time reverse transcription polymerase chain reaction technique was used to study gene expression supported by histomorphometry.
Results:
Administration of growth factors along with mandibular repositioning appliances has induced 5.70-fold expression of matrix metalloproteinase-1 (MMP-1) (P < .0005) and 1.29-fold expression of MMP-13 (P < .0005). In contrast, administration of mandibular repositioning appliances only has induced 2.33-fold expression of MMP-1 (P < .0005) and 0.83-fold expression of MMP-13 (P < .0005). Histomorphometric analysis revealed increased proliferation of the condylar cartilage in the appliance and injection group as compared to the control group.
Conclusion:
The administration of growth factors along with the use of mandibular advancement appliance has increased genetic expression of MMP-1 and MMP-13 supported by histomorphometric evidence indicating growth of condylar cartilage.
Keywords: Transforming growth factor-β (TGF-β), Insulin-like growth factor (IGF-1), Mandibular repositioning appliances, MMP-1, MMP-13, Mandibular condylar cartilage
INTRODUCTION
Since the mandibular condyle plays a significant role in the development of form and elegance of the orofacial complex, it has received special attention in orthodontics.1 Accordingly, besides growth factors, condylar growth modification is induced by mandibular advancement as reported by Petrovic and Stutzmann.2 Most of the studies in this regard have used either histologic, histomorphometric, immuno-histomorphometric, biochemical or auto-radiographic methods as diagnostic tools to evaluate growth at the condyle3–7 or to detect increased expression of some growth factors/biomarkers of mandibular condylar cartilage (MCC) growth. Although these studies have dealt with condylar growth by providing valuable leads at a cellular level, several questions have remained unanswered; these questions could be answered only on a genetic level, elucidated by cellular studies, quantified by molecular markers, and validated by statistical analysis. The present study has attempted precisely such an integrated strategy for evaluation of condylar growth in young rabbits as a function of mandibular anterior repositioning appliances with and without the administration of growth factors (TGF-β and IGF-1).
MATERIALS AND METHODS
In this study, twenty-four 60-day-old (pubertal age group) male and female New Zealand (NZ) white rabbits were used as approved by the Animal Ethical Committee (CPCSEA-01-2009). The rabbits were randomly divided into the following three groups of eight each (four male and four female):
The control group received saline injection in the tempromandibular joint (TMJ). One experimental group received only mandibular anterior repositioning appliances; the second experimental group received mandibular anterior repositioning appliances and were injected with growth factors.
Appliance Fabrication and Cementation
Impressions were taken using alginate impression material for all rabbits, and appliances were fabricated accordingly using heat-cure acrylic material (Ivoclar Vivadent-SR Triplex Hot).
The rabbits fitted with the above bite-jumping devices were closely observed daily at 0600, 0930, 1230,1400, 1630, 2130, and 2400 hours for retention of the appliance, its wear or damage, tissue irritation, somatic growth status (if any), and their tolerance as judged from the quantity of food intake and body weight.
Injection of Growth Factors
Insulin-like growth factor-I (IGF-1, lyophilized powder from mouse recombinant expressed in Escherichia coli; Sigma, St Louis, MO, USA), 25 ng/25 µL, and transforming growth factor-β-1 (TGF-β, lyophilized powder from human platelets, 1 × 106 units/mg; Sigma), 20 ng/25 µL, were injected in the inferior joint space. The needle was directed at 45° in reference to the midsagittal plane, in the fossa behind the posterior orbital ridge until it contacted the condyle. Digital radiographs were taken to confirm the precise location of the needle before injecting both growth factors at the same time. The control group was injected with an equal volume of phosphate buffered saline instead of growth factors. The injections were administered on day 7, day 14, and day 21, and the rabbits were euthanized on day 30. The condyles were immediately recovered, snap frozen in liquid nitrogen, and stored at −80°C until isolation of RNA.
Real-Time Reverse Transcription Polymerase Chain Reaction Technique
The condylar tissue isolated from both groups was treated with RiboPure kit (Ambion, Grand Island, NY, USA), for rapid purification of total high-quality RNA as per its protocol and stored at −20°C. Subsequently, RNA from each control and experimental sample was converted into c-DNA using equal quantity of 2× RT Master Mix in polymerase chain reaction (PCR) tubes and thermocycled as per the protocol by the c-DNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The reaction was set in 48-well plates, with each well containing 10 µL Master Mix TaqMan 2×, 1 µL primer (gene specific), 2 µL c-DNA (sample specific), and 7 µL sterile H2O, making a total 20 µL reaction mixture, with glyceraldehyde-3-phosphate dehydrogenase as the endogenous control. The primers used for the corresponding genes are shown in Table 1. The 48-well plate was set in an Applied Biosystems StepOne Real-Time PCR machine with the TaqMan technique. The results were analyzed for gene expression using StepOne (version 2.1) software.
Table 1.
Profile of Primer Sequence Used for the Corresponding Genes for TaqMan Technique in Real-Time Reverse Transcription Polymerase Chain Reaction
Alcian blue periodic acid Schiff staining and hematoxylin and eosin staining were carried out for histologic sections, and the slides were observed under Leica DM 5000B microscope at 10× magnification. The images were captured with a Leica DFC 320 camera and were analyzed by Leica Application Suite 3.70 image analysis software (Wetzlar, Germany).
Statistical Analysis
Data were statistically analyzed using Statistical Package for Social Sciences (SPSS version 11.5, Chicago, Ill). The P values less than .05 were considered statistically significant. All hypotheses were formulated using two-tailed alternatives against each null hypothesis (hypothesis of no difference). All results were presented as mean ± standard deviation across the study groups and statistical significance of difference of mean proteoglycan (PG) as well as for histology tested using Mann-Whitney U-test.
RESULTS
Effects of Matrix Metalloproteinase Expression
MMP-1 expression
The matrix metalloproteinase-1 (MMP-1) expression as depicted in Figure 1 was 5.70-fold (P value < .0005) as a function of mandibular repositioning appliances along with the administration of IGF-1 and TGF-β group and 2.33-fold (P value < .0005) as a function of mandibular repositioning appliance.
Figure 1.
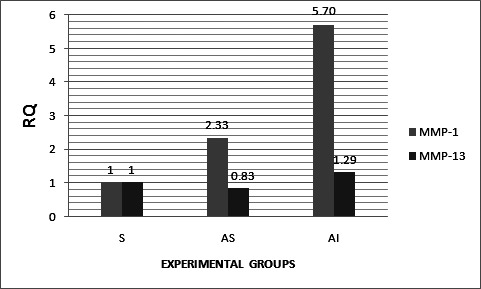
Histogram representing relative quantitation of MMP-1 and MMP-13 gene expression in the control and experimental groups.
MMP-13 expression
At this juncture, it was considered worthwhile to examine if an enhanced expression of the MMP-1 gene has any effect on the expression of the MMP-13 gene. Accordingly, MMP-13 gene expression was 1.29-fold (P value < .0005) as a function of mandibular repositioning appliances along with the administration of IGF-1 and TGF-β and only 0.83-fold (P value < .0005) as a function of mandibular repositioning appliance (Figure 1).
Quantitative Assessment of the PG Content in the Condylar Cartilage and Histomorphometry
For quantitative assessment, a representative measurement frame of 300 × 300 µm was selected with a focus on an area of 4760.992 µm2. Figure 2A,B,C shows that the area reflecting PG, as judged from the magenta color in the combined treatment (Figure 2C) group was approximately two-fold greater than that of the control group (Figure 2A) and greater as compared to the group treated by appliance only (Figure 2B). The histochemical profile (Figure 3) showed an increase in the size of the proliferative layer of the cartilage and an increase in the length of the cartilage. The length of the condylar cartilage from the fibrous layer to the hypertrophic layer was greater in the group treated by appliances and growth factor than that in the other groups (Figure 3).
Figure 2.
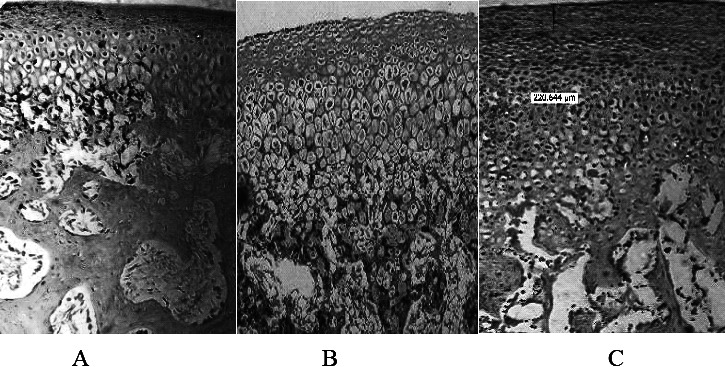
Assessment of PG content. (A) Control group treated with saline only. (B) Experimental group treated with appliance only. (C) Experimental group treated with appliance and growth factor injection.
Figure 3.

Histomorphometric analysis. Increase in the proliferative layer as compared to the hypertrophic layer and a concomitant increase in thickness in the cartilage in experimental groups. (A) Control group treated with saline only. (B) Experimental group treated with appliance only. (C) Experimental group treated with appliance and growth factor injection.
DISCUSSION
Rationale Underlying the Choice of Rabbits as Search Model
NZ rabbits were chosen as a dependable research model in the present study because (1) they are placed as higher mammals than rodents and closer to human beings in an evolutionary scale for extrapolating observations regarding clinical applicability, (2) their TMJs are essentially identical to those in human beings, (3) their jaw apparatus is specialized for a herbiviorous diet as in human beings, and (4) administration of growth factors in the TMJ/ retrodiscal could be more precise because they have large TMJs compare to mice, on which most studies have been conducted so far.
Significance of Condylar Growth at the Genetic Level
Since impaired growth of the condyles contributes to the development of mandibular asymmetries and retrognathia,7 the role of the mandibular condyles in the development of the orofacial complex has received focused attention of orthodontics. Accordingly, earlier studies on growing mice have thrown light on the postnatal craniofacial skeletal growth and development in its entirety by delineating biochemical processes, certainly regulated by a complex network of genes and interacting genetic/epigenetic factors,8,9 which induce secretion of enzymes, growth factors, their receptors, and so on, representing integration of various physiologic subprocesses.10 As cartilage provides endochondral bone as an adaptive growth site during mandibular development,7,11 the present study has attempted to understand the expression of genetic factors (MMP-1 and MMP-13) in a young NZ rabbit model as a function of mandibular anterior repositioning appliances with and without the administration of growth factors (TGF-β and IGF-1).
Quantitative Assessment of the PG Content in the Condylar Cartilage and Histomorphometry
The increasing intensity of staining as a function of treatments observed in the present studies was in concurrence with earlier observations12 while studying the role of mesenchymal cells in the skeletal regeneration. Thus, these studies have pointed out that IGF-1, an anabolic factor, stimulated the PG synthesis observed earlier.13 It is our estimate that although more PG is synthesized, its significant accumulation is not quantifiable, being used immediately in the genesis of different ingredients of extracellular matrix (ECM), namely glycosaminoglycans. The above contention is independently verifiable through biochemical changes in the quantum of PG underlying the growth profile as observed in the histochemical staining pinning down the site and precise nature of the change (Figure 2C). Accordingly, the increase in the PG content in the cartilage is in the descending order AI > AS > S. In totality, these observations on the young rabbits reflected condylar growth, confirming earlier observations on mice and rats that IGF-1 and TGF-β complement the growth of MCC vascular endothelial growth factor.6,14,15
The growth of the mandibular cartilage was evident by an increase in the length of the cartilage and an increase in the proliferative layer of the cartilage as shown in Figure 3 in the descending order AI > AS > S.
Effects of MMP Expression
Having examined the biochemical nature of ECM (especially PGs) in the young rabbits, it was imperative to study those enzymes that are known to participate in the modulation or hydrolysis (partly or fully) to accord desired composition of ECM. In this regard, literature has assigned a significant role to matrix metalloproteinases (MMPs).
Significance of Amplification of MMP-1
The 5.70-fold amplification of the MMP-1 gene unambiguously rules out any possibility of an error, even remotely so, in the interpretation since it is statistically significant. MMP-1 expression selectively at such a high level has definite physiologic significance based on its substrate-specificity, which is corroborated by an intense staining in the histologic observations. It has not only a significant role in prenatal growth, but also in stimulating postnatal longitudinal growth, presumably by digesting a number of ECM and non-ECM proteins.
Rationale Underlying Limited Expression of MMP-13 Gene
The 1.29-fold upregulation of MMP-13 observed in the young rabbits validates earlier in vitro observation in the fetal bovine chondrocytes. Only 25% level of the expression of MMP-13 in comparison with that of MMP-1 (1.29- to 5.70-fold) has clearly indicated that in the young rabbits, there was minimal role of MMP-13 during the remodeling of MCC. This may be due to either its substrate specificity for collagen II, which appears to have a minor role, and/or the modulatory processes needing minimal proteolysis in PGs, which is a major component in the ECM of young rabbits.
In other experimental animals (eg, rat or mouse), activity of MMP-13 has been preponderant over MMP-1 activity for the modulation of ECM proteins by proteolysis. In contrast, in the present study on young rabbits, MMP-1 has shown maximal 5.70-fold upregulation in appliance along with the growth factor, whereas MMP-13 has shown a mere 1.29-fold maximal upregulation only (Figure 1). Similarly, MMP-1 has shown maximal 2.33-fold upregulation in the appliance group, whereas MMP-13 has shown downregulation to 0.83-fold expression only (Figure 1). Being the physiologically need-based inducible enzymes, preferential upregulation of MMP-1 over MMP-13 indicated (1) nature of ECM/non-ECM proteins are different, (2) amount of proteins could be probably less, (3) modulation was maximally by MMP-1 rather than by MMP-13 on the basis of their substrate specificity, and (4) specific proteases (such as cathepsin, acid/alkaline proteases not examined in this study) other than MMP-1/MMP-13 may be playing their legitimate role. Therefore, it was desirable to know their types, localization, and requisites for the function.
Types of MMPs
After the discovery of the first MMP as collagenase in the tail of a tadpole undergoing metamorphosis, more than 20–24 MMPs have been isolated, of which 23 found in human beings are significant due to their specificity for proteins in ECM. Therefore, their main function has been presumed to be remodeling of the ECM. They are classified as collagenases, gelatinases, stromelysins, and matrilysins.16 On the basis of substrate-specificity, they are now classified into five subgroups: collagenases (MMP-1, -8, and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, and -11), membrane type (MT-1 to -6 MMPs), and others.
Activation and Role of MMPs
MMPs being a family of proteolytic enzymes, regulating cell differentiation and tissue remodeling, they are secreted by most types of cells, with their expression transcriptionally regulated by the growth factors and cytokines.17 Their activation from a precursor to an active form is regulated by other MMPs and their inhibitors. Their role during the embryonic development is ECM remodeling by a direct proteolytic cleavage and release of monomers for the recycling required for rapid kinetics of growth. Consequently, they allow (1) cell migration, (2) cell attachment, (3) proliferation, (4) differentiation, (5) alteration in the ECM micro-environment, (6) induction in the altered cellular behavior, and (7) morphogenesis. MMPs also appear to be involved in apoptosis of growth plate chondrocytes, as well as in the modulation of biologic activity of relevant growth factors, their receptors, cytokines, adhesion receptors, and a variety of enzymes.18
Subsequently, mandibular condyle is generated by the endochondral ossification like that of long bone; however, its pattern of development is different from that of the long bone. MCC, often classified as a secondary cartilage, develops from already differentiated cells into periosteum-like cells, rather than from the undifferentiated mesenchymal cells. Naturally, therefore, condylar cartilage is different from the primary cartilage in terms of its rapid progress from progenitor chondrocytes to hypertrophic chondrocytes. For rapid accumulation of hypertrophic chondrocytes, effective degradation of ECM proteins (type II and type X collagen and proteoglycan) is required by MMPs.
Localization of MMP-1
At a cellular level, while MMP-1 (collagenase-1) is not detected in the growth plate, its positive immunostaining in the chondrocytes in the hypertrophic zone did confirm its presence in human postnatal growth. It is more distinctly located in the osteoblasts of the adhering bony tissue, pointing its role in the degradation of type I collagen. In fact, human osteoclasts stained negative for MMP-1. Therefore, detection of MMP-1 in the mice fetal growth plate may be due to erroneous interpretation because mice MMP-1 has a high sequence homology to human MMP-13.19
Physiologic Functions of MMP-1
At a molecular level, MMP-1 cleaves those proteins binding insulin-like growth factor (IGFBP-3) and thereby enhances the biologic activity of IGF-1. It has a significant role not only in prenatal growth but also in stimulating postnatal longitudinal growth, presumably by digesting a number of ECM and non-ECM proteins. This appears to be a reality in the case of young rabbits where amplification of MMP-1 is many fold that of MMP-13, presumably its ECM being rich in PG-derived molecular entities as against richness in collagen as found in mice or rats.20
Localization of MMP-13
At a cellular level, MMP-13 seems to be localized in the lower-most hypertrophic chondrocytes of the growth plate cartilage and distinctly in the osteoblasts. It is often upregulated in the vicinity of bony lesions21 and contributes to (1) rapid chondrocyte enlargement during hypertrophy, (2) matrix calcification in the chondro-osseous region, and (3) endochondral ossification. Paradoxically, MMP-13 is not expressed in any cell types of the mandibles, including condensation of osteoblastic progenitors and periosteal cells in mesenchyme. However, MMP-13 is detected in the fracture healing of bone and human fetal hypertrophic chondrocytes by the immunohistochemical technique.22
Physiologic Functions of MMP-13
MMP-13 seems to be physiologically the most important protease in cartilage remodeling and mineralization by virtue of its substrate preference for (1) cartilage-specific type II collagen, (2) type X collagen, (3) cartilage aggrecans, and (4) Cbfa-1 (a MMP-13 transcription factor), indicating its significance in the chondrocyte maturation and cartilage remodeling.
Molecular and Genetic Regulation of Functions of MMP-13
At a molecular level, MMP-13 activates latent TGF-β secreted by the chondrocytes required for the osteoblastic differentiation.23 At a genetic level, MMP-13 has been shown to be upregulated during the differentiation in fetal bovine chondrocytes in vitro, suggesting that proteolysis catalyzed by MMP-13 is a prerequisite for the chondrocyte differentiation for the degradation of collagenous matrices during the development of cartilage and bone.24
CONCLUSIONS
IGF-1 and TGF-β administration (1) regulates cellular differentiation in the condyles during growth, (2) triggers the onset of cartilage ossification, and (3) catalyzes the growth of matrix of endochondral ossification in the condyle.
Without the knowledge of gene activity and its signaling transduction pathways, elucidation of the mechanisms that control MCC development would be impossible.
Acknowledgments
We acknowledge the assistance of Dr Prashant Khadke, Dr Uday Deshpande, Dr Dada Akolkar, and Mr Mandar from LAB INDIA-RGITBT for collaboration in the gene expression studies, and Mrs Soumya Koppikar in the analysis of PG.
REFERENCES
- 1.Patil A. S, Sable R. B, Kothari R. M. An update on transforming growth factor-β (TGF-β): sources, types, functions and clinical applicability for cartilage/bone healing. J Cell Physiol. 2011;226:3094–3103. doi: 10.1002/jcp.22698. [DOI] [PubMed] [Google Scholar]
- 2.Petrovic A. G, Stutzmann J. J. Further investigations into the functioning of the peripheral comparator of the servosystem in the control of the condylar cartilage growth rate and the lengthening of the jaw. In: McNamara J. A Jr, editor. The Biology of Occlusal Development Monograph 7. Ann Arbor, Mich: University of Michigan; 1977. pp. 255–291. [Google Scholar]
- 3.Tonge E. A, Heath J. K, Meikle M. C. Anterior mandibular displacement and condylar growth: an experimental study in the rat. Am J Orthod. 1982;82:277–287. doi: 10.1016/0002-9416(82)90462-6. [DOI] [PubMed] [Google Scholar]
- 4.Ghafari J, Degroote C. Condylar cartilage response to continuous mandibular displacement in the rat. Angle Orthod. 1986;56:49–57. doi: 10.1043/0003-3219(1986)056<0049:CCRTCM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Basdra E. K, Huber L, Komposch G. Papavassiliou AG. Mechanical loading triggers specific biochemical responses in mandibular condylar chondrocytes. Biochim Biophys Acta. 1994;1222:315–322. doi: 10.1016/0167-4889(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld I, Gasper R, Laufer D, Livne E. Enhancement of toluidine blue staining by transforming growth factor, insulin like growth factors and growth hormone in the temporomandibular joint of aged mice. Cells Tissues Organs. 2000;167:121–129. doi: 10.1159/000016775. [DOI] [PubMed] [Google Scholar]
- 7.Proff P, Gedrange T, Franke R, Schubert H, Fanghanel J, Miehe B, Harzer W. Histological and histomorphometric investigation of the condylar cartilage of juvenile pigs after anterior mandibular displacement. Ann Anat. 2007;189:269–275. doi: 10.1016/j.aanat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Bushang P. H, Hinton R. J. A gradient of potential of modifying craniofacial growth. Semin Orthod. 2005;11:219–226. [Google Scholar]
- 9.Visnapuu V, Peltomaki T, Ronning B, Vahlberg T, Helenius H. Growth hormone and insulin-like growth factor receptors in the temporomandibular joint of the rat. J Dent Res. 2001;80:1903–1907. doi: 10.1177/00220345010800100801. [DOI] [PubMed] [Google Scholar]
- 10.Shen G, Darendeliler M. A. The adaptive remodeling of condylar cartilage—a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- 11.Patil A. S, Sable R. B, Kothari R. M. Occurrence, biochemical profile of vascular endothelial growth factor (VEGF) isoforms and their functions in endochondral ossification. J Cell Physiol. 2012;227:1298–1308. doi: 10.1002/jcp.22846. [DOI] [PubMed] [Google Scholar]
- 12.Rabie A. B. M, She T. T, Hägg U. Functional appliance therapy accelerates and enhances condylar growth. Am J Orthod Dentofacial Orthop. 2003;123:40–48. doi: 10.1067/mod.2003.45. [DOI] [PubMed] [Google Scholar]
- 13.Loser R. F, Pacione C. A, Chubinskaya S. The combination of insulin like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 14.Delatte M, von den Hoff J. W, Malthaa J. C, Kuijpers-Jagtmana A. M. Growth stimulation of mandibular condyles and femoral heads of newborn rats by IGF-I. Arch Oral Biol. 2004;49:165–175. doi: 10.1016/j.archoralbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Patil A. S, Sable R. B, Kothari R. M. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J Cell Physiol. 2012;227:1796–1804. doi: 10.1002/jcp.22905. [DOI] [PubMed] [Google Scholar]
- 16.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 17.D'Angelo M, Billings P. C, Pacifici M, Leboy P. S, Kirsch T. Authentic matrix vesicles contain active metalloproteases: a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- 18.Haeusler G, Walter I, Helmreich M, Egerbacher M. Localization of matrix metalloproteinases, their tissue inhibitors, and vascular endothelial growth factor (VEGF) in growth plates of children and adolescents indicates a role for MMPs in human postnatal growth and skeletal maturation. Calcif Tissue Int. 2005;76:326–335. doi: 10.1007/s00223-004-0161-6. [DOI] [PubMed] [Google Scholar]
- 19.Johansson N, Saarialho-Kere U, Airola K, et al. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells and osteoblasts during human fetal bone development. Dev Dyn. 1997;208:387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Fowlkes J. L, Enghild J. J, Suzuki K, Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269:25742–25746. [PubMed] [Google Scholar]
- 21.Tuckermann J. P, Pittois K, Partridge N. C, Merregaert J, Angel P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: sequential temporal and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J Bone Miner Res. 2000;15:1257–1265. doi: 10.1359/jbmr.2000.15.7.1257. [DOI] [PubMed] [Google Scholar]
- 22.Yamagiwa H, Tokunaga K, Hayami T, Hatano H, Uchida M, Endo N, Takahashi H. E. Expression of metalloproteinase-13 (collagenase-3) is induced during fracture healing in mice. Bone. 1999;25:197–203. doi: 10.1016/s8756-3282(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 23.Porte D, Tuckermann J, Becker M. Both AP-1 and Cbfa1-like factors are required for the induction of interstitial collagenase by parathyroid hormone. Oncogene. 1999;18:667–678. doi: 10.1038/sj.onc.1202333. [DOI] [PubMed] [Google Scholar]
- 24.Wu C. W, Tchetina E. V, Mwale F, et al. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]



