Abstract
Objective:
To evaluate in vitro the influence of topical fluoride application on the mechanical properties of orthodontic cements containing fluoride under pH cycling conditions.
Materials and Methods:
Edgewise brackets for maxillary central incisors were bonded to 192 bovine incisors using Transbond XT (G1), Transbond Plus Color Change (G2), and Fuji Ortho LC (G3) (n = 64 for each group). The specimens of each group were subdivided (n = 16) into different subgroups. Subgroup A received no topical fluoride application during pH cycling, while the experimental subgroups received topical fluoride treatments as follows: B, application three times per day of fluoride dentifrice (1450 ppm F); C, application one time per day of fluoride mouth rinse (250 ppm F); and D, combination of fluoride dentifrice and fluoride mouth rinse. After 14 days of pH cycling, the shear bond strength and Adhesive Remnant Index were evaluated statistically.
Results:
Polarized light microscopy showed that pH cycling induced mineral loss in all specimens. The topical application of fluoride did not have an influence on shear bond strength, although the association of fluoride dentifrice and mouth rinse increased the shear bond strength of the resinous cement without fluoride (P < .01). Regarding the Adhesive Remnant Index, no statistical differences were found within the groups G1 (P = .23), G2 (P = .47), and G3 (P = .74).
Conclusion:
Topical fluoride treatments improved the shear bond strength of resinous cement, regardless of the material's fluoride-releasing capacity, and reached the adhesive fractures.
Keywords: pH cycling, Topical fluoride application, Composite resins, Shear bond strength, Tooth demineralization
INTRODUCTION
In orthodontic practice, white spot lesions are observed with relative frequency around orthodontic appliances, especially when oral hygiene is poor.1–4 White spot lesions can become noticeable around brackets within 1 month of placement, although the formation of caries usually takes at least 6 months.5 Prevention of demineralization during orthodontic treatment is one of the greatest challenges faced by clinicians, despite modern advances in caries prevention.1
Caries lesions adjacent to brackets can be reduced or even eliminated when fluoride compounds are used.6 However, the use of such compounds depends on patient compliance, which is often inadequate.7 Therefore, preventive measures that do not depend on an individual's compliance have been developed to solve this problem, such as bonding dental materials with fluoride-releasing properties,8,9 which excrete an additional source of fluoride near the brackets.3,10 Fluoride-releasing materials present a “burst effect” pattern of fluoride release, with the largest amount of fluoride being released within the first few days of testing, followed by a rapid decline to much lower levels as a result of the small amount of incorporated fluoride.11 Thus, for more efficient caries control in orthodontic patients, the combined use of fluoride-releasing materials and external sources of fluoride has been recommended.12
Orthodontic glass-ionomer cements present a higher caries-preventive effect12 but low mechanical resistance.13,14 For this reason, resin-based materials, which feature improved mechanical properties, that incorporate fluoride-releasing properties have been developed, although these materials present a less pronounced caries-preventive effect than glass ionomers. Many in vitro studies have highlighted the white spot–preventive effect of fluoride,3,15 although it is important to evaluate the properties of bonding materials under conditions that simulate the oral environment. Thus, we tested two main initial hypotheses: external fluoride sources in conditions that simulate oral environment/pH cycling regimens will (1) increase the caries-preventive effect of orthodontic resins and (2) improve mechanical properties, shear bond strength, and Adhesive Remnant Index (ARI) scores.
MATERIALS AND METHODS
Sample Preparation
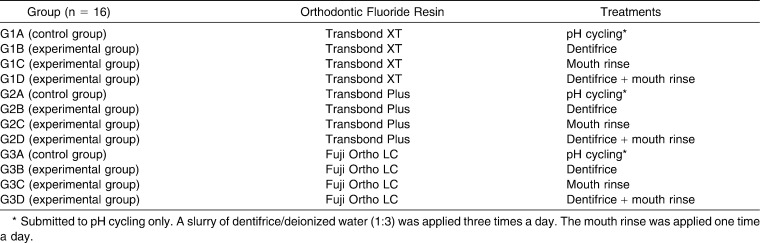
One hundred ninety-two bovine incisors were randomly divided into three groups depending on bonding material (n = 64; Table 1) and sectioned along the cementoenamel junction. Next, the crowns were submerged in acrylic resin, with the buccal surface facing a glass plate. A series of silicon carbide abrasive papers (grit sizes 180, 400, and 600; 3M, Rio de Janeiro, RJ, Brazil) was used to remove excess resin and expose the bonding area. After this, the coronal portion was submitted to prophylaxis with rubber cups (KG Sorensen, Rio de Janeiro, RJ, Brazil) at low speed for 5 seconds. Samples were washed in deionized water for 15 seconds and dried using an oil-free air jet for 15 seconds.
Table 1.
Division of Samples Within the Transbond XT (G1; n = 64), Transbond Plus Color Change (G2; n = 64), and Fuji Ortho LC (G3; n = 64) Groups
Maxillary central incisor brackets (Edgewise System, Morelli, Sorocaba, São Paulo, Brazil) were bonded in the most central area of the middle third of the buccal surface of the bovine incisors with one of three different orthodontic bonding materials (Table 1): Transbond XT (G1, 3M Unitek, Monrovia, Calif), Transbond Plus Color Change (G2, 3M Unitek), or Fuji Ortho LC (G3, GC Corporation, Tokyo, Japan). The materials were used according to the manufacturers' instructions (Table 2). The samples were stored in deionized water at room temperature for one day.
Table 2.
Application Procedures and Lot Numbers of the Tested Materials
pH Cycling Protocol (Cariogenic Challenge)
The samples of each resin group (n = 64) were divided into four treatment groups (n = 16) (Table 1) 24 hours after bonding. During pH cycling, the dentifrice groups (G1B, G2B, G3B) were submitted to fluoride dentifrice slurry (1450 ppm F, Colgate-Palmolive Ind. e Com. Ltda, São Paulo, SP, Brazil). The dentifrice slurry consisted of dentifrice and deionized water (proportion 1∶3) and was applied three times a day. The mouth rinse groups (G1C, G2C, G3C) were submitted to once-daily rinsing for 1 minute with 300 mL of fluoridated mouth rinse (250 ppm F, Oral B, Procter & Gamble do Brasil, São Paulo, SP, Brazil). The concomitant dentifrice/mouth rinse treatment (G1D, G2D, G3D) used the fluoride dentifrice slurry (250 ppm F, Oral B, Procter & Gamble do Brasil) three times per day, and the last application was followed by an application of 300 mL of mouth rinse (250 ppm F, Oral B, Procter & Gamble do Brasil) for 1 minute. The treatments were conducted under mechanical agitation using an orbital shaker (Bio PSU-20i, Grant Instruments, Hillsborough, NJ) during treatment, and each application was followed by washing in deionized water.
The negative control groups (G1A, G2A, and G3A) were submitted to pH cycling only, while the experimental groups alternated between pH cycling and the described fluoride treatments. The pH cycling consisted of artificial remineralizing in neutral saliva (1.54 mmol/L calcium, 1.54 mmol/L phosphate, 20 mmol/L acetic acid, and 0.308 g ammonium acetate, adjusted to pH 7.0 with potassium hydroxide; VETEC, Rio de Janeiro, RJ, Brazil)16 and demineralizing saliva (3 mmol/L calcium, 3 mmol/L phosphate, 50 mL/L acetic acid, and 0.308 g ammonium acetate with the pH adjusted to 4.5 with sodium hydroxide; VETEC).17
To induce a strong cariogenic challenge, the specimens were kept in demineralizing saliva for 22 hours consecutively, and after being washed with deionized water, they were kept in contact with remineralizing saliva for 2 hours to complete a 24-hour cycle. During the pH cycle period, the specimens were kept in an incubator (Fanem Ltda, São Paulo, SP, Brazil), at a constant temperature of 37°C to simulate the oral environment. These dynamics were reproduced for a period of 14 days, during which the artificial saliva (neutral and acid) was changed every 2 days.
After pH cycling, shear bond strength tests, and ARI evaluation, one longitudinal section of approximately 100 µm was selected from each tooth to examine the pattern of demineralization from each group for the visual images. The sections were immersed in water and examined by polarized light microscopy (under 10× magnification); photomicrographs were obtained.18,19
Shear Bond Strength Measurement
Shear bond strength tests were performed in a universal testing machine (EMIC, São José dos Pinhais, SP, Brazil) at a constant speed of 0.5 mm/min. The force required to dislodge the bracket was recorded in Newtons (N) and converted into megapascals (MPa) as a ratio of Newtons to the bracket surface area (MPa = N/mm2).
Adhesive Remnant Index Evaluation
The brackets and enamel surfaces were analyzed by one trained examiner (intraexaminer kappa = 0.92). An optical microscope (Eclipse E600, Nikon, Melville, NY) was used at 4× magnification, with ARI scores given according to Artun and Bergland.20
Statistical Analysis
The results of shear bond strength testing were analyzed with the statistical software program SPSS 16.0 (SPSS Inc, Chicago, Ill) and submitted to analysis of variance and the Tukey test. Evaluation of ARI scores was carried out using the Kruskal-Wallis and Mann-Whitney tests. All statistical tests were performed with a confidence interval of 95% (P < .05).
RESULTS
Cariogenic Challenge Assessment
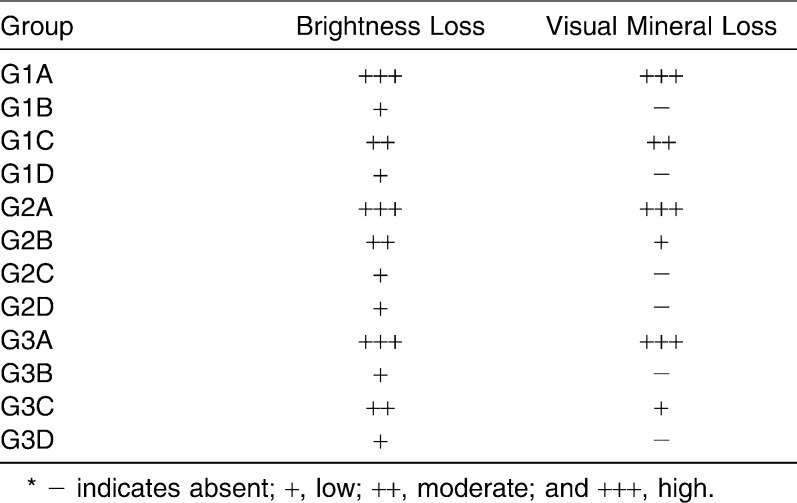
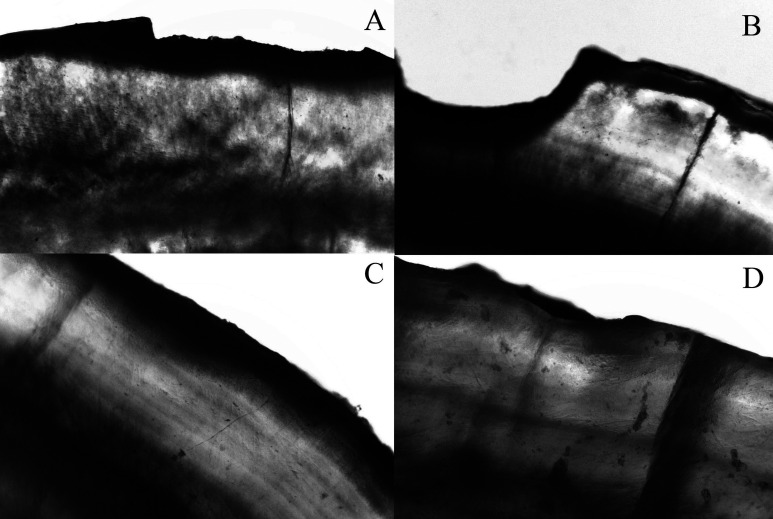
The cariogenic challenge was able to induce white spot lesions around orthodontic brackets (Table 3). Figure 1 shows the patterns of demineralization under polarized light microscopy. The control group showed a microscopic pattern of enamel loss, and the mouth rinse group presented low visual mineral loss. The dentifrice and the dentifrice/mouth rinse groups showed demineralization, but microscopic mineral loss was reduced.
Table 3.
White Spot Characterization*
Figure 1.
Artificial enamel caries formation adjacent to bonding area. Specimens were immersed in water and photographed using polarized light microscopy under 10× magnification. The photomicrography shows representatives images of each group. (A,B) Control group showing enamel loss pattern. (C) Dentifrice/dentifrice and mouth rinse pattern of demineralization with no visual mineral loss. (D) Mouth rinse pattern of demineralization presenting low visual mineral loss.
Shear Bond Strength
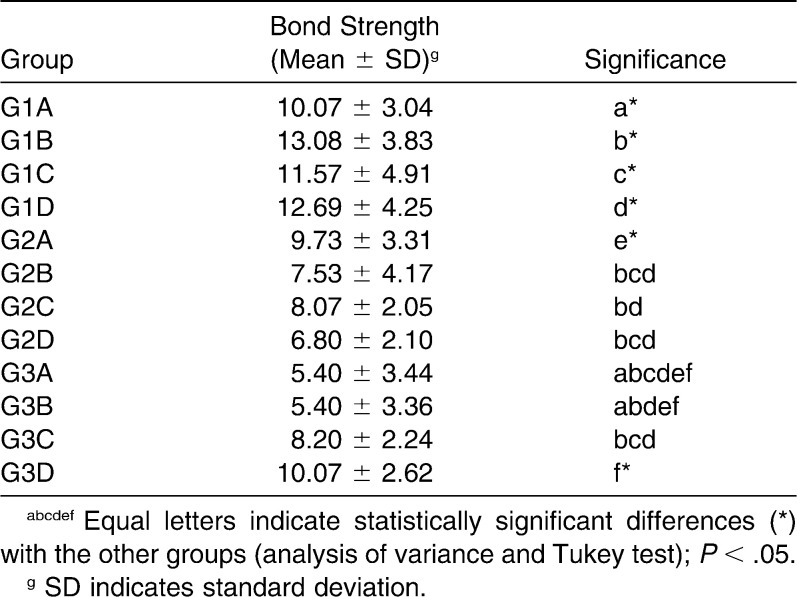
Table 4 summarizes the results of the shear bond strength testing. The results showed that G1 (Transbond XT) presented higher shear bond strength than G2 (Transbond Plus Color Change) and G3 (Fuji Ortho LC) in all evaluated conditions. In the control group (pH cycling only), a statistical difference (P = .03) was found between Transbond XT (G1A; 10.07 ± 3.04 MPa) and Fuji Ortho LC (G3A; 5.40 ± 3.44 MPa); a statistically significant difference was also found between Transbond Plus Color Change (G2A; 9.73 ± 3.31 MPa) and Fuji Ortho LC (P < .01). There was no statistical difference between Transbond XT and Transbond Plus Color Change with the same treatment (P = .99).
Table 4.
Shear Bond Strength Values (MPa) of Different Orthodontic Bond Materials Submitted to High Cariogenic Challenge
When the shear bond strength of samples submitted to the dentifrice protocol was evaluated, Transbond XT presented a statistically significantly higher (P < .01) shear bond strength (G1B; 13.08 ± 3.83 MPa) in comparison to Transbond Plus Color Change (G2B; 7.53 ± 4.17 MPa) and Fuji Ortho LC (G3B; 5.40 ± 3.36 MPa).
The fluoride mouth rinse did not influence the shear bond strength of the materials (P > .05); Transbond XT (G1C; 11.57 ± 4.91 MPa), Transbond Plus Color Change (C2C; 8.07 ± 2.05 MPa), and Fuji Ortho LC (G3C; 8.20 ± 2.24 MPa) showed similar behavior under the applied conditions. However, treatment with fluoride dentifrice and mouth rinse during pH cycling increased the shear bond strength (P = .03) of resinous cement without fluoride (Transbond XT, G1D; 12.69 ± 4.25 MPa) in comparison to Transbond Plus Color Change (G2D; 6.80 ± 2.10 MPa), a resinous material with fluoride-releasing capacity.
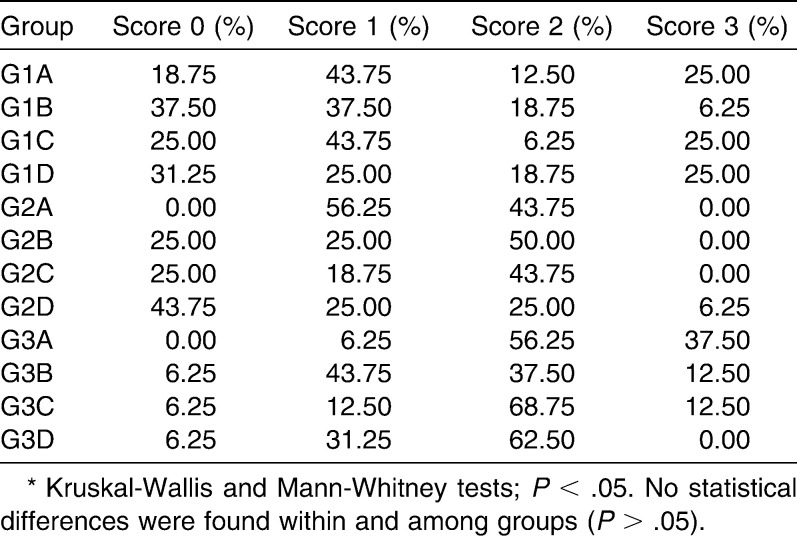
Adhesive Remnant Index
The results of ARI measurement are summarized in Table 5. All GI specimens presented with large amounts of resin adhering to the bracket (score 1), while G2 and G3 presented with greater amounts that had adhered to the enamel (score 2). No statistical difference was found within the groups G1 (P = .23), G2 (P = .47), and G3 (P = .74). Likewise, no statistical differences were found among the different materials (P > .05).
Table 5.
Adhesive Remnant Index (ARI) of Different Orthodontic Bonding Materials and Treatments*
DISCUSSION
The mechanical properties of orthodontic materials are generally overlooked in the literature, and such studies are not usually conducted under a simulated cariogenic challenge.2,21–23 The high prevalence of white spot lesions around orthodontic appliances3 has aroused interest in mechanical property studies that mimic the adverse conditions of the oral environment.12,24 It is important to highlight that high shear bond strength is indispensable in keeping orthodontic brackets adhered to the enamel surface during orthodontic treatment. Consequently, it is essential to evaluate mechanical properties in conditions that simulate the oral environment of patients, which possesses a high susceptibility for the formation of white spot lesions. Thus, a unique feature of the present research is the use of artificial saliva, via demineralizing/remineralizing solutions with different pHs, to simulate the oral environment.
The incorporation of fluoride in dental tissue reduces enamel solubility in acidic environments. This property is based on the capacity of fluoride to be incorporated into a crystalline lattice of the hydroxyapatite of hard dental tissues, resulting in a mineral phase that is less soluble and more acid resistant.25 When the samples were immersed in a solution with a pH that dissolves fluoridated hydroxyapatite, white spot lesions formed around the orthodontic brackets bonded with Transbond XT; fewer lesions were seen around brackets adhered with Transbond Plus Color Change and Fuji Ortho LC owing to the presence of fluoride in these compounds. Nevertheless, the influence of the dynamic of fluoride on the shear bond strength of orthodontic resins under cariogenic challenge is not well known.
In the present study, all evaluated materials presented with satisfactory bond strength. The literature reports that the ideal bond strength to resist orthodontic forces varies from 2.86 to 7.59 MPa.26 All bonding materials, when submitted to cariogenic challenge, presented shear bond strength values capable of resisting orthodontic forces, varying from 5.40 to 13.08 MPa. Transbond XT presented higher shear bond strength in comparison to Transbond Plus Color Change and Fuji Ortho LC under all conditions studied. In the groups submitted to fluoride dentifrice, Transbond XT showed statistically significantly higher shear bond strength in comparison to Transbond Plus Color Change and Fuji Ortho LC. However, it is possible that this is an inherent characteristic of these materials and that the treatment did not influence the results, since the same behavior was noted during cariogenic challenge. With respect to the control groups, evaluating only material properties, it is possible to conclude that Transbond XT presented shear bond strength that was similar to that of Transbond Plus Color Change (P > .05). Fuji Ortho LC presented with lower shear bond strength values vs the resinous materials; this can be explained by the reduced shear bond strength of glass-ionomer cements.13,14 Regarding the treatments, Transbond XT, a resinous material without fluoride, showed increased bond strength (although not statistically significant) following the application of fluoride dentifrice and mouth rinse during pH cycling. It is suggested that remineralization in the presence of an external source of fluoride produces fluoride hydroxyapatite that is more resistant than hydroxyapatite. For this reason this group showed the highest increase in shear bond strength values in comparison with the orthodontic cements containing fluoride.
The present study corroborates the findings of Passalini et al.,12,24 who found that orthodontic bonding materials containing fluoride were able to prevent enamel demineralization under cariogenic challenge without interfering with bond strength. However, the literature shows that fluoride-releasing materials present a “burst effect” pattern of fluoride release, with the largest amount of fluoride being released within the first few days of testing, followed by a rapid decline to much lower levels.11 For this reason, in the current study, only external sources of fluoride were evaluated.
Maintenance of an unblemished enamel surface after removal of brackets is required after orthodontic treatment. Bracket failure at the bracket/adhesive interface presents advantages, since it leaves the enamel surface relatively intact. In the current study, Transbond XT presented higher shear bond strength values. In addition, Transbond XT also presented higher percentages of ARI scores of 0 in comparison to Transbond Plus Color Change and Fuji Ortho LC under all conditions evaluated. For all bonding materials evaluated, the specimens submitted to both fluoride treatments showed increased ARI scores of 0 when compared to the specimens that were submitted to pH cycling only.
It is worth emphasizing that, despite the cariogenic challenge, all bonding materials reached an ARI score of 0 in most specimens of all bonding materials, showing that the adverse oral conditions interfered negatively with the adhesive quality of the material. In addition, the fluoride treatment was not able to reduce the number of samples with a score of 0. When brackets fail at the enamel/adhesive interface, less residual adhesive remains, but the enamel surface may be damaged if cohesive fracture occurs.27
Based on the results of the present study, it is possible to conclude that, with consideration to the high cariogenic challenge endured by the specimens, all tested topical treatments were not able to prevent mineral loss, but they were able to reduce it. In addition, the bonding material should be chosen for specific patient conditions. In normal cases, Transbond XT should be used because of its high shear bond strength. On the other hand, in patients with high susceptibility to white spots, fluoride-containing bonding materials should be used, even though they have a lower shear bond strength.
CONCLUSIONS
Based on the methods applied in the present study, it was shown that:
Transbond XT presented with better shear bond strength but a poorer ARI in comparison to Transbond Plus Color Change and Fuji Ortho LC.
Fluoride treatments did not influence shear bond strength but reached the fractures at the enamel/adhesive interface.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeir (FAPERJ) for financial support.
REFERENCES
- 1.Tufekci E, Dixon J. S, Gunsolley J. C, Lindauer S. J. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81(2):206–210. doi: 10.2319/051710-262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989;96(5):423–427. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 3.Benson P. E, Shah A. A, Millett D. T, Dyer F, Parkin N, Vine R. S. Fluorides, orthodontics and demineralization: a systematic review. J Orthod. 2005;32(2):102–114. doi: 10.1179/146531205225021033. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick L, Geiger A. M, Gwinnett A. J. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982 Feb;81(2):93–98. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 5.Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94(1):68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 6.Ogaard B, Rolla G, Arends J, ten Cate J. M. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am J Orthod Dentofacial Orthop. 1988;94(2):123–128. doi: 10.1016/0889-5406(88)90360-5. [DOI] [PubMed] [Google Scholar]
- 7.Geiger A. M, Gorelick L, Gwinnett A. J, Benson B. J. Reducing white spot lesions in orthodontic populations with fluoride rinsing. Am J Orthod Dentofacial Orthop. 1992;101(5):403–407. doi: 10.1016/0889-5406(92)70112-N. [DOI] [PubMed] [Google Scholar]
- 8.Cook P. A. Direct bonding with glass ionomer cement. J Clin Orthod. 1990;24(8):509–511. [PubMed] [Google Scholar]
- 9.Pascotto R. C, Navarro M. F, Capelozza Filho L, Cury J. A. In vivo effect of a resin-modified glass ionomer cement on enamel demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2004 Jan;125(1):36–41. doi: 10.1016/s0889-5406(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 10.Gorton J, Featherstone J. D. In vivo inhibition of demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2003 Jan;123(1):10–14. doi: 10.1067/mod.2003.47. [DOI] [PubMed] [Google Scholar]
- 11.Cohen W. J, Wiltshire W. A, Dawes C, Lavelle C. L. Long-term in vitro fluoride release and rerelease from orthodontic bonding materials containing fluoride. Am J Orthod Dentofacial Orthop. 2003 Nov;124(5):571–576. doi: 10.1016/s0889-5406(03)00573-0. [DOI] [PubMed] [Google Scholar]
- 12.Passalini P, Fidalgo T. K, Caldeira E. M, Gleiser R, Nojima Mda C, Maia L. C. Preventive effect of fluoridated orthodontic resins subjected to high cariogenic challenges. Braz Dent J. 2010;21(3):211–215. doi: 10.1590/s0103-64402010000300006. [DOI] [PubMed] [Google Scholar]
- 13.Pithon M. M, de Oliveira Ruellas A. C, Sant'Anna E. F, de Oliveira M. V, Alves Bernardes L. A. Shear bond strength of brackets bonded to enamel with a self-etching primer. Effects of increasing storage time after activation. Angle Orthod. 2009 Jan;79(1):133–137. doi: 10.2319/100207-472.1. [DOI] [PubMed] [Google Scholar]
- 14.Pithon M. M, Dos Santos R. L, de Oliveira M. V, Ruellas A. C, Romano F. L. Metallic brackets bonded with resin-reinforced glass ionomer cements under different enamel conditions. Angle Orthod. 2006 Jul;76(4):700–704. doi: 10.1043/0003-3219(2006)076[0700:MBBWRG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Benson P. E, Parkin N, Millett D. T, Dyer F. E, Vine S, Shah A. Fluorides for the prevention of white spots on teeth during fixed brace treatment. Cochrane Database Syst Rev. 2004;(3):CD003809. doi: 10.1002/14651858.CD003809.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Lammers P. C, Borggreven J. M, Driessens F. C. Acid-susceptibility of lesions in bovine enamel after remineralization at different pH values and in the presence of different fluoride concentrations. J Dent Res. 1991 Dec;70(12):1486–1490. doi: 10.1177/00220345910700120301. [DOI] [PubMed] [Google Scholar]
- 17.Damato F. A, Strang R, Stephen K. W. Effect of fluoride concentration on remineralization of carious enamel: an in vitro pH-cycling study. Caries Res. 1990;24(3):174–180. doi: 10.1159/000261262. [DOI] [PubMed] [Google Scholar]
- 18.Hicks M. J, Flaitz C. M, Garcia-Godoy F. Fluoride-releasing sealant and caries-like enamel lesion formation in vitro. J Clin Pediatr Dent. 2000 Spring;24(3):215–219. [PubMed] [Google Scholar]
- 19.Cain K, Hicks J, English J, Flaitz C, Powers J. M, Rives T. In vitro enamel caries formation and orthodontic bonding agents. Am J Dent. 2006 Jun;19(3):187–192. [PubMed] [Google Scholar]
- 20.Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984 Apr;85(4):333–340. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 21.Lowder P. D, Foley T, Banting D. W. Bond strength of 4 orthodontic adhesives used with a caries-protective resin sealant. Am J Orthod Dentofacial Orthop. 2008 Aug;134(2):291–295. doi: 10.1016/j.ajodo.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Fidalgo T. K. S, Pithon M. M, Maciel J. V. B, Bolognese A. M. Friction between different wire bracket combinations in artificial saliva – an in vitro evaluation. J Appl Oral Sci. 2011;19(1):57–62. doi: 10.1590/S1678-77572011000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pithon M. M, dos Santos R. L. Does ozone water affect the bond strengths of orthodontic brackets. Aust Orthod J. 2010 May;26(1):73–77. [PubMed] [Google Scholar]
- 24.Passalini P, Fidalgo T. K, Caldeira E. M, Gleiser R, Nojima Mda C, Maia L. C. Mechanical properties of one and two-step fluoridated orthodontic resins submitted to different pH cycling regimes. Braz Oral Res. 2010 Jun;24(2):197–203. doi: 10.1590/s1806-83242010000200012. [DOI] [PubMed] [Google Scholar]
- 25.Sudjalim T. R, Woods M. G, Manton D. J, Reynolds E. C. Prevention of demineralization around orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop. 2007 Jun;131(6):705.e1–9. doi: 10.1016/j.ajodo.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Keizer S, ten Cate J. M, Arends J. Direct bonding of orthodontic brackets. Am J Orthod. 1976 Mar;69(3):318–327. doi: 10.1016/0002-9416(76)90079-8. [DOI] [PubMed] [Google Scholar]
- 27.Bishara S. E, Ostby A. W, Laffoon J. F, Warren J. Shear bond strength comparison of two adhesive systems following thermocycling. A new self-etch primer and a resin-modified glass ionomer. Angle Orthod. 2007 Mar;77(2):337–341. doi: 10.2319/0003-3219(2007)077[0337:SBSCOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]