Abstract
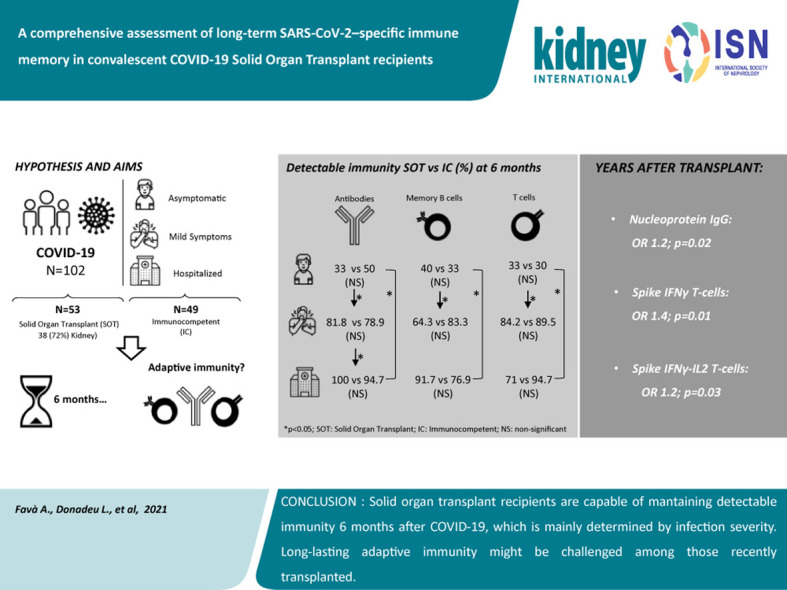
Long-term adaptive immune memory has been reported among immunocompetent individuals up to eight months following SARS-CoV-2 infection. However, limited data is available in convalescent patients with a solid organ transplant. To investigate this, we performed a thorough evaluation of adaptive immune memory at different compartments (serological, memory B cells and cytokine [IFN-γ, IL-2, IFN-γ/IL12 and IL-21] producing T cells) specific to SARS-CoV-2 by ELISA and FluoroSpot-based assays in 102 convalescent patients (53 with a solid organ transplants (38 kidney, 5 liver, 5 lung and 5 heart transplant) and 49 immunocompetent controls) with different clinical COVID-19 severity (severe, mild and asymptomatic) beyond six months after infection. While similar detectable memory responses at different immune compartments were detected between those with a solid organ transplant and immunocompetent individuals, these responses were predominantly driven by distinct COVID-19 clinical severities (97.6%, 80.5% and 42.1%, all significantly different, were seropositive; 84% vs 75% vs 35.7%, all significantly different, showed IgG-producing memory B cells and 82.5%, 86.9% and 31.6%, displayed IFN-γ producing T cells; in severe, mild and asymptomatic convalescent patients, respectively). Notably, patients with a solid organ transplant with longer time after transplantation did more likely show detectable long-lasting immune memory, regardless of COVID-19 severity. Thus, our study shows that patients with a solid organ transplant are capable of maintaining long-lasting peripheral immune memory after COVID-19 infection; mainly determined by the degree of infection severity.
Keywords: adaptive immunity, COVID-19 infection, solid organ transplantation
Graphical abstract
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has challenged global health in an unprecedented manner, resulting in a widespread morbidity and mortality. Even though most people develop mild symptoms or remain asymptomatic after SARS-CoV-2 infection,1 , 2 some patients develop a severe respiratory syndrome that associates with an excessive systemic inflammatory process, ultimately leading to respiratory failure and death.3 , 4 Notably, some specific group of patients seem to be at significantly higher risk of fatal outcomes such as recipients of solid organ transplants (SOT), most likely due to their chronic immunosuppressive therapy that broadly targets adaptive T-cell immunity.5 , 6
Recent important studies have shown that during acute COVID-19 and early convalescence, infected patients develop robust adaptive immune responses by means of high SARS-CoV-2–specific IgG antibody titers and T-cell frequencies, both CD4 and CD8 T cells, in peripheral blood.7 Remarkably, the strength of these adaptive immune responses seems to also vary according to distinct COVID-19 disease severity,8, 9, 10, 11, 12 thus, suggesting a key role of SARS-CoV-2–specific immunity controlling and limiting primary viral replication.13, 14, 15, 16 On the other hand, a long-lasting protective immunity, both serological and cellular, has also been reported among convalescent COVID-19 patients from the general population between 5 and 8 months after infection.17
In the setting of SOT, however, scarce information has been reported regarding the degree, durability, and biological interplay between different adaptive immune mechanisms in response to SARS-CoV-2. In this regard, our group recently showed that SOT patients developing a moderate or severe COVID-19 infection are able to generate, albeit with a notable initial delay, similarly strong SARS-CoV-2–specific serological and T-cell immune responses during early convalescence as compared with immunocompetent (IC) patients with the similar severe infection.18 , 19 Nevertheless, unlike SARS-CoV-2 convalescent immunity, weak adaptive immune responses have been reported in SOT recipients after 2 doses of messenger RNA-based vaccination.20 , 21 Importantly, understanding whether memory immune responses specific to SARS-CoV-2 last for long convalescent periods and how the serological and B and T cellular compartments behave over time is key to establish guided preventing strategies among this high-risk patient population.
Herein, we performed a thorough evaluation of both serological and functional T- and B-cell immune memory against main immunogenic SARS-CoV-2 antigens using functional cell-based immune assays, in a cross-sectional cohort of 102 convalescent SOT (n = 53) and IC healthy individuals (n = 49) with distinct disease severity, beyond 6 months after COVID-19 infection.
Methods
Patients of the study and clinical definitions
One-hundred and two COVID-19 convalescent patients from different European transplant centers were evaluated in this study (Bellvitge University Hospital [N = 67]; Vall d’Hebron University Hospital [N = 13]; Montpellier University Hospital [N = 4]; Fundació Puig-Vert [N = 3]; Lyon University Hospital [N = 2]; Hospital del Mar [N = 2]; Hospital Clinic [N = 1]) and a general medical assistance center (N = 10). A total of 53 SOT recipients (38 kidney, 5 liver, 5 lung, and 5 heart transplants) and 49 IC healthy individuals, in whom peripheral blood mononuclear cells and serum samples could be obtained, with a median follow-up after COVID-19 infection beyond 6 months (199 days; interquartile range [IQR], 170-215), were included in this study. In addition, 35 subjects (21 SOT and 14 IC) having developed a severe COVID-19 were compared with their 1-month postinfection immune memory status. None of the participants had been vaccinated, before or during the study follow-up.
All patients had been tested positive for SARS-CoV-2 infection by a real-time reverse transcription–polymerase chain reaction analysis on nasopharyngeal swab samples and diagnosed for COVID-19 between March and October 2020. Samples from 16 prepandemic uninfected individuals were used as negative controls for T-cell assays, as described elsewhere.18 Additional 10 historic biological samples were employed as controls for the B-cell functional assays.
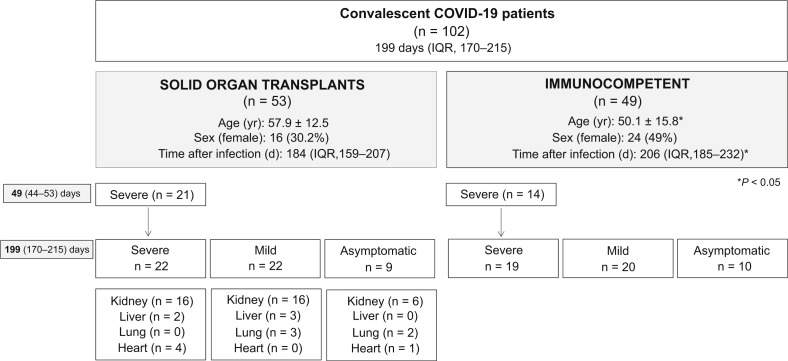
As shown in the flowchart of the study (Figure 1 ), both SOT recipients and IC patients included in the study were classified according to 3 distinct COVID-19 clinical presentations: 41 had been hospitalized for a severe COVID-19 (SEV) requiring oxygen supply (22 SOT and 19 IC), 42 presented with mild symptoms (MILD) and were not hospitalized (22 SOT, 20 IC), and 19 were asymptomatic (ASYMP) and found positive for SARS-CoV-2 by a real-time reverse transcription–polymerase chain reaction on nasopharyngeal swab in routine screening or contact tracking tests (9 SOT and 10 IC). Main clinical, demographic, and immunologic patient characteristics were recorded.
Figure 1.
Flowchart of the study. ∗P < 0.05 (χ2 test and t test). COVID-19, coronavirus disease 2019; IQR, interquartile range.
The study was approved by the ethical review boards (PR115/20) at each center, and patients were recruited in the study after providing a signed informed consent.
Collection and management of serum and peripheral blood mononuclear cell samples
Detailed description is depicted in the Supplementary Methods.
Assessment of SARS-CoV-2–specific humoral immunity
SARS-CoV-2–specific serological memory
Serum IgG antibodies were assessed against 2 main SARS-CoV-2 antigens: the nucleoprotein and spike glycoprotein in 101 of 102 (99%) study patients using 2 distinct enzyme-linked immunosorbent assay platforms. Detailed information of the methodology and interpretation is provided as Supplementary Material.
SARS-CoV-2–specific IgG-producing memory B cells
Circulating SARS-CoV-2–specific IgG-producing memory B-cell (mBC) frequencies was assessed against the receptor binding domain of SARS-CoV-2 spike protein in 71 of 102 (69.6%) individuals of the study using a colorimetric B-cell enzyme-linked immunosorbent spot assay. A thorough description of the method and interpretation (Supplementary Figure S1) of this assay is depicted in the Supplementary Material.
SARS-CoV-2–reactive cytokine-producing memory T cells
Circulating SARS-CoV-2–reactive cytokine-producing memory T-cell frequencies could be assessed in 97 of 101 (95.1%) patients of the study using a multicolor FluoroSpot Immune assay (AID Gmbh), in which 4 distinct cytokine-producing T-cell frequencies were simultaneously assessed: effector (interferon-γ [IFN-γ]), proliferative (interleukin-2 [IL-2]), central (IFN-γ/IL-2) T helper cell 1 and IL-21 T helper cell 1 memory responses. These responses were evaluated against the 4 main structural SARS-CoV-2 proteins: spike glycoprotein (S), membrane protein (M), nucleoprotein (N), and envelope small membrane protein (E) (JPT). A strong positive correlation of T-cell immune responses between all viral antigens was observed but for antigen E, which were barely detectable (Supplementary Table S1). Thus, all the analyses were focused against antigens S, M, and N. Global SARS-CoV-2–reactive T-cell immune responses were calculated by means of the median T-cell frequencies against the 3 main immunogenic antigens (S, M, and N) in each patient. Furthermore, because a hierarchical T-cell immune response was observed and was predominantly driven by IFN-γ-producing T cells against antigen S (Supplementary Figure S2), the qualitative assessment of T-cell immune memory was based on this response. A detailed description of the methodology and interpretation (Supplementary Figure S3) is provided as Supplementary Material.
Statistics
Continuous variables were expressed as mean ± SD or median and IQR, and categorical variables as number of total (n) and percentage (%). A comparison between groups was performed using Pearson’s χ2 test for categorical data. Continuous measurements were compared among groups using the Kruskal-Wallis and Mann-Whitney U test for non-normally distributed data, whereas analysis of variance and t tests were used when data were normally distributed. P values of <0.05 were considered statistically significant. Univariate logistic regression models were used to investigate the influence of clinical covariates (age, gender, symptomatic/asymptomatic infection, and years after transplant) by means of odds ratio (OR) with 95% confidence interval (CI) for humoral and cellular responses. Those covariates that were associated with a P value of <0.1 were introduced into a multivariate binary logistic regression model. SARS-CoV-2–reactive cellular and humoral responses were centered and scaled, and a heatmap was built by means of the pheatmap R package 16 using Euclidean distance and complete method as agglomeration method. R package version 1.0.12 was used (https://CRAN.R-project.org/package=pheatmap). All other analyses were performed using SPSS version 26 software, and graphs were generated using GraphPad Prism version 8.0 software (Graphpad Software).
Results
Patients of the study
As shown in the study flowchart (Figure 1) and Table 1 , 102 COVID-19 convalescent patients after a median time of 199 days (IQR, 170–215 days) after infection were investigated (53 SOT and 49 IC). SOT had a median time after transplantation of 5 years (IQR, 1–12 years), and most of them were receiving a calcineurin-inhibitor-based immunosuppression (83%). All patients were classified and matched according to the clinical severity of COVID-19 infection: 41 SEV (22 SOT, 19 IC), 42 MILD (22 SOT, 20 IC), and 19 ASYM (9 SOT, 10 IC).
Table 1.
Demographic and clinical characteristics of patients infected by SARS-CoV-2
| COVID-19 patients (n = 102) | Severe (n = 41) |
Mild (n = 42) |
Asymptomatic (n = 19) |
P value | |||
|---|---|---|---|---|---|---|---|
| SOT (n = 22) | IC (n = 19) | SOT (n = 22) | IC (n = 20) | SOT (n = 9) | IC (n = 10) | ||
| Age, yr, mean ± SD | 56.7 ± 13.7 | 60.4 ± 9.2 | 60.6 ± 9.6 | 35.2 ± 10.6a | 54.3 ± 15.3 | 60.5 ± 8.9 | <0.001 |
| Sex (female), n (%) | 4 (18.2) | 7 (36.8) | 8 (40) | 11 (55) | 4 (44.4) | 6 (60) | 0.145 |
| Time after infection, d, median (IQR) | 196 (181–213) | 201 (185–206) | 177 (132–203) | 231a (213–252) | 161 (121–168) | 163 (139–185) | <0.001 |
| Transplant organ, n (%) | |||||||
| Kidney | 16 (72.7) | NA | 16 (80) | NA | 6 (66.7) | NA | |
| Liver | 2 (10) | NA | 3 (15) | NA | 0 | NA | 0.161 |
| Heart | 0 (0) | NA | 3 (15) | NA | 2 (22.2) | NA | |
| Lung | 4 (18.2) | NA | 0 | NA | 1 (11.1) | NA | |
| Type of immunosuppression | |||||||
| Calcineurin inhibitors | 16 (72.7) | NA | 20 (100) | NA | 8 (88.9) | NA | 0.241 |
| Mycophenolate mofetil | 21 (94.5) | NA | 19 (95) | NA | 6 (66.7) | NA | 0.099 |
| mTor inhibitors | 4 (18.2) | NA | 2 (10) | NA | 2 (22.2) | NA | 0.566 |
| Steroids | 18 (81.8) | NA | 17 (85) | NA | 9 (100) | NA | 0.304 |
| Time after transplant, yr, mean ± SD | 8.05 ± 7.45 | NA | 5.82 ± 6.79 | NA | 7.56 ± 7.09 | NA | 0.571 |
COVID-19, coronavirus disease 2019; IC, immunocompetent; IQR, interquartile range; mTOR, mammalian target of rapamycin; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant.
Statistical differences were only observed between mild SOT and IC patients.
In general, IC patients were slightly younger (50.1 ± 15.8 vs. 57.9 ± 12.5 mean age, P = 0.017) and their convalescence period was longer (206 [185–232] vs. 184 [159–207] median days, P = 0.005) than SOT (Figure 1), and these differences were mainly driven by the MILD IC group, which was composed of health care workers (Table 1). Among the remaining groups, IC and SOT were matched for age and time after infection. There were no differences regarding the type of immunosuppression, SOT, or time after transplantation between the 3 distinct clinical groups.
None of the included patients was diagnosed of transplant rejection during the acute SARS-CoV-2 infection or the follow-up, but 1 kidney transplant individual who presented a subclinical antibody-mediated rejection in a 12-month protocol biopsy.
Disease severity but not patient condition drives long-lasting immune memory
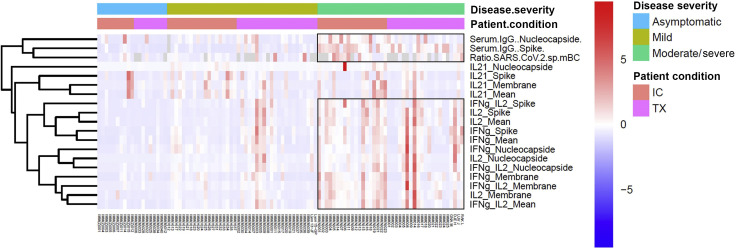
As illustrated in an unsupervised heatmap in Figure 2 , SARS-CoV-2–specific immune memory responses, both at the serological and functional B- and T-cell compartments, were predominantly explained by the clinical severity of COVID-19 infection rather than by the patient condition, either SOT or IC. As shown, all differences were fundamentally driven by the severe clinical groups but not for IL-21-producing T cells, which did not significantly differ across different clinical severities (Supplementary Table S2).
Figure 2.
Heatmaps generated by hierarchical clustering of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific immune responses for solid organ transplant (SOT) and immunocompetent (IC) patients, according to the coronavirus disease 2019 (COVID-19) disease severity (moderate/severe, mild, or asymptomatic). Immune responses used for clustering were differentially expressed (fold change >2, false discovery rate P < 0.05). Gray fields indicate missing values. IFN, interferon; IL, interleukin.
Long-term SARS-CoV-2–specific humoral memory serological memory
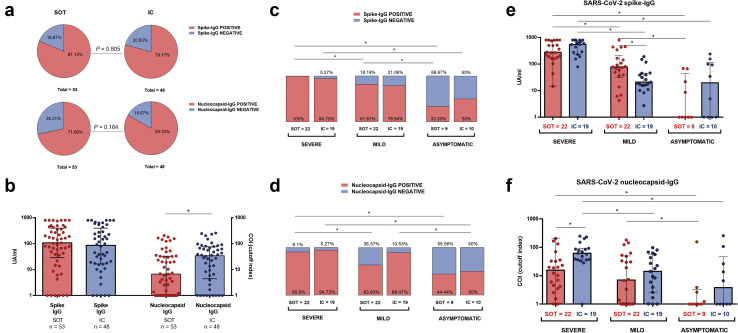
Beyond 6 months after infection, 81 of 101 (80.2%) and 78 of 101 (77.2%) patients showed detectable SARS-CoV-2 IgG antibody levels against antigens spike (S) and nucleoprotein (N), respectively. As illustrated in Figure 3 a and b, there were no differences regarding both seroconversion rates (81.13% vs. 79.17%; P = 0.805) and IgG titters (108 [28.85–396.5] vs. 85.8 [16.5–398.5] UA/ml; P = 0.58) against antigen S between SOT and IC, respectively. Conversely, although similar seroconversion against antigen N was observed between SOT and IC, N-specific IgG titers were lower among SOT patients (6.7 [0.67–33] vs. 34.3 [4.43–75.63] UA/ml; P = 0.027).
Figure 3.
IgG antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike and nucleocapsid proteins. (a) Proportion of solid organ transplants (SOT) and immunocompetent (IC) individuals with detectable IgG antibodies. (b) IgG antibody titters against antigens Spike and nucleoprotein among SOT and IC; ∗P < 0.05. (c,d) Seropositive proportion of patients for spike (c) and nucleoprotein (d), according to infection severity at the onset. In columns, immunosuppression status for every cluster of severity. (e,f) IgG-spike (e) and IgG-nucleoprotein (f) titters according to severity and immunosuppression group; ∗P < 0.05. Detailed data on antibody titters are available in Supplementary Table S3.
A clear seroconversion gradation according to the 3 distinct clinical severities was observed, regardless of patient condition, either SOT or IC (40 of 41 [97.56%] vs. 33 of 41 [80.48%] vs. 8 of 19 [42.1%]; P < 0.001; and 38 of 41 [92.68%] vs. 31 of 41 [75.6%] vs. 9 of 19 [47.36%]; P < 0.001) for SEV, MILD, and ASYMP against antigens S and N, respectively (Figure 3c and d; Supplementary Table S3). The same observation was found for IgG titers (435 [189–775.5] vs. 39.4 [15.85–113] vs. 4.94 [0–68]; P < 0.001; and 35.7 [8.63–81.25] vs. 9.39 [0.89–50.6] vs. 0.08 [0.08–13.7]; P < 0.001) in SEV, MILD, and ASYMP patients against antigens S and N, respectively (Figure 3e and f).
Nonetheless, despite that higher IgG titers against antigen S were observed among MILD-SOT than MILD-IC patients (76.7 [30.4–209.8] vs. 20.9 [15.5–45.2] UA/ml; P = 0.034), most likely due to the later time of analysis of MILD-IC subjects (Table 1), SEV-IC patients displayed numerically higher IgG titers against antigen N than SEV-SOT patients (61.8 [36.2–92.1] vs. 15.7 [4–33.4] UA/ml; P < 0.001).
B-cell memory
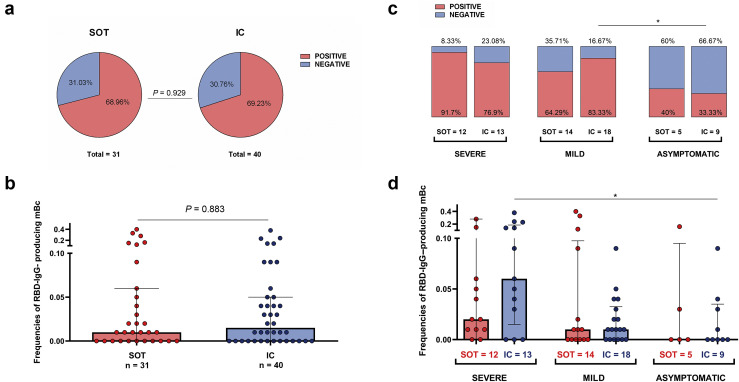
A total of 49 of 71 (69%) patients displayed detectable circulating receptor binding domain of SARS-CoV-2 spike–specific IgG-producing mBC, with similar proportion (22 of 31 vs. 28 of 40; P = 0.929) and frequencies (0.0134 [0–0.0557] vs. 0.0116 [0–0.054]; P = 0.883) between SOT and IC patients, respectively (Figure 4 a and b). Likewise to serology, detection of mBC was highly influenced by the 3 different clinical presentations (84% vs. 75% vs. 35.7%; P = 0.004, in SEV, MILD, and ASYMP, respectively), regardless of patient condition (Figure 4c and d; Supplementary Table S4). Even though no statistical differences were observed regarding IgG-producing mBC frequencies between groups, SEV patients showed numerically higher frequencies, this difference being especially evident between SEV-IC versus ASYMP-IC patients (0.059 [0.013–0.189] vs. 0 [0–0.031]; P = 0.024) (Figure 4d).
Figure 4.
Frequencies of receptor binding domain (RBD)–specific IgG-producing memory B cells (mBCs). (a) Proportion of solid organ transplant (SOT) and immunocompetent (IC) individuals with detectable RBD-IgG–producing mBCs. (b) Frequencies of RBD-IgG–producing mBCs between SOT and IC. (c) Proportion of individuals with detectable RBD-IgG–producing mBCs according to infection severity at the onset. In columns, immunosuppression status for each severity group. (d) Frequencies of RBD-IgG–producing mBCs according to severity and immunosuppression group; ∗P < 0.05. Detailed data on ratio of RBD-IgG–producing mBC are provided in Supplementary Table S4.
Long-term SARS-CoV-2–specific T-cell memory
Overall, there were no differences regarding the proportion of patients with detectable SARS-CoV-2–reactive T cells or their frequencies for any of the evaluated cytokine-producing T cells between SOT and IC patients against the different viral antigens (Supplementary Figures S4 and S5; Supplementary Table S5).
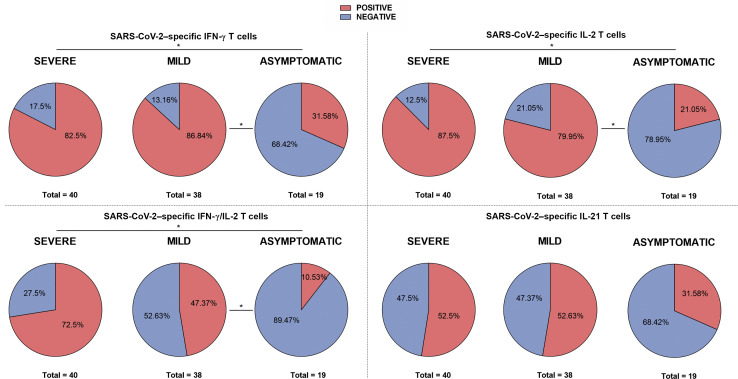
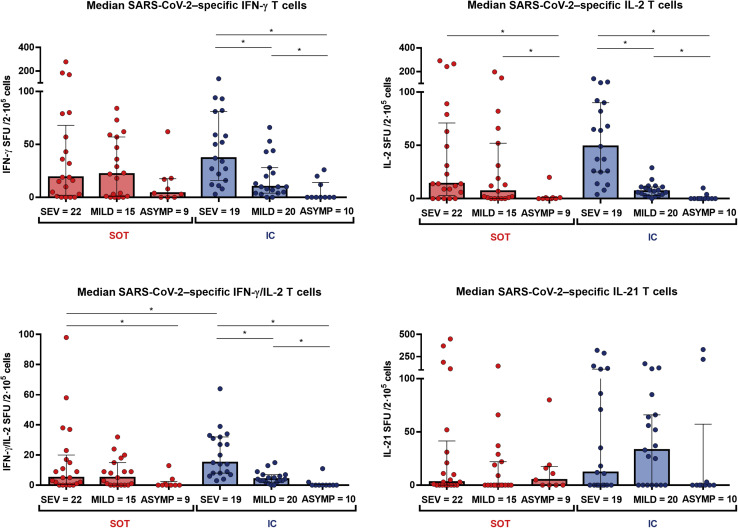
A hierarchical T-cell immune response was observed that was mainly dominated by antigen S (Supplementary Figure S2; Supplementary Table S6). Similar to humoral immunity, the proportion of T-cell responders significantly decreased along with the different clinical presentations (Figure 5 ), and these differences were independent of the patient condition (Supplementary Figure S6). As described in Figure 6 , a clear decrease in all SARS-CoV-2–reactive cytokine-producing T-cell frequencies but not for IL-21-producing T cells was observed in line with the less severe clinical presentation. Of note, a less pronounced SARS-CoV-2–specific T-cell gradient was observed among SOT as compared with IC patients, especially between severe and mild convalescent COVID-19 patients.
Figure 5.
Proportion of patients with detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–reactive cytokine-producing T-cell responses according to infection severity. Interferon-γ (IFN-γ), interleukin-2 (IL-2), IFN-γ/IL-2, and IL-21 were assessed. ∗P < 0.05.
Figure 6.
Global T-cell responses specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; median T-cell frequencies against the 3 SARS-CoV-2 immunogenic antigens: S, M, and N). Significant intra- and intergroup differences (solid organ transplant [SOT] severe symptoms [SEV], SOT mild symptoms [MILD], SOT asymptomatic [ASYMP], immunocompetent [IC] SEV, IC MILD, and IC ASYMP) are shown; ∗P < 0.05. IFN-γ, interferon-γ; IL-2, interleukin-2; SFU, spot forming unit.
Relationship between serological and cellular SARS-CoV-2–specific immunity
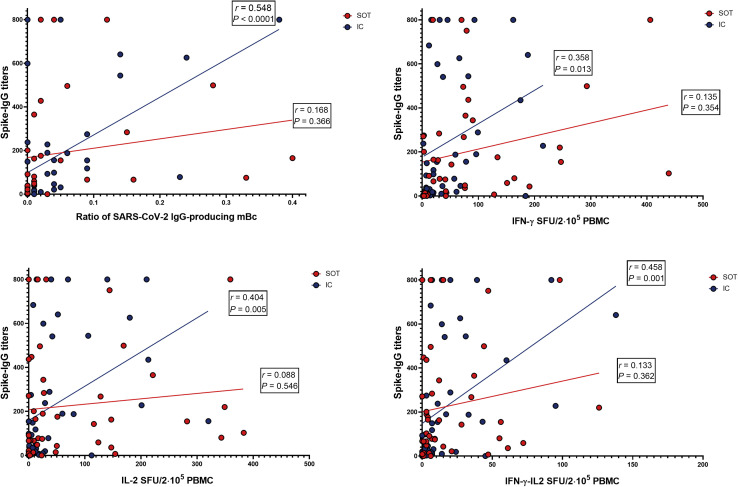
A significant positive correlation between serum IgG titers and frequencies of IgG- and cytokine-producing memory B and T cells, respectively, against protein S was observed, which was more robustly observed within the IC group (Figure 7 ). Conversely, no correlation was found between serologic and cellular responses against protein N and between frequencies of IgG-producing mBC and cytokine-producing T cells.
Figure 7.
Correlations between serologic and cellular immune compartments against (spike) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen. IgG titters against antigen S and circulating (receptor binding domain [RBD]–spike)-specific memory B cell (mBc) frequencies exhibited a significant positive correlation (r = 0.355, P = 0.003), which was fundamentally driven by immunocompetent (IC) subjects (r = 0.548, P < 0.001). A similar pattern was observed between spike-specific IgG titers and the different (spike)SARS-CoV-2–reactive cytokine-producing T-cell frequencies but for IL-21 (data not shown) was mainly observed within IC individuals (interferon-γ [IFN-γ]: r = 0.358, P = 0.013; interleukin-2 [IL-2]: r = 0.404, P = 0.005; and IFN-γ/IL-2: r = 0.458, P = 0.001). PBMC, peripheral blood mononuclear cell; SFU, spot forming unit; SOT, solid organ transplant.
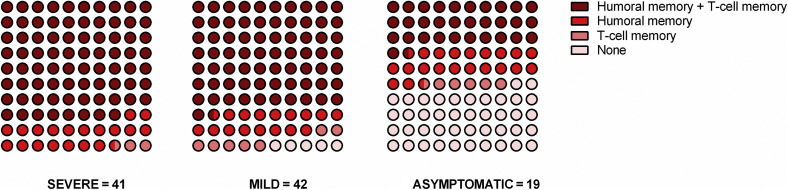
Next, we compared all memory immune compartments in each individual according to the different clinical presentations in all patients in whom the humoral (either mBC or antibodies) and cellular immune responses could be investigated (97 of 102 [95.1%] patients; 40 SEV, 38 MILD, and 19 ASYMP). As shown in Figure 8 , although all SEV patients showed some detectable SARS-CoV-2–specific immune memory, 5.3% (2 of 38) of MILD and up to 42.1% (8 of 19) of ASYMP patients did not show detectable antiviral immunity in any of the 3 immune compartments (P < 0.001). No differences were found between SOT and IC (data not shown).
Figure 8.
Dot plots showing the proportion of subjects with detectable responses at the different immune compartments according to disease severity. Humoral memory (H) + T-cell memory (T) = detectable (receptor binding domain [RBD]–spike)-specific memory B cell (mBC) and/or anti-spike IgG and spike-specific interferon-γ (IFN-γ)–producing T cells. Humoral memory = detectable (RBD-spike)-specific mBC or anti-spike IgG. T-cell memory = detectable spike-specific IFN-γ-producing T cells. None (N): no detectable humoral or cellular immunity. SEVERE group: 80% (H+T), 17.5% (H), 2.5% (T), 0% (N); MILD group: 78.9% (H+T), 7.9% (H), 7.9% (T), 5.3% (N); asymptomatic (ASYMP) group: 26.3% (H+T), 26.3% (H), 5.3% (T), 42.1% (N); P < 0.001.
Longitudinal analysis of SARS-CoV-2 immune memory in severe convalescent COVID-19 patients
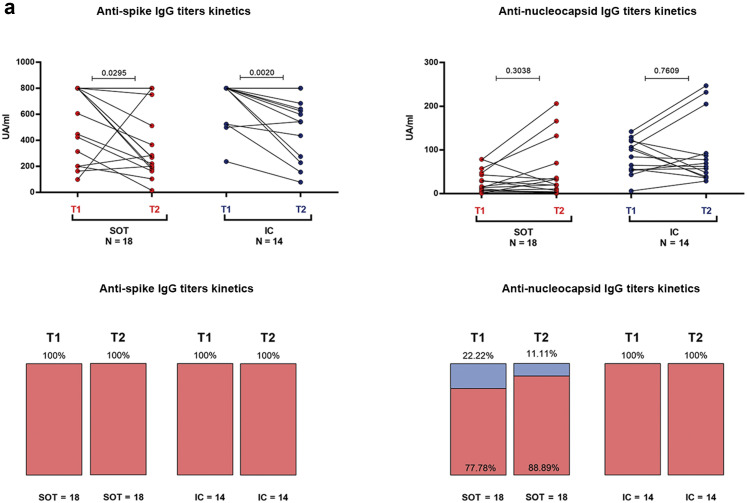
In a subgroup of 35 severe convalescent patients (21 SOT and 14 IC), SARS-CoV-2 immune memory could be compared with a previous initial time point after COVID-19 infection (49 days; IQR, 43–53). As illustrated in Figure 9 a, although no differences were observed regarding percentages of seropositivity against antigens S and N as well as in IgG titers against antigen N between the 2 time points in the 2 groups, anti-S IgG titers significantly dropped in both groups (800 [285–800] vs. 277.5 [186.5–800]; P = 0.029 for SOT; 800 [524–800] vs. 571 [263–713]; P = 0.002 for IC).
Figure 9.
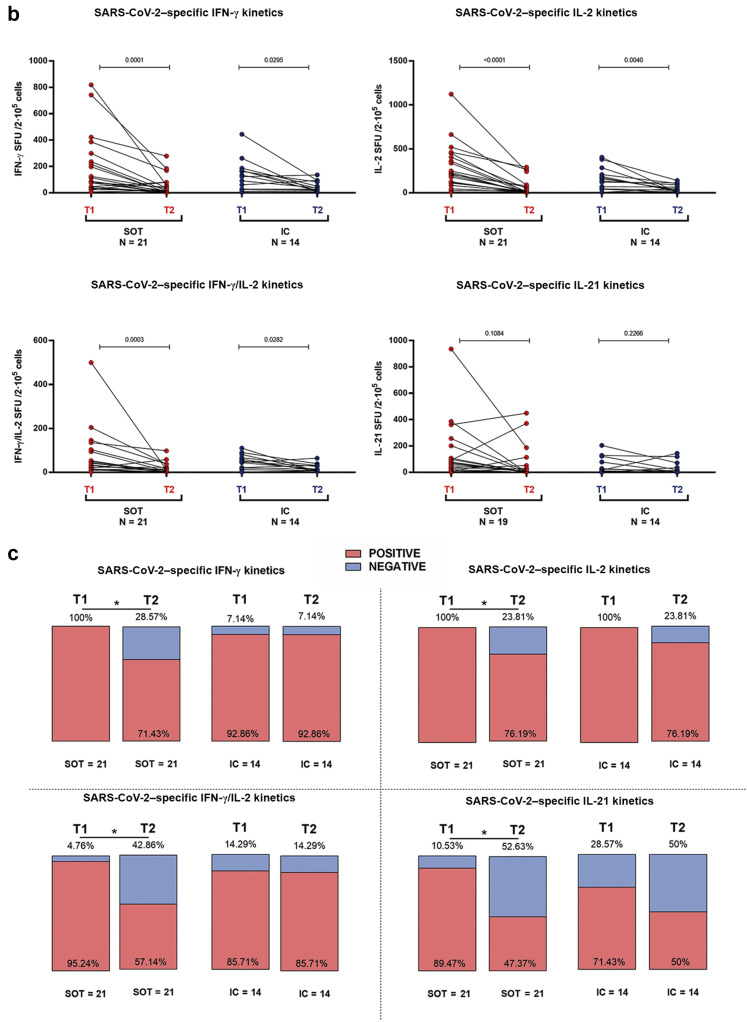
Kinetics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibodies and T-cell responses in severe coronavirus disease2019 individuals between months 1 and 6 after infection. A total of 35 convalescent patients (21 solid organ transplants [SOTs], 14 immunocompetent [IC]) were longitudinally assessed at 2 time points: T1 = 49 (interquartile range [IQR], 44–53) days and T2 = 201 (IQR, 185–208) days after infection. (a) Quantitative and qualitative antibody (spike and nucleoprotein) responses; IgG titters (UA/ml).(b) T-cell frequencies for interferon-γ (IFN-γ), interleukin-2 (IL-2), IFN-γ/IL-2, and IL-21; T-cell frequencies (spot forming unit [SFU]/2 × 105 peripheral blood mononuclear cell [PBMC]). (c) Proportion of patients with detectable T-cell responses for IFN-γ, IL-2, IFN-γ/IL-2, and IL-21. ∗P < 0.05.
Notably, a significant decline was observed in all cytokine-producing SARS-CoV-2–reactive T-cell frequencies but not in IL-21, in both groups (Figure 9b), with a higher proportion of SOT becoming non–T-cell responders than IC (Figure 9c).
Main clinical determinants influencing long-term immune memory
We then investigated whether main demographic characteristics such as age, gender, or time after transplant influenced long-term immune memory at the distinct compartments, adjusting for the type of COVID-19 clinical severity, given its preponderance leading to distinct long-lasting SARS-CoV-2–specific immune responses.
Contrary to infection severity, age and gender did not impact on long-term immunity of IC individuals (data not shown).
Among SOT, however, in addition to COVID-19 disease severity, time (years) since transplantation was also revealed as an independent factor modulating the maintenance of long-term peripheral immune memory (Supplementary Table S7), specifically for anti-N IgG antibodies (OR, 1.2; 95% CI, 1.02–1.40; P = 0.02), IFN-γ, and IFN-γ/IL-2-reactive T cells (OR, 1.4; 95% CI, 1.08–1.83; P = 0.013; and OR, 1.14; 95% CI, 1.01–1.28; P = 0.028, respectively).
Discussion
In this study, we investigated the persistence and magnitude of adaptive immune memory specific to SARS-CoV-2 beyond 6 months after infection in a large cohort of convalescent SOT recipients and IC individuals having experienced 3 distinct clinical presentations, severe, mild, or asymptomatic COVID-19. Herein, we show that SOT patients are capable of maintaining a long-lasting functional immune response specific to SARS-CoV-2 similar to IC individuals both at the serological and T- and B-cell memory immune compartments. Most importantly, we found that the persistence and magnitude of this response is mainly influenced by the degree of COVID-19 clinical severity; thus a high proportion of asymptomatic and some mild convalescent patients did not display any detectable adaptive immune memory in any biological compartment. To note, even though no major differences were generally observed between SOT and IC, SOT individuals displayed weaker humoral responses to SARS-CoV-2 antigen N, a weaker correlation between serologic and cellular responses than convalescent IC patients with the same clinical disease severity, and a more pronounced decline of SARS-CoV-2–reactive cytokine-producing T-cell frequencies over time. Furthermore, our data highlight a more impaired long-term immune preservation among most recently transplanted SOT individuals.
Recent studies have shown that for seasonal coronaviruses, protective immunity seems to be predominantly short-lived.22 However, detectable long-term immune memory against SARS-CoV-2 within 3 main compartments (serological and B- and T-cell memory) has been described in convalescent IC individuals beyond 6 months after COVID-19.8 , 17
In our study, although we confirm that COVID-19 provides detectable peripheral immunity at the 3 main immune compartments (serological and functional B- and T-cell immune responses) beyond 6 months after infection in convalescent IC individuals, we show for the first time that SOT patients are similarly capable of maintaining a long-lasting immune memory response at the serological and functional memory B- and T-cell level. In fact, the robust immune responses detected at the different immune compartments in many SOT with the longest follow-up (up to 355 days after infection) strongly suggest that memory immune responses in this patient population may last even further despite receiving chronic immunosuppression. Rather, the most relevant feature determining the persistence and magnitude of protective immunity was the degree of COVID-19 clinical severity. Notably, a clear gradient of immune responses from the more severe to the mild and asymptomatic groups was clearly delineated in our patients.8, 9, 10, 11, 12 In fact, whereas more than 80% of severe convalescent COVID-19 subjects were seropositive and displayed robust SARS-CoV-2–specific IgG- and cytokine-producing memory B and T cells, respectively, only in 40% of the asymptomatic group were these detectable. A potential explanation for these findings may rely in recent observations showing that severe hospitalized cases, both IC and SOT, display higher viral loads, viremia, and longer viral shedding as compared with milder COVID-19 cases,23, 24, 25, 26 which may lead to higher antigen exposure ultimately triggering stronger and long-lasting immune responses.
In line with previous studies,8 , 27 we also found a high correlation between the different immune compartments specific to SARS-CoV-2. However, these differences were predominantly driven by the IC group, suggesting a more impaired functional immune response of SOT related to chronic immunosuppression. Indeed, the gradient of strength and detection of immune responses at the T-cell level between the distinct clinical COVID-19 presentations within SOT was not pronounced as compared with IC patients, especially between severe and mild patients. Moreover, in a longitudinal analysis of immune response progression within severe convalescent COVID-19 patients, SOT displayed a clearer decline of functional T-cell immune memory than IC patients, again illustrating a certain deleterious effect of chronic immunosuppression on antiviral immunity over time. In addition, despite similar seropositivity rates for both antigens S and N between IC and SOT, the latter displayed significantly lower anti-N IgG titters than IC patients. Unlike in the general population,17 it has recently been described that SOT patients seem to show lower anti-N IgG titers,28 especially those with higher immunosuppressive burden,29 suggesting higher susceptibility of anti-N seroconversion to chronic immunosuppression.
Finally, when we investigated major determinants influencing the presence of SARS-CoV-2–specific immune memory within SOT patients, besides COVID-19 clinical severity, we found that more recently transplanted patients exhibited an independent higher risk of not maintaining detectable serological and T-cell immunity than those with a longer functioning graft. These data highlight the negative effect of the initial immunosuppressive burden challenging adaptive anti-viral immune responses.
Our study has some limitations. First, we have to consider an inherent selection bias in our cohort, because all the included individuals had successfully recovered from SARS-CoV-2 infection, which is not the general COVID-19 outcome among this at-risk patient population. On the other hand, our immune evaluation was restricted to the original SARS-CoV-2 strain, due to the infection time period (March to October 2020), so we are not able to fully ensure whether these data would replicate with the more virulent viral strains. Finally, this study was performed before the successful vaccination campaigns,30 so we cannot completely extrapolate these findings to breakthrough infections in patients after unsuccessful vaccination.
Also, the mild infection group of the study, which was fundamentally based on health care workers, was a bit younger and were analyzed at a later time. However, in general, these differences did not impact on the immune responses compared with the same mild SOT group. Although the number of asymptomatic patients was lower than the other 2 groups, the consistency of the results observed within this group counterbalances this constraint. Finally, we could not describe the predominant T- or B-cell subsets, responsible of these SARS-CoV-2–reactive T and B cells. However, our FluoroSpot assay allowed us to functionally assess the frequencies of different IgG- and cytokine-producing B and T cells specific to SARS-CoV-2 at the single cell level.
In conclusion, our findings show that robust humoral and cellular immune memory persists among IC and SOT convalescent COVID-19 patients for more than 6 months after infection, and these responses are highly dependent on the clinical degree of COVID-19 severity, which might ultimately illustrate a distinct level of viral antigen exposure. However, long-lasting adaptive immunity seems to be challenged to some extent by chronic immunosuppression, especially among those more recently transplanted. Our data may have some relevant implications regarding the long-lasting immune response achieved after vaccination, highlighting the need of an accurate and broader assessment of SARS-CoV-2 immune response to establish guided preventive strategies.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by a national competitive grant by the Instituto de Salud Carlos iii (COV20/00324) and by la Fundació La Marató de TV3 (202101 [64/198]). We acknowledge Biobank HUB-ICO-IDIBELL (PT20/00171) integrated in the Spanish Biobank Network and Xarxa Banc de Tumors de Catalunya (XBTC) for their collaboration. We also acknowledge all the staff of the distinct kidney, heart, liver, and lung transplant units of the different centers for their support and care of the patients, especially in the context of this pandemic.
Footnotes
Supplementary Methods.
Figure S1. Representative images of receptor binding domain (RBD)–specific and polyclonal IgG detection from memory B-cells (mBCs), prior differentiation to antibody secreting cells. No RBD-specific IgG detection was found among healthy donors.
Figure S2. Hierarchical cytokine profile of T-cell responses against main structural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins: spike (S), membrane (M), and nucleoprotein (N).
Figure S3. Representative images of a convalescent solid organ transplant (SOT) from severe coronavirus disease 2019 (COVID-19) and an unexposed individual, including specific interferon-γ (IFN-γ) responses against the spike overlapping peptide pool; pokeweed mitogen (PWM), as internal positive control; isolated medium, as internal negative control; and the readouts after subtraction.
Figure S4. Percentage of solid organ transplant (SOT) and immunocompetent (IC) patients with detectable (spike) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) specific for different cytokine-producing T cells.
Figure S5. Global T-cell responses specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (median [interquartile range] T-cell frequencies against the 3 main SARS-CoV-2 immunogenic antigens: spike [S], membrane [M], and nucleoprotein [N]) for solid organ transplant (SOT) and immunocompetent (IC) patients and for each cytokine assessed (interferon-γ [IFN-γ], interleukin-2 [IL-2], IFN-γ/IL-2, and IL-21).
Figure S6. Proportion of patients with detectable cytokine-producing T-cell responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), according to the immunosuppression status and infection severity.
Table S1. Correlations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific T-cell and B-cell responses.
Table S2. Statistical differences and false discovery rate (fdr) for all the immune responses clustered in Figure 2 heatmap.
Table S3. IgG titters against spike and nucleoprotein (median UA/ml [interquartile range]), according to immunosuppression status and infection severity.
Table S4. Ratio of IgG-producing memory B cells (median [interquartile range]) against receptor binding domain (RBD), according to immunosuppression status and infection severity.
Table S5. Specific T-cell responses (median spots [interquartile range]) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; median of SMN antigens), according to immunosuppression status and infection severity.
Table S6. Hierarchical cytokine profile of T-cell responses against main structural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins: spike (S), membrane (M), and nucleoprotein (N). Frequencies of interferon-γ (IFN-γ)–, interleukin-2 (IL-2)–, IFN-γ/IL-2–, and IL-21–producing T cells were assessed among the 6 groups of study.
Table S7. Univariate and multivariate analyses based on a binary logistic regression model for major determinants influencing persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody and cellular responses among solid organ transplants (SOT) after 6 months (odds ratio [95% confidence interval]).
Supplementary References.
Supplementary Material
References
- 1.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;2019:3–6. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domínguez-Gil B., Coll E., Fernández-Ruiz M., et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant. 2020;20:2593–2598. doi: 10.1111/ajt.15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias M., Pievani D., Randoux C., et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31:2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo J., Dowell A.C., Pearce H., et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanshylla K., Di Cristanziano V., Kleipass F., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29:917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long Q.X., Tang X.J., Shi Q.L., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 11.Sekine T., Perez-Potti A., Rivera-Ballesteros O., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccoli L., Park Y.J., Tortorici M.A., et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042.e21. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren L., Zhang L., Chang D., et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol. 2020;3:780. doi: 10.1038/s42003-020-01526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas C., Klein J., Sundaram M.E., et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med. 2021;27:1178–1186. doi: 10.1038/s41591-021-01355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favà A., Donadeu L., Sabé N., et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21:2749–2761. doi: 10.1111/ajt.16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thieme C.J., Anft M., Paniskaki K., et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression. Transplantation. 2021;105:2156–2164. doi: 10.1097/TP.0000000000003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cucchiari D., Egri N., Bodro M., et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 23.Fajnzylber J., Regan J., Coxen K., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Yan L., Wan L., et al. Viral dynamics in mild and severe cases of COVID-19: the first COVID-19 case in Afghanistan acquired from Iran. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan A.T., Linster M., Tan C.W., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. CellReports. 2021;34:108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benotmane I., Wendling G.G.M., Perrin P., et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3162–3172. doi: 10.1111/ajt.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang X., Wang G., Zhao X., et al. Lasting antibody and T cell responses to SARS-CoV-2 in COVID-19 patients three months after infection. Nat Commun. 2021;12:897. doi: 10.1038/s41467-021-21155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C.C., Vlad G., Vasilescu E.R., et al. Disparity between levels of anti-RBD IgG and anti-nucleocapsid protein IgG antibodies in COVID-19–recovered patients who received a kidney transplant. Kidney Int. 2021;100:240–241. doi: 10.1016/j.kint.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burack D., Pereira M.R., Tsapepas D.S., et al. Prevalence and predictors of SARS-2 antibodies among solid organ transplant recipients with confirmed infection. Am J Transplant. 2021;21:2254–2261. doi: 10.1111/ajt.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bestard O., Jouve T., Castells L., et al. Reconciling short-term clinical and immunological outcomes of SARS-CoV-2 vaccination in solid organ transplant recipients. Am J Transplant. 2022;22:673–675. doi: 10.1111/ajt.16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.